Abstract
Dendritic cell (DC) activation and antigen presentation are critical for efficient priming of T cell responses. Here, we study how lentiviral vectors (LVs) deliver antigen and activate DCs to generate T cell immunization in vivo. We report that antigenic proteins delivered in vector particles via pseudotransduction were sufficient to stimulate an antigen-specific immune response. The delivery of the viral genome encoding the antigen increased the magnitude of this response in vivo but was irrelevant in vitro. Activation of DCs by LVs was independent of MyD88, TRIF, and MAVS, ruling out an involvement of Toll-like receptor or RIG-I–like receptor signaling. Cellular DNA packaged in LV preparations induced DC activation by the host STING (stimulator of interferon genes) and cGAS (cyclic guanosine monophosphate–adenosine monophosphate synthase) pathway. Envelope-mediated viral fusion also activated DCs in a phosphoinositide 3-kinase–dependent but STING-independent process. Pseudotransduction, transduction, viral fusion, and delivery of cellular DNA collaborate to make the DC-targeted LV preparation an effective immunogen.
INTRODUCTION
Dendritic cells (DCs) are targets for immunization purposes because of their superior ability to process antigens and present them to T cells. One strategy uses an HIV-1–derived lentiviral vector (LV) to deliver genes encoding antigen to DCs by pseudotyping the vector with a mutant DC-targeting Sindbis virus glycoprotein (SVGmu) (1, 2). The in vivo administration of this LV resulted in the selective expression of antigen in DCs and efficient priming of antigen-specific CD8+ T cells with antitumor immunity in mice in comparison with methods using recombinant protein antigen or adoptive transfer of antigen-loaded or viral vector–transduced DCs. DC-targeted LV vaccines are under clinical evaluation in humans (3). However, the exact mechanism behind such immunization is unclear. It is evident that antigen delivery methods lacking a DC maturation signal—such as antigen conjugated to the DC-specific anti–DEC-205 antibody—led to effective antigen presentation but promoted tolerance rather than immunity (4, 5). The coadministration of a maturation stimulus was required to break immune tolerance (4). Thus, the efficacy of DC-targeted LV immunization likely requires the coupling of two independent functions: delivery of antigen and activation of DCs.
The first function—LV antigen delivery to DCs—is thought to primarily occur by transduction, which requires overcoming host restriction factors such as SAMHD-1 that block reverse transcription (6). Barriers to transduction are surmountable by using precursor DCs, high multiplicity of infection (MOI), or codelivering Vpx (7–10). Other mechanisms may enable delivery of protein antigens to DCs independent of transduction.
The second function—LV activation of DCs—can occur in well-differentiated DCs and with high MOI (7, 8, 11). LV nucleic acids can be detected by intracellular pathways involving endosomal Toll-like receptors (TLRs) (12–14), mitochondrial antiviral-signaling protein (MAVS) (15), cyclic guanosine monophosphate–adenosine monophosphate synthase (cGAS), and stimulator of interferon genes (STING) (16–18). However, lentivirus-like particles, which were deficient of viral nucleic acids, elicited potent antigen-specific CD8+ T cells responses, suggesting that vector components other than viral nucleic acids contribute to DC activation (19–21).
In this study, we report that LV pseudotransduction was a key mechanism of antigen delivery and immune stimulation. LV transduction contributed to antigen delivery in vivo but was not required for immune stimulation. LVs induced DC activation via two processes. First, viral envelope–mediated fusion itself induced a phosphoinositide 3-kinase (PI3K)–dependent and STING-independent pathway. Second, we find that the human genomic DNA within virion preparations activated the STING and cGAS pathway.
RESULTS
LV pseudotransduction activates DCs
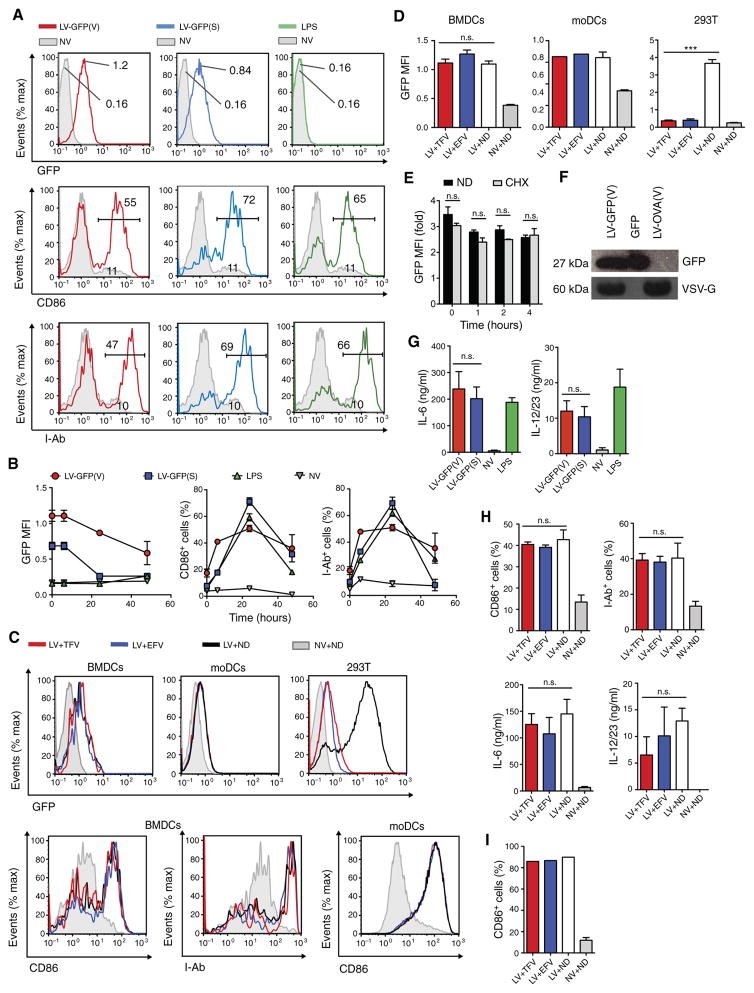
We first sought to understand the mechanism of antigen delivery to DCs generated at day 8 of culture with LV encoding green fluorescent protein (GFP) pseudotyped with vesicular stomatitis virus glycoprotein (VSV-G) or SVGmu (1). Culture of mouse bone marrow cells in granulocyte-macrophage colony-stimulating factor (GM-CSF) generated a heterogeneous population of which 70% were well-differentiated bone marrow–derived DCs (BMDCs) based on the expression of CD11c and CD11b (fig. S1A). Human monocytes cultured in GM-CSF and interleukin-4 (IL-4) generated a cell population composed of 96% monocyte-derived DCs (moDCs) based on negative expression of CD14 and positive expression of DC-SIGN (fig. S1B). Well-differentiated mouse BMDCs and human moDCs were difficult to transduce in vitro, but up to an eightfold increase in GFP mean fluorescence intensity (MFI) was observed [Fig 1, A (top) and B (left)] and undiminished by reverse transcriptase inhibitors (RTIs) [Fig 1, C (top) and D]. By contrast, 293T cells treated with the same dose of LVs were efficiently transduced with up to a 21-fold increase in GFP MFI in a process that was sensitive to RTIs [Fig 1, C (top right) and D (right)]. The GFP expression in BMDCs was dependent on the dose of LVs but not on whether the LVs were from unconcentrated or concentrated preparations (fig. S2A). In addition, GFP expression in BMDCs was highest immediately after LV treatment and then steadily decreased over 48 hours (Fig 1B, left). Although LVs can deliver host cellular mRNA (22), we found that the protein synthesis inhibitor cycloheximide failed to decrease BMDC expression of GFP (Fig 1E), suggesting that the GFP was not produced de novo in DCs. We could detect GFP in lysates of LV particles by Western blot analysis (Fig 1F), finding about 1.53 μg of GFP per microgram of p24 by enzyme-linked immunosorbent assay (ELISA). These results are consistent with previous reports that LVs were capable of pseudotransduction (23, 24).
Fig. 1. LV pseudotransduction delivers proteins and activates DCs.
(A) Representative fluorescence-activated cell sorting (FACS) plots of mouse BMDCs that were treated with LV-GFP(V), LV-GFP(S), LPS, or no vector (NV) and analyzed for expression of GFP, CD86, and I-Ab. GFP geometric MFI was measured immediately after LV spin inoculation, and CD86 and I-Ab expression was measured 24 hours after LV treatment. (B) GFP, CD86, and I-Ab expression of LV-treated BM-DCs was measured over 48 hours. (C) Representative FACS plots of mouse BMDCs, human moDCs, and 293T cells that were incubated with tenofovir (TFV; 40 μM), efavirenz (EFV; 80 μM), or no drug (ND) 6 hours before treatment with LV-GFP(V) and then analyzed 24 hours later. (D) Graph depicts the GFP MFI of BMDCs, moDCs, and 293T cells from (C). (E) Mouse BMDCs were incubated with or without cycloheximide (CHX; 50 μg ml−1) 1 hour before treatment with LV-GFP(V), and then, GFP MFI was presented relative to those BMDCs receiving no LV with or without cycloheximide. (F) Western blot analysis of GFP of lysates from LV-GFP(V) and LV expressing OVA pseudotyped with VSV-G [LV-OVA(V)] and purified GFP protein (40 ng). (G) Mouse BMDCs were treated as in (A) and analyzed for the amount of IL-6 and IL-12/23 in the supernatant by ELISA 24 hours after LV treatment. (H and I) Mouse BMDCs (H) and human moDCs (I) were treated as in (C) and analyzed for expression of CD86, I-Ab, or amount of IL-12/23 and/or IL-6 in the supernatant 24 hours after LV treatment. Data are representative of two (A to D and G to I) or three (E and F) independent experiments. Results are shown as mean ± SEM (B, D, E, G, and H). n.s., not significant. P > 0.05; ***P < 0.001 [one-way ANOVA (D and H) and unpaired Student’s t test (E and G)].
We next sought to determine whether LV pseudotransduction was important to DC activation. Consistent with previous work, we found that mouse BMDCs were activated by LVs, as demonstrated by the increase expression of activation markers CD86 and major histocompatibility complex II (MHC II) molecule and secretion of cytokines IL-6 and IL-12/23 [Fig 1, A (middle and bottom), B (middle and right), and G] (1, 14) in a dose-dependent manner (fig. S2B). DC activation was similar between unconcentrated and concentrated LV preparations (fig. S2B). Pseudotransduction occurred in both well- and less-differentiated BMDCs (fig. S3A), but LV activation occurred only among the well-differentiated DCs (fig. S3B). LV activation of mouse BMDCs and human moDCs was not diminished by RTIs [Fig 1, C (bottom), H, and I], suggesting that LVs activated DCs via a reverse transcriptase–independent mechanism. This lack of sensitivity to RTIs was not due to ineffective inhibition of reverse transcription because the RTIs blocked GFP transduction in LV-infected 293T cells [Fig 1, C (top right) and D (right)].
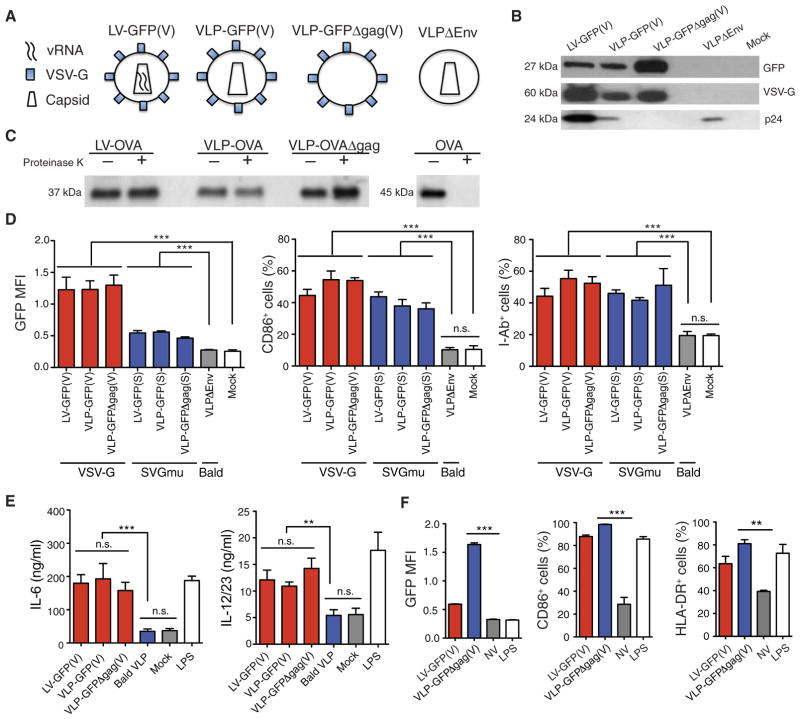
To determine the LV component responsible for DC activation, we generated a platform of genome-less virus-like particles (VLPs) by omitting plasmids encoding the viral genome, capsid, or envelope (Fig 2A) (13, 25). We confirmed the presence of the viral envelope and capsid by detecting VSV-G and p24, respectively, using Western blot analysis (Fig 2B). Similar to LVs, we found that pseudotyped VLPs incorporated the vector-encoded proteins GFP or ovalbumin (OVA) but not “bald” viral particles that were produced by transfecting 293T cells with only the packaging plasmids encoding gag, pol, and rev. To assess whether these proteins were carried within the vectors or associated externally, we treated LV and VLPs incorporating OVA with proteinase K, inactivated the proteinase K with phenyl-methylsulfonyl fluoride (PMSF), lysed the vectors, and still detected OVA in the lysate, indicating that the OVA in vectors was proteinase K–resistant and thus carried within particles (Fig 2C). An OVA suspension, which was not associated with vectors, was not proteinase K–resistant. We next found that the pseudotyped VLPs but not “bald” VLPs delivered GFP and induced the activation of mouse BMDCs (Fig 2, D and E). The inclusion of the vector genome or viral capsid into the VLP had no effect on BMDC activation [Fig 2, D (middle and right) and E]. Furthermore, VSV-G–pseudotyped VLPs capably delivered GFP and activated human moDCs (Fig 2F). Together, these data suggest that the viral envelope, but not the vector genome or capsid, was necessary and sufficient to activate DCs.
Fig. 2. LV envelope is responsible for DC activation.
(A) Schematic of components of LV and VLPs. (B) Western blot analysis for GFP, VSV-G, and p24 on LV and VLP lysates. (C) Western blot analysis detecting OVA in the lysate of the following SVGmu-pseudotyped vectors: LV carrying OVA (LV-OVA), VLP carrying OVA (VLP-OVA), and VLP-carrying OVA deficient of gag (VLP-OVAΔgag). Vectors were treated or not treated with proteinase K, which was inactivated with PMSF before vector lysis. To verify whether proteinase K degradation was effective, we used soluble OVA as a control. (D and E) Mouse BMDCs were treated with VSV-G or SVGmu-pseudotyped LVs and VLPs and then analyzed at 24 hours for GFP, CD86, and I-Ab expression by flow cytometry (D) and for the amount of IL-6 and IL-12/23 in the cell supernatant by ELISA (E). (F) Human moDCs were treated with LV-GFP(V) or VLP carrying GFP deficient of gag [VLP-GFPΔgag(V)] and analyzed at 24 hours for GFP, CD86, and human MHC II molecule human lymphocyte antigen-D–related (HLA-DR) expression by flow cytometry. Data are representative of three (B and C) or two (D to F) independent experiments. Results are shown as mean ± SEM. P > 0.05; **P < 0.005; ***P < 0.001 (unpaired Student’s t test).
LV genome, cDNA, and capsid are not adjuvants in vivo
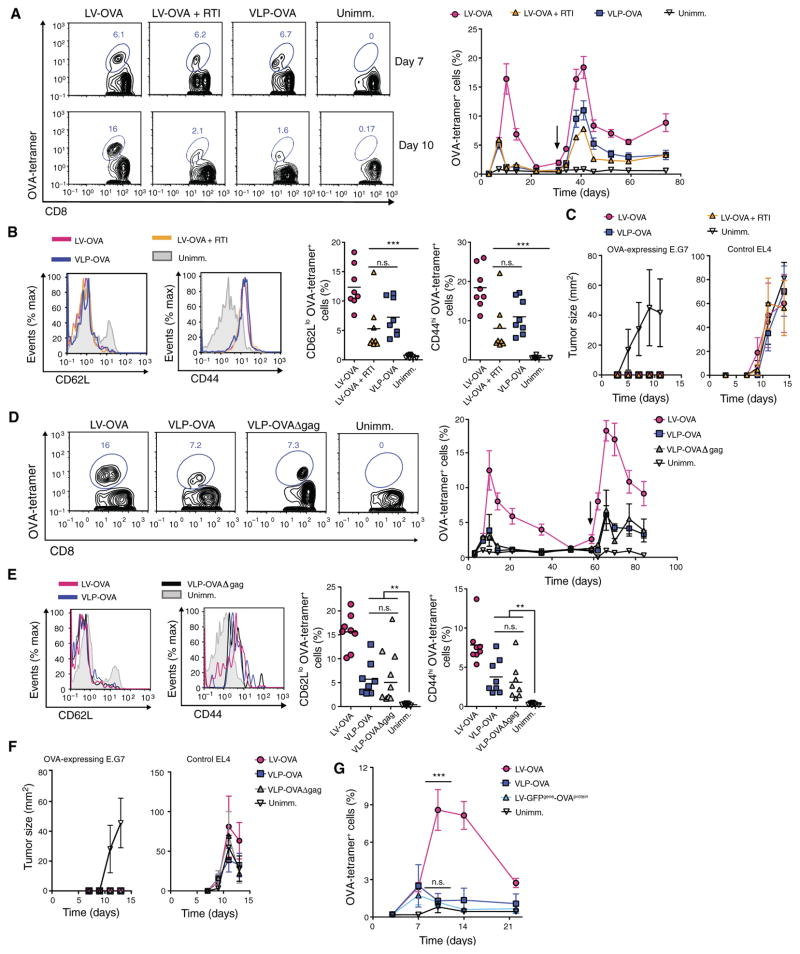
To determine whether these findings were relevant in vivo, we injected into mice the DC-targeted SVGmu-pseudotyped LV-OVA with or without a concurrent oral regimen of RTIs or VLP carrying OVA pseudotyped with SVGmu (VLP-OVA). We initially found that OVA-specific CD8+ T cells were similarly induced in all three groups at 7 days after immunization (Fig 3A and fig. S1C). However, through 10 days after immunization, the magnitude of these cells continued to increase in LV-immunized mice but not in mice immunized with LV-OVA with RTIs or VLP (Fig 3A), suggesting that transduction was enhanced, but was not necessary, for inducing antigen-specific CD8+ T cells. After the second administration of homologous vector, the OVA-specific CD8+ T cells were boosted in all three groups (Fig 3A, right) and expressed similar effector memory phenotypes (CD62LloCD44hi) (Fig 3B). After the secondary immune responses subsided, we injected 5 × 106 OVA-expressing E.G7 thymoma tumor cells and 5 × 106 control non–OVA-expressing EL4 thymoma tumor cells on opposing legs, enabling intra-animal comparison. Mice homologously prime- boosted with LV with or without RTIs or VLPs were protected against the growth of OVA-expressing E.G7 tumors (Fig 3C, left). As expected, non–OVA-expressing EL4 tumors continued to grow in the immunized mice (Fig 3C, right). The antitumor protection of mice immunized with LV with RTI and VLP was not altogether unexpected because 8 to 10% of the CD8+ T cells were OVA-specific after the boost. The similar immunization responses of mice receiving LV with RTIs and VLP demonstrate that a component of LV immunization was independent of reverse transcription and the viral genome, likely due to pseudotransduction. We next found that immunization responses were similar between mice homologously prime-boosted with VLPs carrying OVA with or without the viral capsid (VLP-OVA or VLP-OVAΔgag, respectively) (Fig 3, D and E). Therefore, VLPs, which had the viral envelope as the sole viral component, generated a strong-enough memory CD8+ T cell response to be protective against the growth of OVA-expressing E.G7 tumor cells (Fig 3F). The viral capsid did not enhance immunization responses.
Fig. 3. LV DNA, genome, and capsid are not required for DC activation and CD8+ T cell priming in vivo.
(A) Wild-type mice received homologous prime-boost vaccination of SVGmu-pseudotyped LV-OVA with or without RTI or VLP-OVA (n = 8 mice per group). Representative FACS plots show OVA-tetramer+ cells gated on CD8+ T cells from the blood at 7 and 10 days after primary immunization (left). Graph depicts percentages of OVA-tetramer+ CD8+ T cells from the blood of immunized and unimmunized mice over time (black arrow, boost) (right). (B) Representative FACS plots show expression of CD62L and CD44 on OVA- tetramer+ CD8+ T cells from immunized mice compared with naïve CD8+ T cells from unimmunized mice at 7 days after boost (left). Graphs depict percentages of CD62Llo and CD44hi OVA-tetramer+ cells, with each symbol representing an individual mouse and horizontal bar indicating the mean (right). (C) Seven weeks after boost, mice were injected with 5 × 106 OVA-expressing E.G7 thymoma tumor cells and 5 × 106 EL4 (control) non–OVA-expressing EL4 thymoma tumor cells on opposing legs, and tumor sizes were measured. (D to F) Wild-type mice were homologously prime-boosted with LV-OVA, VLP-OVA, or capsid-less VLP-OVAΔgag. OVA-tetramer+ cells from the blood were analyzed as in (A) (n = 8 mice per group) at 7 days after boost (left) and over time (right) (D). CD62L and CD44 expression of OVA-tetramer+ CD8+ T cells from immunized mice compared with naïve CD8+ T cells from unimmunized mice at 7 days after boost were measured as in (B) (E). Mice were injected with tumor cells, as in (C), and tumor sizes were measured (F). (G) Wild-type mice were immunized with LV-OVA, LV encoding OVA carrying GFP (LV-GFPgene-OVAprotein), or VLP-OVA (n = 8 mice per group), and OVA-tetramer+ CD8+ T cells from the blood were measured over time. Statistical comparisons were made between the LV-GFPgene-OVAprotein– and VLP-OVA– or LV-OVA–immunized mice. Data are representative of two independent experiments (A to G). Results are shown as mean ± SEM (A, C, D, F, and G). P > 0.05; **P < 0.005; ***P < 0.001 (unpaired Student’s t test).
We next questioned why LV-immunized mice had a greater immune response compared with mice treated with LV with RTI or VLP. We generated an SVGmu-pseudotyped LV encoding GFP and carrying the protein OVA (LV-GFPgene-OVAprotein). Because the vector-generated LV DNA did not encode OVA, we could assess whether LV DNA enhances OVA pseudotransduction. This vector induced an OVA-specific CD8+ T cell response similar to VLP-immunized mice but not LV-immunized mice, suggesting that reverse-transcribed LV DNA did not enhance the antigen-specific CD8+ T cell response produced by pseudotransduction (Fig 3G). Together, these results suggest that LV genome transduction amplified antigen delivery but not immune stimulation in vivo.
The direct injection of SVGmu-pseudotyped LV into mice specifically transduces conventional DCs (cDCs) in vivo and not other immune cells (1, 26, 27). To assess whether DCs were pseudotransduced in vivo, we subcutaneously injected SVGmu-pseudotyped VLP carrying GFP into the hindleg of wild-type mice and, after 1 day, harvested lymphoid tissue to analyze cells for GFP expression. GFP-positive cells were detected in cDCs, particularly in the CD11b− and CD11b+ cDC subsets, from the draining inguinal lymph nodes of VLP-immunized mice but not of unimmunized mice (figs. S1D and S4A). There was no obvious difference in GFP expression among the plasmacytoid DCs, B cells, T cells, or macrophages isolated from the lymph nodes of VLP-immunized and unimmunized mice (fig. S4). These results suggest that the DC-targeted vectors pseudotransduced cDCs in vivo.
LVs and VLPs activate DCs and adaptive immunity via the STING and cGAS pathway
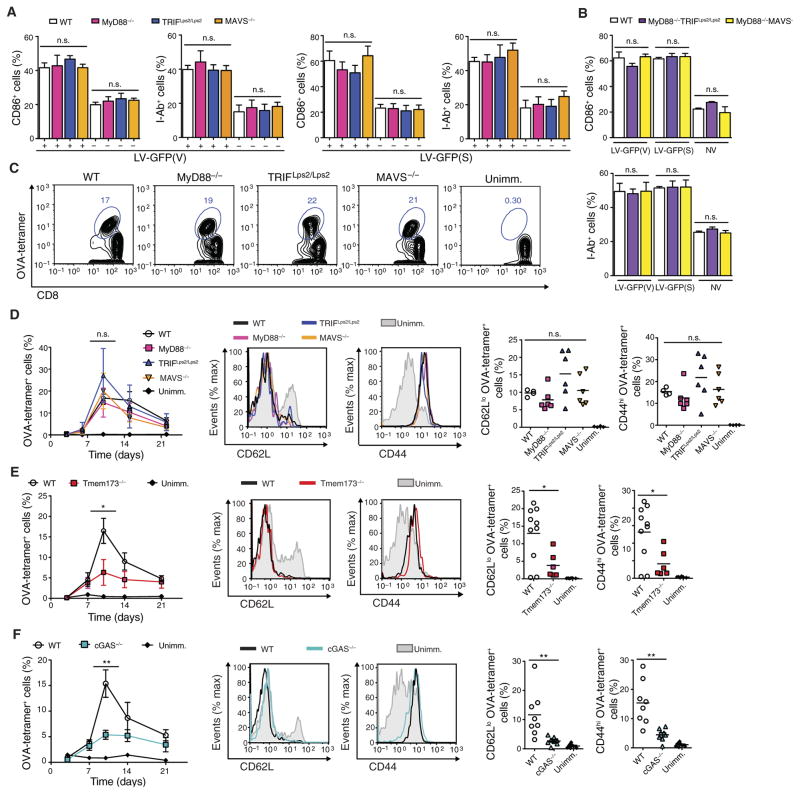
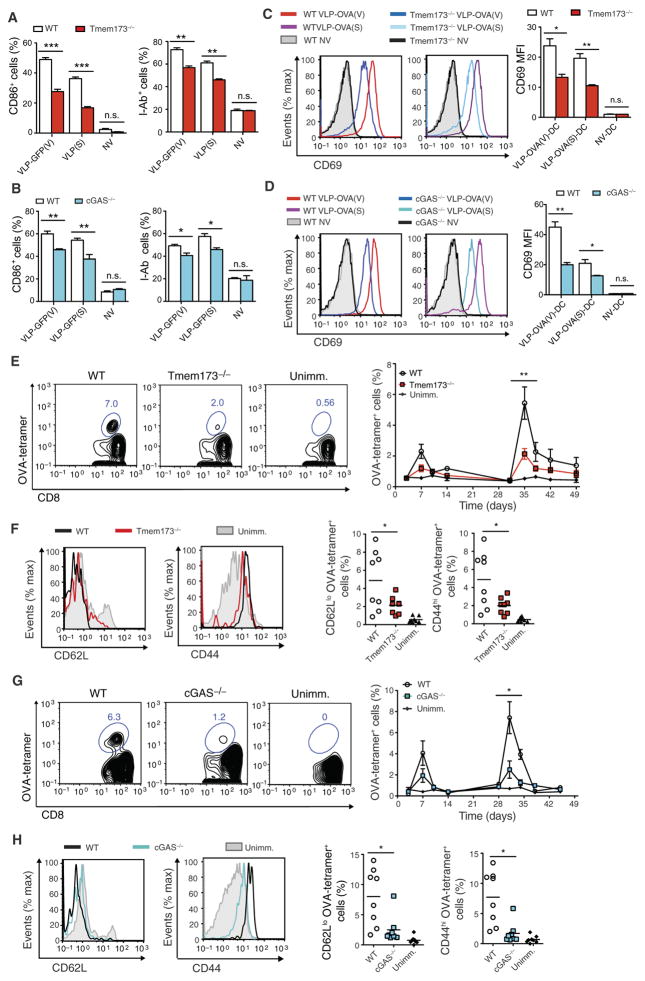
We next set out to identify the innate immune signaling pathway responsible for LV detection. LVs capably activated BMDCs from mice deficient in MyD88, TRIF, or MAVS (Fig 4A). Activation of BMDCs from mice doubly deficient in MyD88 and TRIF or MyD88 and MAVS was still unaffected (Fig 4B). LV-OVA immunization of MyD88-, TRIF-, or MAVS-deficient mice capably induced OVA-specific effector memory CD8+ T cells (Fig 4, C and D). LV-OVA immunization of TLR4-deficient mice was also efficient (fig. S5, A to C). In addition, LVs capably activated BMDCs from mice lacking the interferon-α/β (IFN-α/β) receptor function (fig. S5D), indicating that type I IFN signaling was not required. We then turned to the STING and cGAS pathway and found that LV immunization was significantly decreased up to threefold in these mutant mice (Fig 4, E and F), suggesting that STING and cGAS were important to LV immunization.
Fig. 4. LV activation of DCs and subsequent CD8+ T cell priming are dependent on STING and cGAS but not on MyD88, TRIF, or MAVS.
(A and B) BMDCs from mice singly or doubly deficient in MyD88, TRIF, and MAVS were treated with LV-GFP(V) or LV-GFP(S) and analyzed at 24 hours for expression of CD86 and I-Ab by flow cytometry. (C to F) Mice deficient in MyD88, TRIF, MAVS, STING, or cGAS were immunized with LV-OVA. Unimmunized wild-type (WT) mice were injected with PBS. OVA-tetramer+ cells gated on CD8+ T cells from the blood were demonstrated on representative FACS plot at 10 days after primary immunization (C) or measured over time (D to F) (left). Statistical comparisons were made between the OVA-tetramer+ CD8+ T cell response of the LV-immunized wild-type mice and that of the LV-immunized mutant mice. CD62Llo and CD44hi OVA-tetramer+ CD8+ T cells from LV-immunized mutant and wild-type mice were compared with naïve CD8+ T cells from unimmunized mice at 10 days on representative FACS plots (D to F) (middle) or by group with each symbol representing an individual mouse and horizontal bar indicating the mean (D to F) (right). n = 6 mutant immunized mice per group; n = 4 wild-type immunized and unimmunized mice per group (C and D). n = 6 mice in Tmem173−/− immunized and unimmunized wild-type groups; n = 10 mice in wild-type immunized group (E). n = 8 per group (F). Data are representative of three (A and B) or two (C to E) independent experiments or pooled from two independent experiments (F). Results are shown as mean ± SEM (A, B, and D to F). P > 0.05; *P < 0.05; **P < 0.005 [one way-ANOVA (A, B, and D) and unpaired Student’s t test (E and F)].
In addition, we observed that VLP activation of BMDCs was partially dependent on STING and cGAS (Fig 5, A and B). STING- or cGAS-deficient BMDCs treated with VLP carrying OVA had a reduced ability to up-regulate the activation marker CD69 on CD8+ T cells expressing an OVA-specific T cell receptor (Fig 5, C and D, and fig. S1E). The homologous prime-boost vaccination of STING- or cGAS-deficient mice with the DC-targeted VLP-OVA induced up to threefold less effector memory CD8+ T cells (Fig 5, E to H). Mice deficient in MyD88 (28), TRIF (29), MAVS (30), type I IFN receptor (31), STING (32), or cGAS (33) are born at expected Mendelian ratios and grow without obvious developmental or fertility issues. BMDC populations generated in vitro among these mutants were represented similarly compared with wild-type BMDCs (fig. S6). Together, these results suggest that a component within LVs, which is not the viral genome, triggered the host STING and cGAS pathway.
Fig. 5. VLPs activate DCs and antigen-specific CD8+ T cells via the STING and cGAS pathway.
(A and B) BMDCs from Tmem173−/− and cGAS−/− mice were treated with VLP-GFP(V) or VLP-GFP(S) and analyzed at 24 hours for CD86 and I-Ab expression by flow cytometry. (C and D) Tmem173−/− and cGAS−/− BMDCs were treated with VLP-OVA pseudotyped with VSV-G [VLP-OVA(V)] or SVGmu [VLP-OVA(S)] and then cocultured with OT-1 CD8+ T cells for 24 hours. Representative FACS plots show expression of the T cell activation marker CD69 among the OT-1 CD8+ T cells (C and D) (left). Graph depicts CD69 MFI of the OT-1 CD8+ T cells (C and D) (right). (E to H) Tmem173−/−, cGAS−/−, and wild-type mice were homologously prime-boosted with SVGmu-pseudotyped VLP-OVA (n = 8 per group). OVA-tetramer+ cells gated on CD8+ T cells from the blood were demonstrated on representative FACS plot at 10 days after primary immunization (E and G) (left) or measured over time (E and G) (right) (black arrow, boost). Statistical comparisons were made between the OVA-tetramer+ CD8+ T cell response of the VLP-immunized wild-type and VLP-immunized mutant mice. CD62Llo and CD44hi OVA-tetramer+ CD8+ T cells from LV- immunized Tmem173−/−, cGAS−/−, and wild-type mice were compared with naïve CD8+ T cells from unimmunized wild-type mice at 10 days on representative FACS plots (F and H) (left) or by group with each symbol representing an individual mouse and horizontal bar indicating the mean (F and H) (right). Data are representative of two independent experiments (A to H). Results are shown as mean ± SEM (A to E and G). P > 0.05; *P < 0.05; **P < 0.005; ***P < 0.001 (unpaired Student’s t test).
Viral fusion is required for DC activation
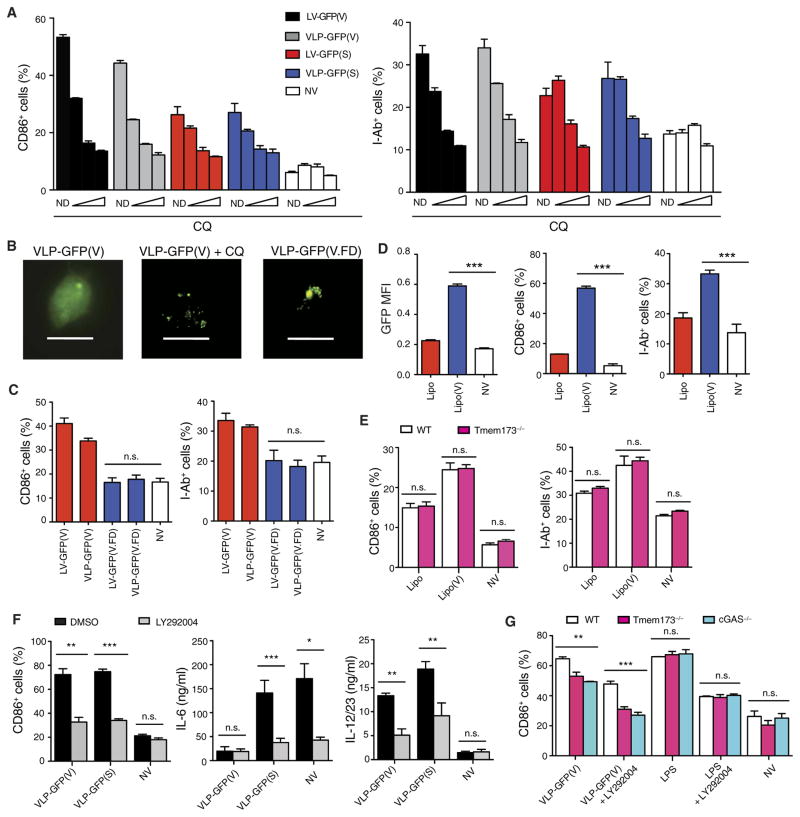
VSV and Sindbis virus release viral contents into the cytosol through pH-dependent fusion between the viral envelope and the host endosomal membrane (34, 35). LV and VLP activation of BMDCs was abolished in the presence of chloroquine, an inhibitor of endosomal acidification and viral fusion (Fig 6A). This effect was not due to lack of vector internalization into endosomes because intracellular punctate GFP was evident in LV- and VLP-treated cells receiving chloroquine by microscopy (Fig 6B). We also generated vectors pseudotyped with an altered VSV-G, in which mutations rendered the envelope fusion-defective without affecting envelope binding or receptor-mediated endocytosis (36). BMDCs treated with fusion-defective VLP showed punctate GFP presence similar to VLP- and chloroquine-treated cells, whereas BMDCs treated with fusion-competent VLP displayed diffuse intracellular GFP presence (Fig 6B). The fusion-defective LV and VLP failed to activate mouse BMDCs (Fig 6C), suggesting that viral fusion was required for the activation of DCs.
Fig. 6. Viral fusion is required for DC activation.
(A) BMDCs from wild-type mice were incubated with chloroquine (CQ) at 25, 75, 100 μM (wedges) or with no drug 1 hour before treatment with LV or VLPs and analyzed at 24 hours for CD86 and I-Ab expression by flow cytometry. (B) Fluorescence microscopy was used to analyze GFP expression in wild-type mouse BMDCs treated with fusion-competent VLP-GFP(V) with or without chloroquine (100 μM) or the fusion-defective VLP, VLP-GFP(V.FD). Magnification, ×400. Scale bars, 10 μm. (C) Wild-type mouse BMDCs were treated with fusion-competent or fusion-defective LVs or VLPs carrying GFP and analyzed for CD86 and I-Ab expression by flow cytometry. (D and E) BMDCs from wild-type and Tmem173−/− mice were treated with naked (Lipo) or VSV-G–enveloped multilamellar liposomes [Lipo(V)] carrying GFP and analyzed at 24 hours for CD86 and I-Ab expression by flow cytometry. (F) Wild-type mouse BMDCs were treated with VSV-G– or SVGmu-pseudotyped VLPs with or without LY292004 (50 μM) and analyzed for CD86 expression by flow cytometry and for the amount of IL-12/23 and IL-6 in the supernatant by ELISA. (G) BMDCs from Tmem173−/−, cGAS−/−, and wild-type mice were treated with VSV-G–pseudotyped VLP or LPS (100 ng ml−1) with or without LY292004 (50 μM) and analyzed for CD86 expression by flow cytometry. Data are representative of three independent experiments (A to D) or two independent experiments (E to G). Results are shown as mean ± SEM (A and C to G). P > 0.05; *P < 0.05; **P < 0.005; ***P < 0.001 [one way-ANOVA (C and G) and unpaired Student’s t test (D to F)].
We next asked whether the viral process of membrane fusion itself and/or the release of a putative activating component into the cytosol represented the activating stimuli. We incorporated VSV-G into the lipid membrane of noncationic multilamellar liposomes, which allowed for the liposomal contents to evade lysosomal degradation and be delivered into the cytosol via viral envelope–directed fusion (37). The delivery of GFP and activation of mouse BMDCs were greatly enhanced if the liposomes were enveloped with VSV-G (Fig 6D), indicating that VSV-G–directed fusion itself was immunostimulatory. DC activation was unaffected in STING-deficient mouse BMDCs treated with VSV-G liposomes (Fig 6E), which suggests that VSV-G–directed fusion induced DC activation independent of STING.
We next examined the role of PI3K in VSV-G fusion–induced DC activation given its role in viral fusion and DC activation (38). We found that DC activation by VSV-G–pseudotyped VLP was, in part, inhibited by the PI3K inhibitor, LY292004 (Fig 6F). In addition, we observed that VSV-G–pseudotyped VLPs were capable of inducing phosphorylation of PI3K but not fusion-defective VLPs (fig. S7A). VSV-G–pseudotyped LVs capably transduced 293T cells treated in the presence of LY292004 (fig. S7B), which is consistent with previous work demonstrating that the entry and fusion of VSV- G–pseudotyped vectors are PI3K-independent (39, 40). Thus, these results suggest that activation of PI3K occurred downstream of viral fusion. To assess whether STING or cGAS was involved in this fusion- induced PI3K-dependent pathway, we treated BMDCs from mice deficient in STING or cGAS with VSV-G–pseudotyped VLPs in the presence of LY292004. Activation was partially decreased in the VLP- treated STING-deficient BMDCs and then further decreased with the addition of LY292004 (Fig 6G), suggesting that the VSV-G fusion and PI3K-dependent pathway were largely independent of STING and cGAS. Together, these data suggest that there are two pathways contributing to VLP activation of DCs: one that is fusion- and PI3K-dependent and one that is STING- and cGAS-dependent.
Human genomic DNA carried by lentiviral particles and VLPs is immunostimulatory
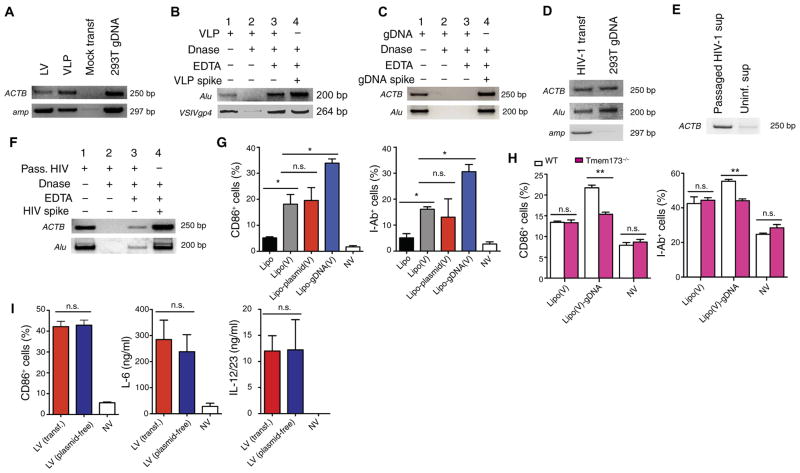
We next sought to identify the stimulatory viral component that was released into the cytoplasm by viral fusion and was responsible for activating STING and cGAS. We amplified plasmid-specific and human genomic DNA sequences from the vector preparations (Fig 7A) (13). We did not find evidence of human DNA in cell-free supernatant collected from 293T cells transfected with a mock plasmid. To assess whether the DNA was carried within or associated externally to the particles, we pretreated VLPs with deoxyribonuclease I (DNase I) to degrade external DNA and then inactivated DNase I with EDTA before the particles were lysed and then analyzed by polymerase chain reaction (PCR). Despite pretreatment with DNase I, we continued to detect plasmid and human DNA in the vector preparations, suggesting that this DNA was contained within the particles (Fig 7B). We used genomic DNA to show that DNase I digestion and EDTA inactivation were effective (Fig 7C). In addition, we detected about 150 ng of double-stranded DNA (dsDNA) per microliter and 12 ng of single-stranded DNA per microliter of the concentrated LV preparation (fig. S8A). The DNA extracted from the vector preparation appeared to be mostly composed of fragments of less than 10 kb, whereas genomic DNA extracted from cells was of longer fragments greater than 10 kb (fig. S8B). Deep sequencing of the DNA extracted from the viral particles demonstrated that about 99% of the reads were mapped to the human genome, whereas about only 1% of the reads were mapped to plasmid DNA (fig. S8C). Of the reads that were mapped to the human genome, there appeared to be a random distribution across the human chromosomes (fig. S8D). These results suggest that the DNA within LV preparations was predominantly double-stranded, fragmented, human genomic in origin, and incorporated into the viral particles randomly.
Fig. 7. LV particles and VLPs contain human genomic DNA recognized by the host STING pathway.
(A) PCR analysis of amplicons of human β-actin (ACTB) and the ampicillin resistance gene (amp) detected in the LV and VLP preparations by PCR. The cell-free supernatant collected from 293T cells transiently transfected with mock plasmid puc19 was used as a negative control. bp, base pairs. (B and C) Amplicons of human Alu element (Alu), ACTB, and VSV-G (VSIVgp4) (lane 1) were analyzed from VLP preparations (B) or genomic DNA (gDNA) (C). VLP preparations and genomic DNA were treated with lysis buffer in the presence of DNase I, leading to DNA degradation (lane 2). DNase I was inactivated with EDTA before lysis treatment (lane 3). DNase I was inactivated with EDTA, and then, VLP lysate or genomic DNA added to show DNase I was effectively inactivated (lane 4). (D) Amplicons of ACTB, Alu, and amp were analyzed on HIV-1 supernatant collected from 293T cells transiently transfected with the plasmid encoding infectious HIV-1 or 293T genomic DNA. (E) HIV-1 was passaged in primary PBMCs, and the cell-free supernatant was collected and analyzed for human ACTB by PCR. As a negative control, cell-free supernatant was collected from uninfected PBMCs. (F) Supernatant primary PBMCs passaged with HIV-1 were treated as in (B) and (C). (G and H) BMDCs from wild-type mice were treated with naked (Lipo) or VSV-G–enveloped multilamellar liposomes carrying plasmid DNA [Lipo-plasmid(V)], genomic DNA extracted from 293T cells [Lipo-gDNA(V)], or nothing [Lipo(V)] (G). BMDCs from Tmem173−/− or wild-type mice were treated with naked (Lipo) or VSV-G–enveloped multilamellar liposomes carrying plasmid DNA [Lipo-plasmid(V)], genomic DNA extracted from 293T cells [Lipo-gDNA(V)], or nothing [Lipo(V)] (H). Cells were analyzed 24 hours after treatment for CD86 and I-Ab expression by flow cytometry. (I) Wild-type mouse BMDCs were treated with LV generated from transient transfection or plasmid-free stable cell line and analyzed for CD86 expression by flow cytometry and for the amount of IL-12/23 and IL-6 in the supernatant by ELISA. Data are representative of three (A to F) or two (G to I) independent experiments. Results are shown as mean ± SEM (G to I). P > 0.05; *P < 0.05; **P < 0.005 (unpaired Student’s t test).
We also amplified plasmid and human DNA in HIV-1 produced from 293T cell transfection (Fig 7D). To determine whether the presence of genomic DNA in vector particles was particular to the transfection process, we passaged HIV-1 in human peripheral blood mononuclear cells (PBMCs) and amplified human DNA from the cell-free HIV-1 supernatant (Fig 7E). Human DNA was not detected in the cell-free supernatant collected from uninfected PBMCs. In addition, some of the DNA detected in the passaged cell-free HIV-1 supernatant was resistant to DNase I (Fig 7F), suggesting that human genomic DNA was also encapsulated inside HIV-1 particles.
We next questioned whether the delivery of plasmid or genomic DNA by viral fusion would enhance the DC activation generated by viral fusion itself. We treated mouse BMDCs with empty VSV-G liposomes or VSV-G liposomes carrying intact plasmid DNA or genomic DNA extracted from 293T cells. Human genomic DNA enhanced the immunogenicity of the fusogenic liposomes in wild-type BMDCs (Fig 7G), which was abrogated in STING-deficient BMDCs (Fig 7H). The addition of intact plasmid DNA to fusogenic liposomes did not enhance BMDC activation (Fig 7G). Furthermore, LVs generated by either transient transfection using plasmids or plasmid-free packaging system similarly stimulated wild-type BMDCs (Fig 7I), suggesting that plasmid DNA within the vector preparations was not likely a dominant activator of DCs. LVs generated by plasmid DNA–free cell lines capably stimulate innate and adaptive immune responses in vivo (41, 42). These findings provide an explanation for the STING and cGAS dependence observed in the innate and adaptive immune responses generated by LVs and VLPs.
DISCUSSION
In the present investigation, we found that vector-encoded protein antigen carried by vector particles via pseudotransduction sufficiently delivered antigen and stimulated the immune system. LV transduction was not inherently immunostimulatory but contributed to antigen delivery. Viral envelope–mediated fusion itself induced DC activation in a PI3K-dependent but STING- and type I IFN signaling–independent manner. Last, cellular DNA packaged from producer cells carried by particles activated the host STING and cGAS pathway.
Our results suggest that DCs were pseudotransduced in vivo and capable of stimulating antigen-specific immunity. Because of the transient and relatively low amount of antigen delivered compared with LV transduction, pseudotransduction has been considered an artifact (23, 24), and LV transduction has been considered the underlying mechanism of antigen delivery and immune stimulation (1, 9, 14, 43). However, we found that reverse-transcribed LV DNA was not inherently immunostimulatory in vivo but contributed to antigen delivery. This is consistent with our in vitro results showing that virtually all of the antigenic stimulation of DCs was due to pseudotransduction because activation was insensitive to RTIs and efficiently occurred with genome-deficient vectors. Neither the dual mechanisms of transduction and pseudotransduction nor the powerful role of pseudotransduction for delivering antigen and activating DCs in vivo has been appreciated. In this study, we presume that transducing and pseudotransducing particles contain the vector-encoded protein, but separation of these particles by size or density has been proven difficult.
Viral fusion by herpes VLPs has been found to activate DCs in a STING-dependent manner (38). We found that DC activation was, in part, a consequence of fusion induced between the vector and endosomal membranes but in a STING-independent manner. Furthermore, PI3K signaling was activated downstream of VSV-G viral fusion because fusion-defective VLPs failed to activate PI3K and LV fusion and transduction was PI3K-independent (39, 40). In contrast, herpes entry and fusion are regulated by PI3K (44–47). Although PI3K is important in VSV-mediated type I IFN production via TLR4 (48), we did not find whether type I IFN or TLR4 signaling was required for LV-mediated DC activation or immunization. How PI3K is activated by VSV-G–mediated viral fusion and whether there are intermediary signaling molecules remain unknown.
We identified cellular DNA packaged from producer cells and carried by vector particles as the dominant activator of the STING and cGAS pathway. Nonviral DNA such as plasmid DNA has been found in LV particles and reported to activate plasmacytoid DCs in an MyD88-dependent manner (13). However, the vast majority of DNA in our LV preparations was human genomic DNA. Further, LVs generated by plasmid-free cell lines capably activated immune responses (41, 42). We did not examine plasmacytoid DCs because type I IFN signaling was not required for DC activation and pseudotransduction of plasmacytoid DCs was not detected in vivo. We assume that vector- encoded proteins such as GFP and OVA were merely encapsulated cytoplasm in the particles, but how genomic DNA is packaged within particles will require further investigation. HIV infection induces cell death by pyroptosis, a process that leads to DNA fragmentation (49). It could be that HIV particles pick up random fragmented DNA from the infected host cell. However, the formation of vector particles by transfection does not typically induce pyroptosis. Liposomal transfection reagents are added to dividing cells and may induce host DNA damage and increase cytosolic dsDNA in cells (50). Therefore, fragmented, cytoplasmic genomic DNA may be available for encapsulation in various cell types via different processes. We found that the human genomic DNA detected in our LV preparation randomly represented the human chromosomes. In addition, viral particles generated from at least two cell types packaged genomic DNA. Whether the incorporation of genomic DNA into vector particles extends to other viruses generated from different cell types remains to be determined.
In conclusion, we identify several important mechanisms involved in DC-targeted LV immunization. We highlight the importance of LV pseudotransduction as a mechanism of antigen delivery and immune stimulation in vivo. Our results suggest that viral fusion itself induces a PI3K-dependent, STING-independent process. In addition, the delivery of cellular DNA by viral particles activates the host STING and cGAS pathway. The development of DNA adjuvants as STING and cGAS agonists could provide new therapeutic strategies for vaccination.
MATERIALS AND METHODS
Study design
This study began as an investigation of understanding how LVs deliver antigen to DCs and provide immune stimulation. For this purpose, we used DCs in vitro and administered in vivo DC-targeted LVs to mice to study the effects of LV on DCs. We used various mechanistic studies involving VLPs and transgenic mice to determine the vector component and intracellular signaling pathway important to elicit DC activation. In mouse experiments, littermate comparisons were used when possible. All mice between 6 and 12 weeks of age were used with sex- and age-matched controls. The investigators were not blinded. In mouse experiments involving tumor injections, mice were euthanized when the tumor size reached 200 mm2. Experimental replication is indicated in the figure legends.
Mice
C57BL/6J, MyD88−/−, C57BL/6J-Ticam1Lps2, Tmem173−/−, C57BL/6- Tg(TcraTcrb)1100Mjb/J (the Jackson Laboratory), MAVS−/− (G. Cheng), and cGAS−/− (Z. Chen) mice were maintained on the C57BL/6J background and used according to the protocols approved by the Institutional Animal Care and Use Committee at California Institute of Technology (Caltech).
Isolation and culture of DCs
Differentiation of BMDCs was achieved by culture for 8 days in media containing GM-CSF (100 ng ml−1) from J558L-conditioned medium (2). Human moDCs were generated by culture for 8 days of CD14+ peripheral blood monocytes [University of California, Los Angeles (UCLA) Center for AIDS Research (CFAR) Virology Core Laboratory] in media containing human GM-CSF (100 ng ml−1) and IL-4 (50 ng ml−1; PeproTech). DCs were cultured in RPMI 1640 supplemented with 10% (v/v) fetal bovine serum (Sigma-Aldrich), 1% (v/v) nonessential amino acids (HyClone), 1 mM sodium pyruvate (Gibco), 10 mM Hepes (Gibco), and 0.05 mM 2-mercaptoethanol (Gibco). DCs were isolated ex vivo from mice using immunomagnetic negative isolation (table S1).
DC treatment with vectors
DCs (1 × 106 to 2 × 106 cells) were centrifuged with vectors at 1050g at 30°C for 90 min and with Polybrene (8 μg ml−1). After centrifugation, the supernatant was removed and replaced with fresh medium and cytokines and incubated at 37°C with 5% CO2. Cycloheximide and chloroquine (Sigma-Aldrich) were added to cell cultures 1 hour before vector treatment. Tenofovir and efavirenz [National Institutes of Health (NIH) AIDS Reagent Program] were added to cell cultures 6 hours before vector treatment.
Mouse immunization and tumor and RTI treatments
LVs and VLPs were injected subcutaneously into the right flank of mice. Prime-boost immunizations contained between 25 and 50 ng of p24. All vectors were normalized to equivalent amounts of OVA and/or p24 by ELISA. Unimmunized mice received equal volume of injections of phosphate-buffered saline (PBS). Blood samples were lysed with red blood cell lysis (BioLegend) before analysis. For the tumor experiments, mice received 5 × 106 EL4 or E.G7 cells injected subcutaneously into the opposing flanks of the mice. Tumor size was measured and shown as a product of the two largest perpendicular diameters a × b (in square millimeters). Tablets containing efavirenz (600 mg), emtricitabine (200 mg), and tenofovir disoproxil fumarate (300 mg; Cipla) were crushed, resuspended in PBS containing 1% (v/v) dimethyl sulfoxide, filtered through a 0.22-μm filter, and stored in aliquots at −80°C. RTIs were added to the fresh drinking water of mice containing efavirenz (10 mg ml−1), emtricitabine (3.6 mg ml−1), and tenofovir (5.4 mg ml−1). Fresh water containing RTIs was replaced two times a week. Mice receiving no RTIs were given similar volumes of drinking water and replaced accordingly. Mice were initiated on RTIs 1 week before immunization and continued throughout the duration of the experiment.
In vitro DC stimulation of OT-1 cells
Mouse BMDCs were spin-infected with VLPs carrying OVA, washed, and resuspended in fresh media and lipopolysaccharide (LPS; 1 μg ml−1). CD8+ T cells were purified using MACS Columns (Miltenyi) from the spleen cells of OT-1 transgenic mice and cultured with the uninfected or VLP-infected BMDCs at a ratio of 1:1 and analyzed 24 hours after coculture.
Liposomes
Multilamellar liposomes were prepared using dioleoylphosphatidylcholine (DOPC), dioleoylphosphatidylglycerol (DOPG), and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidophenyl) butyramide (MPB-PE) (NOF Corporation) and were combined in chloroform at a molar lipid ratio of DOPC/DOPG/MPB-PE = 4:1:5, and the organic solvent in the lipid mixture was evaporated under argon gas (51). The lipid mixture was further dried under vacuum overnight to form dried thin lipid films. The resultant dried film containing 1.12 μg of lipids was hydrated in bis-tris propane (10 mM) at pH 7.0 with GFP (STA-201, Cell Biolabs) at a concentration of 125 ng ml−1 in a total volume of 300 μl. Polyhistidine-tagged VSV-G was expressed and purified from a suspension 293E cells after a 48-hour transfection using Ni-NTA column purification. Purified VSV-G protein (200 μg ml−1) was added to the lipid hydration mixture before sonication. Alternatively, 5 ml of VSV-G–enveloped VLPs, collected from the medium of 293T cell transfected with pVSV-G and purified and concentrated as described above, was added to the lipid hydration mixture, as previously described (37), and DNA was not detectable by PCR in the liposomes made by this method. To add DNA into the liposomes, we extracted genomic DNA from 293T cells using a genomic DNA extraction kit (Thermo Fisher Scientific) or endotoxin-free intact plasmid DNA generated from Escherichia coli cells using a plasmid DNA extraction kit (Qiagen). Genomic or plasmid DNA (10 μg ml−1) was added to the lipid hydration mixture. Lipid film and hydration mixture were vigorously vortexed every 10 min for 1 hour and then applied with four cycles of 15-s sonication (Misonix Microson XL2000) on ice in 1-min intervals for each cycle. To induce divalent-triggered vesicle fusion, we added MgCl2 (10 mM). The resulting multilamellar vesicles were further cross-linked by the addition of dithiothreitol (1.5 mM; Sigma-Aldrich) for 1 hour at 37°C. The resulting vesicles were collected by centrifugation at 14,000g for 4 min and then washed twice with PBS and resuspended in PBS with a final DNA concentration of 250 ng μl−1.
Statistical analyses
Data were analyzed using GraphPad Prism software (GraphPad Software Inc.). The statistical significance of differences for two groups was determined using Student’s t test and one-way analysis of variance (ANOVA) for multiple comparisons, as indicated in the figure legends.
Supplementary Material
Fig. S1. Flow cytometry gating strategies.
Fig. S2. LV-mediated GFP expression and activation of BMDCs are dose-dependent.
Fig. S3. Mouse bone marrow–derived CD11c+CD11b+ cells are pseudotransduced and activated.
Fig. S4. Mouse cDCs are pseudotransduced in vivo.
Fig. S5. LV activation of DCs is independent of TLR4 and type I IFN signaling.
Fig. S6. Wild-type and mutant bone marrow–derived CD11c+CD11b+ cells are generated in GM-CSF culture.
Fig. S7. VSV-G viral fusion activates PI3K.
Fig. S8. Nonviral DNA in vector particle is primarily dsDNA, fragmented, and human genomic in origin.
Table S1. Antibodies used in this study.
Table S2. Primer sets used in this study.
Acknowledgments
We thank G. Cheng (UCLA) for the MAVS−/− mice, Z. Chen (University of Texas Southwestern Medical Center) for the cGAS−/− mice, the UCLA/CFAR Virology Core Laboratory (5P30 AI028697), the UCLA Specialty Training and Advanced Research Program, the AIDS Reagent Program, the National Institute of Allergy and Infectious Diseases Tetramer Core Facility, the UCLA/CFAR Virology Core Laboratory, and the PrimerBank. This work was supported by the Millard and Muriel Jacobs Genetics and Genomics Laboratory at Caltech. We thank M. Bethune and G. Li for the review of the manuscript.
Funding: This work was supported by the NIH (OPPGH5157) and the American Foundation for AIDS Research (108292-51-RGRL). J.T.K. was supported by the NIH (KL2TR001882) and the UCLA Specialty Training and Advanced Research (STAR) Program.
Footnotes
Competing interests: P.W., L.Y., and D.B. are inventors on issued patents for DC-targeted lentiviral technology discussed in this study, which has been licensed to Immune Design Corporation (IDC), and are stock holders in IDC. D.B. is also a member of Board of Directors of IDC.
Data and materials availability: DNA sequence data have been deposited in Sequence Read Archive under accession number SAMN07195639.
Author contributions: J.T.K. designed the research, performed the experiments, analyzed the results, and drafted the manuscript. Y.L., B.D., G.L., R.P.K., K.K.L., and Y.O. performed the experiments. L.Y. and P.W. discussed the results and drafted the manuscript. D.B. designed the research, discussed the results, drafted the manuscript, and provided financial support.
REFERENCES AND NOTES
- 1.Yang L, Yang H, Rideout K, Cho T, Joo Ki, Ziegler L, Elliot A, Walls A, Yu D, Baltimore D, Wang P. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat Biotechnol. 2008;26:326–334. doi: 10.1038/nbt1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai B, Yang L, Yang H, Hu B, Baltimore D, Wang P. HIV-1 Gag-specific immunity induced by a lentivector-based vaccine directed to dendritic cells. Proc Natl Acad Sci USA. 2009;106:20382–20387. doi: 10.1073/pnas.0911742106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Somaiah N, Block MS, Kim JW, Shapiro G, Hwu P, Eder JP, Jones RL, Gnjatic S, Lu H, Hsu FJ, Pollack S. Phase I, first-in-human trial of LV305 in patients with advanced or metastatic cancer expressing NY-ESO-1. J Clin Oncol. 2015;33:3021. [Google Scholar]
- 4.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li DP, Romain G, Flamar AL, Duluc D, Dullaers M, Li XH, Zurawski S, Bosquet N, Palucka AK, Le Grand R, O’Garra A, Zurawski G, Banchereau J, Oh S. Targeting self- and foreign antigens to dendritic cells via DC-ASGPR generates IL-10–producing suppressive CD4+ T cells. J Exp Med. 2012;209:109–121. doi: 10.1084/jem.20110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, Yatim A, Emiliani S, Schwartz O, Benkirane M. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyall J, Latouche JB, Schnell S, Sadelain M. Lentivirus-transduced human monocyte-derived dendritic cells efficiently stimulate antigen-specific cytotoxic T lymphocytes. Blood. 2001;97:114–121. doi: 10.1182/blood.v97.1.114. [DOI] [PubMed] [Google Scholar]
- 8.Breckpot K, Emeagi P, Dullaers M, Michiels A, Heirman C, Thielemans K. Activation of immature monocyte-derived dendritic cells after transduction with high doses of lentiviral vectors. Hum Gene Ther. 2007;18:536–546. doi: 10.1089/hum.2007.006. [DOI] [PubMed] [Google Scholar]
- 9.Arce F, Rowe HM, Chain B, Lopes L, Collins MK. Lentiviral vectors transduce proliferating dendritic cell precursors leading to persistent antigen presentation and immunization. Mol Ther. 2009;17:1643–1650. doi: 10.1038/mt.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger G, Goujon C, Darlix JL, Cimarelli A. SIVMAC Vpx improves the transduction of dendritic cells with nonintegrative HIV-1-derived vectors. Gene Ther. 2009;16:159–163. doi: 10.1038/gt.2008.128. [DOI] [PubMed] [Google Scholar]
- 11.Granelli-Piperno A, Zhong L, Haslett P, Jacobson J, Steinman RM. Dendritic cells, infected with vesicular stomatitis virus-pseudotyped HIV-1, present viral antigens to CD4+ and CD8+ T cells from HIV-1-infected individuals. J Immunol. 2000;165:6620–6626. doi: 10.4049/jimmunol.165.11.6620. [DOI] [PubMed] [Google Scholar]
- 12.Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, Larsson M, Gorelick RJ, Lifson JD, Bhardwaj N. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor–viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pichlmair A, Diebold SS, Gschmeissner S, Takeuchi Y, Ikeda Y, KCollins M, Reis e Sousa C. Tubulovesicular structures within vesicular stomatitis virus G protein-pseudotyped lentiviral vector preparations carry DNA and stimulate antiviral responses via Toll-like receptor 9. J Virol. 2007;81:539–547. doi: 10.1128/JVI.01818-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breckpot K, Escors D, Arce F, Lopes L, Karwacz K, Van Lint S, Keyaerts M, Collins M. HIV-1 lentiviral vector immunogenicity is mediated by Toll-like receptor 3 (TLR3) and TLR7. J Virol. 2010;84:5627–5636. doi: 10.1128/JVI.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg RK, Melchjorsen J, Rintahaka J, Diget E, Søby S, Horan KA, Gorelick RJ, Matikainen S, Larsen CS, Ostergaard L, Paludan SR, Mogensen TH. Genomic HIV RNA induces innate immune responses through RIG-I-dependent sensing of secondary-structured RNA. PLOS ONE. 2012;7:e29291. doi: 10.1371/journal.pone.0029291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakobsen MR, Bak RO, Andersen A, Berg RK, Jensen SB, Tengchuan J, Laustsen A, Hansen K, Østergaard L, Fitzgerald KA, Xiao TS, Mikkelsen JG, Mogensen TH, Paludan SR. IFI16 senses DNA forms of the lentiviral replication cycle and controls HIV-1 replication. Proc Natl Acad Sci USA. 2013;110:E4571–E4580. doi: 10.1073/pnas.1311669110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao D, Wu J, Wu YT, Du F, Aroh C, Yan N, Sun L, Chen ZJ. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341:903–906. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lahaye X, Satoh T, Gentili M, Cerboni S, Conrad C, Hurbain I, El Marjou A, Lacabaratz C, Lelièvre J-D, Manel N. The capsids of HIV-1 and HIV-2 determine immune detection of the viral cDNA by the innate sensor cGAS in dendritic cells. Immunity. 2013;39:1132–1142. doi: 10.1016/j.immuni.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Deml L, Speth C, Dierich MP, Wolf H, Wagner R. Recombinant HIV-1 Pr55gag virus-like particles: Potent stimulators of innate and acquired immune responses. Mol Immunol. 2005;42:259–277. doi: 10.1016/j.molimm.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Kuate S, Stahl-Hennig C, Stoiber H, Nchinda G, Floto A, Franz M, Sauermann U, Bredl S, Deml L, Ignatius R, Norley S, Racz P, Tenner-Racz K, Steinman RM, Wagner R, Überla K. Immunogenicity and efficacy of immunodeficiency virus-like particles pseudotyped with the G protein of vesicular stomatitis virus. Virology. 2006;351:133–144. doi: 10.1016/j.virol.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Buonaguro L, Tornesello ML, Tagliamonte M, Gallo RC, Wang LX, Kamin-Lewis R, Abdelwahab S, Lewis GK, Buonaguro FM. Baculovirus-derived human immunodeficiency virus type 1 virus-like particles activate dendritic cells and induce ex vivo T-cell responses. J Virol. 2006;80:9134–9143. doi: 10.1128/JVI.00050-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beaty SR, Rose CE, Jr, Sung S-sJ. Diverse and potent chemokine production by lung CD11bhigh dendritic cells in homeostasis and in allergic lung inflammation. J Immunol. 2007;178:1882–1895. doi: 10.4049/jimmunol.178.3.1882. [DOI] [PubMed] [Google Scholar]
- 23.Haas DL, Case SS, Crooks GM, Kohn DB. Critical factors influencing stable transduction of human CD34+ cells with HIV-1-derived lentiviral vectors. Mol Ther. 2000;2:71–80. doi: 10.1006/mthe.2000.0094. [DOI] [PubMed] [Google Scholar]
- 24.Nash KL, Lever AML. Green fluorescent protein: Green cells do not always indicate gene expression. Gene Ther. 2004;11:882–883. doi: 10.1038/sj.gt.3302246. [DOI] [PubMed] [Google Scholar]
- 25.Mangeot PE, Dollet S, Girard M, Ciancia C, Joly S, Peschanski M, Lotteau V. Protein transfer into human cells by VSV-G-induced nanovesicles. Mol Ther. 2011;19:1656–1666. doi: 10.1038/mt.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odegard JM, Kelley-Clarke B, Tareen SU, Campbell DJ, Flynn PA, Nicolai CJ, Slough MM, Vin CD, McGowan PJ, Nelson LT, Ter Meulen J, Dubensky TW, Jr, Robbins SH. Virological and preclinical characterization of a dendritic cell targeting, integration-deficient lentiviral vector for cancer immunotherapy. J Immunother. 2015;38:41–53. doi: 10.1097/CJI.0000000000000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albershardt TC, Campbell DJ, Parsons AJ, Slough MM, ter Meulen J, Berglund P. LV305, a dendritic cell-targeting integration-deficient ZVex™-based lentiviral vector encoding NY-ESO-1, induces potent anti-tumor immune response. Mol Ther Oncolytics. 2016;3:16010. doi: 10.1038/mto.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 30.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Sousa CRE, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 31.Muller U, Steinhoff U, Reis LFL, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional-role of type-I and type-II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa H, Barber GN. Sting is an endoplasmic reticulum adaptor that facilitates innate immune signaling. Cytokine. 2009;48:128. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science. 2013;341:1390–1394. doi: 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Florkiewicz RZ, Rose JK. A cell line expressing vesicular stomatitis virus glycoprotein fuses at low pH. Science. 1984;225:721–723. doi: 10.1126/science.6087454. [DOI] [PubMed] [Google Scholar]
- 35.Smit JM, Bittman R, Wilschut J. Low-pH-dependent fusion of Sindbis virus with receptor-free cholesterol- and sphingolipid-containing liposomes. J Virol. 1999;73:8476–8484. doi: 10.1128/jvi.73.10.8476-8484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Ghosh HP. Characterization of the putative fusogenic domain in vesicular stomatitis virus glycoprotein G. J Virol. 1994;68:2186–2193. doi: 10.1128/jvi.68.4.2186-2193.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abe A, Miyanohara A, Friedmann T. Enhanced gene transfer with fusogenic liposomes containing vesicular stomatitis virus G glycoprotein. J Virol. 1998;72:6159. doi: 10.1128/jvi.72.7.6159-6163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holm CK, Jensen SB, Jakobsen MR, Cheshenko N, Horan KA, Moeller HB, Gonzalez-Dosal R, Rasmussen SB, Christensen MH, Yarovinsky TO, Rixon FJ, Herold BC, Fitzgerald KA, Paludan SR. Virus-cell fusion as a trigger of innate immunity dependent on the adaptor STING. Nat Immunol. 2012;13:737–743. doi: 10.1038/ni.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunn EF, Fearns R, Connor JH. Akt inhibitor Akt-IV blocks virus replication through an Akt-independent mechanism. J Virol. 2009;83:11665–11672. doi: 10.1128/JVI.01092-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saeed MF, Kolokoltsov AA, Freiberg AN, Holbrook MR, Davey RA. Phosphoinositide-3 kinase-Akt pathway controls cellular entry of Ebola virus. PLOS Pathog. 2008;4:e1000141. doi: 10.1371/journal.ppat.1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee CL, Chou M, Dai B, Xiao L, Wang P. Construction of stable producer cells to make high-titer lentiviral vectors for dendritic cell-based vaccination. Biotechnol Bioeng. 2012;109:1551–1560. doi: 10.1002/bit.24413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bryson PD, Zhang C, Lee CL, Wang P. A tetracycline-regulated cell line produces high-titer lentiviral vectors that specifically target dendritic cells. J Vis Exp. 2013;76:e50606. doi: 10.3791/50606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esslinger C, Chapatte L, Finke D, Miconnet I, Guillaume P, Lévy F, MacDonald HR. In vivo administration of a lentiviral vaccine targets DCs and induces efficient CD8+ T cell responses. J Clin Invest. 2003;111:1673–1681. doi: 10.1172/JCI17098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tiwari V, Shukla D. Phosphoinositide 3 kinase signalling may affect multiple steps during herpes simplex virus type-1 entry. J Gen Virol. 2010;91:3002–3009. doi: 10.1099/vir.0.024166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacLeod IJ, Minson T. Binding of herpes simplex virus type-1 virions leads to the induction of intracellular signalling in the absence of virus entry. PLOS ONE. 2010;5:e9560. doi: 10.1371/journal.pone.0009560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheshenko N. HSV activates Akt to trigger calcium release and promote viral entry: Novel candidate target for treatment and suppression (vol 27, pg 2584, 2013) Faseb J. 2015;29:355. doi: 10.1096/fj.12-220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicola AV, Straus SE. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J Virol. 2004;78:7508–7517. doi: 10.1128/JVI.78.14.7508-7517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schabbauer G, Luyendyk J, Crozat K, Jiang Z, Mackman N, Bahram S, Georgel P. TLR4/CD14-mediated PI3K activation is an essential component of interferon-dependent VSV resistance in macrophages. Mol Immunol. 2008;45:2790–2796. doi: 10.1016/j.molimm.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doitsh G, Galloway NLK, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Muñoz-Arias I, Greene WC. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knudsen KB, Northeved H, Kumar PE, Permin A, Gjetting T, Andresen TL, Larsen S, Wegener KM, Lykkesfeldt J, Jantzen K, Loft S, Møller P, Roursgaard M. In vivo toxicity of cationic micelles and liposomes. Nanomedicine. 2015;11:467–477. doi: 10.1016/j.nano.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Fang J, Joo KI, Wong MK, Wang P. Codelivery of chemotherapeutics via crosslinked multilamellar liposomal vesicles to overcome multidrug resistance in tumor. PLOS ONE. 2014;9:e110611. doi: 10.1371/journal.pone.0110611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Flow cytometry gating strategies.
Fig. S2. LV-mediated GFP expression and activation of BMDCs are dose-dependent.
Fig. S3. Mouse bone marrow–derived CD11c+CD11b+ cells are pseudotransduced and activated.
Fig. S4. Mouse cDCs are pseudotransduced in vivo.
Fig. S5. LV activation of DCs is independent of TLR4 and type I IFN signaling.
Fig. S6. Wild-type and mutant bone marrow–derived CD11c+CD11b+ cells are generated in GM-CSF culture.
Fig. S7. VSV-G viral fusion activates PI3K.
Fig. S8. Nonviral DNA in vector particle is primarily dsDNA, fragmented, and human genomic in origin.
Table S1. Antibodies used in this study.
Table S2. Primer sets used in this study.