Abstract
Zinc is essential for the maintenance of normal cellular structure and functions. Zinc dyshomeostasis can lead to many diseases, such as cardiovascular disease. However, there are conflicting reports on the relationship between serum zinc levels and heart failure (HF). The purpose of the present study is to explore the relationship between serum zinc levels and HF by using a meta-analysis approach. PubMed, Web of Science, and OVID databases were searched for reports on the association between serum zinc levels and HF until June 2016. 12 reports with 1453 subjects from 27 case-control studies were chosen for the meta-analysis. Overall, the pooled analysis indicated that patients with HF had lower zinc levels than the control subjects. Further subgroup analysis stratified by different geographic locations also showed that HF patients had lower zinc levels than the control subjects. In addition, subgroup analysis stratified by HF subgroups found that patients with idiopathic dilated cardiomyopathy (IDCM) had lower zinc levels than the control subjects, except for patients with ischemic cardiomyopathy (ICM). In conclusion, the results of the meta-analysis indicate that there is a significant association between low serum zinc levels and HF.
1. Introduction
Zinc is an important trace element in the body. It plays a critical role in maintaining cellular structure and functions and is also involved in gene expression and cell growth and differentiation as a catalytic and structural cofactor [1]. Zinc deficiency accompanies many health conditions, such as renal disease, gastrointestinal disorders, alcoholism, sickle cell anemia, some cancer types, AIDS, and aging [2, 3]. The extracellular and intracellular levels of zinc are also related to cardiovascular health [4, 5]. According to Little et al., plasma zinc levels decrease with age and have a strong association with increasing cardiovascular disease (CVD); thus, there is an association between zinc deficiency and increased CVD [4]. Moreover, recent advances in cardiac biology and pathophysiology have highlighted the critical contribution of perturbations in zinc homeostasis to myocardial ischemia/reperfusion injury and the role of zinc signaling in cardioprotection against ischemia/reperfusion injury [2, 6].
Heart failure (HF) is one of the most frequent causes of death worldwide. Recently, there has been emerging evidence suggesting that micronutrient dyshomeostasis is associated with HF [7]. Many studies have attempted to explore the relationship between changes in serum zinc level in HF patients; however, conflicting results have been obtained. Some studies found significantly lower serum zinc levels in HF patients than in the control groups [8–16], whereas other works reported that the serum zinc levels were not significantly different between the HF patients and the control subjects [17–19]. Therefore, a comprehensive and critical meta-analysis of previous studies is carried out in the present work to draw a clearer, evidence-based conclusion on the association between serum zinc levels and HF.
2. Materials and Methods
2.1. Search Strategy
A systematic literature search of PubMed, Web of Science, and OVID databases was done until June 2016 with the use of medical subject headings (MeSH) or free text words. The search keywords were “zinc” or “Zn” and “heart failure.” The references cited in the studies and in review articles were also reviewed to identify additional works that were not captured by the database search. Only published studies with full-text reports were included.
2.2. Inclusion and Exclusion Criteria
Three authors (Xuefang Yu, Lei Huang, and Jinyan Zhao) carried out the search independently. Titles and abstracts were screened for subject relevance, and studies that could not be definitely excluded based on the abstract information were selected for full-text screening. Two authors (Xuefang Yu and Lei Huang) independently selected eligible studies for possible inclusion in the meta-analysis. Any disagreement regarding study inclusion was resolved by discussion with Jinyan Zhao toward reaching a consensus.
The appropriateness of the studies was assessed. The criteria for inclusion in the analysis were (1) human study, (2) case-control or cohort study or randomized clinical trial, (3) focus on the association between serum zinc levels and HF, and (4) providing sufficient data on zinc levels in both HF patients and control subjects. The exclusion criteria were (1) obviously irrelevant study, (2) animal study, (3) review or case report, and (4) not providing data on zinc levels in either HF patients or control subjects.
2.3. Data Extraction and Quality Assessment
All data were extracted independently by two reviewers (Bo Bian and Wei Yao) according to the inclusion and exclusion criteria. Specifically, the following data were extracted: authors' names, year of publication, country, number of subjects, and data on serum zinc levels. Any inconsistencies or discrepancies in the extracted information were resolved by discussion between the two reviewers, with a third author (Zhuoqun Wang) providing input.
The quality of all the included studies was evaluated by using the Newcastle-Ottawa Scale (NOS). This assessment tool focused on three aspects: participant selection, comparability, and exposure. Studies that satisfied all the items on the scale were given nine stars. Two authors (Xianming Wu and Jingjing Huang) assessed the quality of studies independently.
2.4. Statistical Analysis
The statistical analysis was carried out by using Stata 12, and statistical significance was set at p < 0.05. The standardized mean difference (SMD) and 95% confidence intervals (CI) were calculated. A random-effects or fixed-effects model was used to calculate the pooled SMD in the presence or absence of heterogeneity, respectively. Statistical heterogeneity was measured by applying chi-square and I-square tests. If the I2 value was less than 50% or the p value was greater than 0.10, significant heterogeneity was not considered, and a fixed-effects model was applied; otherwise, a random-effects model was used.
Subgroup analysis was done to determine associations between serum zinc levels and other relevant study characteristics, which may be possible sources of heterogeneity. Sensitivity analysis was carried out with one study removed at a time to assess whether the results could be affected markedly by a single study. Publication bias was measured by using Begg's test and visualization of funnel plots.
3. Results
Figure 1 shows the detailed steps of the literature selection. A total of 549 primary reports were identified by using the aforementioned search terms. After a series of assessments, 12 eligible articles with 1453 subjects from 27 case-control studies were chosen for the meta-analysis. Table 1 presents the detailed characteristics of the included studies.
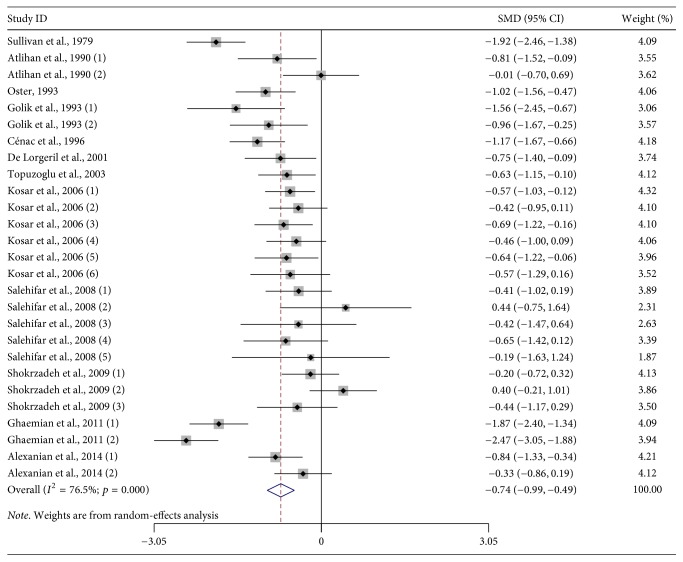
Figure 1.
Forest plot of studies on zinc levels in HF patients versus control subjects. The combined SMD and 95% CIs were calculated by using a random-effects model.
Table 1.
Characteristics of subjects in included studies.
| Studies | Country | Number of participants | Age (year) | Subgroups of HF | Score | ||
|---|---|---|---|---|---|---|---|
| HF | Controls | HF | Controls | ||||
| Sullivan et al., 1979 | USA | 42 | 37 | NA | NA | ICM | 6 |
| Atlihan et al., 1990 (1) | Turkey | 29 | 11 | 2.3 ± 1.5 | 3.1 ± 2.8 | NA | 6 |
| Atlihan et al., 1990 (2) | Turkey | 29 | 11 | 2.3 ± 1.5 | 3.1 ± 2.8 | NA | 6 |
| Oster, 1993 | Germany | 20 | 50 | 53 ± 8 | 50.5 ± 7.2 | IDCM | 8 |
| Golik et al., 1993 (1) | Israel | 9 | 20 | 63.8 ± 6 | 48.7 ± 8 | NA | 8 |
| Golik et al., 1993 (2) | Israel | 15 | 20 | 64.9 ± 5 | 48.7 ± 8 | NA | 8 |
| Cénac et al., 1996 | France | 35 | 36 | NA | NA | NA | 7 |
| De Lorgeril et al., 2001 | France | 21 | 18 | 27–76 | 34–68 | CHF | 7 |
| Topuzoglu et al., 2003 | Turkey | 54 | 20 | 18–75 | 21–73 | IDCM | 6 |
| Kosar et al., 2006 (1) | Turkey | 54 | 30 | 62 ± 10 | 56 ± 8 | CHF | 8 |
| Kosar et al., 2006 (2) | Turkey | 26 | 30 | 62 ± 10 | 56 ± 8 | IDCM | 8 |
| Kosar et al., 2006 (3) | Turkey | 28 | 30 | 62 ± 10 | 56 ± 8 | ICM | 8 |
| Kosar et al., 2006 (4) | Turkey | 24 | 30 | 62 ± 10 | 56 ± 8 | NA | 8 |
| Kosar et al., 2006 (5) | Turkey | 20 | 30 | 62 ± 10 | 56 ± 8 | NA | 8 |
| Kosar et al., 2006 (6) | Turkey | 10 | 30 | 62 ± 10 | 56 ± 8 | NA | 8 |
| Salehifar et al., 2008 (1) | Iran | 18 | 27 | 49.06 ± 8.88 | 42.30 ± 8.99 | IDCM | 8 |
| Salehifar et al., 2008 (2) | Iran | 3 | 27 | 49.06 ± 8.88 | 42.30 ± 8.99 | IDCM | 8 |
| Salehifar et al., 2008 (3) | Iran | 4 | 27 | 49.06 ± 8.88 | 42.30 ± 8.99 | IDCM | 8 |
| Salehifar et al., 2008 (4) | Iran | 9 | 27 | 49.06 ± 8.88 | 42.30 ± 8.99 | IDCM | 8 |
| Salehifar et al., 2008 (5) | Iran | 2 | 27 | 49.06 ± 8.88 | 42.30 ± 8.99 | IDCM | 8 |
| Shokrzadeh et al., 2009 (1) | Iran | 30 | 27 | 57.17 ± 8.88 | 42.3 ± 8.99 | ICM | 7 |
| Shokrzadeh et al., 2009 (2) | Iran | 17 | 27 | 57.17 ± 8.88 | 42.3 ± 8.99 | ICM | 7 |
| Shokrzadeh et al., 2009 (3) | Iran | 10 | 27 | 57.17 ± 8.88 | 42.3 ± 8.99 | ICM | 7 |
| Ghaemian et al., 2011 (1) | Turkey | 38 | 40 | 70.1 ± 9.2 | 64.9 ± 4.7 | NA | 8 |
| Ghaemian et al., 2011 (2) | Turkey | 40 | 40 | 66.7 ± 11.5 | 64.9 ± 4.7 | NA | 8 |
| Alexanian et al., 2014 (1) | Greece | 81 | 21 | 69.22 ± 11.09 | 57.0 ± 19.11 | NA | 8 |
| Alexanian et al., 2014 (2) | Greece | 44 | 21 | 67.50 ± 11.17 | 57.0 ± 19.11 | NA | 8 |
3.1. Serum Zinc Levels and HF
There were 12 studies that assessed the association between serum zinc levels and HF. First, the heterogeneity among the included studies was assessed. The results showed a high statistical heterogeneity (I2 = 76.5%; p < 0.001) (Table 2); thus, a random-effects model was used. The results of the random-effects meta-analysis indicated that HF patients had lower zinc levels than the control subjects [SMD: −0.740; 95% CI: −0.987, −0.493] (Figure 1).
Table 2.
Summary of studies included in the analysis of serum zinc levels for subjects with HF versus control subjects.
| Author | HF | Controls | Weight (%) | SMD (95% CI) | ||
|---|---|---|---|---|---|---|
| Number | Zn concentration (mean ± SD) | Number | Zn concentration (mean ± SD) | |||
| Sullivan et al., 1979 | 42 | 0.74 ± 0.11 μg/ml | 37 | 0.97 ± 0.13 μg/ml | 4.09 | −1.920 (−2.456, −1.384) |
| Atlihan et al., 1990 (1) | 29 | 92.9 ± 18.9 µg/100 ml | 11 | 107.5 ± 15.7 µg/100 ml | 3.55 | −0.806 (−1.523, −0.089) |
| Atlihan et al., 1990 (2) | 29 | 107.4 ± 17.2 µg/100 ml | 11 | 107.5 ± 15.7 µg/100 ml | 3.62 | −0.006 (−0.700, 0.688) |
| Oster, 1993 | 20 | 745 ± 195 µg/l | 50 | 931 ± 178 µg/l | 4.06 | −1.017 (−1.563, −0.4710) |
| Golik et al., 1993 (1) | 9 | 11.5 ± 1.53 μmol/L | 20 | 16 ± 3.3 μmol/L | 3.06 | −1.557 (−2.446, −0.667) |
| Golik et al., 1993 (2) | 15 | 13.2 ± 2.3 μmol/L | 20 | 16 ± 3.3 μmol/L | 3.57 | −0.960 (−1.668, −0.251) |
| Cénac et al., 1996 | 35 | 0.9 ± 0.21 μg/ml | 36 | 1.17 ± 0.25 μg/ml | 4.18 | −1.168 (−1.672, −0.664) |
| De Lorgeril et al., 2001 | 21 | 0.82 ± 0.12 mg/l | 18 | 0.9 ± 0.09 mg/l | 3.74 | −0.746 (−1.398, −0.094) |
| Topuzoglu et al., 2003 | 54 | 81.42 ± 15.4 µg/dl | 20 | 92.51 ± 22.8 µg/dl | 4.12 | −0.628 (−1.151, −0.105) |
| Kosar et al., 2006 (1) | 54 | 555 ± 104 µg/l | 30 | 620 ± 130 µg/l | 4.32 | −0.571 (−1.026, −0.116) |
| Kosar et al., 2006 (2) | 26 | 568 ± 116 µg/l | 30 | 620 ± 130 µg/l | 4.1 | −0.420 (−0.951, 0.111) |
| Kosar et al., 2006(3) | 28 | 542 ± 92 µg/l | 30 | 620 ± 130 µg/l | 4.1 | −0.689 (−1.219, −0.158) |
| Kosar et al., 2006 (4) | 24 | 565 ± 107 µg/l | 30 | 620 ± 130 µg/l | 4.06 | −0.457 (−1.001, 0.087) |
| Kosar et al., 2006 (5) | 20 | 543 ± 106 µg/l | 30 | 620 ± 130 µg/l | 3.96 | −0.636 (−1.216, −0.056) |
| Kosar et al., 2006 (6) | 10 | 553 ± 66 µg/l | 30 | 620 ± 130 µg/l | 3.52 | −0.568 (−1.295, 0.159) |
| Salehifar et al., 2008 (1) | 18 | 0.97 ± 0.25 mg/l | 27 | 1.12 ± 0.42 mg/l | 3.89 | −0.414 (−1.017, 0.189) |
| Salehifar et al., 2008 (2) | 3 | 1.3 ± 0.09 mg/l | 27 | 1.12 ± 0.42 mg/l | 2.31 | 0.444 (−0.754, 1.642) |
| Salehifar et al., 2008 (3) | 4 | 0.95 ± 0.26 mg/l | 27 | 1.12 ± 0.42 mg/l | 2.63 | −0.418 (−1.474, 0.637) |
| Salehifar et al., 2008 (4) | 9 | 0.87 ± 0.24 mg/l | 27 | 1.12 ± 0.42 mg/l | 3.39 | −0.649 (−1.419, 0.121) |
| Salehifar et al., 2008 (5) | 2 | 1.04 ± 0.06 mg/l | 27 | 1.12 ± 0.42 mg/l | 1.87 | −0.194 (−1.631, 1.243) |
| Shokrzadeh et al., 2009 (1) | 30 | 1.05 ± 0.28 mg/l | 27 | 1.12 ± 0.42 mg/l | 4.13 | −0.198 (−0.719, 0.323) |
| Salehifar et al., 2009 (2) | 17 | 1.27 ± 0.29 mg/l | 27 | 1.12 ± 0.42 mg/l | 3.86 | 0.399 (−0.214, 1.012) |
| Shokrzadeh et al., 2009 (3) | 10 | 0.95 ± 0.27 mg/l | 27 | 1.12 ± 0.42 mg/l | 3.5 | −0.439 (−1.172, 0.294) |
| Ghaemian et al., 2011 (1) | 38 | 24.7 ± 27.6 µg/dl | 40 | 70.9 ± 21.6 µg/dl | 4.09 | −1.870 (−2.405, −1.336) |
| Ghaemian et al., 2011 (2) | 40 | 23.2 ± 16.8 µg/dl | 40 | 70.9 ± 21.6 µg/dl | 3.94 | −2.465 (−3.050, −1.881) |
| Alexanian et al., 2014 (1) | 81 | 74.27 ± 15.87 µg/dl | 21 | 87.9 ± 17.85 µg/dl | 4.21 | −0.837 (−1.331, −0.343) |
| Alexanian et al., 2014 (2) | 44 | 81.51 ± 19.73 µg/dl | 21 | 87.9 ± 17.85 µg/dl | 4.12 | −0.334 (−0.857, 0.189) |
3.2. Subgroup Analysis
The subgroup analysis showed that the geographic locations and etiologies of HF [idiopathic dilated cardiomyopathy (IDCM) and ischemic cardiomyopathy (ICM)] had some influence on the serum zinc levels in HF patients and control subjects.
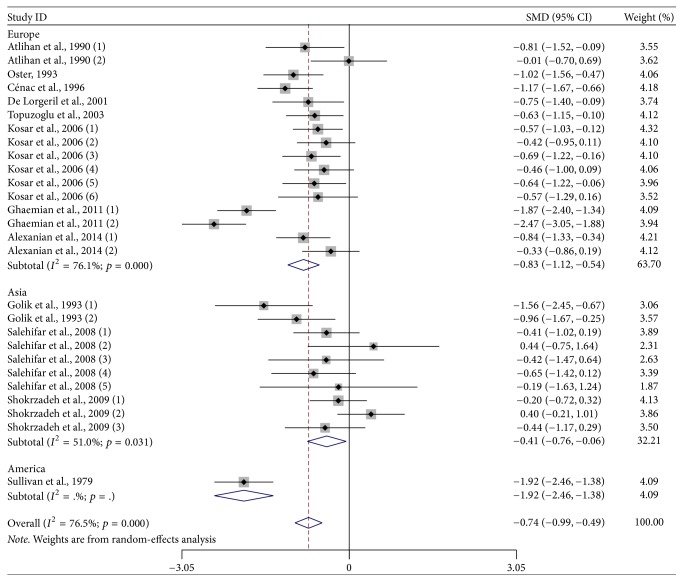
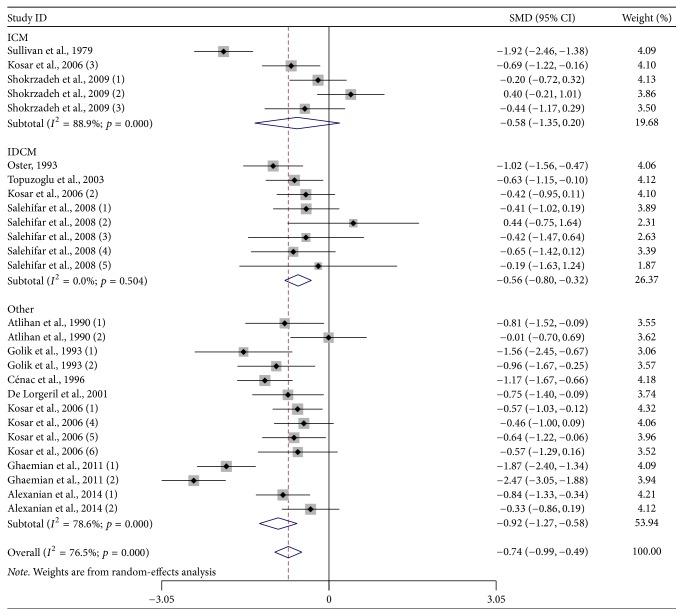
Further subgroup analysis stratified by different geographic locations found that HF patients had lower zinc levels than the control subjects [Europe: SMD: −0.832 and 95% CI: −1.119, −0.545; Asia: SMD: −0.408 and 95% CI: −0.761, −0.055; America: SMD: −1.920 and 95% CI: −2.456, −1.384] (Figure 2). In addition, the subgroup analysis stratified by HF subgroups found that patients with IDCM [SMD: −0.562; 95% CI: −0.804, −0.320] and other HF patients [SMD: −0.924; 95% CI: −1.267, −0.581] had lower zinc levels than the control subjects, except for ICM patients [SMD: −0.577; 95% CI: −1.353, 0.199] (Figure 3). Table 3 provides a summary of subgroup analysis results.
Figure 2.
Subgroup analysis of studies on zinc levels in HF patients versus control subjects stratified by geographic location.
Figure 3.
Subgroup analysis of studies on zinc levels in HF patients versus control subjects stratified by HF subgroups.
Table 3.
Subgroup analyses of zinc level and heart failure (HF).
| Subgroup | Numberof studies | SMD (95% CI) | Test of SMD = 0 | Heterogeneity | ||
|---|---|---|---|---|---|---|
| Z | p for Z | I 2 | p for I2 | |||
| Geographical location | ||||||
| Europe | 16 | −0.832 (−1.119, −0.545) | <0.001 | 5.68 | 76.1% | <0.001 |
| Asia | 10 | −0.408 (−0.761, −0.055) | 2.27 | 0.023 | 51.0% | 0.031 |
| America | 1 | −1.920 (−2.456, −1.384) | <0.001 | 7.02 | NA | NA |
| Subgroups of HF | ||||||
| ICM patients | 5 | −0.577 (−1.353, 0.199) | 1.46 | 0.145 | 88.9% | <0.001 |
| IDCM patients | 8 | −0.562 (−0.804, −0.320) | 4.55 | <0.001 | 0.0% | 0.504 |
| Other HF patients | 14 | −0.924 (−1.267, −0.581) | 5.28 | <0.001 | 78.6% | <0.001 |
3.3. Sensitivity Analysis and Publication Bias
The sensitivity analysis showed that no individual study had an extreme influence on the pooled effect (Table 4). The publication bias was measured by using Begg's test, which showed no evidence of significant publication bias (p = 0.868), and by visualizing the funnel plot, which was symmetrical (Figure 4).
Table 4.
The heterogeneity of the included studies through sensitivity analysis.
| Excluded studies | SMD (95% CI) | I 2 | p value |
|---|---|---|---|
| Sullivan et al., 1979 | −0.692 (−0.929, −0.456) | 72.90% | <0.001 |
| Atlihan et al., 1990 (1) | −0.737 (−0.993, −0.482) | 77.40% | <0.001 |
| Atlihan et al., 1990 (2) | −0.768 (−1.018, −0.518) | 76.30% | <0.001 |
| Oster, 1993 | −0.728 (−0.985, −0.470) | 77.20% | <0.001 |
| Golik et al., 1993 (1) | −0.715 (−0.965, −0.464) | 76.70% | <0.001 |
| Golik et al., 1993 (2) | −0.732 (−0.987, −0.476) | 77.30% | <0.001 |
| Cénac et al., 1996 | −0.721 (−0.977, −0.465) | 76.90% | <0.001 |
| De Lorgeril et al., 2001 | −0.739 (−0.996, −0.483) | 77.40% | <0.001 |
| Topuzoglu et al., 2003 | −0.744 (−1.002, −0.486) | 77.30% | <0.001 |
| Kosar et al., 2006 (1) | −0.747 (−1.006, −0.487) | 77.20% | <0.001 |
| Kosar et al., 2006 (2) | −0.753 (−1.010, −0.497) | 77.00% | <0.001 |
| Kosar et al., 2006 (3) | −0.741 (−1.000, −0.483) | 77.40% | <0.001 |
| Kosar et al., 2006 (4) | −0.752 (−1.008, −0.495) | 77.10% | <0.001 |
| Kosar et al., 2006 (5) | −0.744 (−1.001, -0.486) | 77.30% | <0.001 |
| Kosar et al., 2006 (6) | −0.746 (−1.001, −0.491) | 77.30% | <0.001 |
| Salehifar et al., 2008 (1) | −0.753 (−1.008, −0.497) | 77.10% | <0.001 |
| Salehifar et al., 2008 (2) | −0.769 (−1.016, −0.521) | 76.50% | <0.001 |
| Salehifar et al., 2008 (3) | −0.749 (−1.001, −0.496) | 77.30% | <0.001 |
| Salehifar et al., 2008 (4) | −0.743 (−0.998, −0.488) | 77.40% | <0.001 |
| Salehifar et al., 2008 (5) | −0.751 (−1.001, −0.500) | 77.20% | <0.001 |
| Shokrzadeh et al., 2009 (1) | −0.764 (−1.017, −0.511) | 76.30% | <0.001 |
| Salehifar et al., 2009 (2) | −0.788 (−1.028, −0.549) | 73.90% | <0.001 |
| Salehifar et al., 2009 (3) | −0.751 (−1.005, −0.496) | 77.20% | <0.001 |
| Ghaemian et al., 2011 (1) | −0.694 (−0.932, −0.456) | 73.30% | <0.001 |
| Ghaemian et al., 2011 (2) | −0.676 (−0.891, −0.460) | 67.60% | <0.001 |
| Alexanian et al., 2014 (1) | −0.735 (−0.994, −0.476) | 77.40% | <0.001 |
| Alexanian et al., 2014 (2) | −0.757 (−1.013, −0.502) | 76.80% | <0.001 |
Figure 4.
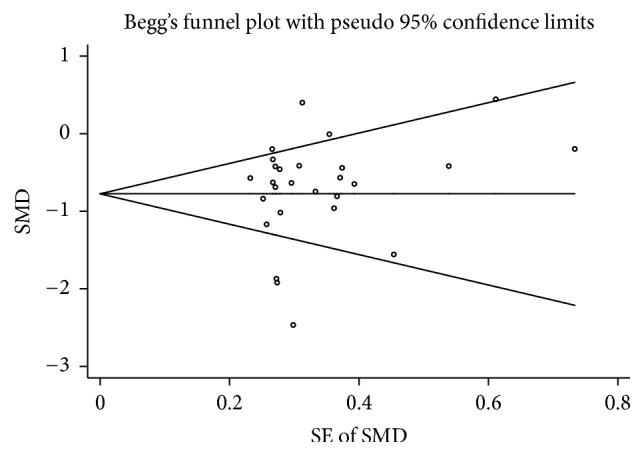
Funnel plot of studies on zinc levels in HF patients versus control subjects.
4. Discussion
Our meta-analysis included 12 reports with 1453 subjects from 27 case-control studies. The results showed that the serum zinc levels in HF patients were significantly lower than those in control subjects. This supported the supposition that there is some difference in zinc levels between HF patients and controls. In the subgroup analysis, the lower serum zinc levels in HF patients compared with control subjects were observed in different geographic locations (Europe, Asia, and America) and in IDCM and other HF patients. However, no significant difference in serum zinc levels was found in ICM patients, which could be due to the limited number of studies included in the analysis.
The mechanisms underlying the association between serum zinc levels and HF are still not fully understood. One underlying explanation for this meta-analysis showing is that micronutrient dyshomeostasis (such as zinc, copper, and Zn/Cu ratio dyshomeostasis) is associated with HF. The zinc and copper levels in the body affect each other, and decreased zinc levels are associated with deterioration of copper homeostasis and function [20]. For instance, decreased zinc levels, increased copper levels, and decreased Zn/Cu ratios have been observed in many diseases, such as rheumatoid arthritis, ICM, and thyroid carcinoma [19, 21, 22]. Moreover, a decreased Zn/Cu ratio and the subsequent systemic oxidative stress explained the more extensive atherosclerosis in some aged patients [23]. Indeed, previous studies have suggested that HF incidence and prevalence rates increased with advancing age [24]. Plasma zinc levels were found to decrease with age [3] and plasma cu to zinc ratio (CZr) was higher in hospitalized elderly subjects than in their healthy counterparts [25]. According to Malavolta et al., increments of CZr reflect increased inflammatory status or decreased nutritional status with subsequent appearance of some degenerative age-related diseases [26]. Thus, we speculated that lower levels of zinc in HF patients may be associated with advancing age or increasing of CZr due to age.
Zinc is an important component of superoxide dismutase (SOD). Manganese SOD and copper-zinc SOD, as metal-binding proteins and enzymes, have highly efficient antioxidant mechanisms and a preventive effect on the occurrence of free radical induced injury. Zinc deficiency has been found to be associated with lower SOD activity [27] and greater susceptibility to oxidative injury [28]. In HF, irrespective of the etiology, oxidative stress is important and correlates with the severity of symptoms and signs [29, 30]. Furthermore, some studies have reported a reduction of antioxidant defenses in HF patients [31–33]. Thus, in HF patients, these defenses (such as copper-zinc SOD) could be overwhelmed, creating an antioxidant deficit, particularly in cases when the activity of these oxidoreductases is dependent on the zinc concentrations.
According to Tousoulis et al., proinflammatory cytokines, such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor-alpha (TNF-α), are elevated in states of HF and are related to long-term prognosis [34]. IL-1, IL-6, and TNF-α have been found to increase metallothioneins (MTs), which bind zinc from plasma and tissues, resulting in reduced zinc bioavailability [35]. Excessive catecholamine- and PTH-induced intracellular and intramitochondrial Ca2+ accumulation in HF has been found to be coupled with increased intracellular Zn2+ due to increased Zn2+ entry and nitric oxide-induced release of inactive Zn2+ bound to MT-1 [16, 36]. Thus, we speculated that elevated inflammatory markers in HF further induce MT-1 and consequently lower zinc levels.
In addition, HF may be associated with zinc deficiency through other mechanisms: reduced dietary intake (due to anorexia, nausea, and premature satiety with eating), reduced absorption due to gastrointestinal edema and impaired motility, increased intestinal zinc losses (protein-losing enteropathy), and excessive urinary excretion due to the use of diuretics [37]. Moreover, zinc deficiency frequently accompanies many health conditions, such as diabetes mellitus (DM), aging, and hypertension [2, 38]. The majority of HF patients are older, and many of them have various comorbidities, such as DM and hypertension, which may further impair zinc homeostasis. Cellular senescence was discovered as strong driver of atherosclerosis and heart failure [39, 40]. Recent observation performed on long-term culture of primary endothelial cells suggests that zinc deficiency may lead to accumulation of senescent cells and to vascular pathology as well as to heart failure [41]. Also, in HF patients, treatment with angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) results in increased urinary zinc excretion and zinc deficiency [37].
To the best of our knowledge, this is the first meta-analysis to evaluate the association between zinc levels and HF. The sensitivity analysis showed that excluding any study from the pooled analysis did not vary the results substantially. Publication bias was also absent, as determined by funnel plot visualization and Begg's test. However, the possible limitations of our study should be considered. First, some too-old studies were included in the meta-analysis, which might weaken the quality of the results. Second, heterogeneity could not be eliminated because of methodological diversity between studies; thus, the conclusion should be conservative. In addition, because of limited data, we did not analyze the zinc levels in other tissues; this might affect the comprehensive interpretation of the zinc levels in HF patients. Therefore, better designed studies are required to verify the results and further assess the role of zinc in the progress of HF.
5. Conclusions
In conclusion, the results of the meta-analysis indicate that there is a significant association between low serum zinc levels and HF. Meanwhile better designed studies are required to verify the results and further assess the role of zinc in the progress of HF.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Berg J. M., Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science. 1996;271(5252):1081–1085. doi: 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]
- 2.Chasapis C. T., Loutsidou A. C., Spiliopoulou C. A., Stefanidou M. E. Zinc and human health: an update. Archives of Toxicology. 2012;86(4):521–534. doi: 10.1007/s00204-011-0775-1. [DOI] [PubMed] [Google Scholar]
- 3.Prasad A. S. Discovery of human zinc deficiency: Its impact on human health and disease. Advances in Nutrition. 2013;4(2):176–190. doi: 10.3945/an.112.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Little P. J., Bhattacharya R., Moreyra A. E., Korichneva I. L. Zinc and cardiovascular disease. Nutrition Journal . 2010;26(11-12):1050–1057. doi: 10.1016/j.nut.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Foster M., Samman S. Zinc and redox signaling: Perturbations associated with cardiovascular disease and diabetes mellitus. Antioxidants & Redox Signaling. 2010;13(10):1549–1573. doi: 10.1089/ars.2010.3111. [DOI] [PubMed] [Google Scholar]
- 6.Xu Z., Zhou J. Zinc and myocardial ischemia/reperfusion injury. BioMetals. 2013;26(6):863–878. doi: 10.1007/s10534-013-9671-x. [DOI] [PubMed] [Google Scholar]
- 7.Weber K. T., Weglicki W. B., Simpson R. U. Macro- and micronutrient dyshomeostasis in the adverse structural remodelling of myocardium. Cardiovascular Research. 2009;81(3):500–508. doi: 10.1093/cvr/cvn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan J. F., Blotcky A. J., Jetton M. M., Hahn H. K., Burch R. E. Serum levels of selenium, calcium, copper magnesium, manganese and zinc in various human diseases. Journal of Nutrition. 1979;109(8):1432–1437. doi: 10.1093/jn/109.8.1432. [DOI] [PubMed] [Google Scholar]
- 9.Atlihan F., Soylemezoglu T., Gokce A., Guvendik G., Satici O. Zinc and copper in congestive heart failure. Turkish Journal of Pediatrics. 1990;32:33–38. [PubMed] [Google Scholar]
- 10.Oster O. Trace element concentrations (Cu, Zn, Fe) in sera from patients with dilated cardiomyopathy. Clinica Chimica Acta. 1993;214(2):209–218. doi: 10.1016/0009-8981(93)90112-H. [DOI] [PubMed] [Google Scholar]
- 11.Cénac A., Simonoff M., Djibo A. Nutritional status and plasma trace elements in peripartum cardiomyopathy. A comparative study in Niger. Journal of Cardiovascular Risk. 1996;3(6):483–487. doi: 10.1097/00043798-199612000-00001. [DOI] [PubMed] [Google Scholar]
- 12.De Lorgeril M., Salen P., Accominotti M., et al. Dietary and blood antioxidants in patients with chronic heart failure. Insights into the potential importance of selenium in heart failure. European Journal of Heart Failure. 2001;3(6):661–669. doi: 10.1016/S1388-9842(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 13.Topuzoglu G., Erbay A. R., Karul A. B., Yensel N. Concentations of Copper, Zinc, and Magnesium in Sera from Patients with Idiopathic Dilated Cardiomyopathy. Biological Trace Element Research. 2003;95(1):11–17. doi: 10.1385/BTER:95:1:11. [DOI] [PubMed] [Google Scholar]
- 14.Kosar F., Sahin I., Taskapan C., et al. Trace element status (Se, Zn, Cu) in heart failure. Anadolu Kardiyol Derg 6. 2006:216–220. [PubMed] [Google Scholar]
- 15.Ghaemian A., Salehifar E., Jalalian R., et al. Zinc and copper levels in severe heart failure and the effects of atrial fibrillation on the zinc and copper status. Biological Trace Element Research. 2011;143(3):1239–1246. doi: 10.1007/s12011-011-8956-6. [DOI] [PubMed] [Google Scholar]
- 16.Alexanian I., Parissis J., Farmakis D., et al. Clinical and echocardiographic correlates of serum copper and zinc in acute and chronic heart failure. Clinical Research in Cardiology. 2014;103(11):938–949. doi: 10.1007/s00392-014-0735-x. [DOI] [PubMed] [Google Scholar]
- 17.Golik A., Cohen N., Ramot Y., et al. Type II diabetes mellitus, congestive heart failure, and zinc metabolism. Biological Trace Element Research. 1993;39(2-3):171–175. doi: 10.1007/BF02783187. [DOI] [PubMed] [Google Scholar]
- 18.Salehifar E., Shokrzadeh M., Ghaemian A., Aliakbari S., Saeedi Saravi S. S. The study of Cu and Zn serum levels in idiopathic dilated cardiomyopathy (IDCMP) patients and its comparison with healthy volunteers. Biological Trace Element Research. 2008;125(2):97–108. doi: 10.1007/s12011-008-8151-6. [DOI] [PubMed] [Google Scholar]
- 19.Shokrzadeh M., Ghaemian A., Salehifar E., Aliakbari S., Saravi S. S. S., Ebrahimi P. Serum zinc and copper levels in ischemic cardiomyopathy. Biological Trace Element Research. 2009;127(2):116–123. doi: 10.1007/s12011-008-8237-1. [DOI] [PubMed] [Google Scholar]
- 20.Milne D. B., Davis C. D., Nielsen F. H. Low dietary zinc alters indices of copper function and status in postmenopausal women. Nutrition Journal . 2001;17(9):701–708. doi: 10.1016/S0899-9007(01)00560-3. [DOI] [PubMed] [Google Scholar]
- 21.Zoli A., Altomonte L., Caricchio R., et al. Serum zinc and copper in active rheumatoid arthritis: Correlation with interleukin 1β and tumour necrosis factor α. Clinical Rheumatology. 1998;17(5):378–382. doi: 10.1007/BF01450895. [DOI] [PubMed] [Google Scholar]
- 22.Kucharzewski M., Braziewicz J., Majewska U., Góźdź S. Copper, zinc, and selenium in whole blood and thyroid tissue of people with various thyroid diseases. Biological Trace Element Research. 2003;93(1-3):9–18. doi: 10.1385/BTER:93:1-3:9. [DOI] [PubMed] [Google Scholar]
- 23.Mezzetti A., Pierdomenico S. D., Costantini F., et al. Copper/zinc ratio and systemic oxidant load: Effect of aging and aging- related degenerative diseases. Free Radical Biology & Medicine. 1998;25(6):676–681. doi: 10.1016/S0891-5849(98)00109-9. [DOI] [PubMed] [Google Scholar]
- 24.Wu J. R., Moser D. K., Dewalt D. A., Rayens M. K., Dracup K. Health Literacy Mediates the Relationship between Age and Health Outcomes in Patients with Heart Failure. Circulation: Heart Failure. 2016;9(1) doi: 10.1161/CIRCHEARTFAILURE.115.002250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belbraouet S., Biaudet H., Tébi A., Chau N., Gray-Donald K., Debry G. Serum Zinc and Copper Status in Hospitalized vs. Healthy Elderly Subjects. Journal of the American College of Nutrition. 2007;26(6):650–654. doi: 10.1080/07315724.2007.10719643. [DOI] [PubMed] [Google Scholar]
- 26.Malavolta M., Piacenza F., Basso A., Giacconi R., Costarelli L., Mocchegiani E. Serum copper to zinc ratio: Relationship with aging and health status. Mechanisms of Ageing and Development. 2015;151:93–100. doi: 10.1016/j.mad.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Ruz M., Cavan K. R., Bettger W. J., Fischer P. W. F., Gibson R. S. Indices of iron and copper status during experimentally induced, marginal zinc deficiency in humans. Biological Trace Element Research. 1992;34(2):197–212. doi: 10.1007/BF02785247. [DOI] [PubMed] [Google Scholar]
- 28.Powell S. R. The Antioxidant Properties of Zinc. Journal of Nutrition. 2000;130(5):1447S–1454S. doi: 10.1093/jn/130.5.1447S. [DOI] [PubMed] [Google Scholar]
- 29.Ungvári Z., Gupte S. A., Recchia F. A., Bátkai S., Pacher P. Role of oxidative-nitrosative stress and downstream pathways in various forms of cardiomyopathy and heart failure. Current Vascular Pharmacology. 2005;3(3):221–229. doi: 10.2174/1570161054368607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wykretowicz A., Furmaniuk J., Smielecki J., et al. The oxygen stress index and levels of circulating interleukin-10 and interleukin-6 in patients with chronic heart failure. International Journal of Cardiology. 2004;94(2-3):283–287. doi: 10.1016/j.ijcard.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Demirbag R., Yilmaz R., Erel O., Gultekin U., Asci D., Elbasan Z. The relationship between potency of oxidative stress and severity of dilated cardiomyopathy. Canadian Journal of Cardiology. 2005;21(10):851–855. [PubMed] [Google Scholar]
- 32.Takano H., Zou Y., Hasegawa H., Akazawa H., Nagai T., Komuro I. Oxidative Stress-Induced Signal Transduction Pathways in Cardiac Myocytes: Involvement of ROS in Heart Diseases. Antioxidants & Redox Signaling. 2003;5(6):789–794. doi: 10.1089/152308603770380098. [DOI] [PubMed] [Google Scholar]
- 33.Itoh M., Oh-Ishi S., Hatao H., et al. Effects of dietary calcium restriction and acute exercise on the antioxidant enzyme system and oxidative stress in rat diaphragm. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2004;287(1):R33–R38. doi: 10.1152/ajpregu.00598.2003. [DOI] [PubMed] [Google Scholar]
- 34.Tousoulis D., Papageorgiou N., Briasoulis A., Antoniades C., Stefanadis C. The failure of immunomodulation therapy in heart failure: Does the statins "paradigm" prove the rule? Current Vascular Pharmacology. 2010;8(1):114–121. doi: 10.2174/157016110790226589. [DOI] [PubMed] [Google Scholar]
- 35.Mocchegiani E., Muzzioli M., Cipriano C., Giacconi R. Zinc, T-cell pathways, aging: Role of metallothioneins. Mechanisms of Ageing and Development. 1998;106(1-2):183–204. doi: 10.1016/S0047-6374(98)00115-8. [DOI] [PubMed] [Google Scholar]
- 36.Efeovbokhan N., Bhattacharya S. K., Ahokas R. A., et al. Zinc and the prooxidant heart failure phenotype. Journal of Cardiovascular Pharmacology. 2014;64(4):393–400. doi: 10.1097/FJC.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen N., Golik A. Zinc balance and medications commonly used in the management of heart failure. Heart Failure Reviews. 2006;11(1):19–24. doi: 10.1007/s10741-006-9189-1. [DOI] [PubMed] [Google Scholar]
- 38.Afridi H. I., Kazi T. G., Kazi N., et al. Distribution of copper, iron, and zinc in biological samples of Pakistani hypertensive patients and referent subjects of different age groups. Clinical Laboratory. 2013;59(9-10):959–967. doi: 10.7754/Clin.Lab.2012.120704. [DOI] [PubMed] [Google Scholar]
- 39.Childs B. G., Baker D. J., Wijshake T., Conover C. A., Campisi J., Van Deursen J. M. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354(6311):472–477. doi: 10.1126/science.aaf6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gevaert A. B., Shakeri H., Leloup A. J., et al. Endothelial Senescence Contributes to Heart Failure with Preserved Ejection Fraction in an Aging Mouse Model. Circulation: Heart Failure. 2017;10(6) doi: 10.1161/CIRCHEARTFAILURE.116.003806.e003806 [DOI] [PubMed] [Google Scholar]
- 41.Malavolta M., Costarelli L., Giacconi R., et al. Changes in Zn homeostasis during long term culture of primary endothelial cells and effects of Zn on endothelial cell senescence. Experimental Gerontology. 2017;99:35–45. doi: 10.1016/j.exger.2017.09.006. [DOI] [PubMed] [Google Scholar]