Abstract
Background
The introduction of targeted treatments for subsets of non-small cell lung cancer (NSCLC) has highlighted the importance of accurate molecular diagnosis to determine if an actionable genetic alteration is present. Few data are available for Central and Eastern Europe (CEE) on mutation rates, testing rates, and compliance with testing guidelines.
Methods
A questionnaire about molecular testing and NSCLC management was distributed to relevant specialists in nine CEE countries, and pathologists were asked to provide the results of EGFR and ALK testing over a 1-year period.
Results
A very high proportion of lung cancer cases are confirmed histologically/cytologically (75–100%), and molecular testing of NSCLC samples has been established in all evaluated CEE countries in 2014. Most countries follow national or international guidelines on which patients to test for EGFR mutations and ALK rearrangements. In most centers at that time, testing was undertaken on request of the clinician rather than on the preferred reflex basis. Immunohistochemistry, followed by fluorescent in situ hybridization confirmation of positive cases, has been widely adopted for ALK testing in the region. Limited reimbursement is a significant barrier to molecular testing in the region and a disincentive to reflex testing. Multidisciplinary tumor boards are established in most of the countries and centers, with 75–100% of cases being discussed at a multidisciplinary tumor board at specialized centers.
Conclusions
Molecular testing is established throughout the CEE region, but improved and unbiased reimbursement remains a major challenge for the future. Increasing the number of patients reviewed by multidisciplinary boards outside of major centers and access to targeted therapy based on the result of molecular testing are other major challenges.
Electronic supplementary material
The online version of this article (10.1186/s12885-018-4023-4) contains supplementary material, which is available to authorized users.
Keywords: Non-small cell lung cancer, EGFR mutations, ALK rearrangements, Molecular testing, Central eastern European region
Background
Globally, for several decades, lung cancer has been the most common cancer and the leading cause of cancer deaths. The situation is particularly serious in Central and Eastern European (CEE) countries, which have the highest age-standardized incidence rates in men around the world [1]. Incidence rates in women are generally lower than in men, but are increasing in many countries worldwide. There are some geographical differences in incidence, reflecting in part the different historical exposure to tobacco smoking [1]. The diagnosis is often not made until late in the course of the disease and, as a result, only a minority of patients are cured and the ratio of mortality-to-incidence is very high. Almost 70% of patients have locally advanced or metastatic disease at initial diagnosis [2].
Nowadays, only about 15% of lung cancer cases are small cell lung cancer, with the majority of lung cancer cases classified as non-small cell lung cancer (NSCLC). When the diagnosis is made based on a small biopsy or cytology sample, besides the three common types of NSCLC (squamous cell carcinoma, adenocarcinoma, and non-small cell carcinoma not otherwise specified [NOS]) several additional subtypes can be defined by morphology, immunohistochemistry (IHC), and molecular pathology [2, 3]. Although most lung cancers are attributable to tobacco smoking, approximately 10–15% of cases in Western countries occur in lifelong never-smokers and these are almost exclusively adenocarcinomas [4].
The study of molecular biology of NSCLC has had a major impact on diagnosis and treatment of this disease [5–7]. The work of the Lung Cancer Mutation Consortium and other groups has shown that driver mutations or other oncogene alterations are present in more than half of all adenocarcinomas [8]. The discovery of targetable genetic alterations, such as activating mutations of the epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) rearrangements, has led to the implementation of precision therapy for certain subtypes of lung cancer based on appropriate patient selection [9]. Clinical trials have shown significantly longer progression-free survival in patients with EGFR mutations who are treated with EGFR tyrosine kinase inhibitors (TKIs) compared with chemotherapy [10]. Similarly, ALK TKI treatment of patients with ALK-rearranged tumors prolongs progression-free survival compared with first-line chemotherapy [11]. Patients with a druggable molecular alteration who are treated with a corresponding targeted treatment benefit from significantly higher response rates and longer progression-free survival, although an improvement in overall survival with targeted agents has not been shown by the majority of randomized clinical trials [8]. There are probably multiple reasons for the lack of overall survival advantage seen in clinical trials, one of the most important reasons is a high number of patients crossing over to targeted agents after failure of treatment in the chemotherapy arms.
This move towards biomarker-based treatment approaches has highlighted the importance of accurate molecular diagnosis. In addition to classical morphologic classification, molecular analysis of tumor samples is essential to determine if a druggable oncogenic alteration is present. Consequently, the pathologist is now a key member of the multidisciplinary lung cancer team [12].
Identification of the challenges for personalized lung cancer treatment within the CEE region might facilitate molecular diagnostics and improve patient care. Information about molecular testing practices for NSCLC in the CEE region is relatively limited. The INSIGHT study has provided some information on EGFR mutation rates, testing, and compliance with testing guidelines in several Central European countries [13]. However, only very few data from this region are available on ALK testing. Our study was designed to collect information on both EGFR and ALK testing from a large number of CEE countries.
Methods
A Working Group of oncologists, pulmonologists, and pathologists from the CEE region was established to obtain more information on NSCLC molecular testing in their countries and to raise awareness of the current issues around personalized medicine for lung cancer.
As a first step, a questionnaire (Additional file 1) with 37 questions addressing issues of molecular testing and NSCLC management was distributed in the second quarter of 2014 to 59 specialists (epidemiologists, oncologists, pulmonologists, and pathologists) from nine CEE countries. In June 2015, pathologists were also asked to provide details of the results of EGFR and ALK testing over a 1-year period.
Results
Respondents
There were a total of the 42 responses from nine countries; the number of responders by country are shown in Table 1 (data were not available for some questions; see Additional file 1 for the questionnaire).
Table 1.
Countries participating and number of responders
| Bulgaria | 2 |
| Croatia | 4 |
| Czech Republic | 4 |
| Hungary | 8 |
| Israel | 3 |
| Poland | 7 |
| Slovakia | 7 |
| Slovenia | 3 |
| Turkey | 4 |
Lung cancer types
Table 2 shows the proportion of lung cancer cases that are confirmed morphologically in each country.
Table 2.
Lung cancer cases morphologically confirmed
| Country (number of responses) | Proportion of cases, % |
|---|---|
| Bulgaria | 75* |
| Croatia | 100* |
| Czech Republic | 85† |
| Israel | 100* |
| Slovakia | 83* |
| Slovenia | 92* |
*Registry data; †Best estimate
Figure 1 shows the breakdown of lung cancer types for selected countries. Adenocarcinoma is the most common type in all countries except Bulgaria, whereas small cell lung cancer represented between 10% and 20% of cases in all countries.
Fig. 1.
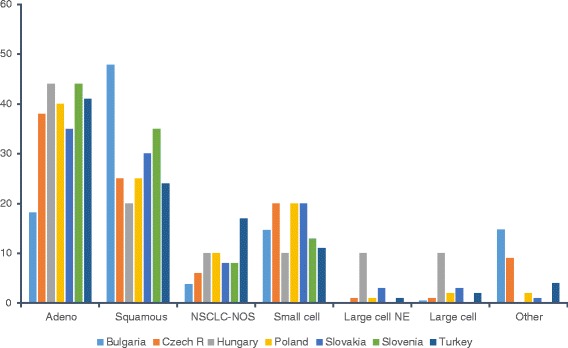
Lung cancer subtypes by country. NE, neuroendocrine carcinoma; NOS, not otherwise specified; NSCLC, non-small cell lung cancer
EGFR testing
Most countries have national guidelines or follow European guidelines on EGFR mutation testing [2]. European guidelines are followed in Bulgaria, Croatia, Israel, Slovakia, Slovenia, and Turkey. Bulgaria, Czech Republic, Hungary, Israel, Poland, and Slovakia also have national guidelines, whereas some centers in Poland follow local guidelines. Some Turkish centers follow American College of Pathologists guidelines [12].
Most centers reported that adenocarcinomas and NSCLC-NOS were tested for activating EGFR mutations. In some countries (Bulgaria, Czech Republic, Israel, Slovakia, Slovenia, and Turkey), large cell and squamous cell tumors may be tested in selected cases (mostly on request from the treating physician). In most countries, only advanced tumors (stage IIIb and IV) were generally tested for EGFR mutations. In the Czech Republic, Slovakia, and Slovenia, as well as in certain individual centers in other countries, other stages were also tested for research purposes.
In most centers, EGFR mutation testing was undertaken when requested by the clinician, usually the oncologist treating the patient. In the Czech Republic, Slovakia, and Slovenia, and some centers in Croatia, testing was reflex (i.e. the pathologist automatically tested all tumors that met the criteria). In Hungary, it is policy to test for KRAS mutations first, and the presence of a KRAS mutation is an exclusion criterion for EGFR/ALK testing. In Turkey, EGFR/ALK testing is not performed in KRAS mutation-positive tumors, although KRAS testing is not routine.
In many countries (Croatia, Czech Republic, Hungary, Israel, Slovakia, and Slovenia), at least 65% of eligible tumors were actually tested for EGFR mutations in 2014. However, a significant proportion of samples (5–25%) were inadequate for testing, usually because the sample was too small or did not contain enough tumor cells.
Usually, more than one method was used in each country for EGFR mutation testing. Real-time polymerase chain reaction (PCR) was used in all countries, direct sequencing in five countries, and other methods were used in addition in only two countries.
The incidence of specific EGFR mutations in selected centers in 2014 is shown in Table 3; the frequency of EGFR-mutated tumors ranged from 6.7 to 15.2%. These numbers cannot be compared because of the different inclusion criteria for testing.
Table 3.
Results of EGFR testing in selected centers in 2014a
| EGFR, n (%) | Non-diagnostic | |||||
|---|---|---|---|---|---|---|
| WT | Mut exon 18 | Mut exon 19 | Mut exon 20 | Mut exon 21 | ||
| Croatia (Zagreb) | 561 (85.9) | 8 (1.2) | 46 (7) | 8 (1.2) | 30 (4.6) | 11 |
| Czech (Prague) | 154 (90.6) | 4 (2.4) | 6 (3.5) | 2 (1.2) | 4 (2.2) | 15 |
| Czech (Hradec Kralove) | 234 (90.0) | 0 | 15 (5.8) | 2 (0.8) | 9 (3.5) | 6 |
| Hungary (Budapest, Timár) | 350 (85.6) | 4 (1.0) | 22 (5.4) | 14 (3.9) | 19 (4.6) | 57 |
| Hungary (Budapest, Toth) | 251 (93.3) | 0 | 9 (3.3) | 1 (0.4) | 8 (3.1) | 2 |
| Hungary (Budapest, Kovalszky) | 500 (88.5) | 6 (1.1) | 27 (4.8) | 0 | 32 (5.7) | – |
| Hungary (Debrecen) | 760 (89.6) | 0† | 61 (7.2) | 0† | 27 (3.2) | 13 |
| Hungary (Pécs) | 112 (86.8) | 0 | 10 (7.8) | 0 | 7 (5.4) | – |
| Hungary (Szeged) | 617 (92.6) | 4 (0.6) | 27 (4.1) | 2 (0.3) | 16 (2.4) | 71 |
| Slovakia | 361 (87.6) | 1 (0.2) | 30 (7) | 2 (0.5) | 16 (3.8) | – |
| Slovenia | 464 (86) | 5 (0.9) | 39 (7.2) | 8 (1.5) | 25 (4.6) | 57 |
| Turkey (Cerrahpaşa) | 714 (87.9) | 17 (2.1) | 52 (6.4) | 3 (0.4) | 26 (3.2) | 38 |
EGFR epidermal growth factor receptor, WT wild-type
aNote that these numbers cannot be compared directly because of the different criteria for selection of samples to test
†Exons 18 and 20 not tested
ALK testing
ALK testing is available in all countries except Bulgaria. Most countries have national guidelines or follow European guidelines on which subtypes to test for ALK rearrangements. European guidelines [2] are followed in Croatia, Slovenia, and Turkey, whereas Czech Republic, Hungary, Israel, Poland, and Slovakia follow national guidelines. In all centers, adenocarcinomas and NSCLC-NOS were tested, usually on request from the treating clinician. As for EGFR testing, reflex ALK testing for all eligible patients was implemented in the Czech Republic, Slovakia, and Slovenia.
In most countries, as for EGFR testing, only advanced tumors (stage IIIb and IV) were tested for ALK rearrangements. At some centers in Croatia, Czech Republic, Slovakia, and Slovenia, other stages were also tested for research purposes. The presence of a known EGFR mutation was an exclusion criterion for ALK testing in all countries. In addition, in countries where some or all samples are tested for KRAS mutations (Turkey, Slovenia, Hungary, and Poland), the presence of a KRAS mutation is also an exclusion criterion. For ALK testing, IHC followed by fluorescent in situ hybridization (FISH) and/or FISH alone were used in all countries. In Israel, other methods, including next-generation DNA sequencing, were also used (Table 4).
Table 4.
ALK testing methods
| Croatia (Zagreb) | IHC (IHC followed by FISH) |
|---|---|
| Czech (Hradec Kralove) | IHC, IHC followed by FISH |
| Czech (Prague) | IHC, IHC followed by FISH, FISH |
| Hungary (Budapest, Timár) | FISH |
| Hungary (Budapest, Toth) | FISH |
| Hungary (Budapest, Kovalszky) | FISH, other |
| Hungary (Debrecen) | FISH |
| Hungary (Pécs) | IHC followed by FISH |
| Hungary (Szeged) | FISH |
| Israel | IHC, IHC followed by FISH, FISH, sequencing |
| Poland (Warsaw) | FISH |
| Slovakia | IHC followed by FISH; FISH |
| Slovenia | IHC followed by FISH |
| Turkey (Cerrahpaşa) | IHC followed by FISH |
FISH fluorescent in situ hybridization, IHC immunohistochemistry
The incidence of ALK rearrangements determined in selected centers in 2014 is shown in Table 5. The substantial differences in the frequency, which ranged from 1.6% to 12%, are most probably due to different testing approaches, excluding KRAS- and EGFR-positive cases from ALK testing or not.
Table 5.
Results of ALK testing in selected centers in 2014a
| ALK, n (%) | Non-diagnostic | ||
|---|---|---|---|
| WT | Rearrangement | ||
| Croatia | 161 (93.1) | 12 (6.9) | 28 |
| Czech (Prague) | 55 (96.5) | 2 (3.5) | 10 |
| Czech (Hradec Kralove) | 260 (95.2) | 13 (4.8) | 2 |
| Hungary (Budapest, Timár) | 332 (94.9) | 18 (5.1) | 0 |
| Hungary (Budapest, Toth) | 122 (98.4) | 2 (1.6) | 4 |
| Hungary (Budapest, Kovalszky) | 415 (95.6) | 19 (4.4) | 0 |
| Hungary (Debrecen) | 226 (91.5) | 21 (8.5) | 35 |
| Hungary (Pécs) | 55 (94.8) | 3 (5.2) | 2 |
| Slovakia | 375 (88) | 51 (12) | 0 |
| Slovenia | 199 (96.5) | 7 (3.5) | 0 |
| Turkey (Cerrahpaşa) | 764 (95.6) | 35 (4.4) | 14 |
ALK anaplastic lymphoma kinase, WT wild-type
aNote that these numbers cannot be compared directly because of the different criteria for selection of samples to test; in some centers only samples negative for KRAS and EGFR mutations were tested for ALK translocations
Testing for other mutations
Tumor samples were tested routinely for KRAS mutations in Hungary and Slovenia; some samples were tested in Turkey and Poland. ROS1 testing was routine in Slovenia and was undertaken on request in Slovakia.
Reimbursement for molecular testing
There are several different sources of funding for EGFR and ALK testing in the region. In the Czech Republic, Hungary, Israel, Poland, Slovakia, Slovenia, and Turkey, testing is partly or fully reimbursed by the national health authority/national health insurance. Private insurance covers some testing in Israel and Turkey.
The pharmaceutical industry supported some testing in Hungary, Poland, and Slovenia, and was the only source of financial support for testing in Bulgaria (EGFR only) and Croatia (EGFR and ALK). The industry did not finance testing in Czech Republic, Israel, Slovakia, or Turkey. However, in the personal experience of the authors, although the molecular testing is stated to be fully reimbursed, this is not the case in practice. Often, there are various forms of budget capping with a limitation on the number of tests performed (e.g. based on the number of samples tested in the previous period). This policy is a disincentive to reflex testing. In Hungary, for example, only 30% of tests were reimbursed.
Multidisciplinary approach
Multidisciplinary lung cancer teams/tumor boards are established in all countries; however, these are often only functioning fully as part of routine clinical practice at specialized lung cancer treatment centers. In Hungary, Poland, and Slovenia, it is mandatory for all cases to be discussed by a multidisciplinary tumor board. In Turkey, it is obligatory for selected cases. When multidisciplinary teams/tumor boards are operational, a pathologist is usually a member.
The proportion of NSCLC cases actually discussed at a multidisciplinary tumor board is 75–100% at most specialist centers; however, there is wide variation and can be as low as 20% in some hospitals. There was a trend towards a higher proportion of cases being discussed at multidisciplinary tumor boards at respondents’ own centers compared with their estimates for the country as a whole. Data on how many patients with druggable EGFR or ALK alterations get access to targeted drugs were not collected, however according to the personal experience of the authors, access to EGFR and/or ALK TKIs is often limited, mainly due to local reimbursement policy restrictions.
Discussion
The survey confirmed that a very high proportion of lung cancer cases are verified by histology or cytology (75–100%) in CEE countries and that, in most countries, the data can be derived from the established cancer registries. Adenocarcinoma is the most common type in all countries except Bulgaria, whereas 10–20% of cases were small cell lung cancer in all countries, which is consistent with global data [14]. The high incidence of squamous cell carcinoma in Bulgaria may reflect the high levels of smoking, relatively late introduction of filtered cigarettes, and pollution levels [15].
It is encouraging that molecular testing of NSCLC samples has been established in all CEE countries evaluated in 2014, and that most countries follow national or international guidelines on which patients to test for EGFR mutations and ALK rearrangements. However, in most centers, EGFR and ALK testing was undertaken on request of the clinician, rather than automatically for eligible samples (reflex testing), an approach which can lead to delays in availability of test results and in the initiation of targeted treatment. There is an increasing focus on shortening the turnaround time for test results, and incorporation of reflex testing at the level of the pathologist can help to avoid such delays [16].
The results showed that a significant proportion of samples were unsuitable for testing for various reasons. Various initiatives, including better communications as well as educational initiatives directed at physicians who collect tissue samples, may improve the situation and help to ensure that samples are of sufficient size and quality for molecular testing [16].
The results of molecular testing presented here (Tables 3 and 5) provide interesting insights into the frequency of EGFR mutations and ALK rearrangements in the region. Yet only very limited general conclusions can be drawn on the overall frequency of EGFR mutations and ALK translocations because of the differences between centers in testing policy, selection of samples for testing based on clinical factors (i.e. not all samples were tested) and sequential testing at some centers resulting in ALK testing being performed only on EGFR- and KRAS-negative samples. Consistent with published data, most EGFR mutations reported were in exons 19 and 21 [17, 18]. Information was not requested on KRAS mutation rate, which would have been particular relevant for Hungary where testing was routine. However, a recent paper reported that the incidence of KRAS mutations was 28.6% in 532 consecutive Caucasian patients tested at Semmelweis University [19].
The results show that IHC, followed by FISH confirmation in positive cases, had been widely adopted for ALK testing in the region in 2014. FISH testing was still regarded as the gold standard for ALK testing; however, this method is relatively costly, time-consuming, and technically difficult to perform for routine use, which has led to extensive evaluation of IHC as an alternative used for screening purposes [20–22]. Data published after our survey showed that both D5F3 and 5A4 antibodies are able to detect ALK rearrangements reliably and are equally well suited for routine diagnostic use [23]. Several studies have shown good concordance between the results of IHC and FISH for ALK testing [24–27]. Indeed, some investigators have shown that IHC can be useful in cases with atypical or borderline FISH results [26, 28].
Acceptance by the United States Food and Drug Administration (FDA) of the Ventana ALK D5F3 IHC as a companion test to identify patients for crizotinib treatment provides additional support for the routine use of IHC. The test provides a fast and accurate method to identify ALK protein expression, with a binary scoring system, and has been validated clinically by retrospective testing of tissue samples from patients screened for inclusion in crizotinib clinical trials [29]. These promising data encourage some centers to use IHC as a primary method replacing FISH. Immunohistochemistry can readily be applied to tissue samples, cell blocks prepared from effusions and Papanicolaou-stained cytologic slides [22, 27].
The European consensus recommended that all non-squamous NSCLC tumors in patients with advanced/recurrent disease be tested for EGFR mutations and ALK translocations. Selected squamous tumors (from patients with minimal or remote smoking history) should be strongly considered for testing. Sequential testing is not recommended and parallel testing of multiple mutations on the same sample is becoming the standard [2]. More recent European recommendations are consistent with these statements. Sanger sequencing, pyrosequencing, and next-generation sequencing are recommended for EGFR testing and validated tests including FISH and IHC may be used for ALK testing [30]. Real-time PCR is also widely used. National guidelines, where they exist, are broadly consistent with these recommendations; in Hungary, KRAS testing is performed before testing for other genetic alterations.
With the increasing number of molecular markers that need to be examined for optimal selection of targeted treatment, the cost of up-to-date molecular testing is rapidly growing. The financial burden of testing the entire cohort of eligible patients can, in fact, reach the cost of the treatment of several individual patients identified by the testing, namely in tumors driven by rare mutations [31]. Thus, routine use of modern approaches, such as next-generation sequencing resulting in dramatic reduction of testing costs, is eagerly awaited.
It should be noted that the data discussed here reflect the status quo in 2014, and molecular testing is evolving fast with changes in testing methods being implemented. The growing use of IHC for ALK testing has already been discussed. In addition, many laboratories in Europe are now adopting next-generation sequencing that can be applied to formalin-fixed paraffin-embedded tissue in routine diagnostic practice. This allows for the detection of many genetic alterations and oncogene targets in parallel, providing the opportunity for fast and deep characterization of tumors as well as for the potential for other targeted therapies [32].
Adherence to the best practices in molecular testing is crucial to ensure accurate diagnoses and appropriate clinical decisions [30, 33]. Quality control is essential to ensure consistent and reliable molecular diagnostic results and to facilitate comparison of results from different laboratories. External quality assessment (EQA) programs have been established for both EGFR and ALK testing [33, 34]. The vast majority of the laboratories contributing to this study already participate in EQA programs. Many laboratories in the region participate in the European Society of Pathology Lung External Quality Assessment Scheme [19]. As the results of molecular testing directly influence the management of individual patients, EQA is essential to guarantee optimal quality of testing. Therefore, each laboratory should prove successful participation in the appropriate EQA program to be included to the network of testing centers [35].
Multidisciplinary tumor boards play a key role in optimizing the diagnosis and treatment of lung cancer [30]. With rapid progress in the molecular profiling of NSCLC and its increasing complexity, it is essential that the tumor boards include specially trained molecular pathologists, as well as molecular biologists, among the tumor board members when discussing the best possible treatment for each individual patient with NSCLC. Although it is positive that all countries studied have implemented lung cancer tumor boards, these are more likely to be operational only in specialized lung cancer treatment centers.
Conclusions
Non-small cell lung cancer molecular testing is established in all CEE countries participating in this study. The responses show that all countries follow guidelines regarding EGFR and ALK testing, with most countries testing only advanced stages of adenocarcinomas, NSCLC-NOS, and NSCLC when an adenocarcinoma component cannot be excluded. Most countries are still undertaking testing on request and not implementing the preferred reflex policy.
Limited reimbursement is a significant barrier to molecular testing in the region and a disincentive to reflex testing. The authors recommend that testing should be independently funded.
The results show that ensuring adequate NSCLC samples and enabling wide access of eligible patients to molecular testing are key issues for the future. Increasing the number of patients reviewed by multidisciplinary boards and the access of patients with druggable molecular alterations to targeted drugs are other major challenges.
Additional file
Questionnaire addressing issues of molecular testing and NSCLC management. Questionnaire was provided to 59 specialists (epidemiologists, oncologists, pulmonologists, and pathologists) from nine CEE countries requesting information. (PDF 191 kb)
Acknowledgments
This work was presented in part at the 16th World Conference on Lung Cancer, 2015. Abstract P3.04-045.
Funding
This work was supported by an unrestricted educational grant from Pfizer; the unrestricted grant supported the work of the medical writer. The funding body had no role in the design of the study or the collection, analysis, and interpretation of data or the writing of the manuscript.
All authors report receiving financial support from Pfizer for advisory roles and nonfinancial support from SuccinctChoice Medical Communications related to the work under consideration.
Medical writing support, provided by Christine Drewienkiewicz of SuccinctChoice Medical Communications, was funded by Pfizer.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Abbreviations
- ALK
Anaplastic lymphoma kinase
- CEE
Central and Eastern European
- EGFR
Epidermal growth factor receptor
- EQA
External quality assessment
- FDA
Food and Drug Administration
- FISH
Fluorescent in situ hybridization
- IHC
Immunohistochemistry
- NOS
Not otherwise specified
- NSCLC
Non-small cell lung cancer
- PCR
Polymerase chain reaction
- TKI
Tyrosine kinase inhibitor
Authors’ contributions
AR, PB, TC, RD, MG, WO, BO, LP and JT designed the study and the questionnaire. AR, PB, LB, TC, RD, MG, IK, WO, BO, LP and JT contributed to data collection for their countries and participated in review and revision of the manuscript. All aforementioned authors read and approve the final manuscript.
Ethics approval and consent to participate
Ethics Committee approval was not needed as the data are purely descriptive and epidemiological. Tables 3 and 5 include retrospective data on the results of prior routine diagnostic molecular testing; no patient level data are included and no additional procedures, tests or samples were required. All respondents were clearly informed that the information collected was to form the basis for a manuscript describing molecular testing for NSCLC in the CEE region.
Consent for publication
Not applicable.
Competing interests
AR reports receiving grants and personal fees from AstraZeneca and MSD, honoraria for advisory board participation AstraZeneca, MSD, Novartis, Boehringer Ingelheim and personal fees and honoraria for advisory board participation and lectures from BMS, outside the submitted work. PB reports receiving honoraria from AstraZeneca, Boehringer Ingelheim, Pfizer, MSD, and Roche, and has participated at advisory boards for Boehringer Ingelheim, Pfizer, and MSD. LB reports receiving personal fees from Roche, AstraZeneca, Pfizer, Boehringer Ingelheim, outside the submitted work. TC reports receiving consulting fees from Pfizer, Boehringer Ingelheim, BMS outside the submitted work. RD reports personal fees from Pfizer, Roche, AstraZeneca, Boehringer Ingelheim, Novartis, outside the submitted work. MG reports receiving consulting fees from Pfizer, Boehringer Ingelheim, BMS, MSD. IK reports receiving personal fees from Pfizer (participation in 2015 advisory board preparing the manuscript) related to the work under consideration. WO reports receiving honoraria or consulting fees from MSD, Boehringer Ingelheim, Pfizer and Abbott outside the submitted work. OB reports receiving consulting fees from Pfizer, Boehringer Ingelheim, BMS and MSD outside the submitted work. LP reports receiving grants from Slovak Research and Development Agency, Pfizer Slovakia, Ministry of Health Slovakia related to the work under consideration and grants from Novartis Slovakia, Takeda Slovakia, and AstraZeneca Slovakia outside the submitted work. JT reports receiving personal fees from BMS, Lilly, Pfizer, Roche, and Boehringer Ingelheim outside the submitted work. All authors report receiving financial support from Pfizer and non-financial support from SuccinctChoice Medical Communications (funded by Pfizer) related to the work under consideration.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12885-018-4023-4) contains supplementary material, which is available to authorized users.
Contributor Information
Ales Ryska, Email: ryskaale@gmail.com.
Peter Berzinec, Email: peterberzinec@yahoo.com.
Luka Brcic, Email: luka.brcic@medunigraz.at.
Tanja Cufer, Email: Tanja.Cufer@klinika-golnik.si.
Rafal Dziadziuszko, Email: rafald@gumed.edu.pl.
Maya Gottfried, Email: Maya.Gottfried@clalit.org.il.
Ilona Kovalszky, Email: koval@korb1.sote.hu.
Włodzimierz Olszewski, Email: wtolszewski@coi.waw.pl.
Buge Oz, Email: ozbuge@gmail.com.
Lukas Plank, Email: Lukas.Plank@jfmed.uniba.sk.
Jozsef Timar, Email: jtimar@gmail.com.
References
- 1.GLOBOCAN . 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase no. 11. Lyon: International Agency for Research on Cancer; 2013. [Google Scholar]
- 2.Kerr KM, Bubendorf L, Edelman MJ, Marchetti A, Mok T, Novello S, O'Byrne K, Stahel R, Peters S, Felip E. Second ESMO consensus conference on lung cancer: pathology and molecular biomarkers for non-small-cell lung cancer. Ann Oncol. 2014;25(9):1681–1690. doi: 10.1093/annonc/mdu145. [DOI] [PubMed] [Google Scholar]
- 3.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung Adenocarcinoma. J Thorac Oncol. 2011;6(2n):244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couraud S, Zalcman G, Milleron B, Morin F, Souquet PJ. Lung cancer in never smokers – a review. Eur J Cancer. 2012;48(9):1299–1311. doi: 10.1016/j.ejca.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Black RC, Khurshid H. NSCLC: an update of driver mutations, their role in pathogenesis and clinical significance. R I Med J (2013) 2015;98(10):25–28. [PubMed] [Google Scholar]
- 6.Dacic S. Molecular genetic testing for lung adenocarcinomas: a practical approach to clinically relevant mutations and translocations. J Clin Pathol. 2013;66(10):870–874. doi: 10.1136/jclinpath-2012-201336. [DOI] [PubMed] [Google Scholar]
- 7.Popper HH, Ryska A, Timar J, Olszewski W. Molecular testing in lung cancer in the era of precision medicine. Transl Lung Cancer Res. 2014;3(5):291–300. doi: 10.3978/j.issn.2218-6751.2014.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, Varella-Garcia M, Franklin WA, Aronson SL, Su PF, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Besse B, Adjei A, Baas P, Meldgaard P, Nicolson M, Paz-Ares L, Reck M, Smit EF, Syrigos K, Stahel R, et al. 2nd ESMO consensus conference on lung cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease. Ann Oncol. 2014;25(8):1475–1484. doi: 10.1093/annonc/mdu123. [DOI] [PubMed] [Google Scholar]
- 10.Zhang WQ, Li T, Li H. Efficacy of EGFR tyrosine kinase inhibitors in non-small-cell lung cancer patients with/without EGFR-mutation: evidence based on recent phase III randomized trials. Med Sci Monit. 2014;20:2666–2676. doi: 10.12659/MSM.892476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 12.Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, Jenkins RB, Kwiatkowski DJ, Saldivar JS, Squire J, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Mol Diagn. 2013;15(4):415–453. doi: 10.1016/j.jmoldx.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Ramlau R, Cufer T, Berzinec P, Dziadziuszko R, Olszewski W, Popper H, Bajcic P, Dusek L, Zbozinkova Z, Pirker R. Epidermal growth factor receptor mutation-positive non-small-cell lung cancer in the real-world setting in Central Europe: the INSIGHT study. J Thorac Oncol. 2015;10(9):1370–1374. doi: 10.1097/JTO.0000000000000621. [DOI] [PubMed] [Google Scholar]
- 14.Wahbah M, Boroumand N, Castro C, El-Zeky F, Eltorky M. Changing trends in the distribution of the histologic types of lung cancer: a review of 4,439 cases. Ann Diagn Pathol. 2007;11(2):89–96. doi: 10.1016/j.anndiagpath.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 15.van Dorn A. Bulgaria lags behind Europe in pollution and smoking targets. Lancet Respir Med. 2014;2(3):182–183. doi: 10.1016/S2213-2600(14)70056-6. [DOI] [PubMed] [Google Scholar]
- 16.Lim C, Tsao MS, Le LW, Shepherd FA, Feld R, Burkes RL, Liu G, Kamel-Reid S, Hwang D, Tanguay J, et al. Biomarker testing and time to treatment decision in patients with advanced nonsmall-cell lung cancer. Ann Oncol. 2015;26(7):1415–1421. doi: 10.1093/annonc/mdv208. [DOI] [PubMed] [Google Scholar]
- 17.Li AR, Chitale D, Riely GJ, Pao W, Miller VA, Zakowski MF, Rusch V, Kris MG, Ladanyi M. EGFR mutations in lung adenocarcinomas: clinical testing experience and relationship to EGFR gene copy number and immunohistochemical expression. J Mol Diagn. 2008;10(3):242–248. doi: 10.2353/jmoldx.2008.070178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shikhrakab H, Elamin YY, O'Brien C, Gately K, Finn S, O'Byrne K, Osman N. Epidermal growth factor receptor (EGFR) mutation testing, from bench to practice: a single institute experience. Ir Med J. 2014;107(7):201–204. [PubMed] [Google Scholar]
- 19.Lohinai Z, Klikovits T, Moldvay J, Ostoros G, Raso E, Timar J, Fabian K, Kovalszky I, Kenessey I, Aigner C, et al. KRAS-mutation incidence and prognostic value are metastatic site-specific in lung adenocarcinoma: poor prognosis in patients with KRAS mutation and bone metastasis. Sci Rep. 2017;7:39721. doi: 10.1038/srep39721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pekar-Zlotin M, Hirsch FR, Soussan-Gutman L, Ilouze M, Dvir A, Boyle T, Wynes M, Miller VA, Lipson D, Palmer GA, et al. Fluorescence in situ hybridization, immunohistochemistry, and next-generation sequencing for detection of EML4-ALK rearrangement in lung cancer. Oncologist. 2015;20(3):316–322. doi: 10.1634/theoncologist.2014-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zwaenepoel K, Van Dongen A, Lambin S, Weyn C, Pauwels P. Detection of ALK expression in non-small-cell lung cancer with ALK gene rearrangements – comparison of multiple immunohistochemical methods. Histopathology. 2014;65(4):539–548. doi: 10.1111/his.12414. [DOI] [PubMed] [Google Scholar]
- 22.Savic S, Bode B, Diebold J, Tosoni I, Barascud A, Baschiera B, Grilli B, Herzog M, Obermann E, Bubendorf L. Detection of ALK-positive non-small-cell lung cancers on cytological specimens: high accuracy of immunocytochemistry with the 5A4 clone. J Thorac Oncol. 2013;8(8):1004–1011. doi: 10.1097/JTO.0b013e3182936ca9. [DOI] [PubMed] [Google Scholar]
- 23.Savic S, Diebold J, Zimmermann AK, Jochum W, Baschiera B, Grieshaber S, Tornillo L, Bisig B, Kerr K, Bubendorf L. Screening for ALK in non-small cell lung carcinomas: 5A4 and D5F3 antibodies perform equally well, but combined use with FISH is recommended. Lung Cancer. 2015;89(2):104–109. doi: 10.1016/j.lungcan.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 24.von Laffert M, Warth A, Penzel R, Schirmacher P, Kerr KM, Elmberger G, Schildhaus HU, Buttner R, Lopez-Rios F, Reu S, et al. Multicenter immunohistochemical ALK-testing of non-small-cell lung cancer shows high concordance after harmonization of techniques and interpretation criteria. J Thorac Oncol. 2014;9(11):1685–1692. doi: 10.1097/JTO.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 25.Park HS, Lee JK, Kim DW, Kulig K, Kim TM, Lee SH, Jeon YK, Chung DH, Heo DS. Immunohistochemical screening for anaplastic lymphoma kinase (ALK) rearrangement in advanced non-small cell lung cancer patients. Lung Cancer. 2012;77(2):288–292. doi: 10.1016/j.lungcan.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Houang M, Toon CW, Clarkson A, Sioson L, Watson N, Farzin M, Selinger CI, Chou A, Morey AL, Cooper WA, et al. Reflex ALK immunohistochemistry is feasible and highly specific for ALK gene rearrangements in lung cancer. Pathology. 2014;46(5):383–388. doi: 10.1097/PAT.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Tang Y, Li J, Jiang L, Jiang Y, Su X. Detection of ALK rearrangements in malignant pleural effusion cell blocks from patients with advanced non-small cell lung cancer: a comparison of Ventana immunohistochemistry and fluorescence in situ hybridization. Cancer Cytopathol. 2015;123(2):117–122. doi: 10.1002/cncy.21510. [DOI] [PubMed] [Google Scholar]
- 28.von Laffert M, Stenzinger A, Hummel M, Weichert W, Lenze D, Warth A, Penzel R, Herbst H, Kellner U, Jurmeister P, et al. ALK-FISH borderline cases in non-small cell lung cancer: implications for diagnostics and clinical decision making. Lung Cancer. 2015;90(3):465–471. doi: 10.1016/j.lungcan.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 29.VENTANA ALK (D5F3) CDx Assay - P140025. https://www.accessdata.fda.gov/cdrh_docs/pdf14/p140025b.pdf. Accessed 25 Jan 2018.
- 30.Dietel M, Bubendorf L, Dingemans AM, Dooms C, Elmberger G, Garcia RC, Kerr KM, Lim E, Lopez-Rios F, Thunnissen E, et al. Diagnostic procedures for non-small-cell lung cancer (NSCLC): recommendations of the European expert group. Thorax. 2016;71(2):177–184. doi: 10.1136/thoraxjnl-2014-206677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atherly AJ, Camidge DR. The cost-effectiveness of screening lung cancer patients for targeted drug sensitivity markers. Br J Cancer. 2012;106(6):1100–1106. doi: 10.1038/bjc.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dietel M, Johrens K, Laffert MV, Hummel M, Blaker H, Pfitzner BM, Lehmann A, Denkert C, Darb-Esfahani S, Lenze D, et al. A 2015 update on predictive molecular pathology and its role in targeted cancer therapy: a review focussing on clinical relevance. Cancer Gene Ther. 2015;22(9):417–430. doi: 10.1038/cgt.2015.39. [DOI] [PubMed] [Google Scholar]
- 33.von Laffert M, Penzel R, Schirmacher P, Warth A, Lenze D, Hummel M, Dietel M. Multicenter ALK testing in non-small-cell lung cancer: results of a round robin test. J Thorac Oncol. 2014;9(10):1464–1469. doi: 10.1097/JTO.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 34.Patton S, Normanno N, Blackhall F, Murray S, Kerr KM, Dietel M, Filipits M, Benlloch S, Popat S, Stahel R, et al. Assessing standardization of molecular testing for non-small-cell lung cancer: results of a worldwide external quality assessment (EQA) scheme for EGFR mutation testing. Br J Cancer. 2014;111(2):413–420. doi: 10.1038/bjc.2014.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Krieken JH, Siebers AG, Normanno N. European consensus conference for external quality assessment in molecular pathology. Ann Oncol. 2013;24(8):1958–1963. doi: 10.1093/annonc/mdt153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Questionnaire addressing issues of molecular testing and NSCLC management. Questionnaire was provided to 59 specialists (epidemiologists, oncologists, pulmonologists, and pathologists) from nine CEE countries requesting information. (PDF 191 kb)
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.


