Abstract
Cancer is a leading cause of death in both developed and developing countries. Metabolic reprogramming is an emerging hallmark of cancer. Glucose homeostasis is reciprocally controlled by the catabolic glycolysis and anabolic gluconeogenesis pathways. Previous studies have mainly focused on catabolic glycolysis, but recently, FBPase, a rate-limiting enzyme in gluconeogenesis, was found to play critical roles in tumour initiation and progression in several cancer types. Here, we review recent ideas and discoveries that illustrate the clinical significance of FBPase expression in various cancers, the mechanism through which FBPase influences cancer, and the mechanism of FBPase silencing. Furthermore, we summarize some of the drugs targeting FBPase and discuss their potential use in clinical applications and the problems that remain unsolved.
Keywords: FBPase, Cancer, Clinical significance, Mechanism
Background
Cancer is one of the leading causes of death worldwide [1]. In the past decade, reprogramming of energy metabolism has emerged as new hallmarks of cancer [2]. There is increasing epidemiological evidence that links the risk of cancer with metabolic disorders, such as diabetes and obesity [3]. However, the complete regulatory network of metabolism reprogramming remains to be elucidated.
Glucose homeostasis is reciprocally controlled by the catabolic glycolysis and anabolic gluconeogenesis pathways. Under normoxic conditions, normal cells process glucose first to pyruvate via glycolysis in the cytosol and thereafter to carbon dioxide in the mitochondria (oxidative phosphorylation, OXPHOS); under anaerobic conditions, glycolysis is favoured, and relatively little pyruvate is dispatched to the oxygen-consuming mitochondria [4, 5]. However, as first observed by Otto Warburg in the 1920s, some cancer cells preferentially rely on glycolysis, even in conditions of high oxygen tension (‘‘aerobic glycolysis” or “the Warburg effect’’) [6, 7]. Aerobic glycolysis was validated with the wide use of 18F-deoxyglucose positron emission tomography (FDG-PET) in most cancers [8, 9]. Aerobic glycolysis leads to several advantages for tumour growth by increasing glucose intake, enhancing lactate production and secretion, and diverting glycolytic intermediates to anabolic reactions such as ribose synthesis, serine and glycine synthesis, phosphoglycerol synthesis, and protein glycosylation [10]. Moreover, recent studies have developed promising individualized therapeutic strategies by targeting the altered energy metabolism for the treatment of cancer [11].
Though our understanding of metabolic reprogramming in cancer is progressing at an unprecedented pace, previous studies have mainly focused on glycolysis. Recently, fructose-1,6-bisphosphatase (FBPase), a rate-limiting enzyme responsible for gluconeogenesis, was found to play critical roles in tumour initiation and progression in several cancer types [12–28]. Here, we search Pubmed, EMbase, Web of Science using keywords “FBPase” and “cancer” (the most recent published report search date: 1 August 2017) and review recent ideas and discoveries regarding the role of FBPase in cancer.
FBPase location and regulation
FBPase (EC 3.1.3.11), which catalyses the hydrolysis of fructose 1,6-bisphosphate to fructose 6-phosphate and inorganic phosphate, is a rate-limiting enzyme responsible for gluconeogenesis and glyconeogenesis and, more generally, for the control of energy metabolism and glucose homeostasis [29]. In mammals, two separate genes, FBP1 and FBP2, encode the liver and muscle isoforms of FBPase, respectively. The FBP1 gene, which is mainly expressed in gluconeogenic organs, such as the liver and kidneys, is located at chromosome region 9q22.3. FBP1 consists of seven exons which span more than 31 kb that encode 338 amino acids and six introns [30]. The FBP1 promoter has been well characterized since 2000 [31]. The FBP2 gene, which was initially isolated from muscle tissues and later found to be expressed in all cells, is located at chromosome region 1p36.1 [32]. FBP2 encodes a 339-amino acid protein that shares 77% identity with the FBP1 protein [33]. However, the promoter of the FBP2 gene has not yet been characterized. All FBPases are homotetrameric proteins with a molecular weight of approximately 37 kDa per subunit (Fig. 1). FBP1 has been identified as the regulatory enzyme of gluconeogenesis, but the physiological role of FBP2 is far from clear.
Fig. 1.
Amino acid sequences and characteristic homotetrameric structure of human FBPase. This figure provides the human FBP1 and FBP2 amino acid sequence. Human FBP2 is 77% identical to human FBP2 [34]. The sequence alignments were run through BLAST. The characteristic homotetrameric structure of human FBPase was shown [35]
Acute regulation of FBPase is achieved mainly through its oscillation between two conformational states: the inactive T form when in complex with AMP and the active R form [29, 36–40]. By modulating pyruvate kinase activity, fructose-1,6-bisphosphate (F1,6BP) affects substrate cycling in the pyruvatel/phosphoenolpyruvate (PEP) substrate cycle [41]. Phosphofructokinase-1 (PFK1), the “gatekeeper” of glycolysis, converts fructose-6-phosphate (F6P) to F1,6BP and has the opposite effect of FBPase in regulating F1,6BP [42]. Fructose-2,6-bisphosphate (F2,6BP) is a potent allosteric activator of PFK1 and a competitive inhibitor of FBPase [5, 41–44]. When the level of F2,6BP is low, the rates of gluconeogenesis are high (such as in starvation and diabetes). Conversely, when the level of F2,6BP is high, the rate of gluconeogenesis is low (such as during refeeding and insulin administration) [5]. The synthesis and hydrolysis of this regulator are catalysed by the bifunctional enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFK-2/FBPase-2) [45]. Phosphorylation of this enzyme by cAMP-dependent protein kinase results in inhibition of the kinase and activation of the bisphosphatase, whereas dephosphorylation has the opposite effect [36, 40]. TIGAR (TP53-induced glycolysis and apoptosis regulator), a recently identified enzyme, shares similarities with the bisphosphatase domain of PFK-2/FBPase-2 [46]. TIGAR acts to degrade intracellular F2,6BP [47]. Interestingly, FBP1 and FBP2 differ significantly in their kinetic properties. FBP2 is approximately 100 times more susceptible to the allosteric inhibitors AMP and NAD+ [48] and approximately 1000 times more sensitive to inhibition by Ca2+ than FBP1 [49, 50]. Crystal structures have shown that in contrast to the well-studied R form of FBP1, which is flat, the R form of FBP2 was diametrically different, with a perpendicular orientation of the upper and lower dimers [37].
Chronic regulation of hepatic glucose metabolism occurs through transcriptional and hormonal mechanisms [51]. The master hormone that promotes gluconeogenesis is glucagon. Increased glucagon stimulates gluconeogenesis via the induction of intracellular cAMP. cAMP triggers protein kinase A (PKA), leading to the phosphorylation of PFK-2/FBPase-2, which activates FBPase-2, leading to the dephosphorylation of F2,6BP and concomitant increases in FBPase activity and gluconeogenesis [45]. In addition, PKA activates cAMP response element-binding protein (CREB), the CREB coactivator CRTC2 [52], and the class II histone deacetylases (HDACs)/FOXO pathways [53, 54] and promotes the expression of key gluconeogenic genes; however, it suppresses the expression of glycolytic genes. Glucocorticoid hormones and glucagon plays essential synergistic roles in the regulation of gluconeogenesis [55]. The response to glucocorticoids is mediated by the glucocorticoid receptor (GR), which binds to glucocorticoid responsive elements (GREs) in the promoters of gluconeogenic genes [56]. In contrast, insulin acts to repress the transcription of gluconeogenic enzymes. This repression of the expression of gluconeogenic enzymes is achieved via the activation of the insulin-phosphoinositide 3-kinase (PI3K)-Akt pathway or suppression of the cAMP/PKA/CREB pathway [57] (Fig. 2).
Fig. 2.
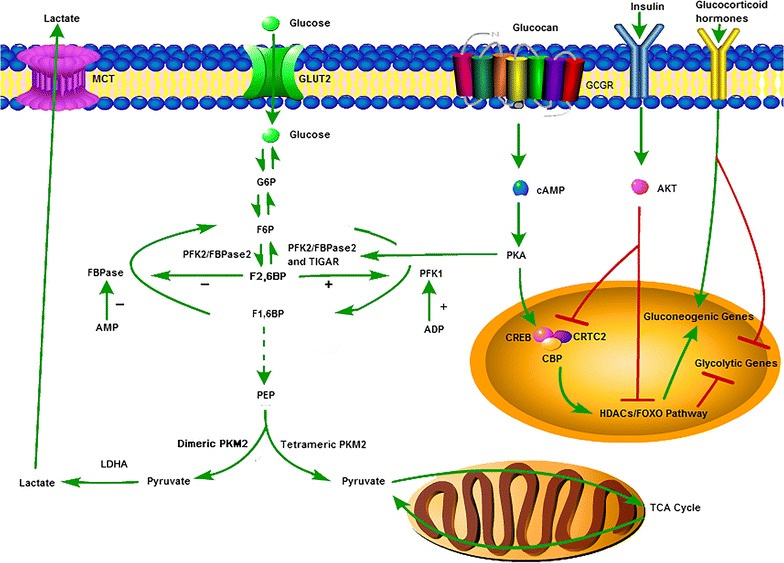
Regulation of FBPase. Acute regulation of FBPase is achieved mainly through allosteric regulator. Chronic regulation of FBPase occurs through transcriptional and hormonal regulation mainly by glucagon, glucocorticoid hormones and insulin. ADP adenosine diphosphate, AMP adenosine monophosphate, AKT protein kinase B, cAMP cyclic adenosine monophosphate, CBP CREB-binding protein, CREB cAMP response element-binding protein, CRTC2 CREB coactivator, G6P glucose-6-phosphate, GLUT2 glucose transportor 2, GCGR glucagon receptor, F1,6BP fructose-1,6-bisphosphate, F2,6BP fructose-2,6-bisphosphate, F6P fructose-6-phosphate, FBPase fructose-1,6-bisphosphatase, FOXO forkhead box O protein, HDAC histone deacetylase, LDHA lactate dehydrogenase A, MCT monocarboxylate transporters, PFK2/FBPase2 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase, PEP phosphoenolpyruvate, PFK1 phosphofructokinase-1, PKA protein kinase A, PKM2 pyruvate kinase M2, TIGAR TP53-induced glycolysis and apoptosis regulator, TCA tricarboxylic acid
FBPase and non-cancerous diseases
FBP1 deficiency
Mutations in the FBP1 gene cause FBP1 deficiency, an inherited autosomal recessive disorder, which leads to the impairment of glucose synthesis from all gluconeogenic precursors [58]. This deficiency was first described by Baker and Winegrad in 1970 [59]. This disorder is characterized by recurrent episodes of hypoglycaemia and metabolic acidosis during fasting, with symptoms usually manifesting during the first days of life [60–62]. If not treated appropriately, FBP1 deficiency leads to unexpected infant death [63]. However, with diet control and avoidance of prolonged fasting, most adult patients exhibit relatively normal clinical profiles.
FBPase and type 2 diabetes
Blood glucose levels are elevated in type 2 diabetes (T2DM) due to impaired insulin secretion resulting from declining β-cell function; decreased glucose uptake by tissues such as muscle, liver, and fat; and increased hepatic glucose production (HGP) [64]. Gluconeogenesis contributes approximately 50% of the total HGP in humans following overnight fasting and is primarily responsible for the increase in fasting HGP in individuals with T2DM [64–66]. The rate-limiting enzymes of gluconeogenesis have been raised as potential targets for combating T2DM. FBPase is an attractive target as it functions within only the gluconeogenesis pathway [67]. In animal models, the inhibition of FBPase markedly inhibited gluconeogenesis and increased glucose sensitivity and utilization [68]. Upregulation of FBPase in pancreatic islet cells, as examined in transgenic mice or stably transfected pancreatic cell lines and occurring in states of T2DM, decreased the cell proliferation rate and significantly suppressed glucose-induced insulin secretion (GSIS) [69]. Downregulation of FBP1 in mouse pancreatic β-cells by small interfering RNA enhanced glucose utilization and GSIS, whereas overexpression of FBP1 decreased GSIS [70]. Phase 2 clinical studies of some inhibitors of FBP1in T2DM are in progress [71–73].
FBPase and cancer
Accumulating evidence has disclosed the role of FBPase in the carcinogenesis, development and progression of various cancer types. Lower FBPase expression frequently correlated significantly with an advanced tumour stage, a highly malignant phenotype, and worse prognoses in cancer patients. All these data implied that FBPase might be a novel biomarker and potential target for the treatment of cancer (Table 1).
Table 1.
FBPase expression in cancers (listed in alphabetical order)
| Type of cancer | FBPase expression | Change in expression over disease progression | Prognostic significance | Reference(s) |
|---|---|---|---|---|
| Breast cancer | Lower in animal model, human breast cancer [74–76], basal-like breast cancer cell lines [20], triple-negative breast cancer but not in luminal cell lines [19] and brain metastatic cells [21]. Data mining shown FBP1 over-expression were common in breast cancer irrespective of histological type in cell lines and human breast cancer [20] | Expression inhibited tumorigenicity in vitro and tumor-formation in vivo [20, 22] but promoted the growth of brain metastasis [21]. FBP1 expression associated with nuclear grade and tumor stage [18] | Loss of FBP1 expression associated with poor survival [18, 20, 22]. But data mining shown no correlation between FBP1 and prognosis in triple-negative breast cancer [20] | [18–22, 74–76] |
| Colon cancer | Lower in cancer cell lines and in human colon cancer [17] | Overexpression reduced cancer cell colony formation and inhibited the growth of cancer cells [17] | [17] | |
| Gastric cancer | Downregulated in gastric cancer cell lines and gastric carcinomas [17, 25, 26] | Overexpression inhibited proliferation inhibition in vitro as well as xenograft tumor growth in vivo [25, 26] | Absent or low FBP2 expression correlated with poor survival [25] | [17, 25, 26] |
| Liver cancer | Decreased in 3-methyl-4-dimethyl aminoazobenzene (3MeDAB) induced [77] and choline-deficient diet-induced hepatocellular carcinoma model [78]; Decreased in most human liver cancer cell lines [14, 17] and in human hepatocellular carcinoma [15–17, 77, 79–82] | Low expression correlated with highly malignant phenotype, including large tumor size, poor differentiation, advanced tumor stage [15, 80–82], vascular cell invasion and high pathological grade [14] | Loss of FBP1 expression associated with poor overall survival and higher tumor recurrence rates [14, 15, 79, 81, 82] | [14–17, 77–82] |
| Lung cancer | Loss in lung cancer cells [12, 13] and in human lung cancer tissues [13, 83, 84] | Forced expression inhibited tumorigenesis and invasion in lung cancer cells [12, 13] and cancer progression in human lung cancer [13] | Low FBP1 expression correlated with poor overall survival [13] | [12, 13, 83, 84] |
| Pancreatic cancer | Lower in pancreatic cancer tissues [27, 28] | FBP1 expression inversely correlated with tumor grades and prognosis [27, 28] | [27, 28] | |
| Renal carcinoma | Ubiquitous loss in clear cell renal cell carcinoma [23, 24, 85, 86] | FBP1 expression in several renal cancer cell lines inhibited their growth. Suppression of FBP1 correlated with advanced tumour stage [24]. But no correlation was found between clinicopathological factors, including age, gender, T stage, Fuhrman grade and expression of FBP1 expression in another study [23] | Suppression correlated with worse patient prognosis [24] | [23, 24, 85, 86] |
| Small intestinal neuroendocrine tumour | Comprehensive integrated genomic analysis shown epigenetically dysregulation [87] | [87] |
Breast cancer
FBPase was found to decrease significantly in animal models, basal-like breast cancer (BLBC) cell lines, triple-negative breast cancer (TNBC), and human breast cancer but not in luminal cell lines and brain metastatic cells [19, 21, 22, 74–76]. FBPase inhibits tumourigenicity in vitro and tumour formation in vivo [20, 23] as well as the growth of brain metastasis [21]. FBP1 expression was associated with the nuclear grade and tumour stage [18]. Loss of FBP1 expression was associated with poor survival [18, 20, 22] and was strongly related to palindromia after tamoxifen treatment in patients with breast cancer [88].
However, some contradictory results existed. FBP1 over-expression was found to be a common event, irrespective of histological type, in cell lines and human breast cancer. Furthermore, there was no correlation between FBP1 and the prognosis of TNBC [20]. These contradictory findings indicated that the prognostic value of FBP1 in breast cancer may be molecular type- and tissue type-dependent.
Gastric cancer
Expression of FBP1 and FBP2 was significantly downregulated in gastric cancer cell lines and gastric carcinomas (GCs) due to promoter hypermethylation [17, 25, 26]. Ectopic expression of FBPase in GC cells led to significant inhibition of proliferation in vitro, as well as xenograft tumour growth in vivo [25, 26]. Absent or low FBP2 expression [25] and FBP1 promoter methylation [26] in GC tissues was correlated with the poor survival of GC patients. However, no significant correlation between the methylation of the FBP1 promoter and clinicopathological features such as age, gender, Helicobacter pylori status, Lauren type, differentiation or pathologic stage was found [26].
Liver cancer
FBPase suppression in liver cancer has been widely validated in animal models [77, 78], cancer cell lines [14, 17] and clinical specimens [14, 15, 17, 24, 78–82]. Restoration of FBP1 expression by agents such as plumbagin [77], 5-aza-2′-deoxycytidine (Aza) [17], dexamethasone [89], or bortezomib [80] or by ectopic lentiviral transfection [14] could significantly inhibit cell growth and colony-formation ability in vitro [14, 17, 80], as well as xenograft tumour growth in vivo [15, 81, 89]. Hepatocellular carcinoma (HCC) exhibiting low expression of FBP1 had a highly malignant phenotype, including large tumour size, poor differentiation, and advanced tumour stage [15, 80–82], as well as vascular cell invasion and a high pathological grade (stages III–IV) [14]. Loss of FBP1 expression was associated with poor overall survival and higher tumour recurrence rates [14, 15, 79, 81, 82].
In addition, proteomic techniques have recently provided new evidence. Proteomic analysis has revealed significant downregulation of FBPase in carcinogenic processes in rats after as early as 3 weeks of exposure, indicating its potential utility as an early predictive biomarker for liver carcinogenicity [90]. In a review including a total of 16 proteomic studies, FBP1 was one of 27 proteins identified as differentially expressed proteins with consistent directions of change in at least three studies, that were found to be potential biomarkers for HCC [91].
Lung cancer
FBP1 was absent in lung cancer cells, and forced expression of FBP1 led to inhibition of tumorigenesis and invasion, especially under hypoxic conditions (0.1% oxygen) [12, 13]. In human lung cancer tissues, FBP1 mRNA and proteins were found to poorly express when compared to paired normal lung tissues [13, 83, 84]. Low FBP1 expression correlates with poor overall survival and cancer progression [13].
Renal carcinoma
Recently, a ubiquitous loss of FBP1 expression has been identified in clear cell renal cell carcinoma (ccRCC) [23, 24, 85, 86]. Ectopic FBP1 expression in several ccRCC cell lines significantly inhibited their growth, while FBP1 depletion promoted the growth of kidney proximal tubule cells, the presumptive cells-of-origin for ccRCC. Lower FBP1 expression correlated significantly with advanced tumour stage and worse patient prognosis [24]. However, in another study, no correlation was found between clinicopathological factors, including age, gender, T stage, and Fuhrman grade and the expression of FBP1. This finding may be partially due to the high proportion of patients with low T stages and low Fuhrman grades in that cohort [23].
Other cancer types
There is an even bigger knowledge gap in the role of FBPase in some cancer types due to only one to two publications, such as in colon cancer, pancreatic cancer and small intestinal neuroendocrine tumor.
Downregulation of FBP1 was also observed in HT29, SW480, SW620, HCT116, LoVo and RKO colon cancer cell lines when compared to human normal adult colon tissue, and this downregulation correlated well with the promoter methylation status of FBP1. FBP1 overexpression reduced the colony formation abilities of cancer cells and inhibited their growth. In human colon cancer, FBP1 expression was significantly downregulated in 80% (4/5) of the colon tumour tissues when compared with adjacent non-tumour tissues [17].
Recently, one study reported their data of integrated molecular analysis of small intestinal neuroendocrine tumour (SINETs) in 97 tumours and 25 normal tissue samples from 85 individuals. 21 epigenetically dysregulated genes were identified, one of the most significant genes was FBP1 (84%). 82% of tumour specimens demonstrate altered methylation in at least 4 of the top 5 frequent altered candidates including caudal type homeobox 1 (CDX1), FBP1, transmembrane protein 171 (TMEM171), ganglioside induced differentiation associated protein 1 like 1 (GDAP1L1), and cadherin, EGF LAG seven-pass G-type receptor (CELSR3) [87].
FBP1 was consistently reported to be expressed at low levels in pancreatic cancer tissues; on the contrary, FBP1 was always at high level in normal pancreatic tissues. FBP1 expression inversely correlated with tumour grade and prognosis [27, 28].
Mechanism through which FBPase influences cancer
The mechanism through which FBP1 influences cancer cells were summarized in Fig. 3. At present, the role of FBP1 in regulating the Warburg effect in cancer cells was best understood.
Fig. 3.
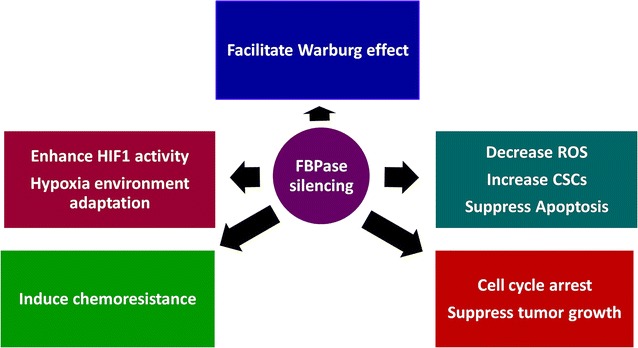
Mechanisms through which FBPase influence cancer
Loss of FBPase facilitates the Warburg effect in cancer
Glucose uptake and lactate secretion are two common indicators of glycolysis. Upon FBPase silencing, glucose uptake and lactate secretion were significantly increased in various cancer cells (BLBC [20, 22], ccRCC [24], gastric cancer [26], HCC [14, 15, 80–82], lung cancer [12, 13], and pancreatic cancer cell lines [28]). TXNIP, a commonly used intracellular glucose sensor [92], and insulin, the major hormone regulating glucose uptake [53], were tested in cancer cell lines. FBP1 expression decreased glucose uptake, TXNIP induction and insulin sensitivities, whereas the loss of FBP1 enhanced glucose uptake, TXNIP induction and insulin sensitivities [13, 16, 23, 24]. These finding indicated that FBP1 was critical in inhibiting glucose uptake, as exemplified by the downregulation of glucose and insulin sensitivities. Accumulation of lactate is another common feature of cancer cells and is involved in the progression of malignancies [93]. Lactate secretion was significantly decreased in FBP1-expressing cells [12, 13, 15, 22, 26, 80]. The extracellular acidification rate (ECAR) is the glycolysis rate after glucose treatments and is equal to the glycolysis capacity after oligomycin treatment. In HCC, studies have shown that FBP1 significantly reduced the ECAR, while FBP1 suppression did the opposite [14].
Besides, certain regulators in carcinogenesis were found to reprogram cancer cell metabolism by suppressing FBP1. NMP1, a multifaceted nucleolar protein, was found to stimulate glucose uptake and lactate generation in pancreatic cancer cells by directly inhibiting FBP1 expression. Restoring FBP1 in pancreatic cancer cells reversed the NPM1-induced dysfunction of glucose metabolism [28]. TRIM proteins, members of a subfamily of the RING type E3 ubiquitin ligases [94], were found to increase glucose consumption and lactate production in HCC cells by promoting FBP1 degradation. Importantly, the effect of TRIM28 was largely inhibited by the co-expression of FBP1 [80]. c-Myc, a crucial downstream factor of Wnt/β-catenin signalling, was found to be negatively correlated with the level of FBP1 in breast cancer cells [20]. As c-Myc is also a transcription factor involved in metabolic reprogramming [95], it is reasoned that the inhibitory effect of FBP1 in glycolysis might be mediated partially by the downregulation of c-Myc. However, the precise mechanism by which FBP1 regulates Wnt/β-catenin signalling warrants further investigation [20].
In addition, the consuming oxygen during OXPHOS and aerobic glycolysis is totally different. The reliance of tumour cells on glycolysis for energy production causes them to decrease oxygen consumption to adapt to the hypoxia tumour microenvironment. The basal oxygen consumption rate (OCR) was found to be significantly decreased in FBP1-knockdown cells, whereas it was significantly increased in FBP1-expressing cells. Similar results were obtained in the analyses of ATP-linked and maximal OCR [12, 13, 22], indicating that FBP1 is involved in the switch from glycolysis to OXPHOS.
FBPase silencing helped maintain energy homeostasis in cancer cells. For every glucose molecule a cell consumes, aerobic glycolysis produces 2 ATP, whereas OXPHOS produces 36 ATP. Under normoxic conditions, expression or knockdown of FBP1 did not alter the steady-state level of ATP in BLBC or luminal cells. However, under hypoxia, knockdown of FBP1 helped maintain ATP production, whereas expression of FBP1 significantly decreased ATP production in BCLC and HCC [15, 22]. In gastric cancer cells, studies have found that FBP2 overexpression significantly reduces the levels of ATP and lactate through interference of the Akt-mTOR pathway [25].
Stable isotope-resolved metabolomic (SIRM) analysis was used to investigate the metabolic fate of [U-13C]-glucose, which directly produces glycolytic intermediates that contain six or three 13C atoms (M6/M3 species) and the intermediates of the first turn of the tricarboxylic acid (TCA) cycle that contain two 13C atoms (M2 species). When FBP1 was overexpressed, M3 enrichment of lactate was significantly inhibited, and the levels of the glycolytic intermediates F-1,6-BP (M6 species), dihydroxyacetone phosphate (M3 species), and glucose-6-phosphate were decreased. Ectopic FBP1 tended to inhibit M2 enrichment of TCA cycle intermediates, such as succinate, fumarate and malate, as well as M4 enrichment of citrate [15, 22, 96]. Furthermore, FBP1 expression reduced M5 enrichment of the ribosyl unit of ribonucleotides and their derivatives (i.e., NAD+, and UDPG), suggesting that FBP1 suppressed de novo nucleic acid synthesis through the pentose phosphate pathway [15, 22, 24]. In line with these findings, the ratio of NADP+/NADPH was increased in FBP1-expressing cells, whereas this ratio was decreased in FBP1-knockdown cells. The production of the M3 isotopologues of glycerol-3-phosphate (G3P) and serine was significantly reduced in FBP1-expressing cells [22]. Reduced glucose-dependent TCA flux is known to increase anaplerotic glutamine flux [97]; elevated glutamine uptake and enrichment of glutamine-derived TCA cycle intermediates (M4 species) were observed upon forced FBPase expression [21, 24]. Increased oxidation of branched chain amino acids (BCAAs) (valine, leucine, and isoleucine), except for glutamine, was also found in brain metastatic cancer cells, with upregulation of FBP2, but not FBP1, and enhanced gluconeogenesis in the absence of glucose [21].
All these data imply that loss of FBPase facilitates glycolytic flux and decreases OXPHOS in cancer cells.
How cancer cells switch OXPHOS to aerobic glycolysis by FBPase silencing
Key enzymes of glucose uptake and aerobic glycolysis in cancer cells were found to decrease significantly when FBPase was expressed. In HCC, FBP1 expression was found to significantly decrease the levels of glucose transporter 1 (GLUT1) and lactate dehydrogenase A (LDHA) [82]. Three enzymes are involved in catalysing the irreversible steps of glycolysis: hexokinase (HK) [98, 99], phosphofructokinase (PFK) [100], and pyruvate kinase (PK) [101]. While dimeric PKM2 diverts glucose metabolism towards anabolism through glycolysis, tetrameric PKM2 promotes the flux of glucose-derived carbons for ATP production via oxidative phosphorylation [101]. FBP1 expression significantly decreased the tetrameric PKM2, whereas knockdown of FBP1 increased the formation of tetrameric PKM2. In addition, FBP1 expression was found to significantly decrease the HK2 and PFK1 levels in HCC [15]. All these results indicated that the loss of FBP1 activated GLUT1, PKM2, HK2, PFK1 and LDHA, which facilitated glucose uptake and lactate production and triggered the switch to aerobic glycolysis.
In addition, FBP1 expression was found to be correlated with higher complex I activity. TFB1M is a nuclear gene, encoding mitochondrial transcription factor, which is essential for mitochondrial biogenesis and OXPHOS [102]. When FBP1 was expressed, TFB1M and its targets from mitochondrial complex I, ND1 and ND5, were found to be increased, indicating that the increase in complex I activity is the main factor underlying the increase in OXPHOS [22].
FBPase increased ROS generation, reduced cancer stem cells, and induced apoptosis
Along with increases in complex I activity and mitochondrial OCR, ROS levels increased when FBPase was expressed [12, 17, 19, 22, 25]. ROS amplify tumourigenic phenotypes, such as cancer stem cells (CSCs) [103]. Breast CSCs are enriched in cells with a CD44 high/CD24 low/EpCAM+ phenotype [104]. In BLBC cell lines, FBP1 expression significantly reduced the percentage of CD44 high/CD24 low/EpCAM+ populations and decreased tumour sphere formation. Conversely, FBP1 silencing resulted in an increased CSC-like phenotype [22]. Mechanically, increased ROS levels induced by FBP1 would shift the interaction of β-catenin from TCF4 to FOX O3a and thus inhibit tumourigenicity in vitro and tumour formation in vivo.
Moreover, ROS induces mitochondrial apoptosis [105]. Forced FBP1 expression in lung cancer stem cells and breast cancer or FBP2 expression in gastric cancer increased apoptosis by reducing the Bax/Bcl-2 ratio, inducing poly ADP-ribose polymerase (PARP), caspase-3 and caspase-9 activation and suppressing endogenous ROS scavenging systems such as superoxide dismutase (SOD) [12, 19, 25]. In breast cancer cells, FBP1 limited the efficient removal of diseased mitochondria and reduced the expression of hypoxia-induced factor 1α (HIF1α), BCL2/adenovirus E1B 19 kDa interacting protein 3-like (BNIP3L/NIX), and BCL2/adenovirus E1B 19 kDa interacting protein 3 (BNIP3), which disrupts BNIP3/NIX-Bcl-2 complex formation under normal conditions but promotes complex formation between Bcl-2 and Beclin 1 [19]. Annexin V+/propidium iodide (PI)− and Annexin V+/PI+ cells represent early apoptotic cells and late apoptotic/necrotic cells, respectively [106]. FBP2 expression increased both subpopulations in gastric cancer cells [25]. In brain metastatic breast cancer cells, knocking down FBP2 resulted in a significant amount of apoptotic cell death (as indicated by the increase incleaved PARP), whereas exogenous FBP2 significantly rescued cell death [21].
FBPase induced cell cycle arrest to suppress tumour growth
The ability to sustain chronic proliferation is the most fundamental trait of cancer cells. Normal tissues carefully maintain the homeostasis of cell number, normal tissue architecture and function by rigorous regulation of progression signals through the cell cycle as well as cell growth. But cancer cells, by hijacking these regulating mechanisms, become masters of their own destinies [2]. Cell cycle arrests if the numbers of cells in G2 and M phase increase, whereas it proceeds if the numbers of cells in S phase increase. Cell cycle distribution was investigated to understand the molecular mechanisms by which FBP1 suppressed colony formation and cell proliferation. In hepatocellular carcinoma and colon cancer, forced FBP1 expression was found to increase the number of G2–M phase cells but decrease the number of S phase cells [17]. In lung cancer, FBP2 accumulated in cell nuclei during the S and G2 phases and interacted with histone family members and with several proteins involved in cell-cycle regulation and RNA processing [83, 107]. All these data indicated that the growth suppression induced by forced FBP1 expression might be partly due to cell cycle arrest. However, the detailed mechanism remains unclear.
FBPase inhibits HIF1 activity and influences adaption to hypoxia tumour microenvironment of cancer
All cancer cells suffer hypoxia microenvironment due to rapid proliferation, differentiation and poorly formed tumour vasculature [108]. HIF1, a well-known transcriptional regulator, is the most important aspect of how cancer cells respond to the unfriendly microenvironment. HIF1 promotes glycolytic metabolic alterations by the activation of multiple glycolytic genes. Under conditions of persistent hypoxia, the induction of HIF1 leads to adaptive mechanisms for the reduction of ROS and re-establishment of homeostasis [109].
Knockdown of FBP1 significantly reduced growth inhibition in luminal cell lines under hypoxic condition (0.1% O2) but not at normoxic condition (21% O2). Similarly, FBP1 expression induced a drastic growth inhibition in BLBC cell lines under hypoxic condition but not at normoxic condition [22]. The expression of FBP1, but not of FBP2, was inversely correlated with HIF1α activity in RCC [23, 24]. FBP1 was also found to be negative correlated with HIF1α activity in HCC and breast cancer [18, 82]. Ectopic expression of FBP1 suppressed HIF activity and reduced the expression of HIF target genes, whereas loss of FBP1 enhanced HIF activity [24]. FBP1 inhibited HIF1α activity in the nucleus via a direct interaction with the HIF-inhibitory domain in an enzyme-activity-independent manner [24]. ALL these results indicated that the regulation of FBP1 are important when oxygen and glucose levels are limiting, as often occurs in solid tumours [110].
FBPase antagonizes cancer chemoresistance
Gemcitabine is the first-line chemotherapy for pancreatic cancer [111]. However, gemcitabine fails to significantly improve prognosis of pancreatic carcinoma patients due to acquisition of chemoresistance in patients. It is well documented that gemcitabine treatment results in undesirable activation of the RAS-RAF-MAPK pathway [112]. IQ motif-containing GTPase-activating protein 1 (IQGAP1) is a MAPK scaffold that directly regulates the activation of RAF, MEK, and extracellular signalling-regulated kinases (ERKs) [113]. In pancreatic cancer, FBP1 expression impeded gemcitabine-induced ERK activation through inhibition of the IQGAP1-ERK1/2 signalling axis in a manner independent of its enzymatic activity. Co-treatment of FBP1-derived small peptide inhibitor FBP1 E4 enhanced the anti-cancer efficacy of gemcitabine in pancreatic cancer [27]. However, the molecular mechanisms by which FBP1 antagonizes cancer chemoresistance in pancreatic cancer warrant further investigation. Moreover, whether FBP1 exerts similar effect in chemoresistance in other cancer types needs to be elucidated.
Regulation of aberrant expression of FBP1 in cancer
The mechanisms for the aberrant expression of FBP1 are mainly focused on the epigenetic regulation of the FBP1 promoter. However, recently, ubiquitin-mediated degradation and copy number loss of FBP1 were also shown to explain the loss of FBPase in cancer (Table 2).
Table 2.
Regulation of aberrant expression of FBP1 in cancer
| Regulator | Interaction | Reference(s) |
|---|---|---|
| LSD1 | Promoter methylation | [114] |
| NF-kappaB | Promoter methylation | [26] |
| NPM1 | Promoter methylation | [28] |
| Snail-G9a-Dnmt1 | Promoter methylation | [22] |
| ZEB1 | Promoter methylation | [13] |
| HDAC1/2 | Histone deacetylation | [81] |
| Copy number loss | Genomic alterations | [15, 86] |
| TRIM28 | Ubiquitination degradation | [80] |
Epigenetic changes
Epigenetic changes, including DNA methylation and histone modifications, have been shown to alter patterns of gene expression and to be involved in carcinogenesis [115]. The methylation of the FBPase promoter has been observed in various cancers [13, 15, 17, 22, 25, 26, 75, 114]. NF-ΚB functioning downstream of the Ras pathway promoted the epigenetic downregulation of FBP1 in gastric cancer [26]. The Snail-G9a-Dnmt1 complex, which is critical for E-cadherin promoter silencing and the corresponding increase in H3K9me2 and DNA methylation, was also required for the promoter-methylation of FBP1 in BLBC [22]. Lysine (K)-specificdemethylase 1A (LSD1)-mediated demethylation of H3K4me2 at FBP1 promoters suppressed FBP1 expression in HepG2 cells [114]. Zinc finger E-box-binding homeobox 1 (ZEB1) bound to the FBP1 promoter to enhance DNA methylation in lung cancer [13]. NPM1 bound directly to the FBP1 promoter region to suppress the expression of FBP1 in pancreatic cancer [28]. Demethylation with 5-aza-deoxycytidine (5AZA), Ras inhibitor or LSD inhibitor restored FBP1 expression, implying that hypermethylation is directly responsible for the loss or downregulation of FBP1 expression [13, 26, 75]. A study has shown that additional inhibition of histone deacetylase inhibitor further increased FBPase expression compared to 5aza alone [75], suggesting that histone deacetylation may contribute synergistically to the silencing of FBPase. In line with this finding, histone deacetylase 1 (HDAC1) and HDAC2 together induced the suppression of FBP1 expression by decreasing histone H3 lysine 27 acetylation (H3K27Ac) of the FBP1 enhancer in HCC. Treatment with HDAC inhibitors or knockdown of HDAC1 and/or HDAC2 restored FBP1 expression [81].
Copy number loss
Copy number loss of FBP1 was observed in ccRCC cases [86]. Copy number loss of FBP1 was significantly associated with lower FBP1 expression in HCC [15]. These data indicated that genomic alterations were also responsible for FBP1 inhibition.
Ubiquitin-mediated degradation
Recently, the E3 ubiquitin ligase TRIM28 has been found to play a critical role in regulating FBP1 protein levels through a post-translational mechanism in HCC. TRIM28 was found to directly bind to and promote the ubiquitination and degradation of FBP1. MAGE-A3 and MAGE-C2, which are known to specifically bind to TRIM28 [94, 116], can enhance TRIM28-dependent degradation of FBP1 by forming ubiquitin ligase complexes with TRIM28 [80].
Chemical inhibitors utilized to restore FBP1 expression
Several chemical inhibitors have been shown to successfully restore the expression of FBP1. Drugs targeting promoter methylation, HDAC and the upstream regulator of FBPase, have been proven efficacious. Their interactions are detailed below (Fig. 4).
Fig. 4.
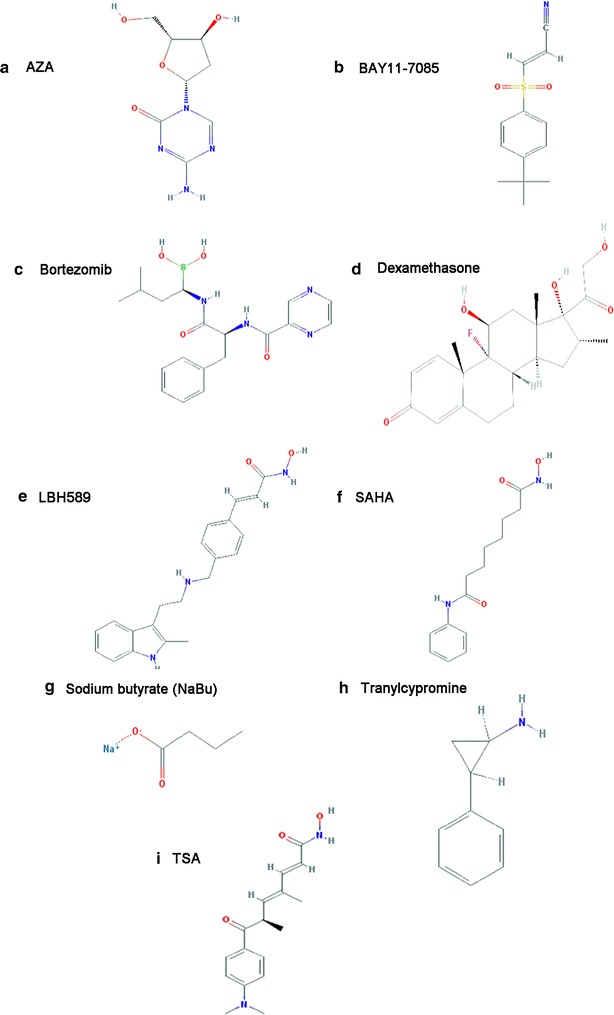
Structures of chemical inhibitors restoring FBPase. a 5-Aza-deoxycytidin (5AZA) [117], b BAY11-7085 [118], c bortezomib [119], d dexamethasone [120], e LBH589 [121], f SAHA [122], g sodium butyrate (NaBu) [123], h tranylcypromine (TCP) [124], i trichostatin A (TSA) [125]
Recently, 5AZA, a hypomethylating agent [126], together with HDAC inhibitors, such as trichostatin A (TSA), sodium butyrate (NaBu) [127], SAHA [128] and LBH589 [129], have been approved by the Food and Drug Administration (FDA) of the USA for cancer therapy in myelodysplastic syndrome (MDS) and acute myeloid leukaemia (AML) [130]. A combination of DNA methyltransferase inhibitors and HDAC inhibitors has also shown promising synergistic effects in the treatment of MDS, AML and non-Hodgkin’s lymphoma [131]. Treatment of cells with 5AZA resulted in a significant increase in the expression of FBPase mRNA in breast cancer, gastric cancer and lung cancer [13, 25, 75], while treatment with sodium butyrate, SAHA and LBH589 upregulated FBP1 protein and mRNA expression in HCC [81]. Restoration of FBP1 expression by HDAC inhibitors led to a switch from glycolysis to gluconeogenesis, altered energy metabolism and inhibition of tumour growth [81]. All these data indicated that the silencing of FBP1 can be a target of methyltransferase inhibitors and HDAC inhibitors for the potential treatment of cancer. However, we are still a long way from the clinical application of these epigenetic drugs for solid tumours [132].
LSD1, the first histone demethylase identified, converts active H3K4me2/3 to the less active H3K4me1 mark, leading to gene activation [133]. Meanwhile, LSD1 also converts the inactive H3K9me3 into the less repressive H3K9me1 or H3K9me2 marks, thereby leading to gene derepression [134]. Thus, the functional outcome of LSD1 activity depends on the balance between the modification of H3K4me2/3 or H3K9me3. Tranylcypromine (TCP) is a potent inhibitor of the demethylation activity of LSD1 [135]. FBP1 was robustly and quickly induced by TCP treatment in HepG2 cells [114]. However, before using LSD1 inhibitors, their pleiotropic actions should be taken into careful consideration.
Bortezomib, a 26S proteasome inhibitor, has been approved by the FDA for the treatment of multiple myeloma and mantle cell lymphoma [136–139]. In HCC, a TRIM28-induced decrease in FBP1 protein levels (but not mRNA levels) was completely inhibited by bortezomib treatment. Meanwhile, bortezomib-induced decreases in glucose consumption, lactate levels and cell growth inhibition were largely diminished by the knockdown of FBP1. These data indicated that bortezomib could regulate the Warburg effect by inhibiting the proteasome-dependent degradation of FBP1 [80], which might be harnessed in combination therapy [140], but not bortezomib monotherapy, for cancer [141].
In addition, drugs targeting upstream regulators of FBPase have shown efficacy in restoring FBPase expression. Ras, the first oncogene isolated from human tumour cells, was aberrantly activated in many cancers [142]. In gastric cancer, Ras-induced FBP1 downregulation was reversed after the inhibition of NF-ΚB activity by either a chemical inhibitor of NF-ΚB, BAY11-7085, or a genetic suppressor of NF-ΚB, IkB-alpha M [26, 143]. Dexamethasone, an active form of synthesized glucocorticoids, restored the expression of gluconeogenesis genes, including FBP1, thereby antagonizing the Warburg effect and leading to therapeutic efficacy in the treatment of hepatocarcinoma [89]. Nevertheless, considering the complex regulatory networks of these regulators, adverse effects and toxicity caused by poor specificity may always be potential problems in their clinical applications.
Conclusions and perspectives
Research on metabolic reprogramming in cancer is progressing at an unprecedented pace. FBPase, one of the rate-limiting enzymes responsible for gluconeogenesis, is usually found to be downregulated in many cancers and is treated as a tumour-suppressor gene. Meanwhile, these results should be interpreted with caution due to only one to two publications in some cancer types such as in colon cancer, pancreatic cancer and small intestinal neuroendocrine tumor. In most cancers, FBPase goes far beyond its enzymatic function, as it is located inside the cell nucleus and is efficacious through a catalytic-activity-independent mechanism via direct interaction with other genes. In our opinion, to maintain rapid proliferation and differentiation, all cancer cells will confront a microenvironmental energy crisis, including the intrinsic shortage of metabolic substrates and energy as well as the external deterioration of the microenvironment due to factors such as hypoxia, acidosis and hypoglycaemia. To overcome all these unfavourable growth conditions, cancer cells have to reprogram their metabolic and epigenetic phenotypes through the activation of oncogenes and inactivation of tumour-suppressor genes, altering the pattern of epigenetic modification and leading to the aberrant expression of numerous genes, including those involved in metabolic rate-limiting, metastasis or differentiation. Therefore, it is not surprising to find an aberrant epigenetic modification of FBPase in cancer. Furthermore, it is also not surprising to find a close correlation between FBPase expression and transcriptional factors such as HIF, EMT transcriptional factors and cancer differentiation. However, the complete mechanism underlying the interplay of FBPase and other genes is complicated and unclear. Does noncoding RNA play an important role in the regulation of FBPase expression? What is the meaning and intrinsic mechanism of the interplay between aberrant FBPase and other cancer hallmarks? Investigating the role of FBPase in CSCs and apoptosis, for example, will promote our understanding of cell differentiation and programmed cell death and reveal new clues for combating cancer.
Several problems remain to be solved for the use of FBPase as a target in cancer treatment. One main obstacle is the identification of distinct regulatory markers exclusive to aberrant FBPase. Without this knowledge, drugs targeting aberrant FBPase with higher specificity but fewer side effects can’t be found or designed. Another problem is identifying the optimal timing for the use of FBPase-targeting drugs, as these drugs may have completely different effects at different stages of progression of different types of cancer. These drugs may induce a switch from aerobic glycolysis to OXPHOS and inhibit the aggressive phenotypes of some cancers; meanwhile, in other circumstances, they may help promote the survival of cancer cells and sustain metastasis. Co-treatment of drugs targeting FBPase with conventional chemotherapy is promising, as expression of FBPase promotes differentiation and apoptosis and inhibits the chemoresistance of cancer cells. However, the efficacy of cancer drugs targeting FBPase warrants further investigation in the real world.
Authors’ contributions
G-ML was responsible for reviewing the literature, summarizing data and was a major contributor in writing the manuscript. Y-MZ conceptualized and developed an outline for the manuscript. Both authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This work was supported by Medical Scientific Research Foundation of Guangdong Province, China (2016102120351019).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- 5AZA
5-aza-deoxycytidin
- AML
acute myeloid leukaemia
- AMP
adenosine monophosphate
- AKT
protein kinase B
- ATP
adenosine triphosphate
- BCAAs
branched chain amino acids
- BLBC
basal-like breast cancer
- BNIP3L/NIX
adenovirus E1B 19 kDa interacting protein 3-like
- cAMP
cyclic adenosine monophosphate
- ccRCC
clear cell renal cell carcinoma
- CBP
CREB-binding protein
- CREB
cAMP response element-binding protein
- CRTC2
CREB coactivator
- CSCs
cancer stem cells
- EpCAM
epithelial cell adhesion molecule
- H3K27Ac
histone H3 lysine 27 acetylation
- HDAC
histone deacetylase
- Dnmt1
DNA methyltransferase 1
- ECAR
extracellular acidification rate
- ERKs
extracellular signalling-regulated kinases
- F1,6BP
fructose-1,6-bisphosphate
- F2,6BP
fructose-2,6-bisphosphate
- FOXO
forkhead box O protein
- FOX O3
forkhead box O3 protein
- F6P
fructose-6-phosphate
- FBPase
fructose-1,6-bisphosphatase
- FDG-PET
18F-deoxyglucose positron emission tomography
- G3P
glycerol-3-phosphate
- GCs
gastric carcinomas
- GLUT
glucose transporter
- GCGR
glucagon receptor
- GR
glucocorticoid receptor
- GREs
glucocorticoid responsive elements
- GSIS
glucose-induced insulin secretion
- HCC
hepatocellular carcinoma
- HGP
hepatic glucose production
- HIF1
hypoxia-inducible factor-1
- HK
hexokinase
- IQGAP1
IQ motif-containing GTPase-activating protein 1
- LDHA
lactate dehydrogenase A
- LSD1
lysine (K)-specificdemethylase 1A
- MAPK
mitogen-activated protein kinase
- MDS
myelodysplastic syndrome
- MMP7
matrix metalloproteinase-7
- mTOR
mammalian target of rapamycin
- NAD+
nicotinamide adenine dinucleotide
- NADP
nicotinamide adenine dinucleotide phosphate
- NADPH
triphosphopyridine nucleotide
- OCR
oxygen consumption rate
- OXPHOS
oxidative phosphorylation
- PARP
poly ADP-ribose polymerase
- PEP
pyruvatel/phosphoenolpyruvate
- PFK
phosphofructokinase
- PFK1
phosphofructokinase-1
- PFK-2/FBPase-2
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase
- PI3K
phosphoinositide 3-kinase
- PI
propidium iodide
- PKA
protein kinase A
- PK
pyruvate kinase
- PKM2
pyruvate kinase M2
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- SIRM
stable isotope-resolved metabolomic
- T2DM
type 2 diabetes
- TCA
tricarboxylic acid
- TCF4
transcription factor 4
- TCP
tranylcypromine
- TIGAR
TP53-induced glycolysis and apoptosis regulator
- TNBC
triple-negative breast cancer
- TSA
trichostatin A
- UDPG
uridine diphosphate glucose
- ZEB1
zinc finger E-box-binding homeobox 1
Contributor Information
Gao-Min Liu, Phone: +0086-13750560390, Email: liugaom88@foxmail.com.
Yao-Ming Zhang, Phone: +0086-13750578911, Email: zhangyaominht@foxmail.com.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60:207–221. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- 4.Dhup S, Dadhich RK, Porporato PE, Sonveaux P. Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Curr Pharm Des. 2012;18:1319–1330. doi: 10.2174/138161212799504902. [DOI] [PubMed] [Google Scholar]
- 5.Pilkis SJ, Granner DK. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol. 1992;54:885–909. doi: 10.1146/annurev.ph.54.030192.004321. [DOI] [PubMed] [Google Scholar]
- 6.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 8.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 9.Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491:364–373. doi: 10.1038/nature11706. [DOI] [PubMed] [Google Scholar]
- 10.DeNicola Gina M, Cantley Lewis C. Cancer’s fuel choice: new flavors for a picky eater. Mol Cell. 2015;60:514–523. doi: 10.1016/j.molcel.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oronsky BT, Oronsky N, Fanger GR, Parker CW, Caroen SZ, Lybeck M, Scicinski JJ. Follow the ATP: tumor energy production: a perspective. Anticancer Agents Med Chem. 2014;14:1187–1198. doi: 10.2174/1871520614666140804224637. [DOI] [PubMed] [Google Scholar]
- 12.Dai J, Ji Y, Wang W, Kim D, Fai LY, Wang L, Luo J, Zhang Z. Loss of fructose-1,6-bisphosphatase induces glycolysis and promotes apoptosis resistance of cancer stem-like cells: an important role in hexavalent chromium-induced carcinogenesis. Toxicol Appl Pharmacol. 2017;331:164–173. doi: 10.1016/j.taap.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Wang J, Xing H, Li Q, Zhao Q, Li J. Down-regulation of FBP1 by ZEB1-mediated repression confers to growth and invasion in lung cancer cells. Mol Cell Biochem. 2016;411:331–340. doi: 10.1007/s11010-015-2595-8. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Wang C, Zhao F, Luo X, Qin M, Arunachalam E, Ge Z, Wang N, Deng X, Jin G, et al. Loss of FBP1 facilitates aggressive features of hepatocellular carcinoma cells through the Warburg effect. Carcinogenesis. 2017;38:134–143. doi: 10.1093/carcin/bgw109. [DOI] [PubMed] [Google Scholar]
- 15.Hirata H, Sugimachi K, Komatsu H, Ueda M, Masuda T, Uchi R, Sakimura S, Nambara S, Saito T, Shinden Y, et al. Decreased expression of fructose-1,6-bisphosphatase associates with glucose metabolism and tumor progression in hepatocellular carcinoma. Cancer Res. 2016;76:3265–3276. doi: 10.1158/0008-5472.CAN-15-2601. [DOI] [PubMed] [Google Scholar]
- 16.da Silva EF, Krause GC, Lima KG, Haute GV, Pedrazza L, Mesquita FC, Basso BS, Velasquez AC, Nunes FB, de Oliveira JR. Rapamycin and fructose-1,6-bisphosphate reduce the HEPG2 cell proliferation via increase of free radicals and apoptosis. Oncol Rep. 2016;36:2647–2652. doi: 10.3892/or.2016.5111. [DOI] [PubMed] [Google Scholar]
- 17.Chen M, Zhang J, Li N, Qian Z, Zhu M, Li Q, Zheng J, Wang X, Shi G. Promoter hypermethylation mediated downregulation of FBP1 in human hepatocellular carcinoma and colon cancer. PLoS ONE. 2011;6:e25564. doi: 10.1371/journal.pone.0025564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi L, Zhao C, Pu H, Zhang Q. FBP1 expression is associated with basal-like breast carcinoma. Oncol Lett. 2017;13:3046–3056. doi: 10.3892/ol.2017.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Jiang Y, Wang N, Jin Q, Ji F, Zhong C, Zhang Z, Yang J, Ye X, Chen T. Invalidation of mitophagy by FBP1-mediated repression promotes apoptosis in breast cancer. Tumour Biol. 2017;39:1010428317708779. doi: 10.1177/1010428317708779. [DOI] [PubMed] [Google Scholar]
- 20.Li K, Ying M, Feng D, Du J, Chen S, Dan B, Wang C, Wang Y. Fructose-1,6-bisphosphatase is a novel regulator of Wnt/beta-catenin pathway in breast cancer. Biomed Pharmacother. 2016;84:1144–1149. doi: 10.1016/j.biopha.2016.10.050. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Lee HJ, Wu X, Huo L, Kim SJ, Xu L, Wang Y, He J, Bollu LR, Gao G, et al. Gain of glucose-independent growth upon metastasis of breast cancer cells to the brain. Cancer Res. 2015;75:554–565. doi: 10.1158/0008-5472.CAN-14-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, Lin Y, Yao J, Shi J, Kang T, et al. Loss of FBP1 by snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013;23:316–331. doi: 10.1016/j.ccr.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ning XH, Li T, Gong YQ, He Q, Shen QI, Peng SH, Wang JY, Chen JC, Guo YL, Gong K. Association between FBP1 and hypoxia-related gene expression in clear cell renal cell carcinoma. Oncol Lett. 2016;11:4095–4098. doi: 10.3892/ol.2016.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B, Qiu B, Lee DS, Walton ZE, Ochocki JD, Mathew LK, Mancuso A, Gade TP, Keith B, Nissim I, Simon MC. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature. 2014;513:251–255. doi: 10.1038/nature13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Wang J, Xu H, Xing R, Pan Y, Li W, Cui J, Zhang H, Lu Y. Decreased fructose-1,6-bisphosphatase-2 expression promotes glycolysis and growth in gastric cancer cells. Mol Cancer. 2013;12:110. doi: 10.1186/1476-4598-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Wang X, Zhang J, Lam EK, Shin VY, Cheng AS, Yu J, Chan FK, Sung JJ, Jin HC. Warburg effect revisited: an epigenetic link between glycolysis and gastric carcinogenesis. Oncogene. 2010;29:442–450. doi: 10.1038/onc.2009.332. [DOI] [PubMed] [Google Scholar]
- 27.Jin X, Pan Y, Wang L, Ma T, Zhang L, Tang AH, Billadeau DD, Wu H, Huang H. Fructose-1,6-bisphosphatase inhibits ERK activation and bypasses gemcitabine resistance in pancreatic cancer by blocking IQGAP1-MAPK interaction. Cancer Res. 2017;77:4328–4341. doi: 10.1158/0008-5472.CAN-16-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu Y, Shi M, Chen H, Gu J, Zhang J, Shen B, Deng X, Xie J, Zhan X, Peng C. NPM1 activates metabolic changes by inhibiting FBP1 while promoting the tumorigenicity of pancreatic cancer cells. Oncotarget. 2015;6:21443–21451. doi: 10.18632/oncotarget.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benkovic SJ, Demaine MM. Mechanism of action of fructose 1,6-bisphosphatase. Adv Enzymol Relat Areas Mol Biol. 1982;53:45. doi: 10.1002/9780470122983.ch2. [DOI] [PubMed] [Google Scholar]
- 30.El-Maghrabi MR, Lange AJ, Jiang W, Yamagata K, Stoffel M, Takeda J, Fernald AA, Le Beau MM, Bell GI, Baker L, et al. Human fructose-1,6-bisphosphatase gene (FBP1): exon-intron organization, localization to chromosome bands 9q22.2-q22.3, and mutation screening in subjects with fructose-1,6-bisphosphatase deficiency. Genomics. 1995;27:520–525. doi: 10.1006/geno.1995.1085. [DOI] [PubMed] [Google Scholar]
- 31.Herzog B, Waltner-Law M, Scott DK, Eschrich K, Granner DK. Characterization of the human liver fructose-1,6-bisphosphatase gene promoter. Biochem J. 2000;351(Pt 2):385–392. doi: 10.1042/bj3510385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tillmann H, Stein S, Liehr T, Eschrich K. Structure and chromosomal localization of the human and mouse muscle fructose-1,6-bisphosphatase genes. Gene. 2000;247:241–253. doi: 10.1016/S0378-1119(00)00079-2. [DOI] [PubMed] [Google Scholar]
- 33.Tillmann H, Eschrich K. Isolation and characterization of an allelic cDNA for human muscle fructose-1,6-bisphosphatase. Gene. 1998;212:295–304. doi: 10.1016/S0378-1119(98)00181-4. [DOI] [PubMed] [Google Scholar]
- 34.Human FBP1 and FBP2. HumanFBP1 and FBP2: sequence. http://www.uniprot.org/uniprot/ P09467 and O00757and alignments run through NIH BLAST http://blast.st-va.ncbi.nlm.nih.gov/Blast.cgi.
- 35.Cubrilovic D, Haap W, Barylyuk K, Ruf A, Badertscher M, Gubler M, Tetaz T, Joseph C, Benz J, Zenobi R. Determination of protein-ligand binding constants of a cooperatively regulated tetrameric enzyme using electrospray mass spectrometry. ACS Chem Biol. 2014;9:218–226. doi: 10.1021/cb4007002. [DOI] [PubMed] [Google Scholar]
- 36.Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. 2017;13:572. doi: 10.1038/nrendo.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barciszewski J, Wisniewski J, Kolodziejczyk R, Jaskolski M, Rakus D, Dzugaj A. T-to-R switch of muscle fructose-1,6-bisphosphatase involves fundamental changes of secondary and quaternary structure. Acta Crystallogr D Struct Biol. 2016;72:536–550. doi: 10.1107/S2059798316001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ke HM, Liang JY, Zhang YP, Lipscomb WN. Conformational transition of fructose-1,6-bisphosphatase: structure comparison between the AMP complex (T form) and the fructose 6-phosphate complex (R form) Biochemistry. 1991;30:4412–4420. doi: 10.1021/bi00232a007. [DOI] [PubMed] [Google Scholar]
- 39.Taketa K, Pogell BM. Allosteric inhibition of rat liver fructose 1,6-diphosphatase by adenosine 5′-monophosphate. J Biol Chem. 1965;240:651–662. [PubMed] [Google Scholar]
- 40.Pilkis SJ, El-Maghrabi MR, Claus TH. Hormonal regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Biochem. 1988;57:755–783. doi: 10.1146/annurev.bi.57.070188.003543. [DOI] [PubMed] [Google Scholar]
- 41.Van Schaftingen E, Hers HG. Inhibition of fructose-1,6-bisphosphatase by fructose 2,6-biphosphate. Proc Natl Acad Sci USA. 1981;78:2861–2863. doi: 10.1073/pnas.78.5.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webb BA, Forouhar F, Szu FE, Seetharaman J, Tong L, Barber DL. Structures of human phosphofructokinase-1 and atomic basis of cancer-associated mutations. Nature. 2015;523:111–114. doi: 10.1038/nature14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Claus TH, El-Maghrabi MR, Pilkis SJ. Modulation of the phosphorylation state of rat liver pyruvate kinase by allosteric effectors and insulin. J Biol Chem. 1979;254:7855–7864. [PubMed] [Google Scholar]
- 44.Pilkis SJ, El-Maghrabi MR, Claus TH. Fructose-2,6-bisphosphate in control of hepatic gluconeogenesis. From metabolites to molecular genetics. Diabetes Care. 1990;13:582–599. doi: 10.2337/diacare.13.6.582. [DOI] [PubMed] [Google Scholar]
- 45.Rider MH, Bertrand L, Vertommen D, Michels PA, Rousseau GG, Hue L. 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis. Biochem J. 2004;381:561–579. doi: 10.1042/BJ20040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, Gottlieb E, Vousden KH. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 47.Lee P, Vousden KH, Cheung EC. TIGAR, TIGAR, burning bright. Cancer Metab. 2014;2:1. doi: 10.1186/2049-3002-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rakus D, Tillmann H, Wysocki R, Ulaszewski S, Eschrich K, Dzugaj A. Different sensitivities of mutants and chimeric forms of human muscle and liver fructose-1,6-bisphosphatases towards AMP. Biol Chem. 2003;384:51–58. doi: 10.1515/BC.2003.006. [DOI] [PubMed] [Google Scholar]
- 49.Rakus D, Gizak A, Kasprzak AA, Zarzycki M, Maciaszczyk-Dziubinska E, Dzugaj A. The mechanism of calcium-induced inhibition of muscle fructose 1,6-bisphosphatase and destabilization of glyconeogenic complex. PLoS ONE. 2013;8:e76669. doi: 10.1371/journal.pone.0076669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zarzycki M, Maciaszczyk E, Dzugaj A. Glu 69 is essential for the high sensitivity of muscle fructose-1,6-bisphosphatase inhibition by calcium ions. FEBS Lett. 2007;581:1347–1350. doi: 10.1016/j.febslet.2007.02.051. [DOI] [PubMed] [Google Scholar]
- 51.Jitrapakdee S. Transcription factors and coactivators controlling nutrient and hormonal regulation of hepatic gluconeogenesis. Int J Biochem Cell Biol. 2012;44:33–45. doi: 10.1016/j.biocel.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 52.Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12:141–151. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang B, Moya N, Niessen S, Hoover H, Mihaylova MM, Shaw RJ, Yates JR, 3rd, Fischer WH, Thomas JB, Montminy M. A hormone-dependent module regulating energy balance. Cell. 2011;145:596–606. doi: 10.1016/j.cell.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mihaylova MM, Vasquez DS, Ravnskjaer K, Denechaud PD, Yu RT, Alvarez JG, Downes M, Evans RM, Montminy M, Shaw RJ. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. 2011;145:607–621. doi: 10.1016/j.cell.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hill MJ, Suzuki S, Segars JH, Kino T. CRTC2 is a coactivator of GR and couples GR and CREB in the regulation of hepatic gluconeogenesis. Mol Endocrinol. 2016;30:104–117. doi: 10.1210/me.2015-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldstein I, Hager GL. Transcriptional and chromatin regulation during fasting—the genomic era. Trends Endocrinol Metab. 2015;26:699–710. doi: 10.1016/j.tem.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Titchenell PM, Lazar MA, Birnbaum MJ. Unraveling the regulation of hepatic metabolism by insulin. Trends Endocrinol Metab. 2017;28:497–505. doi: 10.1016/j.tem.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santer R, du Moulin M, Shahinyan T, Vater I, Maier E, Muntau AC, Steinmann B. A summary of molecular genetic findings in fructose-1,6-bisphosphatase deficiency with a focus on a common long-range deletion and the role of MLPA analysis. Orphanet J Rare Dis. 2016;11:44. doi: 10.1186/s13023-016-0415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baker L, Winegrad AI. Fasting hypoglycaemia and metabolic acidosis associated with deficiency of hepatic fructose-1,6-diphosphatase activity. Lancet. 1970;2:13–16. doi: 10.1016/S0140-6736(70)92474-8. [DOI] [PubMed] [Google Scholar]
- 60.Li N, Chang G, Xu Y, Ding Y, Li G, Yu T, Qing Y, Li J, Shen Y, Wang J, Wang X. Clinical and molecular characterization of patients with fructose 1,6-bisphosphatase deficiency. Int J Mol Sci. 2017;18:857. doi: 10.3390/ijms18040857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamate M, Jambagi M, Gowda P, Sonoli S. Fructose-1,6-diphosphatase deficiency: a treatable neurometabolic disorder. BMJ Case Rep. 2014;2014:bcr2013201553. doi: 10.1136/bcr-2013-201553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pagliara AS, Karl IE, Keating JP, Brown BI, Kipnis DM. Hepatic fructose-1,6-diphosphatase deficiency. A cause of lactic acidosis and hypoglycemia in infancy. J Clin Invest. 1972;51:2115–2123. doi: 10.1172/JCI107018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Emery J, Variend S, Howat A, Vawter G. Investigation of inborn errors of metabolism in unexpected infant deaths. Lancet. 1988;332:29–31. doi: 10.1016/S0140-6736(88)92955-8. [DOI] [PubMed] [Google Scholar]
- 64.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383:1068–1083. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992;90:1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rothman DL, Magnusson I, Katz LD, Shulman RG, Shulman GI. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science. 1991;254:573–576. doi: 10.1126/science.1948033. [DOI] [PubMed] [Google Scholar]
- 67.Dang Q, Kasibhatla SR, Reddy KR, Jiang T, Reddy MR, Potter SC, Fujitaki JM, van Poelje PD, Huang J, Lipscomb WN, Erion MD. Discovery of potent and specific fructose-1,6-bisphosphatase inhibitors and a series of orally-bioavailable phosphoramidase-sensitive prodrugs for the treatment of type 2 diabetes. J Am Chem Soc. 2007;129:15491–15502. doi: 10.1021/ja074871l. [DOI] [PubMed] [Google Scholar]
- 68.van Poelje PD, Potter SC, Chandramouli VC, Landau BR, Dang Q, Erion MD. Inhibition of fructose 1,6-bisphosphatase reduces excessive endogenous glucose production and attenuates hyperglycemia in Zucker diabetic fatty rats. Diabetes. 2006;55:1747–1754. doi: 10.2337/db05-1443. [DOI] [PubMed] [Google Scholar]
- 69.Kebede M, Favaloro J, Gunton JE, Laybutt DR, Shaw M, Wong N, Fam BC, Aston-Mourney K, Rantzau C, Zulli A, et al. Fructose-1,6-bisphosphatase overexpression in pancreatic—cells results in reduced insulin secretion: a new mechanism for fat-induced impairment of—cell function. Diabetes. 2008;57:1887–1895. doi: 10.2337/db07-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y, Xie Z, Zhou G, Zhang H, Lu J, Zhang WJ. Fructose-1,6-bisphosphatase regulates glucose-stimulated insulin secretion of mouse pancreatic β-cells. Endocrinology. 2010;151:4688–4695. doi: 10.1210/en.2009-1185. [DOI] [PubMed] [Google Scholar]
- 71.Poelje PDV, Potter SC, Erion MD. Fructose-1, 6-bisphosphatase inhibitors for reducing excessive endogenous glucose production in type 2 diabetes. Handb Exp Pharmacol. 2011;203:279. doi: 10.1007/978-3-642-17214-4_12. [DOI] [PubMed] [Google Scholar]
- 72.Yoshida T, Okuno A, Izumi M, Takahashi K, Hagisawa Y, Ohsumi J, Fujiwara T. CS-917, a fructose 1,6-bisphosphatase inhibitor, improves postprandial hyperglycemia after meal loading in non-obese type 2 diabetic Goto-Kakizaki rats. Eur J Pharmacol. 2008;601:192–197. doi: 10.1016/j.ejphar.2008.10.050. [DOI] [PubMed] [Google Scholar]
- 73.Dang Q, Kasibhatla SR, Jiang T, Fan K, Liu Y, Taplin F, Schulz W, Cashion DK, Reddy KR, van Poelje PD. Discovery of phosphonic diamide prodrugs and their use for the oral delivery of a series of fructose 1,6-bisphosphatase inhibitors. J Med Chem. 2008;51:4331–4339. doi: 10.1021/jm8001235. [DOI] [PubMed] [Google Scholar]
- 74.Nandakumar N, Rengarajan T, Balamurugan A, Balasubramanian MP. Modulating effects of hesperidin on key carbohydrate-metabolizing enzymes, lipid profile, and membrane-bound adenosine triphosphatases against 7,12-dimethylbenz(a)anthracene-induced breast carcinogenesis. Hum Exp Toxicol. 2014;33:504–516. doi: 10.1177/0960327113485252. [DOI] [PubMed] [Google Scholar]
- 75.Bigl M, Jandrig B, Horn LC, Eschrich K. Aberrant methylation of human l- and M-fructose 1,6-bisphosphatase genes in cancer. Biochem Biophys Res Commun. 2008;377:720–724. doi: 10.1016/j.bbrc.2008.10.045. [DOI] [PubMed] [Google Scholar]
- 76.Jayasri K, Padmaja K, Saibaba M. Altered oxidative stress and carbohydrate metabolism in canine mammary tumors. Vet World. 2016;9:1489–1492. doi: 10.14202/vetworld.2016.1489-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parimala R, Sachdanandam P. Effect of plumbagin on some glucose metabolising enzymes studied in rats in experimental hepatoma. Mol Cell Biochem. 1993;125:59–63. doi: 10.1007/BF00926835. [DOI] [PubMed] [Google Scholar]
- 78.Wang B, Hsu SH, Frankel W, Ghoshal K, Jacob ST. Stat3-mediated activation of microRNA-23a suppresses gluconeogenesis in hepatocellular carcinoma by down-regulating glucose-6-phosphatase and peroxisome proliferator-activated receptor gamma, coactivator 1 alpha. Hepatology. 2012;56:186–197. doi: 10.1002/hep.25632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen SS, Yu KK, Ling QX, Huang C, Li N, Zheng JM, Bao SX, Cheng Q, Zhu MQ, Chen MQ. The combination of three molecular markers can be a valuable predictive tool for the prognosis of hepatocellular carcinoma patients. Sci Rep. 2016;6:24582. doi: 10.1038/srep24582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jin X, Pan Y, Wang L. MAGE-TRIM28 complex promotes the Warburg effect and hepatocellular carcinoma progression by targeting FBP1 for degradation. Oncogenesis. 2017;6:312. doi: 10.1038/oncsis.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang J, Jin X, Yan Y, Shao Y, Pan Y, Roberts LR, Zhang J, Huang H, Jiang J. Inhibiting histone deacetylases suppresses glucose metabolism and hepatocellular carcinoma growth by restoring FBP1 expression. Sci Rep. 2017;7:43864. doi: 10.1038/srep43864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang L, Ravichandran R, Potts PR, Jiang J, Wu H, Huang H, Chen R, Li J, Zhou X, Liu J, Huang G. Fructose-1,6-bisphosphatase 1 reduces 18F FDG uptake in hepatocellular carcinoma. Oncogenesis. 2017;284(3):844–853. doi: 10.1148/radiol.2017161607. [DOI] [PubMed] [Google Scholar]
- 83.Wang D, Moothart DR, Lowy DR, Qian X. The expression of glyceraldehyde-3-phosphate dehydrogenase associated cell cycle (GACC) genes correlates with cancer stage and poor survival in patients with solid tumors. PLoS ONE. 2013;8:e61262. doi: 10.1371/journal.pone.0061262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pastor MD, Nogal A, Molina-Pinelo S, Melendez R, Salinas A, Gonzalez De la Pena M, Martin-Juan J, Corral J, Garcia-Carbonero R, Carnero A, Paz-Ares L. Identification of proteomic signatures associated with lung cancer and COPD. J Proteomics. 2013;89:227–237. doi: 10.1016/j.jprot.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 85.Dondeti VR, Wubbenhorst B, Lal P, Gordan JD, D’Andrea K, Attiyeh EF, Simon MC, Nathanson KL. Integrative genomic analyses of sporadic clear cell renal cell carcinoma define disease subtypes and potential new therapeutic targets. Cancer Res. 2012;72:112–121. doi: 10.1158/0008-5472.CAN-11-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moore LE, Jaeger E, Nickerson ML, Brennan P, Vries SD, Roy R, Toro J, Li H, Karami S, Lenz P. Genomic copy number alterations in clear cell renal carcinoma: associations with case characteristics and mechanisms of VHL gene inactivation. Oncogenesis. 2012;1:e14. doi: 10.1038/oncsis.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karpathakis A, Dibra H, Pipinikas C, Feber A, Morris T, Francis J, Oukrif D, Mandair D, Pericleous M, Mohmaduvesh M, et al. Prognostic impact of novel molecular subtypes of small intestinal neuroendocrine tumor. Clin Cancer Res. 2016;22:250–258. doi: 10.1158/1078-0432.CCR-15-0373. [DOI] [PubMed] [Google Scholar]
- 88.Johansson HJ, Sanchez BC, Forshed J, Stal O, Fohlin H, Lewensohn R, Hall P, Bergh J, Lehtio J, Linderholm BK. Proteomics profiling identify CAPS as a potential predictive marker of tamoxifen resistance in estrogen receptor positive breast cancer. Clin Proteomics. 2015;12:8. doi: 10.1186/s12014-015-9080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ma R, Zhang W, Tang K, Zhang H, Zhang Y, Li D, Li Y, Xu P, Luo S, Cai W, et al. Switch of glycolysis to gluconeogenesis by dexamethasone for treatment of hepatocarcinoma. Nat Commun. 2013;4:2508. doi: 10.1038/ncomms3508. [DOI] [PubMed] [Google Scholar]
- 90.Fella K, Gluckmann M, Hellmann J, Karas M, Kramer PJ, Kroger M. Use of two-dimensional gel electrophoresis in predictive toxicology: identification of potential early protein biomarkers in chemically induced hepatocarcinogenesis. Proteomics. 2005;5:1914–1927. doi: 10.1002/pmic.200401067. [DOI] [PubMed] [Google Scholar]
- 91.Liu Z, Ma Y, Yang J, Qin H. Upregulated and downregulated proteins in hepatocellular carcinoma: a systematic review of proteomic profiling studies. OMICS. 2011;15:61–71. doi: 10.1089/omi.2010.0061. [DOI] [PubMed] [Google Scholar]
- 92.Peterson CW, Stoltzman CA, Sighinolfi MP, Han KS, Ayer DE. Glucose controls nuclear accumulation, promoter binding, and transcriptional activity of the MondoA-Mlx heterodimer. Mol Cell Biol. 2010;30:2887–2895. doi: 10.1128/MCB.01613-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hirschhaeuser F, Sattler UG, Mueller-Klieser W. Lactate: a metabolic key player in cancer. Cancer Res. 2011;71:6921–6925. doi: 10.1158/0008-5472.CAN-11-1457. [DOI] [PubMed] [Google Scholar]
- 94.Hatakeyama S. TRIM proteins and cancer. Nat Rev Cancer. 2011;11:792–804. doi: 10.1038/nrc3139. [DOI] [PubMed] [Google Scholar]
- 95.Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fan TW, Yuan P, Lane AN, Higashi RM, Wang Y, Hamidi AB, Zhou R, Guitart X, Chen G, Manji HK, Kaddurah-Daouk R. Stable isotope-resolved metabolomic analysis of lithium effects on glial-neuronal metabolism and interactions. Metabolomics. 2010;6:165–179. doi: 10.1007/s11306-010-0208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wolf A, Agnihotri S, Micallef J, Mukherjee J, Sabha N, Cairns R, Hawkins C, Guha A. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208:313–326. doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Patra KC, Wang Q, Bhaskar PT, Miller L, Wang Z, Wheaton W, Chandel N, Laakso M, Muller WJ, Allen EL, et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213–228. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yi W, Clark PM, Mason DE, Keenan MC, Hill C, Goddard WA, 3rd, Peters EC, Driggers EM, Hsieh-Wilson LC. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 2012;337:975–980. doi: 10.1126/science.1222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wong N, Ojo D, Yan J, Tang D. PKM2 contributes to cancer metabolism. Cancer Lett. 2015;356:184–191. doi: 10.1016/j.canlet.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 102.Metodiev MD, Lesko N, Park CB, Cámara Y, Shi Y, Wibom R, Hultenby K, Gustafsson CM, Larsson NG. Methylation of 12S rRNA is necessary for in vivo stability of the small subunit of the mammalian mitochondrial ribosome. Cell Metab. 2009;9:386–397. doi: 10.1016/j.cmet.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 103.Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer. 2014;14:709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Blick T, Hugo H, Widodo E, Waltham M, Pinto C, Mani SA, Weinberg RA, Neve RM, Lenburg ME, Thompson EW. Epithelial mesenchymal transition traits in human breast cancer cell lines parallel the CD44(hi/)CD24(lo/−) stem cell phenotype in human breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:235–252. doi: 10.1007/s10911-010-9175-z. [DOI] [PubMed] [Google Scholar]
- 105.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-I. [DOI] [PubMed] [Google Scholar]
- 107.Mamczur P, Sok AJ, Rzechonek A, Rakus D. Cell cycle-dependent expression and subcellular localization of fructose 1,6-bisphosphatase. Histochem Cell Biol. 2012;137:121–136. doi: 10.1007/s00418-011-0884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Can Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- 109.Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20:51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alderton GK. Tumorigenesis: FBP1 is suppressed in kidney tumours. Nat Rev Cancer. 2014;14:575. doi: 10.1038/nrc3810. [DOI] [PubMed] [Google Scholar]
- 111.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 112.Chai X, Chu H, Yang X, Meng Y, Shi P, Gou S. Metformin increases sensitivity of pancreatic cancer cells to gemcitabine by reducing CD133+ cell populations and suppressing ERK/P70S6K signaling. Sci Rep. 2015;5:14404. doi: 10.1038/srep14404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roy M, Li Z, Sacks DB. IQGAP1 is a scaffold for mitogen-activated protein kinase signaling. Mol Cell Biol. 2005;25:7940–7952. doi: 10.1128/MCB.25.18.7940-7952.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pan D, Mao C, Wang YX. Suppression of gluconeogenic gene expression by LSD1-mediated histone demethylation. PLoS ONE. 2013;8:e66294. doi: 10.1371/journal.pone.0066294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.You JS, Jones PA. Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell. 2012;22:9–20. doi: 10.1016/j.ccr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Doyle JM, Gao J, Wang J, Yang M, Potts PR. MAGE-RING protein complexes comprise a family of E3 ubiquitin ligases. Mol Cell. 2010;39:963–974. doi: 10.1016/j.molcel.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.PubChem Compound Database; CID = 451668. https://pubchem.ncbi.nlm.nih.gov/compound/451668. Accessed 24 Sept 2017.
- 118.PubChem Compound Database; CID = 5353432. https://pubchem.ncbi.nlm.nih.gov/compound/5353432. Accessed 24 Sept 2017.
- 119.PubChem Compound Database; CID = 387447. https://pubchem.ncbi.nlm.nih.gov/compound/387447. Accessed 24 Sept 2017.
- 120.PubChem Compound Database; CID = 5743. https://pubchem.ncbi.nlm.nih.gov/compound/5743. Accessed 24 Sept 2017.
- 121.PubChem Compound Database; CID = 6918837. https://pubchem.ncbi.nlm.nih.gov/compound/6918837. Accessed 24 Sept 2017.
- 122.PubChem Compound Database; CID = 5311. https://pubchem.ncbi.nlm.nih.gov/compound/5311. Accessed 24 Sept 2017.
- 123.PubChem Compound Database; CID = 5222465. https://pubchem.ncbi.nlm.nih.gov/compound/5222465. Accessed 24 Sept 2017.
- 124.PubChem Compound Database; CID = 5530. https://pubchem.ncbi.nlm.nih.gov/compound/5530. Accessed 24 Sept 2017.
- 125.PubChem Compound Database; CID = 444732. https://pubchem.ncbi.nlm.nih.gov/compound/444732. Accessed 24 Sept 2017.
- 126.Fandy TE. Development of DNA methyltransferase inhibitors for the treatment of neoplastic diseases. Curr Med Chem. 2009;16:2075–2085. doi: 10.2174/092986709788612738. [DOI] [PubMed] [Google Scholar]
- 127.West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest. 2014;124:30–39. doi: 10.1172/JCI69738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang C, Richon V, Ni X, Talpur R, Duvic M. Selective induction of apoptosis by histone deacetylase inhibitor SAHA in cutaneous T-cell lymphoma cells: relevance to mechanism of therapeutic action. J Invest Dermatol. 2005;125:1045–1052. doi: 10.1111/j.0022-202X.2005.23925.x. [DOI] [PubMed] [Google Scholar]
- 129.Crisanti MC, Wallace AF, Kapoor V, Vandermeers F, Dowling ML, Pereira LP, Coleman K, Campling BG, Fridlender ZG, Kao GD, Albelda SM. The HDAC inhibitor panobinostat (LBH589) inhibits mesothelioma and lung cancer cells in vitro and in vivo with particular efficacy for small cell lung cancer. Mol Cancer Ther. 2009;8:2221–2231. doi: 10.1158/1535-7163.MCT-09-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Piekarz RL, Bates SE. Epigenetic modifiers: basic understanding and clinical development. Clin Cancer Res. 2009;15:3918–3926. doi: 10.1158/1078-0432.CCR-08-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li L, Li W. Epithelial-mesenchymal transition in human cancer: comprehensive reprogramming of metabolism, epigenetics, and differentiation. Pharmacol Ther. 2015;150:33–46. doi: 10.1016/j.pharmthera.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 132.Falkenberg KJ, Johnstone RW. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discov. 2014;13:673–691. doi: 10.1038/nrd4360. [DOI] [PubMed] [Google Scholar]
- 133.Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 134.Metzger E, Wissmann M, Yin N, Muller JM, Schneider R, Peters AH, Gunther T, Buettner R, Schule R. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437:436–439. doi: 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- 135.Schmidt DM, McCafferty DG. trans-2-Phenylcyclopropylamine is a mechanism-based inactivator of the histone demethylase LSD1. Biochemistry. 2007;46:4408–4416. doi: 10.1021/bi0618621. [DOI] [PubMed] [Google Scholar]
- 136.Hambley B, Caimi PF, William BM. Bortezomib for the treatment of mantle cell lymphoma: an update. Ther Adv Hematol. 2016;7:196–208. doi: 10.1177/2040620716648566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pal A, Young MA, Donato NJ. Emerging potential of therapeutic targeting of ubiquitin-specific proteases in the treatment of cancer. Cancer Res. 2014;74:4955–4966. doi: 10.1158/0008-5472.CAN-14-1211. [DOI] [PubMed] [Google Scholar]
- 138.Wang C, Gao D, Guo K, Kang X, Jiang K, Sun C, Li Y, Sun L, Shu H, Jin G, et al. Novel synergistic antitumor effects of rapamycin with bortezomib on hepatocellular carcinoma cells and orthotopic tumor model. BMC Cancer. 2012;12:166. doi: 10.1186/1471-2407-12-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kane RC, Farrell AT, Sridhara R, Pazdur R. United States Food and Drug Administration approval summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin Cancer Res. 2006;12:2955–2960. doi: 10.1158/1078-0432.CCR-06-0170. [DOI] [PubMed] [Google Scholar]
- 140.Wahl K, Siegemund M, Lehner F, Vondran F, Nussler A, Langer F, Krech T, Kontermann R, Manns MP, Schulze-Osthoff K, et al. Increased apoptosis induction in hepatocellular carcinoma by a novel tumor-targeted TRAIL fusion protein combined with bortezomib. Hepatology. 2013;57:625–636. doi: 10.1002/hep.26082. [DOI] [PubMed] [Google Scholar]
- 141.Kim GP, Mahoney MR, Szydlo D, Mok TS, Marshke R, Holen K, Picus J, Boyer M, Pitot HC, Rubin J, et al. An international, multicenter phase II trial of bortezomib in patients with hepatocellular carcinoma. Invest New Drugs. 2012;30:387–394. doi: 10.1007/s10637-010-9532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–531. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


