Abstract
Background
The Rocky Mountain elk (Cervus elaphus nelsoni) prion protein gene (PRNP) is polymorphic at codon 132, with leucine (L132) and methionine (M132) allelic variants present in the population. In elk experimentally inoculated with the chronic wasting disease (CWD) agent, different incubation periods are associated with PRNP genotype: LL132 elk survive the longest, LM132 elk are intermediate, and MM132 elk the shortest. The purpose of this study was to investigate potential mechanisms underlying variations in incubation period in elk of different prion protein genotypes. Elk calves of three PRNP genotypes (n = 2 MM132, n = 2 LM132, n = 4 LL132) were orally inoculated with brain homogenate from elk clinically affected with CWD.
Results
Elk with longer incubation periods accumulated relatively less PrPSc in the brain than elk with shorter incubation periods. PrPSc accumulation in LM132 and MM132 elk was primarily neuropil-associated while glial-associated immunoreactivity was prominent in LL132 elk. The fibril stability of PrPSc from MM132 and LM132 elk were similar to each other and less stable than that from LL132 elk. Real-time quaking induced conversion assays (RT-QuIC) revealed differences in the ability of PrPSc seed from elk of different genotypes to convert recombinant 132 M or 132 L substrate.
Conclusions
This study provides further evidence of the importance of PRNP genotype in the pathogenesis of CWD of elk. The longer incubation periods observed in LL132 elk are associated with PrPSc that is more stable and relatively less abundant at the time of clinical disease. The biochemical properties of PrPSc from MM132 and LM132 elk are similar to each other and different to PrPSc from LL132 elk. The shorter incubation periods in MM132 compared to LM132 elk may be the result of genotype-dependent differences in the efficiency of propagation of PrPSc moieties present in the inoculum. A better understanding of the mechanisms by which the polymorphisms at codon 132 in elk PRNP influence disease pathogenesis will help to improve control of CWD in captive and free-ranging elk populations.
Keywords: Chronic wasting disease, Conformational stability, Elk, RT-QuIC, Prion protein
Background
Chronic wasting disease (CWD) is a transmissible spongiform encephalopathy (TSE) that affects a number of cervid species including elk, moose, mule deer, white-tailed deer and reindeer. The TSE’s are a group of neurodegenerative diseases that are characterized by the accumulation of disease-associated prion protein (PrPSc) in the nervous system and other body tissues. In cervids, CWD infection is associated with clinical signs including behavioral abnormalities, excess salivation, emaciation, and eventually death [49].
The host prion protein (PrP) amino acid sequence that is encoded by the prion protein gene (PRNP) influences the susceptibility of both humans and animals to TSE’s. Rocky Mountain elk (Cervus elaphus nelsoni) are polymorphic at PRNP codon 132, encoding either methionine (M) or leucine (L) [30]. The elk PRNP codon 132 polymorphism is homologous to the human PRNP codon 129 polymorphism that encodes either methionine (M) or valine (V) [39, 40]. In TSE-affected humans, the MM129 genotype is associated with susceptibility to kuru [23] and variant Creutzfeldt-Jakob disease (vCJD) [38]. Some studies have found that elk expressing prion protein homozygous for methionine at codon 132 (hereafter referred to as MM132 elk) are over-represented among CWD-affected elk [11, 12, 31, 41], while another study concluded that elk of all 3 genotypes (MM132, LM132, LL132) show equivalent susceptibility [36]. In experimental studies, LL132 elk orally inoculated with CWD have incubation periods approximately 1.5 times longer than LM132 elk, and 3 times longer than MM132 elk [14, 28]. A better understanding of the biological effects of polymorphisms at elk PRNP codon 132 may help to clarify the role of this locus in the spread of CWD in North American elk populations.
Here, we provide further histopathologic characterization of experimental CWD infection in MM132, LM132 [14] and LL132 [28] elk. We examine the intersection of host genotype, incubation period, PrPSc fibril stability, and amyloid formation rate and demonstrate that genotype-dependent differences in PrPSc stability and amyloid formation rate may contribute to the observed variation in incubation periods of elk of different genotypes. These results may help us to better understand the influence of the PRNP 129 polymorphism in human prion diseases.
Methods
Ethics statement
This experiment was carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Academy of Sciences, Washington, DC) and the Guide for the Care and Use of Agricultural Animals in Research and Teaching (Federation of Animal Science Societies, Champaign, IL). The Institutional Animal Care and Use Committee at the National Animal Disease Center reviewed and approved the animal use protocol (protocol number: 3833).
Inoculum preparation and animal procedures
The source, genotyping, husbandry and oral inoculation of the eight elk in this study has been described previously [14]. Briefly, elk were obtained from a captive elk game farm on which 79 cases of CWD were diagnosed between 1997 and 2001. All CWD-positive elk were of the MM132 or LM132 genotypes; no cases were found in LL132 elk [28]. MM132 and LM132 elk calves were sourced from the 2000 birth cohort of 1 of the 3 premises operated by the captive elk game farm; LL132 elk calves were sourced from the 2001 birth cohort of a different premises to the MM132 and LM132 calves. Genotype analysis was conducted on nucleic acid extracted from live animal blood samples as described previously [8]. The inoculum was prepared from pooled brain material from one MM132 and one LM132 elk (equal parts MM132 and LM132 donor tissue), both of which had showed clinical signs of CWD. At 8 months of age four LL132 elk, two LM132 elk and two MM132 elk were inoculated orally with 3 mL of inoculum daily for five consecutive days (total dose equivalent to 15 g of pooled brain) [14, 28]. Elk were housed in a biosafety level-2 isolation barn at the National Animal Disease Center (Ames, IA). This barn had not previously housed CWD-infected animals and entry and exit procedures were in place to eliminate potential cross-contamination from any source. Health was monitored twice daily. Sentinel LL132 animals were not included in the study design.
Animals were necropsied after being found dead, or euthanized upon showing clinical signs or at the conclusion of the experiment at 64 months postinoculation (MPI). Two sets of tissue samples were collected. One set of tissues included representative sections of: brain, eye (retina), optic nerve, sciatic nerve, trigeminal ganglion, peripheral nerves (optic, sciatic), lymph nodes (retropharyngeal, mesenteric, popliteal, prescapular), tonsils (palatine, pharyngeal), 3rd eyelid, foregut (esophagus, reticulum, omasum, rumen, abomasum), jejunum, ileum, recto-anal mucosa-associated lymphoid tissue (RAMALT), salivary gland, liver, pancreas, kidney, urinary bladder, spleen, adrenal, pituitary, thyroid, skeletal muscles (diaphragm, biceps femoris, masseter, psoas major, triceps), heart muscle, tongue, turbinate, lung, trachea, skin. These tissues were fixed in 10% buffered formalin, embedded in paraffin wax, and sectioned at 5 μm for microscopy examination after hematoxylin and eosin staining. The second set of tissues, comprising subsamples of all tissues collected into formalin, was frozen.
Immunohistochemistry
All paraffin-embedded tissues were immunostained by an automated immunohistochemical method for detection of PrPSc as described previously [9] using the anti-PrP monoclonal antibody F99/96.7.1 [29].
Antigen-capture enzyme immunoassay (EIA)
The IDEXX HerdChek BSE-Scrapie Ag EIA plate (Westbrook, ME) was used with modifications for the EIA-based fibril stability assay and the determination of PrPSc levels. Brain samples from elk were recovered from archived frozen brainstem stored at either − 20 °C or − 80 °C. Brainstem samples were mixed with 1X PBS (phosphate-buffered saline, lacking calcium and magnesium) and homogenized in a bead beater.
EIA-based fibril stability assay
PrPSc fibril stability was determined using an EIA-based assay as described previously ([9]. This assay is a protease-free method to monitor PrPSc unfolding that exposes the epitopes for the antibodies used in the IDEXX assay. The capture surface of the IDEXX EIA is a proprietary ligand that is specific for misfolded protein with detection of bound protein by a PrP specific antibody, and does not require protease digestion to distinguish PrPSc from PrPC. Briefly, dilutions of elk brain samples were incubated at concentrations of guanidine hydrochloride (GdnHCl) over a range from 0.25 M to 4.0 M. Neither brainstem samples nor intact brain were available for MM132 elk #2 so spinal cord was used for a comparison of elk #2 and elk #1; sections of gray matter from the cervical spinal cord were excised and homogenized as for the brainstem samples. The relative amount of PrPSc remaining was assessed by the EIA optical density (OD450) after dilution of treated brain homogenates to a final [GdnHCl] of 0.25 M and application to the IDEXX plate. The amount of PrPSc remaining was then plotted against GdnHCl concentration. The midpoint of the curve, or [GdnHCl]1/2, is defined as the concentration of GdnHCl at which the PrPSc signal was reduced by half of the signal at 0.25 M GdnHCl; PrPSc with a smaller [GdnHCl]1/2 is less stable. As described previously [44], due to variations in the upper baseline shape, the Smooth Line function in Microsoft Excel was used to connect data points in each curve and visualize the midpoint.
Calculation of amount of PrPSc versus incubation period
To determine the relative amount of PrPSc in brain from elk at clinical disease, 1% w/v brain homogenates were serially diluted in 1X PBS and tested using the EIA assay and diluted until the OD450 readings were in the linear range of detection. To provide a normalization metric across multiple samples, the 1% (w/v) homogenate was assigned a brain unit equivalent (BU) value of 100 and equivalent BU’s were calculated for each dilution, i.e. 1:2 dilution = 50 BU, 1:4 dilution = 25 BU. For each sample, the EIA OD reading in the linear range (minus the negative control value) was divided by the BU of the dilution at which the linear range OD was measured, to generate an OD/BU value. We then calculated the ratio of the OD/BU values for each sample compared to the sample with the lowest OD/BU value. Ratio values were plotted against incubation period.
Recombinant prion protein production and purification
E. coli (BL21(λDE3)) was transformed with the pET28a vector containing the elk PrP gene corresponding to mature length PrP (amino acids 23–231, GenBank accession number AAC12860.2), and elk recombinant PrP was expressed and purified as described for bovine PrP [17, 46]. The concentration of filtered protein eluent was determined by UV absorbance at 280 nm using an extinction coefficient of 59,485 M− 1 cm− 1 as calculated for mature length elk prion protein.
Real-time quaking induced conversion (RT-QuIC) protocol
RT- QuIC was performed on 10% (w/v) brainstem homogenized in PBS from elk #1 (MM132), elk #4 (LM132) and elk #7 (LL132) as described previously [17]. The reaction mix was composed of 10 mM phosphate buffer (pH 7.4), 400 mM NaCl, 0.1 mg/ml recombinant mature length elk prion protein (132 L, [23–231]; 132 M, [23–231]), 10 μM thioflavin T (ThT), 1 mM ethylenediaminetetraacetic acid tetrasodium salt (EDTA). The positive threshold was calculated as the mean value of normal elk brain homogenates plus 10 standard deviations. Previously described criteria were applied for classification of positive samples of RT-QuIC [5, 32, 34].
Results
Differences in incubation period were associated with polymorphisms at PRNP codon 132
At approximately 23 MPI MM132 elk (animals #1 and #2) developed loss of appetite and body condition. Both elk rapidly became unable to stand without assistance and were euthanized. At 38 (#3) and 40 (#4) MPI respectively, LM132 elk developed similar clinical signs and were euthanized (average incubation period = 39 MPI) (Table 1). The first LL132 elk (#5) to succumb to CWD was found dead at 59 MPI. This elk had previously been noted to be smaller and thinner than the other LL132 elk. During month 63 post-inoculation elk #6 developed muscle fasciculations, staggering, tremor, anorexia, mental dullness, head pressing and loss of bladder control, and was euthanized. The two remaining elk (#7 and #8) were euthanized at 64 MPI after displaying early signs of clinical disease, including subtle behavior changes, mild loss of body condition, and roughened hair coat (Table 1). The average incubation period for the four LL132 elk was 62.8 MPI.
Table 1.
Animal information and results for study elk
| Animal number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Genotype codon 132 | MM | MM | LM | LM | LL | LL | LL | LL |
| Incubation period (MPI) | 23 | 23 | 38 | 40 | 60 | 63 | 64 | 64 |
| Clinical presentation | LBC, Rec | LBC, Rec | LBC, ataxia | LBC | LBC | Neuro | FD | LBC |
| Tissue results | ||||||||
| Brain | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ |
| Retina | + | + | + | + | + | + | + | + |
| Peripheral NS | + | + | + | – | + | + | – | + |
| Lymphoid head | + | + | + | + | + | + | + | + |
| Lymphoid other | + | + | + | + | + | + | + | n/a |
| Intestines | + | + | + | + | – | + | + | + |
| Spleen | + | + | + | – | + | – | – | + |
| Pituitary | n/a | n/a | + | + | n/a | + | – | n/a |
| Foregut | n/a | – | – | + | – | – | – | + |
| Adrenal | n/a | + | – | – | n/a | – | + | n/a |
M methionine, L leucine, MPI months post inoculation, LBC Loss of body condition, Rec recumbency, Neuro neurological signs (for more detail see Results), FD found dead. Tissue results: brain, vacuolation/PrPSc; other tissues, PrPSc; n/a, tissue not available for examination
Spongiform change was more prominent in the gray matter in MM132 and LM132 elk, while in LL132 elk the white matter was more severely affected
To investigate the patterns of spongiform change in the brain, hematoxylin and eosin stained coronal sections of brain and spinal cord were examined by light microscopy. Pathologic changes in MM132 and LM132 elk have been described previously [14]. Microscopic lesions of spongiform encephalopathy were present in all elk. In LM132 and MM132 elk, microcavitation of the gray matter was more prevalent than intraneuronal vacuolation and neuronal degeneration, and there was mild astrocytosis [14]. In all LM132 and MM132 elk, moderate to severe spongiform change was present in the dorsal motor nucleus of the vagus nerve (Fig. 1a) and surrounding nuclei. In LL132 elk, vacuolation of white matter tracts (Fig. 1b) was more prevalent than microcavitation of the gray matter.
Fig. 1.
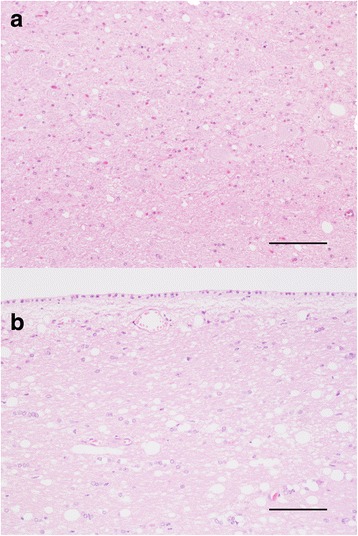
Spongiform change observed in elk inoculated with the CWD agent. a Spongiform change in the dorsal motor nucleus of the vagus nerve in elk #2 (MM132). (Hematoxylin and eosin, original magnification 20×). b White matter vacuolation in the corpus callosum in elk #8 (LL132). (Hematoxylin and eosin, original magnification 10×)
In summary, microcavitation of gray and white matter was observed in all elk. Spongiform change was more prominent in the gray matter of LM132 and MM132 elk and more prominent in the white matter of LL132 elk.
PrPSc accumulation in LM132 and MM132 elk was primarily neuropil-associated while intra-glial immunoreactivity was prominent in LL132 elk
To investigate the patterns of PrPSc deposition in the brain, immunolabeled sections of brain, spinal cord, and peripheral tissues were examined by light microscopy. Subjectively, the total amount of PrPSc immunoreactivity was greater in MM132 and LM132 elk compared to LL132 elk. In LM132 and MM132 elk, PrPSc immunoreactivity in the brain appeared as coarse granular material that was scattered throughout the neuropil. Perineuronal immunolabeling was common while intraneuronal immunolabeling was rare [14].
In LL132 elk, coarse granular and perineuronal immunolabeling were common, as was intraneuronal immunolabeling (Fig. 2a). In addition, there was granular to punctate immunolabeling that was often associated with astrocytes. This astrocyte-associated immunolabeling was most prominent in white matter (Fig. 2b) but also was observed in gray matter (Fig. 2c).
Fig. 2.
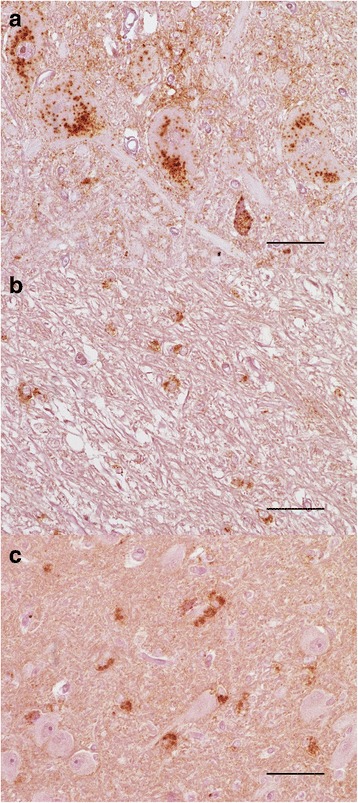
Spongiform change and patterns of PrPSc immunoreactivity observed in elk inoculated with the CWD agent. a Intraneuronal immunoreactivity in the hypoglossal nucleus in elk #5 (LL132) elk. b Glial associated immunoreactivity in the cerebellar white matter of elk #5 (LL132). c Glial associated immunoreactivity in the lateral geniculate nucleus of elk #5 (LL132). a-c immunostained using monoclonal anti-PrP antibody F99/96.7.1, original magnification 40×
In elk of all genotypes, PrPSc was abundant in the lymphoid follicles of the palatine tonsil, retropharyngeal lymph node and gut-associated lymphoid tissue. The skeletal muscles (M. biceps femoris, M. masseter, M. psoas major, M. triceps), diaphragm, kidney, urinary bladder, nose skin, turbinate, trachea, lung, tongue, liver, pancreas, salivary gland, and thyroid were negative in all samples examined.
PrPSc immunoreactivity was widespread in the central nervous system and peripheral lymphoid tissues of all elk. Intraneuronal immunolabeling was less prominent in LM132 and MM132 elk compared to LL132 elk. Glial-associated immunolabeling observed in LL132 elk was not seen in LM132 or MM132 elk.
PrPSc fibrils from LL132 elk are more stable than fibrils from LM132 and MM132 elk
To determine whether there is an association between fibril stability of PrPSc and incubation period in CWD-affected elk, we assessed the stability of PrPSc using an EIA-based stability assay.
When the fibril stability of PrPSc in homogenized brainstem of elk of each genotype was measured, two clusters of curves were evident (Fig. 3a). Samples from MM132 and LM132 elk exhibited lower fibril stability, with a [GdnHCl]1/2 of ≈2.75, while samples from LL132 elk exhibited higher fibril stability, with a [GdnHCl]1/2 of ≈3.2–3.3. When fibril stability data from samples from MM132 and LM132 elk are combined and compared to LL132 elk samples (Fig. 3b), average values of LL132 versus M132-containing groups (MM132 and LM132) exhibited statistically significant differences at 2.5, 3, and 3.5 M GdnHCl (p < 0.004, t-test with unequal variances). Since unfixed brainstem tissue was unavailable for the second MM132 elk (elk #2) spinal cord homogenate was used to determine the fibril stability of PrPSc from this elk. The stability of PrPSc from the elk #2 spinal cord sample was similar to PrPSc from brainstem homogenate from the other MM132 elk (#1) in the study (data not shown).
Fig. 3.
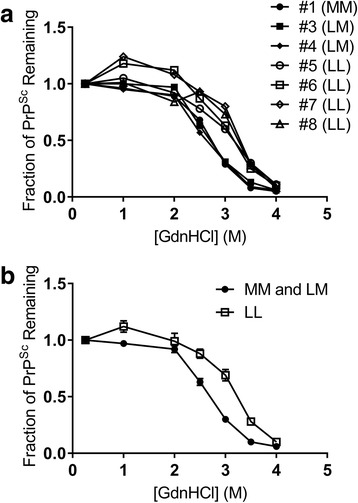
The fibril stability of PrPSc from MM132 and LM132 elk was lower than the fibril stability of PrPSc from LL132 elk. Homogenates of infected elk brain were incubated in GdnHCl at the indicated concentration as described in Methods, with remaining PrPSc, as detected by EIA, expressed as a fraction of the signal after treatment with 0.25 M GdnHCl. a Comparison of individual animals of MM132, LM132 and LL132 genotype elk, as indicated. Data were averaged across 4–6 technical replicates to generate each curve. b Average curves for LL132 elk (closed symbols) as compared to MM132 and LM132 elk (open symbols) from (a); error bars depict +/− the standard error of the mean (SEM) of the biological replicates
In summary, PrPSc from samples from MM132 and LM132 elk that have short and intermediate incubation periods, respectively, was less stable than PrPSc from samples from LL132 elk that have the longest incubation periods.
Relative amount of PrPSc in comparison to incubation period in elk
To investigate the relationship between the incubation period and relative amount of PrPSc accumulation in the brain, the amount of PrPSc in brain homogenates was quantified using EIA.
The relative amount of PrPSc in the brain was lowest for LL132 elk, intermediate for LM132 elk, and highest for MM132 elk. When the relative amount of PrPSc in the brain was plotted against elk incubation period, a strong negative correlation between these two variables was apparent (Fig. 4).
Fig. 4.
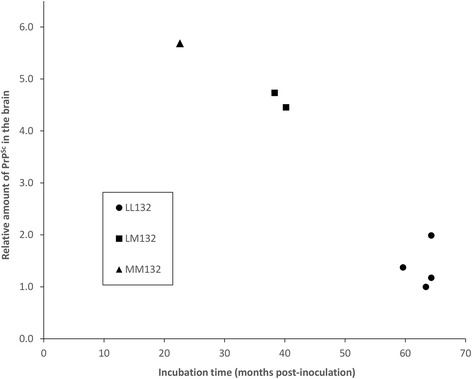
The relative amount of PrPSc in the brainstem (obex) is strongly associated with incubation period. The amount of PrPSc in the brain was calculated using optical density readings from an antigen-capture enzyme immunoassay (EIA). The relative amount of PrPSc in the brain of the elk with the lowest EIA result in the linear range was designated a baseline value of 1.0. Results for other elk are expressed as a ratio relative to the baseline elk. Frozen obex was not available for elk #2 (MM132) so this animal is not included in the figure
Real-time quaking induced conversion assays seeded with samples from LM132 and MM132 elk produced shorter lags times in 132 M substrate and longer lag times in 132 L substrate
To investigate if RT-QuIC can be used to detect differences in either conversion efficiency of the substrate or the prion seeding activity from CWD infected elk brain of different genotypes, we used infected and normal elk brain homogenates as seed for mature length recombinant 132 L or 132 M elk prion protein substrates. To allow for comparison between substrates, all assays were performed in the same reaction conditions as described in the Materials and Methods.
Using the 132 L substrate (Fig. 5a) and 132 M substrate (Fig. 5b) an increase in Thioflavin-T fluorescence, indicating the presence of misfolded prion protein, was observed in each quadruplicate reaction seeded with 10− 2 dilution of normalized elk brain homogenate, but no increase in fluorescence was observed in reactions seeded with normal brain homogenates. The lag times in assays using the 132 L substrate were similar for seeds from elk of all three genotypes (LM132 = 21 h, MM132 = 23 h, LL132 = 20.5 h). For LM132 and MM132 seeds, the lag times in assays using 132 M substrate (LM132 = 12 h, MM132 = 12.5 h) were shorter than the lag times in assays using 132 L substrate (LM132 = 21 h, MM132 = 23 h). The lag time for the LL132 seed in 132 L substrate (19 h) was similar to the lag time in 132 M substrate (20.5 h).
Fig. 5.
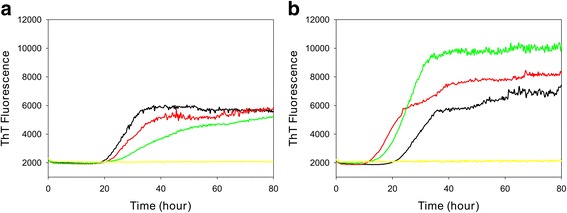
RT-QuIC detection of seeding activity in elk brain samples using mature length recombinant elk prion protein 132 L (a) and 132 M (b) substrates. RT-QuIC assays seeded with MM132 (green), LM132 (red), LL132 (black) or negative seed (yellow) are shown. RT-QuIC reaction mixtures were seeded with 10− 2 dilutions of normalized brain homogenate. A final 400 mM NaCl was used with each substrate. Data are presented as mean ThT fluorescence of quadruplicate reactions
Discussion
We demonstrate that the shorter incubation periods of elk that are homozygous for methionine at PRNP codon 132 (MM132) or heterozygous for leucine and methionine (LM132) elk are associated with PrPSc that is less stable than PrPSc from elk that are homozygous for leucine (LL132), which have the longest incubation periods. Subjectively, the amount of PrPSc immunoreactivity in the brain was similar across elk of all genotypes using IHC. However, serial dilution studies using EIA revealed that the brains of LL132 elk contain relatively lower amounts of PrPSc than LM132 and MM132 elk. Although the interpretation of results from this study is limited by the small number of elk of each genotype that were available for inoculation, this study nevertheless provides valuable baseline data on the relationship between PRNP codon 132 genotype and disease pathogenesis in elk with chronic wasting disease.
We observed a strong negative association between incubation period and the relative amount of PrPSc in the brain in elk of different genotypes, i.e. elk with longer incubation periods accumulate less PrPSc. Differences in the relative amount of PrPSc in the brain were detected using EIA on frozen brain tissue and IHC on formalin-fixed paraffin-embedded brain tissue. This suggests that MM132 elk may be more permissive to PrPSc accumulation than LM132 elk. This observation is supported by a previous study in transgenic mice that showed that the L132 polymorphism severely restricts propagation of CWD prions [7]. Since PrPSc from MM132 and LM132 elk show similar fibril stability profiles and RT-QuIC conversion profiles using recombinant 132 L and 132 M elk prion protein, one explanation for the rapid PrPSc accumulation in MM132 elk may be a potential difference in the effective concentration of PrPC-132M. In heterozygous sheep, both allelic variants of PrPC are present in equal amounts [27]. It is assumed that this relationship is similar in heterozygous elk, which means that the amount of PrPC-132M in MM132 elk is twice that of LM132 elk. In transgenic mice higher expression levels of PrPC result in reduced incubation times (reviewed in [47]). Therefore, the relatively higher proportion of PrPC-132M in MM132 elk compared to LM132 elk may contribute to the relatively shorter incubation times observed in MM132 elk.
In sheep, conversion of PrPC to PrPSc is more efficient when the PRNP genotype of the inoculum and substrate are the same [2, 3, 21]. Furthermore, in heterozygous animals there is preferential conversion of the PrPC moiety of the allele associated with a higher susceptibility to disease [18, 27]. Since the biological behavior of scrapie prions in sheep and CWD prions in cervids are similar, it seems reasonable to assume that the conversion efficiency of elk CWD prions has a sequence dependence similar to sheep scrapie prions. The brain homogenate used to inoculate the elk was prepared from pooled brain material from one MM132 and one LM132 elk. Titration of brain homogenate was not performed prior to pooling. Based on observations in sheep [18, 27] it is probable that the PrPSc in the LM132 brain was predominantly PrPSc-132M and therefore that the pooled brain homogenate contained mostly PrPSc-132M. This PrPSc-132M would propagate more efficiently in elk expressing PrPC-132M than those expressing PrPC-132L or a mixture of PrPC-132M and PrPC-132L. Experimental challenge of elk of each genotype with brain homogenates from homozygous and heterozygous donors may help to elucidate the relative contribution of donor and recipient PRNP genotypes to incubation time in CWD-affected elk.
If relative incubation period reflects the relative permissibility of elk of different PRNP genotypes to PrPSc accumulation and by extension, their susceptibility to disease, our findings support previous CWD surveys that have shown that MM132 elk are most susceptible to CWD, the susceptibility of LM132 elk is intermediate, and LL132 elk are least susceptible to CWD [31, 41]. These findings suggest that genetic selection for the L132 allele has the potential to reduce the impact of CWD in captive and free-ranging elk populations, although it should be kept in mind that the protective effects of the L132 allele against CWD prions are not absolute [4, 7]. The elk breeding facility from which the elk calves for this experiment were obtained was known to have a high prevalence of CWD [14] so infection of elk calves with CWD prior to being moved to the quarantine facility at 8 months of age cannot be ruled out. However, since incubation periods for elk within each genotype group were similar to each other and different to elk of different genotypes, it appears that potential infection at the breeding facility did not influence the outcome of experimental infection at the quarantine facility in this study.
We have shown that the fibril stability of PrPSc from elk with shorter incubation periods (i.e. MM132 and LM132) is lower, while PrPSc fibrils from elk with longer incubation periods (LL132) are more stable. These observations are in agreement with previous observations in mice challenged with synthetic [24] and mouse-adapted [1, 25] prion strains, and in sheep challenged with different scrapie isolates [45]. It is hypothesized that lower fibril stability leads to increased PrPSc fibril fragmentation that facilitates the conversion of PrPC to PrPSc and results in faster replication of PrPSc and reduced incubation periods [42, 51]. However, an inverse relationship between incubation period and fibril stability – that is, PrPSc from animals with shorter incubation periods is more stable – has been observed in Syrian hamsters challenged with hamster adapted scrapie or transmissible mink encephalopathy strains [35], sheep with naturally occurring classical or Nor98 scrapie [37, 48], and cattle challenged with classical or atypical (H-type) bovine spongiform encephalopathy [44]. These variable relationships between incubation period and fibril stability suggest that factors other than, or in addition to, fibril stability of PrPSc can influence incubation periods.
Western blot analyses of brain samples from elk in this study have been published previously [14, 28]. The three characteristic bands of the proteinase-resistant core of PrPSc were observed in all elk and samples from MM132 and LM132 elk showed similar migration profiles, glycoform ratios, and N-terminal cleavage sites [14, 28]. However, samples from LL132 elk showed a significantly lower mean apparent molecular mass compared to MM132 and LM132 elk; this was associated with cleavage near residues 98–113 [28], as compared to cleavage at residues 78 and 82 in MM132 elk [50]. Therefore, similar to fibril stability and amyloid formation rate, western blot phenotype does appear to be a strongly associated with differences in incubation periods in MM132 and LM132 elk.
Until now, RT-QuIC applications in cervids have mainly focused on detection of small amounts of prions in fluids and tissues relevant to pre-clinical diagnosis of disease or disease transmission: urine [15, 20], feces [4, 20], saliva [15, 16], blood [6], cerebrospinal fluid [13], rectal biopsy and nasal brush samples [11, 12]. RT-QuIC has also been utilized for the discrimination of subtypes of bovine spongiform encephalopathy (BSE) [17, 26, 33].
To investigate if conversion efficiency of PrPSc influences incubation period, real-time quaking induced conversion (RT-QuIC) was performed using recombinant mature length elk prion protein (132 L and 132 M) seeded with brain homogenates from one elk of each genotype. These experiments revealed differences in the ability of PrPSc seed from CWD-infected elk of different genotypes to convert recombinant elk prion substrate. The MM132 or LM132 seeds convert 132 M substrate protein readily, whereas LL132 seed is much slower to do so. In contrast, all seeds convert 132 L substrate protein although the LL132 seed exhibited the fastest conversion. This conversion data suggests two potential hypotheses: (a) there are two distinct and stably propagating conformations of elk PrPSc present, one that is adopted more readily by 132 M protein and one that is adopted more readily by 132 L protein; or (b) the differences in conversion rate (both in the animal and in RT-QuIC) are the result of genotype mismatches between seed PrPSc and substrate. The similar lag phases observed with MM132 and LM132 seed are consistent with previously reported RT-QuIC analyses [10] and the fibril stability results reported here, and may provide further evidence that the LM132 seed contains a relatively large proportion of PrPSc-132M. The results of the stability assay also provide evidence that there are two conformations with distinct molecular properties, but future investigations are needed to explore this question. Inoculation of both the MM132 and LL132 seeds into transgenic mice carrying the elk prion gene will be useful in assessing differences in PrPSc fibril stability and incubation times upon serial passage into mice of a single PRNP genotype.
PrPSc from CWD-infected LL132 elk shares a number of immunohistochemical features with the ovine scrapie strain CH1641, namely a loss of the epitope for the anti-PrP monoclonal antibody P4 that binds PrP residues 93–99 [43], and reduced but detectable immunoreactivity with the monoclonal antibody 8G8 (that binds residues 98–113 [22]) [19, 28]. The phenotype of PrPSc accumulation in the brain of sheep with CH1641 is characterized by prominent intracellular immunoreactivity in neurons and glial cells, and relatively little extracellular immunoreactivity [19]. Intraneuronal PrPSc accumulation is rare in MM132 and LM132 elk with CWD [14] but was commonly observed in the LL132 elk in this study. Furthermore, glial-associated immunolabeling was prominent in LL132 elk and not observed in MM132 or LM132 elk.
Conclusions
This study provides further evidence of the importance of PRNP genotype in the pathogenesis of CWD of elk. We have shown that the biochemical properties of PrPSc from MM132 and LM132 elk are similar to each other and different to PrPSc from LL132 elk. The shorter incubation periods in MM132 compared to LM132 elk may be the result of genotype-dependent differences in the efficiency of propagation of PrPSc moieties present in the inoculum. Further work is needed to develop a better understanding of the underlying mechanisms by which the polymorphisms at codon 132 in elk PRNP influence disease pathogenesis, with a view to improving control of CWD in captive and free-ranging elk populations.
Acknowledgements
We would like to thank Trudy Tatum for optimizing and performing the EIA assays, and Joe Lesan, Leisa Mandell, Kevin Hassall and Semakelang Lebepe-Mazur for excellent technical support.
Funding
This research was funded in its entirety by congressionally appropriated funds to the United States Department of Agriculture, Agricultural Research Service. The funders of the work did not influence study design, data collection and analysis, decision to publish, or the preparation of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Disclaimer
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Abbreviations
- CWD
Chronic wasting disease
- EIA
Antigen-capture enzyme immunoassay
- MPI
Months postinoculation
- PRNP
Prion protein gene
- PrPC
Cellular prion protein, not associated with disease
- PrPSc
Disease-associated prion protein
- RAMALT
Recto-anal lymphoid tissue
- RT-QuIC
Real-time quaking induced conversion
- TSE
Transmissible spongiform encephalopathy
- vCJD
variant Creutzfeldt-Jakob disease
Authors’ contributions
SJM drafted the manuscript and performed the microscopic examinations. CEV performed fibril stability assays and assisted with the drafting of the manuscript. SH performed the RT-QuIC experiments and assisted with the drafting of the manuscript. MHWG contributed to data analysis and critically revised the manuscript. JJG and EN designed the study, supervised the work, and critically revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This experiment was carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Academy of Sciences, Washington, DC) and the Guide for the Care and Use of Agricultural Animals in Research and Teaching (Federation of Animal Science Societies, Champaign, IL). The Institutional Animal Care and Use Committee at the National Animal Disease Center reviewed and approved the animal use protocol (protocol number: 3833).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
S. Jo Moore, Email: Jo.Moore@ARS.USDA.GOV.
Catherine E. Vrentas, Email: Catherine.Vrentas@ARS.USDA.GOV
Soyoun Hwang, Email: Soyoun.Hwang@ARS.USDA.GOV.
M. Heather West Greenlee, Email: mheather@iastate.edu.
Eric M. Nicholson, Email: Eric.Nicholson@ARS.USDA.GOV
Justin J. Greenlee, Email: Justin.Greenlee@ARS.USDA.GOV
References
- 1.Bett C, Joshi-Barr S, Lucero M, Trejo M, Liberski P, Kelly JW, Masliah E, Sigurdson CJ. Biochemical properties of highly neuroinvasive prion strains. PLoS Pathog. 2012;8(2):e1002522. doi: 10.1371/journal.ppat.1002522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bossers A, Belt P, Raymond GJ, Caughey B, de Vries R, Smits MA. Scrapie susceptibility-linked polymorphisms modulate the in vitro conversion of sheep prion protein to protease-resistant forms. Proc Natl Acad Sci U S A. 1997;94(10):4931–4936. doi: 10.1073/pnas.94.10.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bossers A, de Vries R, Smits MA. Susceptibility of sheep for scrapie as assessed by in vitro conversion of nine naturally occurring variants of PrP. J Virol. 2000;74(3):1407–1414. doi: 10.1128/JVI.74.3.1407-1414.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng YC, Hannaoui S, John TR, Dudas S, Czub S, Gilch S. Early and non-invasive detection of chronic wasting disease prions in elk feces by real-time quaking induced conversion. PLoS One. 2016;11(11):e0166187. doi: 10.1371/journal.pone.0166187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dassanayake RP, Orru CD, Hughson AG, Caughey B, Graca T, Zhuang D, Madsen-Bouterse SA, Knowles DP, Schneider DA. Sensitive and specific detection of classical scrapie prions in the brains of goats by real-time quaking-induced conversion. J Gen Virol. 2016;97(3):803–812. doi: 10.1099/jgv.0.000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elder AM, Henderson DM, Nalls AV, Wilham JM, Caughey BW, Hoover EA, Kincaid AE, Bartz JC, Mathiason CK. In vitro detection of prionemia in TSE-infected cervids and hamsters. PLoS One. 2013;8(11):e80203. doi: 10.1371/journal.pone.0080203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green KM, Browning SR, Seward TS, Jewell JE, Ross DL, Green MA, Williams ES, Hoover EA, Telling GC. The elk PRNP codon 132 polymorphism controls cervid and scrapie prion propagation. J Gen Virol. 2008;89(Pt 2):598–608. doi: 10.1099/vir.0.83168-0. [DOI] [PubMed] [Google Scholar]
- 8.Greenlee JJ, Smith JD, Kunkle RA. White-tailed deer are susceptible to the agent of sheep scrapie by intracerebral inoculation. Vet Res. 2011;42(1):107. doi: 10.1186/1297-9716-42-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenlee JJ, Smith JD, West Greenlee MH, Nicholson EM. Clinical and pathologic features of H-type bovine spongiform encephalopathy associated with E211K prion protein polymorphism. PLoS One. 2012;7(6):e38678. doi: 10.1371/journal.pone.0038678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haley NJ, Rielinger R, Davenport KA, O'Rourke K, Mitchell G, Richt JA. Estimating chronic wasting disease susceptibility in cervids using real-time quaking-induced conversion. J Gen Virol. 2017;98(11):2882–2892. doi: 10.1099/jgv.0.000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haley NJ, Siepker C, Hoon-Hanks LL, Mitchell G, Walter WD, Manca M, Monello RJ, Powers JG, Wild MA, Hoover EA, Caughey B, Richt JA. Seeded amplification of chronic wasting disease prions in nasal brushings and recto-anal mucosa-associated lymphoid tissues from elk by real-time quaking-induced conversion. J Clin Microbiol. 2016;54(4):1117–1126. doi: 10.1128/JCM.02700-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haley NJ, Siepker C, Walter WD, Thomsen BV, Greenlee JJ, Lehmkuhl AD, Richt JA. Antemortem detection of chronic wasting disease prions in nasal brush collections and rectal biopsy specimens from white-tailed deer by real-time quaking-induced conversion. J Clin Microbiol. 2016;54(4):1108–1116. doi: 10.1128/JCM.02699-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haley NJ, Van de Motter A, Carver S, Henderson D, Davenport K, Seelig DM, Mathiason C, Hoover E. Prion-seeding activity in cerebrospinal fluid of deer with chronic wasting disease. PLoS One. 2013;8(11):e81488. doi: 10.1371/journal.pone.0081488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamir AN, Gidlewski T, Spraker TR, Miller JM, Creekmore L, Crocheck M, Cline T, O'Rourke KI. Preliminary observations of genetic susceptibility of elk (Cervus elaphus nelsoni) to chronic wasting disease by experimental oral inoculation. J Vet Diagn Investig. 2006;18(1):110–114. doi: 10.1177/104063870601800118. [DOI] [PubMed] [Google Scholar]
- 15.Henderson DM, Davenport KA, Haley NJ, Denkers ND, Mathiason CK, Hoover EA. Quantitative assessment of prion infectivity in tissues and body fluids by real-time quaking-induced conversion. J Gen Virol. 2015;96(Pt 1):210–219. doi: 10.1099/vir.0.069906-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson DM, Manca M, Haley NJ, Denkers ND, Nalls AV, Mathiason CK, Caughey B, Hoover EA. Rapid antemortem detection of CWD prions in deer saliva. PLoS One. 2013;8(9):e74377. doi: 10.1371/journal.pone.0074377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang S, Greenlee JJ, Nicholson EM. Use of bovine recombinant prion protein and real-time quaking-induced conversion to detect cattle transmissible mink encephalopathy prions and discriminate classical and atypical L- and H-type bovine spongiform encephalopathy. PLoS One. 2017;12(2):e0172391. doi: 10.1371/journal.pone.0172391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs JG, Bossers A, Rezaei H, van Keulen LJ, McCutcheon S, Sklaviadis T, Lantier I, Berthon P, Lantier F, van Zijderveld FG, Langeveld JP. Proteinase K-resistant material in ARR/VRQ sheep brain affected with classical scrapie is composed mainly of VRQ prion protein. J Virol. 2011;85(23):12537–12546. doi: 10.1128/JVI.00448-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeffrey M, Gonzalez L, Chong A, Foster J, Goldmann W, Hunter N, Martin S. Ovine infection with the agents of scrapie (CH1641 isolate) and bovine spongiform encephalopathy: immunochemical similarities can be resolved by immunohistochemistry. J Comp Pathol. 2006;134(1):17–29. doi: 10.1016/j.jcpa.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 20.John TR, Schatzl HM, Gilch S. Early detection of chronic wasting disease prions in urine of pre-symptomatic deer by real-time quaking-induced conversion assay. Prion. 2013;7(3):253–258. doi: 10.4161/pri.24430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirby L, Goldmann W, Houston F, Gill AC, Manson JC. A novel, resistance-linked ovine PrP variant and its equivalent mouse variant modulate the in vitro cell-free conversion of rPrP to PrP(res) J Gen Virol. 2006;87(Pt 12):3747–3751. doi: 10.1099/vir.0.82086-0. [DOI] [PubMed] [Google Scholar]
- 22.Krasemann S, Groschup MH, Harmeyer S, Hunsmann G, Bodemer W. Generation of monoclonal antibodies against human prion proteins in PrP0/0 mice. Mol Med. 1996;2(6):725–734. [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HS, Brown P, Cervenakova L, Garruto RM, Alpers MP, Gajdusek DC, Goldfarb LG. Increased susceptibility to kuru of carriers of the PRNP 129 methionine/methionine genotype. J Infect Dis. 2001;183(2):192–196. doi: 10.1086/317935. [DOI] [PubMed] [Google Scholar]
- 24.Legname G, Nguyen HO, Baskakov IV, Cohen FE, Dearmond SJ, Prusiner SB. Strain-specified characteristics of mouse synthetic prions. Proc Natl Acad Sci U S A. 2005;102(6):2168–2173. doi: 10.1073/pnas.0409079102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Legname G, Nguyen HO, Peretz D, Cohen FE, DeArmond SJ, Prusiner SB. Continuum of prion protein structures enciphers a multitude of prion isolate-specified phenotypes. Proc Natl Acad Sci U S A. 2006;103(50):19105–19110. doi: 10.1073/pnas.0608970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masujin K, Orru CD, Miyazawa K, Groveman BR, Raymond LD, Hughson AG, Caughey B. Detection of atypical H-type bovine spongiform encephalopathy and discrimination of bovine prion strains by real-time quaking-induced conversion. J Clin Microbiol. 2016;54(3):676–686. doi: 10.1128/JCM.02731-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morel N, Andreoletti O, Grassi J, Clement G. Absolute and relative quantification of sheep brain prion protein (PrP) allelic variants by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2007;21(24):4093–4100. doi: 10.1002/rcm.3317. [DOI] [PubMed] [Google Scholar]
- 28.O'Rourke K, Spraker T, Zhuang D, Greenlee J, Gidlewski T, Hamir A. Elk with a long incubation prion disease phenotype have a unique PrPd profile. Cell Mol Dev Neurosci. 2007;18(18):1935–1938. doi: 10.1097/WNR.0b013e3282f1ca2f. [DOI] [PubMed] [Google Scholar]
- 29.O'Rourke KI, Baszler TV, Besser TE, Miller JM, Cutlip RC, Wells GA, Ryder SJ, Parish SM, Hamir AN, Cockett NE, Jenny A, Knowles DP. Preclinical diagnosis of scrapie by immunohistochemistry of third eyelid lymphoid tissue. J Clin Microbiol. 2000;38(9):3254–3259. doi: 10.1128/jcm.38.9.3254-3259.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Rourke KI, Baszler TV, Miller JM, Spraker TR, Sadler-Riggleman I, Knowles DP. Monoclonal antibody F89/160.1.5 defines a conserved epitope on the ruminant prion protein. J Clin Microbiol. 1998;36(6):1750–1755. doi: 10.1128/jcm.36.6.1750-1755.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Rourke KI, Besser TE, Miller MW, Cline TF, Spraker TR, Jenny AL, Wild MA, Zebarth GL, Williams ES. PrP genotypes of captive and free-ranging Rocky Mountain elk (Cervus elaphus nelsoni) with chronic wasting disease. J Gen Virol. 1999;80(Pt 10):2765–2769. doi: 10.1099/0022-1317-80-10-2765. [DOI] [PubMed] [Google Scholar]
- 32.Orru CD, Bongianni M, Tonoli G, Ferrari S, Hughson AG, Groveman BR, Fiorini M, Pocchiari M, Monaco S, Caughey B, Zanusso G. A test for Creutzfeldt-Jakob disease using nasal brushings. N Engl J Med. 2014;371(6):519–529. doi: 10.1056/NEJMoa1315200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orru CD, Favole A, Corona C, Mazza M, Manca M, Groveman BR, Hughson AG, Acutis PL, Caramelli M, Zanusso G, Casalone C, Caughey B. Detection and discrimination of classical and atypical L-type bovine spongiform encephalopathy by real-time quaking-induced conversion. J Clin Microbiol. 2015;53(4):1115–1120. doi: 10.1128/JCM.02906-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orru CD, Groveman BR, Hughson AG, Zanusso G, Coulthart MB, Caughey B. Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. MBio. 2015;6(1) 10.1128/mBio.02451-14. [DOI] [PMC free article] [PubMed]
- 35.Peretz D, Williamson RA, Legname G, Matsunaga Y, Vergara J, Burton DR, DeArmond SJ, Prusiner SB, Scott MR. A change in the conformation of prions accompanies the emergence of a new prion strain. Neuron. 2002;34(6):921–932. doi: 10.1016/S0896-6273(02)00726-2. [DOI] [PubMed] [Google Scholar]
- 36.Perucchini M, Griffin K, Miller MW, Goldmann W. PrP genotypes of free-ranging wapiti (Cervus elaphus nelsoni) with chronic wasting disease. J Gen Virol. 2008;89(Pt 5):1324–1328. doi: 10.1099/vir.0.83424-0. [DOI] [PubMed] [Google Scholar]
- 37.Pirisinu L, Di Bari M, Marcon S, Vaccari G, D'Agostino C, Fazzi P, Esposito E, Galeno R, Langeveld J, Agrimi U, Nonno R. A new method for the characterization of strain-specific conformational stability of protease-sensitive and protease-resistant PrP. PLoS One. 2010;5(9):e12723. [DOI] [PMC free article] [PubMed]
- 38.Saba R, Booth SA. The genetics of susceptibility to variant Creutzfeldt-Jakob disease. Public Health Genomics. 2013;16(1–2):17–24. doi: 10.1159/000345203. [DOI] [PubMed] [Google Scholar]
- 39.Schätzl HM, Da Costa M, Taylor L, Cohen FE, Prusiner SB. Prion protein gene variation among primates. J Mol Biol. 1997;265(2):257. doi: 10.1006/jmbi.1996.0791. [DOI] [PubMed] [Google Scholar]
- 40.Schätzl HM, Wopfner F, Gilch S, von Brunn A, Jager G. Is codon 129 of prion protein polymorphic in human beings but not in animals? Lancet. 1997;349(9065):1603–1604. doi: 10.1016/S0140-6736(05)61632-7. [DOI] [PubMed] [Google Scholar]
- 41.Spraker TR, Balachandran A, Zhuang D, O'Rourke KI. Variable patterns of distribution of PrP(CWD) in the obex and cranial lymphoid tissues of Rocky Mountain elk (Cervus elaphus nelsoni) with subclinical chronic wasting disease. Vet Rec. 2004;155(10):295–302. doi: 10.1136/vr.155.10.295. [DOI] [PubMed] [Google Scholar]
- 42.Sun Y, Makarava N, Lee CI, Laksanalamai P, Robb FT, Baskakov IV. Conformational stability of PrP amyloid fibrils controls their smallest possible fragment size. J Mol Biol. 2008;376(4):1155–1167. doi: 10.1016/j.jmb.2007.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thuring CM, Erkens JH, Jacobs JG, Bossers A, Van Keulen LJ, Garssen GJ, Van Zijderveld FG, Ryder SJ, Groschup MH, Sweeney T, Langeveld JP. Discrimination between scrapie and bovine spongiform encephalopathy in sheep by molecular size, immunoreactivity, and glycoprofile of prion protein. J Clin Microbiol. 2004;42(3):972–980. doi: 10.1128/JCM.42.3.972-980.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vrentas CE, Greenlee JJ, Baron T, Caramelli M, Czub S, Nicholson EM. Stability properties of PrP(Sc) from cattle with experimental transmissible spongiform encephalopathies: use of a rapid whole homogenate, protease-free assay. BMC Vet Res. 2013;9:167. doi: 10.1186/1746-6148-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vrentas CE, Greenlee JJ, Tatum TL, Nicholson EM. Relationships between PrPSc stability and incubation time for United States scrapie isolates in a natural host system. PLoS One. 2012;7(8):e43060. doi: 10.1371/journal.pone.0043060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vrentas CE, Onstot S, Nicholson EM. A comparative analysis of rapid methods for purification and refolding of recombinant bovine prion protein. Protein Expr Purif. 2012;82(2):380–388. doi: 10.1016/j.pep.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Watts JC, Prusiner SB. Mouse models for studying the formation and propagation of prions. J Biol Chem. 2014;289(29):19841–19849. doi: 10.1074/jbc.R114.550707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wemheuer WM, Benestad SL, Wrede A, Schulze-Sturm U, Wemheuer WE, Hahmann U, Gawinecka J, Schutz E, Zerr I, Brenig B, Bratberg B, Andreoletti O, Schulz-Schaeffer WJ. Similarities between forms of sheep scrapie and Creutzfeldt-Jakob disease are encoded by distinct prion types. Am J Pathol. 2009;175(6):2566–2573. doi: 10.2353/ajpath.2009.090623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams ES, Young S. Spongiform encephalopathy of Rocky Mountain elk. J Wildl Dis. 1982;18(4):465–471. doi: 10.7589/0090-3558-18.4.465. [DOI] [PubMed] [Google Scholar]
- 50.Xie Z, O'Rourke KI, Dong Z, Jenny AL, Langenberg JA, Belay ED, Schonberger LB, Petersen RB, Zou W, Kong Q, Gambetti P, Chen SG. Chronic wasting disease of elk and deer and Creutzfeldt-Jakob disease: comparative analysis of the scrapie prion protein. J Biol Chem. 2006;281(7):4199–4206. doi: 10.1074/jbc.M509052200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zampieri M, Legname G, Altafini C. Investigating the conformational stability of prion strains through a kinetic replication model. PLoS Comput Biol. 2009;5(7):e1000420. doi: 10.1371/journal.pcbi.1000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.


