Abstract
Objective
We examined the prognostic significance of left ventricular (LV) mass for cardiovascular disease (CVD) events in older adults with and without metabolic syndrome (MetS) and diabetes (DM).
Background
MetS and DM are associated with increased CVD risk, but it is unclear in these groups whether subclinical CVD evidenced by increased LV mass improves risk prediction over standard risk factors in older individuals.
Methods
We studied 3,724 adults (mean age 72.4 ± 5.4, 61.0% female, 4.4% African American) from the Cardiovascular Health Study who had MetS (but without DM), DM, or neither condition. Cox regression examined the association of LV mass (alone and indexed by height and body surface area [BSA]) determined by echocardiography with CVD events, including coronary heart disease (CHD), stroke, heart failure (HF), and CVD death, as well as total mortality. We also assessed the added prediction, discriminative value and net reclassification improvement (NRI) for clinical utility of LV mass over standard risk factors.
Results
Over a mean follow-up of 14.2 ± 6.3 years, 2,180 subjects experienced CVD events, including 986 CVD deaths. After adjustment for age, gender and standard risk factors, LV mass was positively associated with CVD events in those with MetS (hazard ratio [HR]=1.4, p<0.001) and without MetS (HR=1.4, p<0.001), but not DM (HR=1.0, p=0.62), with similar findings for LV mass indexed for height or BSA. Adding LV mass to standard risk factors moderately improved the prediction accuracy in the overall sample and MetS group from changes in C-statistics (p<0.05). Categorical-free net reclassification improvement increased significantly by 17–19% in those with MetS. Findings were comparable for CHD, CVD mortality and total mortality.
Conclusions
LV mass is associated with increased CVD risk and provides modest added prediction and clinical utility over standard risk factors in older persons with and without MetS, but not with DM.
Keywords: echocardiography, left ventricular mass, metabolic syndrome, diabetes, cardiovascular disease
INTRODUCTION
Persons with metabolic syndrome (MetS) and diabetes (DM) are more likely to have subclinical atherosclerosis and are at a greater risk of suffering cardiovascular disease (CVD) events 1–3 and mortality in older persons 4 Previous studies have identified left ventricular (LV) mass to independently predict CVD events 5–7. While the association of MetS5 and the number of MetS risk factors 7 with LV mass has been demonstrated, and DM adversely impacts hypertropic remodeling through increased LV mass and larger cavity dimensions8, there are limited data examining the value of LV mass for predicting CVD events in persons with MetS or DM. Although a smaller previous study compared the prognosis of increased LV mass in diabetic and non-diabetic hypertensive individuals9, to our knowledge, no population-based study has compared the prognostic significance of LV mass in persons with and without MetS and DM. Although DM is a well-known risk factor for coronary heart disease (CHD), it has been shown by some studies to confer a lower risk of subsequent cardiac complications than CHD.10 There is a need to better identify what further screening methods for subclinical CVD can further improve risk prediction in persons with MetS and DM.11 For instance, it is known such persons demonstrate a greater extent of myocardial ischemia12 and coronary calcium13,14, with the latter providing prognostic value for CVD events.15 Whether subclinical CVD evidenced by higher LV mass provides significant incremental prognostic value in predicting CVD events over standard risk factors in these conditions is unclear, especially in those with MetS and DM and in older persons who have a longer exposure to these conditions. Such information would be useful to judge the utility of LV mass assessment in these groups.
This paper examines whether readily-available echocardiographic measurements of LV mass add to standard CVD risk factors in the prediction of CVD events in older persons with and without MetS or DM. Our analysis addresses the question of whether there is a role for these readily available measurements in risk stratification for these populations.
METHODS
Study Sample and Recruitment
Our analyses include 3724 adults aged 65 to 95 years from the Cardiovascular Health Study (CHS), a prospective National Institutes of Health-sponsored study of older adults focused on studying risk factors and subclinical measures of CVD and their outcomes. Initial enrollment during 1989–1990 recruited 5,201 participants, while a second cohort of 687 African-American participants was recruited in 1992–1993. Specifically, of the initial cohort of 5,201, the current analysis included CHS participants who had baseline measurements of LV mass from two-dimensionally directed M-mode echocardiography as well as information on incidence of CVD events; patients with prior CVD events were excluded. Participants were initially recruited from Heath Care Financing Administration Medicare eligibility lists and other household members from four U.S. geographic regions: Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania. Participant consent was obtained during baseline exam. Baseline exam data were collected from a clinical examination which consisted of medical history, physical examination, and fasting blood analyses. The methodology and design of CHS have been previously reported.15 Up to 22 years of follow-up data were available through June 30, 2004, with vital status known on all the 3,724 subjects included in the study with complete risk factor data (no persons lost to follow-up). This project was exempt from Institutional Review Board review due to the use of de-identified data.
Measurements
Risk factors measured in our study were measured by standardized methodology, as previously described, and included systolic and diastolic blood pressure (BP), low density and high density lipoprotein-cholesterol, triglycerides, glucose, waist circumference, and body mass index (BMI).15 Subjects were classified into three groups as having MetS (without DM) according to American Heart Association/National Heart Lung & Blood Institute (AHA/NHLBI) criteria, DM, or neither condition. MetS (n=1,178) (without DM) was defined according to the AHA/NHLBI definition16 as having any three of the following five criteria: elevated BP (>130 systolic or >85 mmHg) or treatment for hypertension, low high-density lipoprotein cholesterol (HDL-C, <40 mg/dl in males or <50 mg/dl in females), elevated triglycerides (>150 mg/dl), increased waist circumference (>88 cm [35 inches] in females, or >102 cm [40 inches] in males), or impaired fasting glucose (100–125 mg/dl). DM (n=485) was defined as having a fasting glucose level ≥6.99 mmol/L (126 mg/dL), taking oral hypoglycemic medication, or self-reported use of insulin. Subjects with neither condition (n=2,061) were also included in our analyses.
The protocol for performing and reading transthoracic echocardiograms (echo) has been previously described.17 Briefly, a baseline echo was recorded onto super-VHS tape using a standardized protocol, with measurements made at the Echocardiography Reading Center at the University of California, Irvine in 1989–1990 and at Georgetown University in 1992–1993 from digitized images utilizing an off-line image-analysis system equipped with customized computer algorithms. Quality control measures included standardized training of sonographers and readers, periodic sonographer observation by a trained echocardiographer, and blind duplicate readings to establish interreader and intrareader measurement variability. This manuscript focuses on two-dimensionally directed M-mode measurements of LV mass, which was calculated as described by Devereux et al.18 : LV mass (g) = 0.80 × 1.04 [(VSTd + LVIDd + PWTd)3 − (LVIDd)3] + 0.6 cm where VSTd= ventricular septal thickness in diastole, LVIDd=LV internal dimension in diastole, and PWTd=posterior wall thickness in diastole. We also present our data according to LV mass indexed to height (cm)1.7 given that this has been recently proposed to represent more accurate scaling19 than older scaling (such as by a power of 2.7), as well as by body surface area.
CVD and CHD events were adjudicated by the CHS endpoints committee of physician investigators. Incident CVD was defined as CHD, stroke, heart failure (HF), or claudication, with CVD deaths due to either of these incident conditions. CHD events included incident non-fatal myocardial infarction, angina requiring hospitalization, coronary artery angioplasty, coronary bypass surgery or death caused by “atherosclerotic CHD”. CHS criteria for angina required a report of symptoms such as chest pain, chest tightness, or shortness of breath; the diagnosis of angina from a physician; and being under medical treatment for angina (including nitroglycerin, beta blocker, or calcium channel blocker). Total CVD and CHD events, CVD mortality, and total mortality were defined as occurring after the baseline echo assessment of LV mass. The first occurrence of a qualifying event was used as the individual’s “event”, so recurrent events were not included in the analysis. Follow-up time was defined from the baseline LV mass echo assessment to the date of first occurrence of a CVD event (or CHD, CVD death, or total mortality for analyses specific to those endpoints).
Statistical Analysis
Descriptive statistics of proportions for categorical variables and means (±standard deviation [SD]) for continuous variables were presented by disease group and compared by the Chi-square test of proportions or analysis of variance among groups, respectively. Cox proportional hazards regression analysis was used to examine the association of LV mass with time to the primary outcome of a first CVD event and with time to the secondary endpoints of CHD, CVD mortality, and total mortality providing hazard ratios (HRs) and 95% confidence intervals. These analyses were adjusted for age, gender, ethnicity, and standard risk factors (systolic BP, diastolic BP, hypertensive medications, HDL-C, LDL-C, total cholesterol, triglycerides, lipid medications, BMI, and fasting glucose). LV mass was stratified according to gender-specific quartiles in grams, and was also examined continuously per standard deviation (SD) of LV mass indexed by height (grams/meter1.7), per standard deviation of LV mass indexed by body surface area (grams/meter2), and per standard deviation of LV mass of 30 grams. The area under the ROC curve (AUC) was used to examine the incremental value of LV mass above standard risk factors for the prediction of CVD events. We constructed logistic regression models with/without LV mass measures to compare the AUC differences. In addition, to examine the added clinical utility of echo LV mass over standard risk factors, the category-free net reclassification improvement (NRI) was calculated as: NRI= [(number of events reclassified higher risk minus number of events reclassified lower risk)/number of events] + [(number of nonevents reclassified lower risk minus number of nonevents reclassified higher risk)/number of nonevents]. SAS statistical software version 9.4 (SAS Institute, Cary, North Carolina)20 was used for analysis. A p value < 0.05 (and a p value < 0.1 for interaction test) was considered statistically significant.
RESULTS
Subject Characteristics
The mean (± SD) age of our 3274 participants was 72.4 ± 5.4 years, with 61% females and 4.4% African Americans (Table 1). As expected, participants with metabolic syndrome or DM had significantly higher mean BPs, lipid measurements, blood sugar, BMI, and LV mass when compared to persons with neither disease. Unadjusted LV mass for those with MetS, DM, or neither were 155.7 ± 28.3, 163.5 ± 31.6, and 142.4 ± 28.4 grams, respectively ( p<0.0001). Mean LV mass indexed to height (LV mass (g)/height1.7 (m)) for those with MetS, DM, or neither was 66.9 ± 9.8, 69.1 ± 11.4, and 60.8 ± 10.6 gram/meter, respectively (p<0.0001). Mean LV mass indexed to body surface area (LV mass (g)/BSA (m2)) for those with MetS, DM, or neither was 85.6 ± 10.2, 89.0 ± 12.4, and 82.8 ± 11.1 gram/meter2, respectively (p<0.0001).
Table 1.
Baseline Characteristics
| Overall (n=3,724) | Neither (n=2,061) | Metabolic Syndrome (n=1,178) | Diabetes (n=485) | P value | |
|---|---|---|---|---|---|
| Age (year) | 72.4 ± 5.4 | 72.5 ± 5.6 | 72.2 ± 5.1 | 72.6 ± 5.5 | 0.16 |
| Male | 1453(39.0%) | 828(40.2%) | 400(34.0%) | 225(46.4 %) | p <0.0001 |
| African American | 165(4.4%) | 84(4.1%) | 36(3.1%) | 45(9.3%) | p <0.0001 |
| Systolic Blood Pressure(mmHg) | 135.5 ± 21.0 | 131.8 ± 21.1 | 139.8 ± 19.7 | 140.8 ± 20.9 | p <0.0001 |
| Diastolic Blood Pressure(mmHg) | 70.7 ± 11.1 | 69.7 ± 11.0 | 72.1 ±10.7 | 71.9 ± 11.8 | p <0.0001 |
| Triglycerides (mg/dl) | 139.3 ± 73.0 | 110.0 ± 40.7 | 175.8 ± 76.3 | 175.1 ±107.8 | p <0.0001 |
| Total Cholesterol (mg/dl) | 212.7 ±39.0 | 211.3 ± 36.4 | 217.6 ±40.8 | 206.7 ± 43.8 | p <0.0001 |
| LDL-C (mg/dl) | 130.1 ± 35.7 | 128.3 ± 33.8 | 135.3 ± 37.0 | 125.6 ± 39.1 | p <0.0001 |
| HDL-C (mg/dl) | 55.4 ± 15.8 | 61.1 ± 15.7 | 48.3 ± 12.8 | 48.1 ± 13.1 | p <0.0001 |
| Waist Circumference (cm) | 93.4 ± 12.9 | 88.2 ± 11.5 | 99.7 ±11.6 | 99.5 ± 12.1 | p <0.0001 |
| Glucose (mg/dl) | 108.4 ± 31.5 | 96.5 ± 8.3 | 105.0 ± 8.96 | 166.9 ± 55.3 | p <0.0001 |
| Body Mass Index (kg/m2) | 26.4 ± 4.5 | 24.6 ± 3.64 | 28.7 ± 4.49 | 28.3 ± 4.73 | p <0.0001 |
| Hypertension Medication | 1372(36.8%) | 511(24.8%) | 594(50.4%) | 267(55.1 %) | p <0.0001 |
| Lipid-lowering Medication | 156(4.2%) | 70(3.4%) | 63(5.3%) | 23(4.7%) | 0.02 |
| Obesity | 663(17.8%) | 129(6.3%) | 372(31.6%) | 162(33.4 %) | p <0.0001 |
| Smoking status | 0.53 | ||||
| Non-Smoker | 1784(47.9%) | 966(46.9%) | 588(49.9%) | 230(47.4 %) | |
| Former Smoker | 1504(40.4%) | 845(41.0%) | 458(38.9%) | 201(41.4 %) | |
| Current Smoker | 436(11.7%) | 250(6.7%) | 132(3.5%) | 54(11.1%) | |
| LV Mass (g) | 149.3 ± 29.9 | 142.4 ± 28.4 | 155.7 ± 28.3 | 163.6 ± 31.6 | p <0.0001 |
| Indexed LV Mass (g/m1.7) | 63.8 ± 10.6 | 60.8 ± 9.8 | 66.9 ± 9.8 | 69.1 ± 11.4 | p <0.0001 |
| Indexed LV Mass (g/m2) | 84.5± 11.2 | 82.8 ± 11.1 | 85.6 ± 10.2 | 89.0 ± 12.4 | p <0.0001 |
Continuous variables are presented as mean ± SD; Categorical variables are presented as frequencies (percentage).
Cardiovascular Disease Events
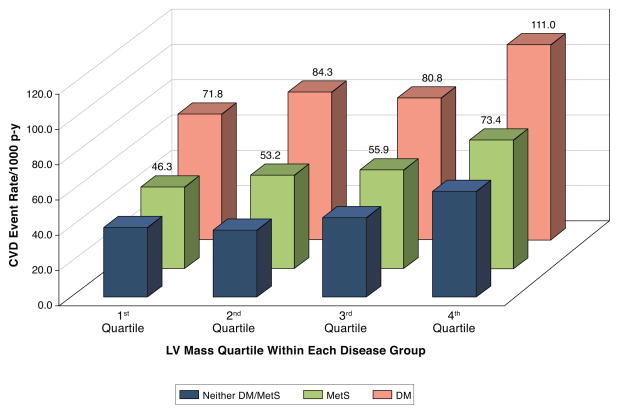
Over a mean follow-up of 14.2 ± 6.3 years, 2,180 participants experienced at least one CVD event. Unadjusted rates of total CVD events per 1000 person years were highest in those with DM. A stepwise increase of unadjusted rates of total CVD events per 1000 person years was observed across quartiles of LV mass for those with MetS, DM and those with neither condition (Figure 1).
Figure 1. Total Cardiovascular Disease Event Rates (per 1000 person years) by LV Mass Quartile within Disease Group.
A higher unadjusted cardiovascular event rate is observed across increasing LV mass quartiles for those with neither MetS/DM, MetS without DM, and for those with DM. MetS=metabolic syndrome. DM=diabetes mellitus. LV = left ventricular mass.
Relationship between LV Mass and Outcomes
Findings from adjusted Cox proportional hazards regression are shown in Table 2 for primary (total CVD) and secondary outcomes (total CHD, CVD mortality and all-cause mortality). In participants with neither MetS nor DM and persons with MetS alone, higher LV mass and indexed LV mass were risk factors for total CVD, total CHD, CVD mortality and all-cause mortality. These associations were not observed in persons with DM. Interaction tests for disease groups and LV mass were significant for total CHD (p=0.049), CVD death (p=0.057) and total mortality (p=0.060); for disease groups and LV mass indices they were only significant for CVD death in relation to LV mass/height 1.7 (p=0.064) and for LV mass/BSA (p=0.029). Similar findings were noted when stratified by gender. Both men and women with neither disease or with MetS alone had a higher risk of total CVD events per standard deviation of increase in LV mass; men and women with DM did not (Table 3).
Table 2.
Standardized Hazard Ratios of LV Mass/LV Mass Indexes with Relation of CVD/CHD Events, CVD Mortality and All-Cause Mortality by Disease Group
| Events No. (percentage) | HR per 1 SD LV Mass(g) (95% CI’s) | HR per 2 SD LV Mass/Height (g/m1.7) (95% CI’s) | HR per 3 SD LV Mass/BSA (g/m2) (95% CI’s) | |
|---|---|---|---|---|
| Total CVD | ||||
| Neither MetS/DM (n= 2,061) | 1098(53.3%) | 1.37(.123–1.53)**** | 1.29(1.18–1.42)**** | 1.23(1.14–1.32)**** |
| MetS (without DM) (n=1,178) | 746(63.3%) | 1.38(1.22–1.57)**** | 1.29(1.16–1.45)**** | 1.25(1.13–1.67)**** |
| DM (n=485) | 336(69.3%) | 0.99(0.86–1.13) | 1.06(0.94–1.19) | 1.04(0.94–1.15) |
| Overall (n=3,724) | 2,180(58.5%) | 1.28(1.20–1.37)**** | 1.25(1.17–1.32)**** | 1.20(1.14–1.26)**** |
| Total CHD | ||||
| Neither MetS/DM (n= 2,061) | 672(32.6%) | 1.37(1.20–1.56)**** | 1.35(1.21–1.51)**** | 1.26(1.16–1.38)**** |
| MetS (without DM) (n=1,178) | 477(40.5%) | 1.29(1.11–1.51)** | 1.22(1.06–1.40)** | 1.19(1.06–1.34)** |
| DM (n=485) | 219(45.2%) | 0.93(0.77–1.12) | 1.07(0.92–1.24) | 1.05(0.93–1.19) |
| Overall (n=3,724) | 1,368(36.7%) | 1.22(1.13–1.32)**** | 1.24(1.15–1.33)**** | 1.19(1.13–1.27)**** |
| CVD Mortality | ||||
| Neither MetS/DM (n= 2,061) | 493(23.9%) | 1.60(1.39–1.86)**** | 1.53(1.35–1.73)**** | 1.39(1.26–1.53)**** |
| MetS (without DM) (n=1,178) | 311(26.4%) | 1.41(1.15–1.73)*** | 1.44(1.22–1.70)**** | 1.37(1.19–1.57)**** |
| DM (n=485) | 182(37.5%) | 1.00(0.82–1.22) | 1.09(0.91–1.29) | 1.07(0.93–1.23) |
| Overall (n=3,724) | 986(26.5%) | 1.39(1.27–1.52)**** | 1.41(1.29–1.53)**** | 1.33(1.24–1.42)**** |
| All-cause Mortality | ||||
| Neither MetS/DM (n= 2,061) | 1565(75.9%) | 1.24(1.13–1.36)**** | 1.22(1.12–1.32)**** | 1.17(1.10–1.24)**** |
| MetS (without DM) (n=1,178) | 919(78.0%) | 1.22(1.08–1.39)** | 1.18(1.06–1.32)** | 1.16(1.06–1.26)** |
| DM (n=485) | 438(90.3%) | 1.07(0.94–1.21) | 1.09(0.91–1.22) | 1.08(0.98–1.19) |
| Overall (n=3,724) | 2922(78.5%) | 1.22(1.15–1.30)**** | 1.20(1.13–1.26)**** | 1.16(1.11–1.21)**** |
HR = hazard ratio, CI = confidence intervals;
SD of LV mass=30 grams.
SD of indexed LV mass/height = 10.6 grams/m.
SD of LV mass/BSA = 11.2 grams/m2.
Adjusted for age, gender, ethnicity, systolic blood pressure, diastolic blood pressure, hypertensive medications, HDL, LDL, total cholesterol, lipid medications, BMI, and fasting glucose.
p<0.05,
p<0.01,
p<0.001,
p<0.0001
Table 3.
Standardized Hazard Ratios of Total CVD events for LV Mass/LV Mass Indexes by Gender
| Events No. (percentage) | HR per 1 SD LV Mass(g) (95% CIs) | HR per 2 SD LV Mass (g)/Height (m1.7) (95% CI’s) | HR per 3 SD LV Mass (g)/BSA (m2) (95% CI’s) | ||
|---|---|---|---|---|---|
| Male vs. Female | |||||
| Neither MetS/DM (n= 2,061) | Male (n=828) | 474(57.2%) | 1.37(1.20–1.56)**** | 1.28(1.14–1.45)**** | 1.22(1.11–1.34)**** |
| Female (n=1,233) | 624(50.6%) | 1.39(1.15–1.68)*** | 1.31(1.13–1.53)**** | 1.23(1.10–1.39)**** | |
| MetS (without DM) (n=1,178) | Male (n=400) | 263(65.8%) | 1.37(1.14–1.65)*** | 1.29(1.09–1.53)** | 1.24(1.08–1.43)** |
| Female (n=778) | 483(62.1%) | 1.36(1.13–1.63)** | 1.29(1.11–1.51)** | 1.25(1.10–1.43)*** | |
| DM (n=485) | Male (n=225) | 166(73.8%) | 1.00(0.85–1.17) | 1.08(0.93–1.26) | 1.07(0.94–1.22) |
| Female (n=260) | 170(65.4%) | 0.99(0.74–1.34) | 1.00(0.80–1.24) | 0.99(0.83–1.18) | |
| Overall (n=3,724) | Male (n=1,453) | 903(62.1%) | 1.27(1.17–1.37)**** | 1.25(1.16–1.36)**** | 1.20(1.13–1.28)**** |
| Female (n=2,271) | 1,277(56.2%) | 1.31(1.17–1.47)**** | 1.24(1.13–1.36)**** | 1.19(1.11–1.29)**** | |
SD of LV mass=30 grams,
SD of indexed LV mass/height = 10.6 grams/m.
SD of LV mass/BSA = 11.2 grams/m2.
Risk factors include: age, gender, ethnicity, systolic blood pressure, diastolic blood pressure, hypertensive medications, HDL, LDL, total cholesterol, triglycerides, lipid medications, BMI, and fasting glucose.
p<0.05,
p<0.01,
p<0.001,
p<0.0001
While unadjusted HRs for CVD events in relation to LV mass (per SD) were significant (p<0.01) in all three disease groups, they were weaker in those with DM (1.16) compared to those with MetS (1.27) or neither condition (1.25), and in those with DM were further attenuated to being nonsignificant after adjustment for gender (higher LV mass and event rates in men with DM), age, systolic blood pressure, and cholesterol in particular. In addition the relation of LV mass with CVD events did not differ between men and women (interaction tests not significant). As less than 5% of our subjects (n=165) were African-American, the sample size was insufficient to show relationships with CVD events in those with MetS or DM; however, in those with neither condition, risks of CVD events (per standard deviation LV mass) appeared to be greater in African-Americans (HR=2.42 [1.26–4.64], p<0.01) than in whites ( HR=1.36 [1.22–1.51], p<0.001) with similar findings for indexed LV mass measures (results not shown).
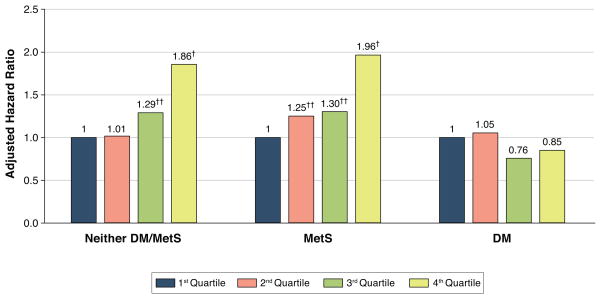
Adjusted Cox proportional hazard models for outcomes were also examined by quartiles of LV mass. Participants with neither MetS nor DM and those with MetS alone who were in the highest quartile of LV mass had significantly increased risks for total CVD [HRs of 1.9 (p<0.0001) and 2.0 (p<0.0001), respectively] when compared to those in the first quartile (Figure 2). Similarly, the highest quartile (versus lowest quartile) of LV mass independently predicted secondary endpoints of total CHD (HR = 2.0, p<0.0001), CVD death (HR = 2.4, p<0.0001), and all-cause mortality (HR=1.5, p<0.01) in those with neither condition. Hazard ratios were increased for total CHD (HR = 1.6, p=0.03) and CVD death (HR = 1.7, p=0.04), but not for all-cause mortality (HR=1.3 p=0.13) in those with MetS in the highest versus lowest quartile of LV mass. In contrast, there was no significant increased risk of both primary and secondary endpoints across quartiles of LV mass in those with DM.
Figure 2. Adjusted Hazard Ratios for Total Cardiovascular Disease Events by Quartiles of LV Mass Within Disease Group.
A higher adjusted hazard for cardiovascular events is observed across increasing LV mass quartiles for those with neither MetS/DM or for those with MetS without DM, but not in those with DM. MetS=metabolic syndrome. DM=diabetes mellitus. LV = left ventricular mass. †p<0.05, ‡p<0.01 compared to first quartile.
The area under the ROC curve (AUC) did not show significant incremental predictive value for total incident CVD events between the base model and the other three models for the prediction of CVD events with LV mass, LV mass/height1.7 or LV mass/BSA across all three disease groups, except modestly (p<0.05) for LV mass/height1.7 and LV mass/BSA in those with MetS (Table 4). There was also a significant (p<0.05) improvement in C-statistic in the overall sample comparing models with LV mass added to those with risk factors alone, although the absolute degree of improvement was minimal (both were 0.63 to the second decimal). We additionally examined the AUC improvement for CVD mortality and total mortality. The results showed that among those with neither disease AUC increased from 0.64 to 0.66 (p<0.05) for all three LV mass scores. The AUCs for total mortality ranged from 0.70–0.74 after including LV mass measures in the model but the improvement was not significant (data not shown).
Table 4.
Area Under the ROC Curve (AUC) for Models with LV Mass and Risk Factors versus Models with Risk Factors Alone for Prediction of Total CVD Events
| Base model (Risk factors) | Base model vs. Model(LV Mass + Risk factors) | Base model vs. Model (LV Mass/height1.7 + Risk factors) | Base model vs. Model (LV Mass/BSA + Risk factors) | |
|---|---|---|---|---|
| Neither MetS/DM (n= 2,061) | 0.62 | 0.62 | 0.62 | 0.62 |
| MetS (without DM) (n=1,178) | 0.61 | 0.63 | 0.63* | 0.63* |
| DM (n=485) | 0.65 | 0.65 | 0.65 | 0.65 |
| Overall(n=3,724) | 0.63 | 0.63* | 0.63* | 0.63* |
Risk factors include: age, gender, ethnicity, systolic blood pressure, diastolic blood pressure, hypertensive medications, HDL, LDL, total cholesterol, triglycerides, lipid medications, BMI, and fasting glucose.
p<0.05,
p<0.01
Analysis from net reclassification improvement (NRI) showed modest added clinical utility for prediction of CVD events and ranged from 4–7% in the non-disease group and 17–19% in the MetS group but was only significant in MetS group (p<0.01) when comparing the base model and models with three forms of LV mass measures; however, in those with DM NRI was not significant (Table 5). In the overall sample, there was a significant 9–10% NRI (p<0.01). The NRI for CVD mortality were 9%–15% in the three disease groups (p<0.05 in MetS and no disease group for LV mass/height1.7 and LV mass/BSA) The NRI for total mortality were greatest in those with MetS (10%, p value not significant) and were less than 5% in the other two groups (p=ns) (data not shown).
Table 5.
Category-Free Net Reclassification Improvement (NRI) for Models with LV Mass and Risk Factors versus Models with Risk Factors Alone for Prediction of CVD Events
| Base model vs. Model(LV Mass + Risk factors) | Base model vs. Model (LV Mass/height1.7 + Risk factors) | Base model vs. Model (LV Mass/BSA + Risk factors) | |
|---|---|---|---|
| Neither MetS/DM (n= 2,061) | 0.04 | 0.07 | 0.07 |
| MetS (without DM) (n=1,178) | 0.17** | 0.19** | 0.19** |
| DM (n=485) | −0.03 | −0.07 | −0.06 |
| Overall(n=3,724) | 0.10** | 0.09** | 0.09** |
Risk factors include: age, gender, ethnicity, systolic blood pressure, diastolic blood pressure, hypertensive medications, HDL, LDL, total cholesterol, triglycerides, lipid medications, BMI, and fasting glucose.
p<0.05,
p<0.01,
p<0.001
DISCUSSION
Our study found that increased LV mass (highest quartile) was associated with increases in risk for total CVD events, total CHD events, as well as CVD and total mortality in those with and without MetS, but not in those with DM. Our paper is the first to report on added discriminative and clinical utility for echocardiographic LV mass over standard CVD risk factors using ROC and NRI techniques.
Our report corroborates earlier findings from shorter-term follow-up regarding the overall relation of echo predictors (including LV mass) to CVD events in the entire CHS cohort by Gardin et al5. In addition, Kuller and colleagues21 previously reported among persons with DM that the general presence of subclinical CVD (from the presence of a low ankle-brachial index, increased carotid intimal medial thickness or stenosis, major ECG abnormalities, or angina) was associated with a two-fold greater risk of incident CHD. More recently, in the longitudinal Multiethnic Study of Atherosclerosis (MESA), LV mass measured by cardiac magnetic resonance imaging was shown to improve the c-statistic over traditional risk factors for the prediction of incident HF,22 although this relationship was not examined in those with MetS and DM.
DM is noted to have an adverse effect on hypertrophic remodeling through promoting increases in LV mass and dimensions7. The Framingham Heart study identified an association between DM and increased LV wall thickness and mass that was independent of traditional risk factors in women, but not in men.23 LV hypertrophy is common in those with DM, but previous screening modalities such as with ECG and NT-proBNP have been noted to be inadequate for detecting LV hypertrophy.24 Our data also suggests echo LV mass has limited utility to stratify risk in persons with DM, at least in older adults which comprised our cohort. While increased LV mass is a well-known marker of end-organ hypertensive damage, a possible explanation for our lack of a relation in those with DM may be their high baseline risk, supported by the common notion that DM is a coronary risk equivalent25 (which would especially be the case in our older cohort), and hypertension or other highly prevalent risk factors in older persons with DM may have obscured our relationships with LV mass in such persons. Of note, we observe that even though the unadjusted relation of LV mass with CVD events is significant in those with DM, it is of lower magnitude than those with MetS or neither condition, and is attenuated to being nonsignficant after adjustment for age, gender (in particular), systolic blood pressure, and cholesterol, whereas the LV mass relation with CVD events remains significant after adjustment for these and other risk factors in those without DM. Alternatively, if smaller LV mass is protective, the prognostic value of LV mass in diabetics may be lost because of their higher baseline LV mass--in particular, in a cohort of older subjects who have had years of exposure to DM and more advanced subclinical CVD. Other measures which more directly reflect atherosclerosis burden may be more important for further risk stratification of the patient with DM, such as coronary calcium which has been shown to add prognostic value in such patients.13–14 MetS, however, is a more heterogeneous condition associated with a wide variation in CVD risk,26 with many persons at intermediate risk where further evaluation such as by echo LV mass, may be helpful for risk stratification; our data support this by showing a modest added value for echo LV mass in risk prediction in such persons and in those without MetS.
Limitations of our study include the fact that the unidimensional nature of our M-mode measurements does not take into account changes in eccentricity based on long-axis and short-axis LV measurements; thus, future studies involving 2D or 3D echocardiographic recordings should investigate whether LV mass is erroneously estimated by M-mode echo in conditions such as obesity, MetS, and DM in which the ventricle may be more spherically-shaped. In fact, Bluemke et al 27 showed that stroke and CHD events were better predicted by abnormal LV geometry (e.g., increased LV mass to volume ratio), whereas HF events were driven primarily by increased LV mass alone. Our study did not have measures of LV geometry, systolic or diastolic function, which are known to be affected in DM. However, other studies have shown that LV mass was the single 2D echo measurement consistently associated with total and individual CVD endpoints 28. The substantially higher overall CVD event rates in the diabetic group, which were not further increased by higher LV mass levels, contrasts with those without DM where increased LV mass added more to CVD event prediction. Of additional note, the majority of our cohort was of Caucasian descent; consequently, our findings may not be generalizable to other racial/ethnic groups and to younger populations.
In conclusion, our study shows in older persons with and without MetS, but not DM, echo LV mass is positively associated increases in total CVD risk, including CHD, HF, and stroke, and adds modest clinical utility for CVD prediction over standard risk factors. Thus, measurement of LV mass, while possibly useful to stratify risk in older persons without DM, may be of limited clinical utility in those with DM, who are already at significant CVD risk.
Clinical Perspectives.
Competency in Medical Knowledge
The evaluation of left ventricular mass using two-dimensionally guided M-mode echocardiography is known to provide risk stratification for future cardiovascular events beyond information provided by standard risk factors. This study confirms these findings in older adults generally, and in those without diabetes, including those with and without metabolic syndrome. The weaker role of left ventricular mass for improving risk prediction in those with diabetes may be due to the important effect of other risk factors.
Translational Outlook
Older persons and especially those with diabetes have significant, but often varied risks for developing cardiovascular events. Future studies might examine the role of other structural and functional characteristics, especially measured by newer technologies such as cardiac magnetic resonance imaging (MRI) for further refining cardiovascular risk prediction in such patients.
Acknowledgments
Presented in part at the American Heart Association Scientific Sessions, Orlando, Fl, November 2009. This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Abbreviations
- LV
left ventricular
- CVD
cardiovascular disease
- CHD
coronary heart disease
- MetS
metabolic syndrome
- DM
diabetes mellitus
- HR
hazard ratio
- AUC
area under the curve
- NRI
net reclassification index
- BP
blood pressure
- LDL-C
LDL-cholesterol
- BMI
body mass index
- BSA
body surface area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wong ND. Metabolic syndrome: cardiovascular risk assessment and management. Am J Cardiovasc Drugs. 2007;7:259–72. doi: 10.2165/00129784-200707040-00004. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 3.Malik S, Wong ND, Franklin SS, Kamath TV, L’Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–50. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Kamineni A, Prineas RJ, Siscovick DS. Metabolic syndrome and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med. 2008;168:969–78. doi: 10.1001/archinte.168.9.969. [DOI] [PubMed] [Google Scholar]
- 5.Gardin JM, McClelland R, Kitzman D, et al. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (The Cardiovascular Health Study) Am J Cardiol. 2001;87:1051–7. doi: 10.1016/s0002-9149(01)01460-6. [DOI] [PubMed] [Google Scholar]
- 6.Mule G, Nardi E, Cottone S, et al. Impact of metabolic syndrome on left ventricular mass in overweight and obese hypertensive subjects. Int J Cardiol. 2007;121:267–75. doi: 10.1016/j.ijcard.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Burchfiel CM, Skelton TN, Andrew ME. Metabolic syndrome and echocardiographic left ventricular mass in blacks; the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2006;112:819–27. doi: 10.1161/CIRCULATIONAHA.104.518498. [DOI] [PubMed] [Google Scholar]
- 8.Lindman BR, Arnold SV, Madrazo JA, et al. The adverse impact of diabetes mellitus on left ventricular remodeling and function in patients with severe aortic stenosis. Circ Heart Fail. 2011;4:286–92. doi: 10.1161/CIRCHEARTFAILURE.110.960039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eguchi K, Ishikawa J, Hoshide S, et al. Differential impact of left ventricular mass and relative wall thickness on cardiovascular prognosis in diabetic and nondiabetic hypertensive subjects. Am Heart J. 2007;154:79.e9–15. doi: 10.1016/j.ahj.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Evans JM, Wang J, Morris AD. Comparison of cardiovascular risk between patients with type 2 diabetes and those who had had a myocardial infarction: cross sectional and cohort studies. BMJ. 2002;324(7343):939–42. doi: 10.1136/bmj.324.7343.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alipour N, Wong ND, Malik S. Diagnosis of coronary artery disease in persons with diabetes mellitus. Curr Diab Rep. 2012;12:286–293. doi: 10.1007/s11892-012-0273-8. [DOI] [PubMed] [Google Scholar]
- 12.Wong ND, Rozanski A, Gransar H, et al. Metabolic syndrome and diabetes are associated with an increased likelihood of inducible myocardial ischemia among patients with subclinical atherosclerosis. Diabetes Care. 2005;28:1445–50. doi: 10.2337/diacare.28.6.1445. [DOI] [PubMed] [Google Scholar]
- 13.Wong ND, Sciammarella MG, Polk D, et al. The metabolic syndrome, diabetes, and subclinical atherosclerosis assessed by coronary calcium. J Am Coll Cardiol. 2003;41:1547–53. doi: 10.1016/s0735-1097(03)00193-1. [DOI] [PubMed] [Google Scholar]
- 14.Malik S, Budoff MJ, Katz R, et al. Impact of subclinical atherosclerosis on cardiovascular disease events in persons with metabolic syndrome and diabetes: the Multiethnic Study of Atherosclerosis. Diabetes Care. 2011;34:2285–90. doi: 10.2337/dc11-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fried LP, Borhanni NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittlemark MB, Newman A, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 16.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and Management of the Metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735– 2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 17.Gardin JM, Wong ND, Bommer W, Klopfenstein HS, Smith V-E, Tabatznik B, Siscovick D, Lobodzinski S, Anton-Culver H, Manolio TA. Echocardiographic design of a multi-center investigation of free-living elederly subjects: the Cardiovascular Health Study. J Am Soc Echocardiog. 1992;5:63–72. doi: 10.1016/s0894-7317(14)80105-3. [DOI] [PubMed] [Google Scholar]
- 18.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 19.Chirinos JA, Segers P, De Buyzere ML, Kronmal RA, Raja MW, De Bacquer D, Claessens T, Gillebert TC, St John-Sutton M, Rietzschel ER. Left ventricular mass: allometric scaling, normative values, effect of obesity, and prognostic performance. Hypertension. 2010;56:91–8. doi: 10.1161/HYPERTENSIONAHA.110.150250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SAS Procedures Guide, version 6.12. 3. Cary, NC: SAS Institute; 1995. [Google Scholar]
- 21.Kuller LH, Velentgas P, Barzilay J, et al. Diabetes mellitus: subclinical cardiovascular disease and risk of incident cardiovascular disease and all-cause mortality. Arterioscler Throm Vasc Biol. 2000;20:823–29. doi: 10.1161/01.atv.20.3.823. [DOI] [PubMed] [Google Scholar]
- 22.Jain A, McClelland RL, Polak JF, et al. Cardiovascular imaging for assessing cardiovascular risk in asyptomatic men versus women: the multi-ethnic study of atherosclerosis (MESA) Circ Cardiovasc Imaging. 2011;4:8–15. doi: 10.1161/CIRCIMAGING.110.959403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galderisi M, Anderson KM, Wilson PW, Levy D. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study) Am J Cardiol. 1991;68:85–9. doi: 10.1016/0002-9149(91)90716-x. [DOI] [PubMed] [Google Scholar]
- 24.Somaratne JB, Whalley GA, Poppe KK, et al. Screening for left ventricular hypertrophy in patients with type 2 diabetes mellitus in the community. Cardiovasc Diabet. 2011;10:29. doi: 10.1186/1475-2840-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 26.Hoang K, Ghandehari H, Lopez VA, Barboza MG, Wong ND. Global coronary heart disease risk assessment of individuals with the metabolic syndrome in the U. S Diabetes Care. 2008;31:1405–9. doi: 10.2337/dc07-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–55. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy D, Savage DD, Garrison RJ, Anderson KM, Kannel WB, Castelli WP. Echocardiographic criteria for left ventricular hypertrophy: the Framingham Heart Study. Am J Cardiol. 1987;59(9):956–60. doi: 10.1016/0002-9149(87)91133-7. [DOI] [PubMed] [Google Scholar]