Abstract
Background
Laparoscopic resection (LLR) of colorectal liver metastases (CRLM) located in the posterosuperior liver (segments 4a, 7, and 8) is challenging but has become more practical recently due to progress in operative technique. We aimed to compare tumor-specific, perioperative and short-term oncological outcomes after LLR and open liver resection (OLR) for CRLM.
Methods
Patients who underwent curative resection of CRLM with at least 1 tumor in posterosuperior liver during 2012–2015 were analyzed. Tumor-specific factors associated with adoption of LLR were analyzed by logistic regression model. One-to-one propensity score matching was used to match baseline characteristics between patients with LLR and OLR.
Results
The original cohort included 30 patients with LLR and 239 with OLR. Median follow-up time was 23.8 months. Logistic regression analysis showed that multiple, diameter ≥30 mm, deep location, and closeness to major vessels were associated with OLR. None of 24 patients with none or 1 of these factors were converted from LLR to OLR. After matching, 29 patients with LLR and 29 with OLR were analyzed. The 2 groups had similar preoperative factors. The LLR and OLR groups did not differ with respect to operative time, intraoperative bleeding, incidence of blood transfusion, surgical margin positivity, incidence of postoperative complications, and unplanned readmission within 45 days. Median length of postoperative hospital stay was significantly shorter for LLR vs. OLR (4 days [1–12] vs. 5 days [4–18]; p=0.0003). Median recurrence-free survival was similar for patients who underwent LLR vs. OLR (10.6 months for LLR vs. 13.4 months for OLR; p=0.87).
Conclusions
Compared to OLR, LLR of posterosuperior CRLM is associated with significantly shorter postoperative hospital stay but otherwise similar perioperative and short-term oncological outcomes. Tumor-specific factors associated with safe and routine LLR approach despite challenging location are superficial, solitary, and small (<30mm) CRLM not associated with major vessels.
Keywords: laparoscopic hepatectomy, colorectal liver metastasis, posterosuperior segment
Introduction
Liver resection is the standard therapy for patients with colorectal liver metastases (CRLM) and the only potentially curative treatment approach. The 5-year overall survival rate after resection of CRLM has been reported to be 33% to 55% [1–4], whereas the median overall survival time of patients with untreated CRLM is less than 1 year [5].
CRLM can be resected using an open or laparoscopic approach. Laparoscopic liver resection (LLR) was first reported in the early 1990s, and use of this procedure has steadily increased since then [6,7]. Previous studies have reported benefits of LLR over open liver resection (OLR), including decreased blood loss, shorter length of hospital stay, and decreased overall morbidity [8,9]. For resection of CRLM, studies have shown that LLR results in superior perioperative outcomes and similar oncological outcomes (recurrence-free and overall survival) in selected patients compared to OLR [10–13]. Although the International Consensus Conference held in 2014 in Morioka, Japan, concluded that minor LLR had become a standard procedure [14], the difficulty of LLR varies with anatomic tumor location [15].
LLR for tumors located in the posterosuperior liver (segments 4a, 7, and 8) in particular has been considered especially challenging because of the limited surgical view and restricted handling of laparoscopic instruments as shown in Fig. 1 [16,17]. However, recent progress in operative technique, including the introduction of transthoracic port placement, has reduced the difficulty of LLR of tumors in the posterosuperior liver [18]. A prior study examined the perioperative course and oncological outcome after LLR vs. OLR of hepatocellular carcinoma (HCC) located in posterosuperior segments, and demonstrated superior outcomes for LLR with regards to length of hospital stay, postoperative complications, and blood loss; oncologic outcomes were similar to those following OLR [19]. To our knowledge, no previous study has directly compared LLR to OLR of only CRLM located in the posterosuperior liver in terms of the operative and postoperative course and oncological outcome.
Fig. 1.
(a) Posterosuperior segments of the liver (segments 4a, 7, 8) are located cranial to the costal arch. (b) Sagittal view: the liver fulcrum impacts access to inferior tumors less (c) Sagittal view: Liver fulcrum and costal arch prevent optimal access to the posterosuperior liver. Shaded area shows posterosuperior segments.
The purpose of this study was to identify tumor-specific factors associated with adoption of LLR for CRLM in the posterosuperior liver, and to evaluate the operative and short-term oncological outcomes after LLR and OLR of CRLM in the posterosuperior liver using propensity score (PS) matching analysis.
Methods
Patients
This study was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center. Patients who underwent liver resection for CRLM at MD Anderson Cancer Center from January 2012 through December 2015 were identified from an institutional database. Patients who underwent 2-stage hepatectomy or non-curative resection were excluded, as were patients with missing data. For the remaining patients, we reviewed computed tomography and magnetic resonance imaging scans to identify the location of CRLM in the liver, including depth, relation to major blood vessels, and distribution. Patients with at least 1 tumor in posterosuperior segments (segments 4a, 7, and 8) were included in the analysis.
Definitions
A tumor was considered deep when the center of the tumor was located more than 30 mm from the liver surface. If at least 1 tumor existed close to a main or second branch of portal triad, major hepatic veins, or the inferior vena cava, we defined the tumor as adjacent to a major vessel. LLR included pure LLR, hand-assisted LLR, and LLR converted to OLR. Major resection was defined as liver resection including 3 or more liver segments. A positive surgical margin was defined as a tumor-free margin narrower than 1 mm [20]. Postoperative complication within 90 days after surgery was classified using Clavien-Dindo classification [21]. Unplanned readmission was defined as readmission within 45 days after discharge due to all causes [22]. Postoperative mortality was death within 90 days after surgery [23]. RAS mutation status, whether wild-type or mutant, was determined by a sample from primary tumor or CRLM. Single mutations in the various codons of KRAS and NRAS were reported as RAS mutations.
Surgical procedure
Most indications for surgical resection were determined at a multidisciplinary tumor board with the decision to perform LLR or OLR at the discretion of the operating surgeon. During both LLR and OLR, tumor location was evaluated using intraoperative ultrasonography. In pure LLR, a 12-mm trocar was placed in the right upper quadrant as the first port. Additional ports (at least 3) were placed in appropriate locations below the costal arch. When a transthoracic port was needed to obtain adequate surgical visualization, a balloon-tipped trocar was inserted though the intercostal space and through the diaphragm. The liver transection was performed using bipolar forceps and ultrasonic shears. In hand-assisted LLR, a blunt trocar was used to generate a supraumbilical port as the first port. After intraabdominal evaluation, trocars were placed at the left upper quadrant and right midabdomen, and a 7-cm incision was made in the right subcostal position to serve as a hand port. Liver parenchymal dissection was performed with an ultrasonic surgical aspirator and ultrasonic shears. In OLR, parenchymal transection was performed with 2-surgeon technique using an ultrasonic surgical aspirator and saline-linked cautery under total or selective hepatic inflow occlusion [24]. An enhanced recovery program was systematically available and applied at the discretion of the surgeon [25].
PS matching
We used PS matching to minimize differences in baseline characteristics between the patients who underwent LLR and those who underwent OLR. The PS was calculated by using a logistic regression model including variables that were considered to be directly associated with either undergoing LLR or undergoing OLR. The following valuables were included to establish the model: age, sex, body mass index, American Society of Anesthesiologists (ASA) score, RAS status, primary tumor location, primary lymph node metastases, timing of metastases, tumor number, largest tumor diameter, extrahepatic metastases, tumor location (depth and relation to major blood vessels), extent of liver resection, repeated hepatectomy, preoperative portal vein embolization, resection with radiofrequency ablation, and preoperative chemotherapy. After PS generation, patients treated with LLR and those treated with OLR underwent 1:1 nearest available matching of the logit of the propensity score with a caliper width of 0.20 of the standard deviation of the score. When both LLR patients and OLR patients did not meet matching criteria, these patients were excluded.
Statistical analysis
Data were analyzed using Wilcoxon signed-rank test, χ2 test, or Fisher’s exact test where appropriate. Recurrence-free survival and overall survival were calculated from the date of hepatectomy and estimated using the Kaplan–Meier method. The log-rank test was used to compare survival curves. A multivariate analysis based on the logistic regression model was used to identify the factors associated with adoption of LLR. All variables with a p value of less than 0.10 in univariate analysis were included in the logistic regression model for multivariate analysis. All tests were 2-tailed, and p<0.05 was considered significant. All statistical computations were performed using JMP pro 12.1 software (SAS Institute Inc., Cary, NC); PS matching was performed using the JMP add-in program.
Results
Patient characteristics
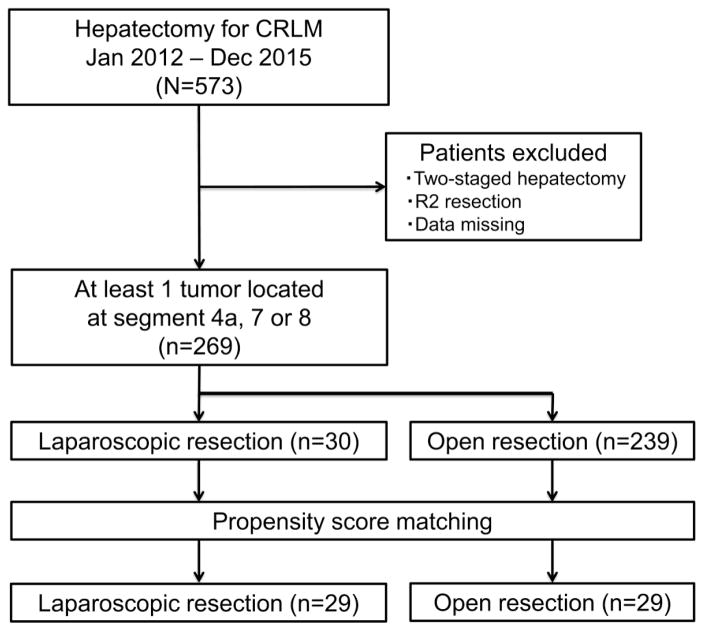
A total of 269 patients were included in the study (Fig. 2). Within this cohort, 30 patients (11.2%) underwent LLR, and 239 patients (88.8%) underwent OLR. Before evaluating the PS-matched data, we compared the patient characteristics between the LLR group and the OLR group (Table 1). The proportion of patients with ASA score of 3 or greater was significantly greater in the LLR group than in the OLR group. Patients who underwent OLR had significantly more tumors and larger tumors than patients who underwent LLR. Compared to patients who underwent OLR, those who underwent LLR had higher incidences of superficial location tumor, tumor away from major vessels, unilobar tumors, and minor hepatectomy. An enhanced recovery program was applied to 10 of the 30 patients (33%) who underwent LLR and 44 of the 239 patients (18.4%) who underwent OLR (p=0.08) (Table 1).
Fig. 2.
Patient Selection
Table 1.
Patient characteristics of overall cohort
| Characteristic | Laparoscopic resection (n=30) | Open resection (n=239) | p | |
|---|---|---|---|---|
| Age, median (range), y | 55 (27–79) | 54 (27–78) | 0.46 | |
| Sex | Female | 16 (53.3) | 103 (43.1) | 0.33 |
| Male | 14 (46.7) | 136 (56.9) | ||
| BMI, median (range), kg/m2 | 27.2 (19.9–40.3) | 27.6 (16.4–54.7) | 0.98 | |
| ASA | <3 | 1 (3.3) | 54 (22.6) | 0.01 |
| ≥3 | 29 (96.7) | 185 (77.4) | ||
| RAS status | Wild-type | 15 (50.0) | 95 (39.8) | 0.32 |
| Mutant | 9 (30.0) | 106 (44.3) | ||
| Unknown | 9 (30.0) | 38 (15.9) | ||
| Primary tumor location | Colon | 25 (83.3) | 182 (76.2) | 0.49 |
| Rectum | 5 (16.7) | 57 (23.8) | ||
| Primary lymph node metastases | Absent | 8 (26.7) | 86 (35.0) | 0.41 |
| Present | 22 (73.3) | 153 (64.0) | ||
| Timing of metastases | Metachronous | 11 (36.7) | 54 (22.6) | 0.11 |
| Synchronous | 19 (63.3) | 185 (77.4) | ||
| Tumor number, median (range) | 1 (1–5) | 2 (1–41) | <0.0001 | |
| Largest tumor diameter, median (range), mm | 15.5 (1.6–60) | 26 (1–180) | <0.0001 | |
| Extrahepatic metastases | Absent | 27 (90.0) | 202 (84.5) | 0.59 |
| Present | 3 (10.0) | 37 (15.5) | ||
| Tumor location | Superficial | 25 (83.3) | 108 (45.2) | <0.0001 |
| Deep | 5 (16.7) | 131 (54.8) | ||
| Tumor located at segment 1 | No | 30 (100) | 222 (92.9) | 0.23 |
| Yes | 0 (0) | 17 (7.1) | ||
| Tumor adjacent to major vessels | No | 26 (86.7) | 127 (53.1) | 0.0003 |
| Yes | 4 (13.3) | 112 (46.9) | ||
| Tumor distribution | Unilobar | 24 (80.0) | 139 (58.2) | 0.028 |
| Bilobar | 6 (20.0) | 100 (41.8) | ||
| Extent of resection | Minor | 26 (86.7) | 109 (45.6) | <0.0001 |
| Major | 4 (13.3) | 130 (54.4) | ||
| Hepatectomy concomitant with primary resection | No | 27 (90.0) | 215 (90.0) | 0.99 |
| Yes | 3 (10.0) | 24 (10.0) | ||
| Repeated hepatectomy | No | 28 (93.3) | 214 (89.5) | 0.75 |
| Yes | 2 (6.7) | 25 (10.5) | ||
| Preoperative PVE | No | 29 (96.7) | 219 (91.6) | 0.49 |
| Yes | 1 (3.3) | 20 (8.4) | ||
| Resection with RFA | No | 30 (100) | 217 (90.8) | 0.15 |
| Yes | 0 (0) | 22 (9.2) | ||
| Pre-hepatectomy chemotherapy | No | 5 (16.7) | 17 (7.1) | 0.08 |
| Yes | 25 (83.3) | 222 (92.9) | ||
| Application of ERAS program | No | 20 (66.7) | 195 (81.6) | 0.08 |
| Yes | 10 (33.3) | 44 (18.4) |
ASA, American Society of Anesthesiologists; BMI, body mass index; ERAS, enhanced recovery after surgery; PVE, portal vein embolization; RFA, radiofrequency ablation.
Details of LLR
Of the 30 patients who underwent LLR, 19 (63%) underwent pure LLR and 11 (37%) underwent hand-assisted LLR. Four patients (13%) had LLR performed using a transthoracic approach [26,27]; all 4 had pure LLR. Four patients (13%) had conversion to OLR, including 3 patients with unfavorable tumor location and 1 with insufficient resection margin. Four patients (13%) who underwent LLR had major hepatectomy, and 2 patients who underwent LLR (7%) had repeated hepatectomy for intrahepatic recurrence (Table 1). No patient who underwent LLR underwent vascular reconstruction due of concomitant resection of major vessels.
Tumor- specific factors for LLR
To identify the tumor-specific factors associated with surgeons’ decision to perform either LLR or OLR, logistic regression analysis was performed using only preoperative tumor factors. In multivariate analysis, multiple tumors (odds ratio (OR): 2.78, p=0.039) and tumor diameter ≥30 mm (OR: 5.54, p=0.0017) were the independent factors associated with OLR. Although not statistically significant, deep location of the tumor (OR: 2.54, p=0.09) and tumor abutting major vessels (OR: 2.8, p=0.07) were also associated with OLR (Table 2). Six patients treated with LLR had 2 or more of these 4 factors, and 4 patients of them were converted to OLR. None of 24 patients with 0 or 1 of these 4 factors experienced conversion to OLR.
Table 2.
Preoperative tumor factors associated with surgeon’s decision for open liver resection
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Odds ratio | 95% CI | p | Odds ratio | 95% CI | p | ||
| Tumor number | Single | ||||||
| Multiple | 4.34 | 1.99–9.90 | 0.0002 | 2.78 | 1.05–7.89 | 0.039 | |
| Largest tumor diameter, mm | <30 | ||||||
| ≥30 | 7.05 | 2.41–30.1 | 0.0001 | 5.54 | 1.80–24.3 | 0.0017 | |
| Tumor location | Superficial | ||||||
| Deep | 6.06 | 2.43–18.4 | <0.0001 | 2.54 | 0.86–8.64 | 0.09 | |
| Adjacent to major vessels | No | ||||||
| Yes | 5.73 | 2.15–19.9 | 0.0002 | 2.8 | 0.91–10.6 | 0.07 | |
| Tumor distribution | Unilobar | ||||||
| Bilobar | 2.88 | 1.20–8.00 | 0.016 | 1.4 | 0.46–4.57 | 0.56 | |
PS-matched patient characteristics
The 1:1 PS-matched cohort comprised 29 patients who underwent LLR and 29 who underwent OLR. One patient in the initial LLR cohort did not match the patient in the OLR group and was excluded from the analysis. The differences in patient characteristics between the LLR and OLR groups in the original cohort analysis were alleviated after matching (Table 3). An enhanced recovery program was applied to 10 patients (34%) who underwent LLR and 6 patients (21%) who underwent OLR (p=0.38).
Table 3.
Patient characteristics after matching
| Characteristic | Laparoscopic resection (n=29) | Open resection (n=29) | p | |
|---|---|---|---|---|
| Age, median (range), y | 54 (29–78) | 54 (33–70) | 0.94 | |
| Sex | Female | 15 (51.7) | 14 (48.3) | 1.00 |
| Male | 14 (48.3) | 15 (51.7) | ||
| BMI, median (range), kg/m2 | 27.6 (19.9–40.3) | 29.0 (18.6–34.9) | 0.79 | |
| ASA | <3 | 1 (3.5) | 0 (0) | 1.00 |
| ≥3 | 28 (96.5) | 29 (100) | ||
| RAS status | Wild-type | 14 (48.3) | 14 (48.3) | 0.74 |
| Mutant | 9 (31.0) | 11 (37.9) | ||
| Unknown | 6 (20.7) | 4 (13.8) | ||
| Primary tumor location | Colon | 24 (82.8) | 23 (79.3) | 1.00 |
| Rectum | 5 (17.2) | 6 (20.7) | ||
| Primary lymph node metastases | Absent | 8 (27.6) | 10 (34.5) | 0.78 |
| Present | 21 (72.4) | 19 (65.5) | ||
| Timing of metastases | Metachronous | 11 (37.9) | 10 (34.5) | 1.00 |
| Synchronous | 18 (62.1) | 19 (65.5) | ||
| Tumor number, median (range) | 1 (1–5) | 1 (1–4) | 0.52 | |
| Largest tumor diameter, median (range), mm | 16 (1.6–60) | 17 (2–50) | 0.74 | |
| Extrahepatic metastases | Absent | 26 (89.7) | 26 (89.7) | 1.00 |
| Present | 3 (10.3) | 3 (10.3) | ||
| Tumor location | Superficial | 24 (82.8) | 23 (79.3) | 1.00 |
| Deep | 5 (17.2) | 6 (20.7) | ||
| Tumor located at segment 1 | No | 0 (0) | 0 (0) | 1.00 |
| Yes | 29 (100) | 29 (100) | ||
| Tumor adjacent to major vessels | No | 25 (86.2) | 23 (79.3) | 0.73 |
| Yes | 4 (13.8) | 6 (20.7) | ||
| Tumor distribution | Unilobar | 23 (79.3) | 24 (82.8) | 1.00 |
| Bilobar | 6 (20.7) | 5 (17.2) | ||
| Extent of resection | Minor | 25 (86.2) | 26 (89.7) | 1.00 |
| Major | 4 (13.8) | 3 (10.3) | ||
| Hepatectomy concomitant with primary resection | No | 26 (89.7) | 24 (82.8) | 0.45 |
| Yes | 3 (10.3) | 5 (17.2) | ||
| Repeated hepatectomy | No | 27 (93.1) | 27 (93.1) | 1.00 |
| Yes | 2 (6.9) | 2 (6.9) | ||
| Preoperative PVE | No | 28 (96.5) | 28 (96.5) | 1.00 |
| Yes | 1 (3.5) | 1 (3.5) | ||
| Resection with RFA | No | 29 (100) | 29 (100) | 1.00 |
| Yes | 0 (0) | 0 (0) | ||
| Pre-hepatectomy chemotherapy | No | 4 (13.8) | 4 (13.8) | 1.00 |
| Yes | 25 (86.2) | 25 (86.2) | ||
| Application of ERAS program | No | 19 (65.2) | 23 (79.3) | 0.38 |
| Yes | 10 (34.5) | 6 (20.7) |
ASA, American Society of Anesthesiologists; BMI, body mass index; ERAS, enhanced recovery after surgery; PVE, portal vein embolization; RFA, radiofrequency ablation.
Perioperative outcomes
Perioperative outcomes after PS matching are shown in Table 4. Length of hospital stay was significantly shorter in the LLR group (median: 4 days, range: 1–12 days) than in the OLR group (median: 5 days, range: 4–18 days) (p=0.0003). Other perioperative factors, including operative time, blood loss, incidence of blood transfusion, surgical margin positivity, incidence of overall and major (Clavien-Dindo 3 and over) postoperative complications, and rate of unplanned postoperative readmission, were similar between the groups. No death within 90 days after hepatectomy was observed in either group.
Table 4.
Perioperative outcomes after matching
| Outcome | Laparoscopic resection (n=29) | Open resection (n=29) | p | |
|---|---|---|---|---|
| Operative time, median (range), min | 217 (62–586) | 251 (90–465) | 0.34 | |
| Blood loss, median (range), ml | 100 (10–800) | 150 (30–1300) | 0.21 | |
| Blood transfusion | No | 28 (96.5) | 29 (100) | 1.00 |
| Yes | 1 (3.5) | 0 (0) | ||
| Surgical margin | Negative | 25 (86.2) | 23 (79.3) | 0.73 |
| Positive | 4 (13.8) | 6 (20.7) | ||
| Postoperative complication | Absent | 23 (79.3) | 17 (58.6) | 0.16 |
| Present | 6 (20.7) | 12 (41.4) | ||
| Postoperative complication, Clavien-Dindo grade ≥3 | Absent | 26 (89.7) | 27 (93.1) | 1.00 |
| Present | 3 (10.3) | 2 (6.9) | ||
| Length of stay, median (range), d | 4 (1–12) | 5 (4–18) | 0.0003 | |
| Unplanned readmission ≤45 days | No | 26 (89.7) | 26 (89.7) | 1.00 |
| Yes | 3 (10.3) | 3 (10.3) | ||
| Postoperative death ≤90 days | No | 29 (100) | 29 (100) | 1.00 |
| Yes | 0 (0) | 0 (0) |
Oncological outcome
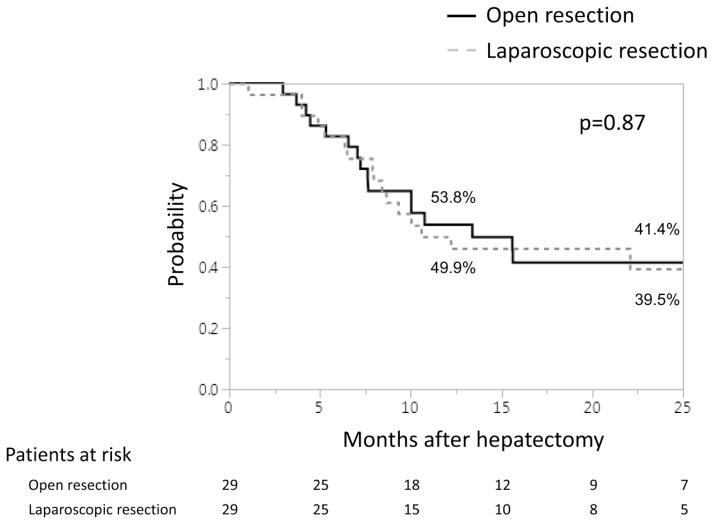
The median follow-up period for all 269 patients in the overall cohort was 23.8 months, with 24.2 months for the OLR group and 18.7 months for the LLR group individually. After PS matching, median follow-up period was 23.8 months for the OLR group and 22.8 months for the LLR group. One-year and 2-year recurrence-free survival rates and median recurrence-free survival time were 49.9%, 39.5%, and 10.6 months in the LLR group and 53.8%, 41.4%, and 13.4 months in the OLR group, respectively. There were no significant differences in recurrence-free survival between the groups (p=0.87) (Fig. 3). One-year and 2-year overall survival rates were 100% and 94.1% in the LLR group and 100% and 95% in the OLR group, respectively. Although the follow-up period was not sufficient for evaluation, no significant difference was observed in overall survival between the groups (p=0.57).
Fig. 3.
Recurrence-free survival based on type of hepatectomy after PS matching
Discussion
This study of patients with CRLM located in the posterosuperior liver (segments 4a, 7, and 8) who were treated with LLR compared to a propensity-matched group of patients who underwent OLR found that length of hospital stay was significantly shorter in the patients who had LLR and that other perioperative factors, as well as recurrence-free and overall survival, were similar in the two groups.
Previous studies have compared perioperative outcomes after LLR and OLR in patients with CRLM, and many of those studies reported that LLR was associated with shorter hospital stay, a lower rate of postoperative complications, and a lower incidence of blood transfusion [10,11,13,28]. Other studies have compared oncological outcome after LLR and OLR in patients with CRLM, and all have identified equivalent outcomes [11,13,12,29,28]. However, those studies were not specifically focused on CRLM located in the posterosuperior liver, an anatomically and technically challenging location for LLR.
The results of the current study are reflective of previous studies that examined perioperative and oncological outcomes after LLR of HCC or other tumor types [30,31,19]. Several previous studies have evaluated the perioperative and oncological outcomes after LLR of HCC located in posterosuperior segments, demonstrating that this procedure is feasible and is associated with oncological outcomes similar to those found after LLR of HCC located in the anterolateral liver [31,30]. Xiao et al. directly compared LLR and OLR of HCC in posterosuperior segments and found that LLR was associated with lower blood loss, a lower incidence of postoperative complications, and shorter hospital stay as well as with similar oncological outcomes [19].
Recently several studies have determined that transthoracic port placement for LLR of liver tumors located in posterosuperior segments can permit excellent visualization and a sufficient workspace [18,32,26,33]. As described by Ogiso et al. [26], we considered deep tumor location, cranial location, large diameter, and proximity to major vessels to be especially important considerations favoring intercostal port placement, and permitting LLR even for tumors located in posterosuperior segments (Fig. 1b and c). Therefore, we applied transthoracic port placement to the selected group of patients (13.3% of those undergoing liver resection) described herein.
Our results for the overall patient group before PS matching provide insight into surgeons’ decisions to perform OLR or LLR. The patients treated with OLR had more tumors, larger tumors, and a higher incidence of bilobar tumors, indicating higher liver tumor burden. Additionally, a larger proportions of patients treated with OLR had unfavorable tumor location—i.e., deep tumor(s), or a location adjacent to major vessels. These results may help explain the fact that the rate of major resection was higher among patients who underwent OLR. We found that only 13% of patients with LLR, compared to 54% of those with OLR, had a major resection. This means that surgeons can accurately select patients for laparoscopic liver resection for tumors located in the posterosuperior liver based on preoperatively identifiable factors. Our result also showed that tumors >30mm, multiple, deeply and adjacent to major vessels located CRLM were factors associated with OLR. Among the patients treated with LLR, none of the patients with 0 or 1 of these 4 factors had to be converted to OLR. In contrast, 4 of 6 patients who had 2 or more of these 4 factors were converted to OLR. These results suggest the accurate identification of patients who are good candidates for LLR based on preoperative available factors. Interestingly, 97% of patient with LLR but only 77% of those with OLR had high preoperative risk (ASA score ≥3). When tumors are located in the posterosuperior liver, OLR usually requires a muscle-cutting incision (subcostal or transverse L-shape incision), even in the case of a limited, partial hepatectomy. As these incisions have been reported to be associated with higher rates of postoperative infectious complications and pleural effusion [34,35], knowledge of the risk associated with these incisions in high-risk patients may in part explain the lower incidence of patients with ASA score ≥3 in the OLR group.
The main limitation of this study relates to generalizability of the findings. Our LLR group included few patients with deep tumors, tumors adjacent to major vessels, or bilobar tumors. Therefore, after PS matching, both the LLR group and the OLR group had relatively low tumor burden, which generally requires limited partial hepatectomy without the need to employ advanced surgical techniques such as segmental resection or vascular reconstruction. Thus, our results apply only to the limited group of patients with relatively low tumor burden. To evaluate the feasibility and efficacy of LLR for CRLM with high tumor burden located in posterosuperior segments, careful patient selection and additional investigation will be needed. Secondly, the study is comprised of a relatively small cohort with short-term follow up period, non-randomized stratification and retrospective analysis. Thirdly, there was a statistically non-significant higher incidence of patients treated with an enhanced recovery program in the LLR group. This might affect the shorter length of hospital stay although, as stated above, this difference was not statistically significant. Further, above-mentioned limitations were effectively addressed using PS matching for comparison of the survival between both groups. Additionally, although the follow up period was short, it may be sufficient to evaluate recurrence-free survival since 70% of recurrences develop within 2 years after hepatectomy [36].
In conclusion, we evaluated the perioperative and short-term oncologic outcomes after LLR and OLR of CRLM located in the posterosuperior liver. Our results found that compared to OLR, LLR was associated with shorter hospital stay and similar oncologic outcome when patients are accurately stratified to OLR vs. LLR. Even if the CRLM are located in the posterosuperior liver, if solitary, less than 30mm, in a superficial location and not abutting major vessels, they can be excellent candidates for LLR.
Acknowledgments
Research support for this study:
The University of Texas MD Anderson Cancer Center is supported in part by the NIH/NCI under award number P30CA016672.
Footnotes
Conflicts of Interest:
Drs. Okuno, Gourmand, Mizuno, Omichi, Tzeng, Chun, Aloia, Fleming, Lee, Vauthey, and Conrad have no conflicts of interest or financial ties to disclose.
References
- 1.Minagawa M, Makuuchi M, Torzilli G, Takayama T, Kawasaki S, Kosuge T, Yamamoto J, Imamura H. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg. 2000;231(4):487–499. doi: 10.1097/00000658-200004000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–318. doi: 10.1097/00000658-199909000-00004. discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, Giacchetti S, Paule B, Kunstlinger F, Ghemard O, Levi F, Bismuth H. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240(4):644–657. doi: 10.1097/01.sla.0000141198.92114.f6. discussion 657–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreou A, Aloia TA, Brouquet A, Dickson PV, Zimmitti G, Maru DM, Kopetz S, Loyer EM, Curley SA, Abdalla EK, Vauthey JN. Margin status remains an important determinant of survival after surgical resection of colorectal liver metastases in the era of modern chemotherapy. Ann Surg. 2013;257(6):1079–1088. doi: 10.1097/SLA.0b013e318283a4d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 6.Azagra JS, Goergen M, Gilbart E, Jacobs D. Laparoscopic anatomical (hepatic) left lateral segmentectomy-technical aspects. Surg Endosc. 1996;10(7):758–761. doi: 10.1007/BF00193052. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg. 2009;250(5):831–841. doi: 10.1097/SLA.0b013e3181b0c4df. [DOI] [PubMed] [Google Scholar]
- 8.Rao A, Rao G, Ahmed I. Laparoscopic or open liver resection? Let systematic review decide it. Am J Surg. 2012;204(2):222–231. doi: 10.1016/j.amjsurg.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Wakabayashi G, Cherqui D, Geller DA, Han HS, Kaneko H, Buell JF. Laparoscopic hepatectomy is theoretically better than open hepatectomy: preparing for the 2nd International Consensus Conference on Laparoscopic Liver Resection. J Hepatobiliary Pancreat Sci. 2014;21(10):723–731. doi: 10.1002/jhbp.139. [DOI] [PubMed] [Google Scholar]
- 10.Allard MA, Cunha AS, Gayet B, Adam R, Goere D, Bachellier P, Azoulay D, Ayav A, Navarro F, Pessaux P. Early and Long-term Oncological Outcomes After Laparoscopic Resection for Colorectal Liver Metastases: A Propensity Score-based Analysis. Ann Surg. 2015;262(5):794–802. doi: 10.1097/sla.0000000000001475. [DOI] [PubMed] [Google Scholar]
- 11.Beppu T, Wakabayashi G, Hasegawa K, Gotohda N, Mizuguchi T, Takahashi Y, Hirokawa F, Taniai N, Watanabe M, Katou M, Nagano H, Honda G, Baba H, Kokudo N, Konishi M, Hirata K, Yamamoto M, Uchiyama K, Uchida E, Kusachi S, Kubota K, Mori M, Takahashi K, Kikuchi K, Miyata H, Takahara T, Nakamura M, Kaneko H, Yamaue H, Miyazaki M, Takada T. Long-term and perioperative outcomes of laparoscopic versus open liver resection for colorectal liver metastases with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci. 2015;22(10):711–720. doi: 10.1002/jhbp.261. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa Y, Nitta H, Sasaki A, Takahara T, Itabashi H, Katagiri H, Otsuka K, Nishizuka S, Wakabayashi G. Long-term outcomes of laparoscopic versus open liver resection for liver metastases from colorectal cancer: A comparative analysis of 168 consecutive cases at a single center. Surgery. 2015;157(6):1065–1072. doi: 10.1016/j.surg.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Cipriani F, Rawashdeh M, Stanton L, Armstrong T, Takhar A, Pearce NW, Primrose J, Abu Hilal M. Propensity score-based analysis of outcomes of laparoscopic versus open liver resection for colorectal metastases. Br J Surg. 2016;103(11):1504–1512. doi: 10.1002/bjs.10211. [DOI] [PubMed] [Google Scholar]
- 14.Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, O’Rourke N, Tanabe M, Koffron AJ, Tsung A, Soubrane O, Machado MA, Gayet B, Troisi RI, Pessaux P, Van Dam RM, Scatton O, Abu Hilal M, Belli G, Kwon CH, Edwin B, Choi GH, Aldrighetti LA, Cai X, Cleary S, Chen KH, Schon MR, Sugioka A, Tang CN, Herman P, Pekolj J, Chen XP, Dagher I, Jarnagin W, Yamamoto M, Strong R, Jagannath P, Lo CM, Clavien PA, Kokudo N, Barkun J, Strasberg SM. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015;261(4):619–629. doi: 10.1097/sla.0000000000001184. [DOI] [PubMed] [Google Scholar]
- 15.Ishizawa T, Gumbs AA, Kokudo N, Gayet B. Laparoscopic segmentectomy of the liver: from segment I to VIII. Ann Surg. 2012;256(6):959–964. doi: 10.1097/SLA.0b013e31825ffed3. [DOI] [PubMed] [Google Scholar]
- 16.Ban D, Tanabe M, Ito H, Otsuka Y, Nitta H, Abe Y, Hasegawa Y, Katagiri T, Takagi C, Itano O, Kaneko H, Wakabayashi G. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci. 2014;21(10):745–753. doi: 10.1002/jhbp.166. [DOI] [PubMed] [Google Scholar]
- 17.Ogiso S, Nomi T, Araki K, Conrad C, Hatano E, Uemoto S, Fuks D, Gayet B. Laparoscopy-Specific Surgical Concepts for Hepatectomy Based on the Laparoscopic Caudal View: A Key to Reboot Surgeons’ Minds. Ann Surg Oncol. 2015;22(Suppl 3):S327–333. doi: 10.1245/s10434-015-4661-6. [DOI] [PubMed] [Google Scholar]
- 18.Lee W, Han HS, Yoon YS, Cho JY, Choi Y, Shin HK. Role of intercostal trocars on laparoscopic liver resection for tumors in segments 7 and 8. J Hepatobiliary Pancreat Sci. 2014;21(8):E65–68. doi: 10.1002/jhbp.123. [DOI] [PubMed] [Google Scholar]
- 19.Xiao L, Xiang LJ, Li JW, Chen J, Fan YD, Zheng SG. Laparoscopic versus open liver resection for hepatocellular carcinoma in posterosuperior segments. Surg Endosc. 2015;29(10):2994–3001. doi: 10.1007/s00464-015-4214-x. [DOI] [PubMed] [Google Scholar]
- 20.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, Curley SA, Loyer EM, Muratore A, Mentha G, Capussotti L, Vauthey JN. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241(5):715–722. doi: 10.1097/01.sla.0000160703.75808.7d. discussion 722–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brudvik KW, Mise Y, Conrad C, Zimmitti G, Aloia TA, Vauthey JN. Definition of Readmission in 3,041 Patients Undergoing Hepatectomy. J Am Coll Surg. 2015;221(1):38–46. doi: 10.1016/j.jamcollsurg.2015.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mise Y, Vauthey JN, Zimmitti G, Parker NH, Conrad C, Aloia TA, Lee JE, Fleming JB, Katz MH. Ninety-day Postoperative Mortality Is a Legitimate Measure of Hepatopancreatobiliary Surgical Quality. Ann Surg. 2015;262(6):1071–1078. doi: 10.1097/sla.0000000000001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aloia TA, Zorzi D, Abdalla EK, Vauthey JN. Two-surgeon technique for hepatic parenchymal transection of the noncirrhotic liver using saline-linked cautery and ultrasonic dissection. Ann Surg. 2005;242(2):172–177. doi: 10.1097/01.sla.0000171300.62318.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Day RW, Cleeland CS, Wang XS, Fielder S, Calhoun J, Conrad C, Vauthey JN, Gottumukkala V, Aloia TA. Patient-Reported Outcomes Accurately Measure the Value of an Enhanced Recovery Program in Liver Surgery. J Am Coll Surg. 2015;221(6):1023–1030. e1021–1022. doi: 10.1016/j.jamcollsurg.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Ogiso S, Conrad C, Araki K, Nomi T, Anil Z, Gayet B. Laparoscopic Transabdominal With Transdiaphragmatic Access Improves Resection of Difficult Posterosuperior Liver Lesions. Ann Surg. 2015;262(2):358–365. doi: 10.1097/sla.0000000000001015. [DOI] [PubMed] [Google Scholar]
- 27.Yamashita S, Loyer E, Kang HC, Aloia TA, Chun YS, Mehran RJ, Eng C, Lee JE, Vauthey JN, Conrad C. Total Transthoracic Approach Facilitates Laparoscopic Hepatic Resection in Patients with Significant Prior Abdominal Surgery. Ann Surg Oncol. 2016 doi: 10.1245/s10434-016-5685-2. [DOI] [PubMed] [Google Scholar]
- 28.Schiffman SC, Kim KH, Tsung A, Marsh JW, Geller DA. Laparoscopic versus open liver resection for metastatic colorectal cancer: a metaanalysis of 610 patients. Surgery. 2015;157(2):211–222. doi: 10.1016/j.surg.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 29.Lewin JW, O’Rourke NA, Chiow AK, Bryant R, Martin I, Nathanson LK, Cavallucci DJ. Long-term survival in laparoscopic vs open resection for colorectal liver metastases: inverse probability of treatment weighting using propensity scores. HPB (Oxford) 2016;18(2):183–191. doi: 10.1016/j.hpb.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiang L, Xiao L, Li J, Chen J, Fan Y, Zheng S. Safety and feasibility of laparoscopic hepatectomy for hepatocellular carcinoma in the posterosuperior liver segments. World J Surg. 2015;39(5):1202–1209. doi: 10.1007/s00268-015-2946-3. [DOI] [PubMed] [Google Scholar]
- 31.Lee W, Han HS, Yoon YS, Cho JY, Choi Y, Shin HK, Jang JY, Choi H, Jang JS, Kwon SU. Comparison of laparoscopic liver resection for hepatocellular carcinoma located in the posterosuperior segments or anterolateral segments: A case-matched analysis. Surgery. 2016;160(5):1219–1226. doi: 10.1016/j.surg.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Chiow AK, Lewin J, Manoharan B, Cavallucci D, Bryant R, O’Rourke N. Intercostal and transthoracic trocars enable easier laparoscopic resection of dome liver lesions. HPB (Oxford) 2015;17(4):299–303. doi: 10.1111/hpb.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ichida H, Ishizawa T, Tanaka M, Terasawa M, Watanabe G, Takeda Y, Matsuki R, Matsumura M, Hata T, Mise Y, Inoue Y, Takahashi Y, Saiura A. Use of intercostal trocars for laparoscopic resection of subphrenic hepatic tumors. Surg Endosc. 2016 doi: 10.1007/s00464-016-5107-3. [DOI] [PubMed] [Google Scholar]
- 34.Pessaux P, van den Broek MA, Wu T, Olde Damink SW, Piardi T, Dejong CH, Ntourakis D, van Dam RM. Identification and validation of risk factors for postoperative infectious complications following hepatectomy. J Gastrointest Surg. 2013;17(11):1907–1916. doi: 10.1007/s11605-013-2226-1. [DOI] [PubMed] [Google Scholar]
- 35.Uchiyama H, Harimoto N, Itoh S, Yoshizumi T, Ikegami T, Maehara Y. Pleural Effusion After Hepatectomy for Hepatocellular Carcinoma: Risk Factor Analyses and Its Impact on Oncological Outcomes. World J Surg. 2016 doi: 10.1007/s00268-016-3826-1. [DOI] [PubMed] [Google Scholar]
- 36.Hallet J, Sa Cunha A, Adam R, Goere D, Bachellier P, Azoulay D, Ayav A, Gregoire E, Navarro F, Pessaux P. Factors influencing recurrence following initial hepatectomy for colorectal liver metastases. Br J Surg. 2016;103(10):1366–1376. doi: 10.1002/bjs.10191. [DOI] [PubMed] [Google Scholar]