Abstract
The mosquito-borne disease dengue is caused by four serologically and genetically related flaviviruses termed DENV-1 to DENV-4. Dengue is a global public health concern, with both the geographical range and burden of disease increasing rapidly. Clinically, dengue ranges from a relatively mild self-limiting illness to a severe life-threatening and sometimes fatal disease. Infection with one DENV serotype produces life-long homotypic immunity, but incomplete and short-term heterotypic protection. The development of small-animal models that recapitulate the characteristics of the disseminated disease seen clinically has been difficult, slowing the development of vaccines and therapeutics. The AG129 mouse (deficient in interferon alpha/beta and gamma receptor signalling) has proven to be valuable for this purpose, with the development of models of disseminated DENV-2,-3 and -4 disease. Recently, a DENV-1 AG129 model was described, but it requires antibody-dependent enhancement (ADE) to produce lethality. Here we describe a new AG129 model utilizing a non-mouse-adapted DENV-1 strain, West Pacific 74, that does not require ADE to induce lethal disease. Following high-titre intraperitoneal challenge, animals experience a virus infection with dissemination to multiple visceral tissues, including the liver, spleen and intestine. The animals also become thrombocytopenic, but vascular leakage is less prominent than in AG129 models with other DENV serotypes. Taken together, our studies demonstrate that this model is an important addition to dengue research, particularly for understanding the pathological basis of the disease between DENV serotypes and allowing the full spectrum of activity to test comparisons for putative vaccines and antivirals.
Keywords: dengue virus, Flavivirus, mouse model, pathogenicity, AG129 mouse
Introduction
Members of the genus Flavivirus include the causative agents of multiple important mosquito-borne diseases, including yellow fever, Japanese encephalitis, West Nile encephalitis and Zika, as well as the four serologically and genetically distinct viruses (DENV-1 to DENV-4) that cause dengue. Estimates of the global burden of dengue vary considerably, but recent reports suggest a figure of between 60–96 million clinical cases annually worldwide [1, 2]. Currently, over three billion people are at risk for dengue [3] and the disease continues to expand globally [4]. The reasons for this expansion include the increasing geographical distribution of Aedes aegypti and Ae. albopictus, the two principal arthropod transmission vectors of the viruses [5], increasing global population with continued concentration of the population in urban centres, and the continuing expansion of international trade and travel.
The severity of clinical dengue can vary considerably. Typically, infection is followed by a 4–10-day incubation period before the onset of a flu-like illness, sometimes accompanied by high fever, severe headache, joint and muscle pain, nausea, vomiting and rash. Usually these symptoms last for several days and then the patient slowly recovers. However, the disease may also progress to more severe forms, with one or more of a variety of clinical features, including persistent vomiting, rapid breathing, plasma leakage, fluid accumulation, respiratory distress, severe bleeding, neurological involvement, organ impairment and sometimes death. The World Health Organization (WHO) has classified severe dengue to include dengue haemorrhagic fever and dengue shock syndrome [6]. Following recovery from infection, there is lifelong immunity against the causative DENV serotype, but protection against the other three serotypes is incomplete and typically wanes over time [7]. Further, immune enhancement is believed to contribute to the increased severity of disease often seen following infection with a second DENV serotype [8, 9]. The perceived risk of immune enhancement following secondary DENV infections has been a driving force in the quest to develop a tetravalent vaccine to protect against all four DENV serotypes simultaneously. The first such vaccine, a recombinant, chimeric live-attenuated vaccine based on a backbone of yellow fever virus vaccine strain 17D with the prM and E proteins replaced by the corresponding proteins from the four DENV serotypes, has undergone extensive clinical trials [10, 11] and is now licensed in 16 countries. This is a major step forward in the fight against dengue. However, in the clinical trials vaccine efficacy was limited in those who were dengue-naïve at entry and the vaccine showed relatively modest efficacy against disease caused by DENV-1 and DENV-2. In addition, safety concerns in young children have led to recommendations that vaccine use be limited to those aged 9 years and older. Further, the recent WHO dengue vaccine position paper recommends that the vaccine only be used in countries with high endemicity [12]. Thus, there is still a need for further vaccine development and there are still no effective antiviral drugs to treat dengue.
One of the challenges in this area has been the difficulty of developing animal models that closely recapitulate human disease for preclinical testing studies. A particular problem has been that many small animal species, including immunocompetent mice, fail to develop clinical signs of disease following DENV challenge, which limits the capacity for early-phase testing studies (reviewed in [13, 14]). However, a number of models using immunocompromised mouse strains have been described. In particular, the AG129 mouse, which is deficient in interferon α/β and γ receptors, has proved to be a valuable model. The first report for this model showed that intraperitoneal (i.p.) inoculation with mouse brain-adapted DENV-2 strain New Guinea C produced viraemia and that the animals developed clinical signs culminating in death by day 12 post-inoculation (p.i.) [15]. One drawback of this model was that the mice mainly showed neurological signs. Although neurological involvement is seen clinically, generalized disseminated infection is a more characteristic presentation. Subsequently, a model of lethal disseminated DENV-2 disease in AG129 mice was developed following studies in which a second DENV-2 strain, PL046, that produced lethal neurological disease in mice, was passaged alternately between mosquito cells and AG129 mice to mimic the normal viral transmission cycle. This led to the development of a new strain, D2S10, which produced a rapidly lethal disseminated disease without neurological signs, better modelling clinical dengue [16]. Subsequently, other DENV-2 strains have been generated, including S221, a triple-plaque purified derivative of D2S10, and D2Y98P, a non-mouse adapted virus derived by passage in mosquito cells from a human Singaporean virus isolate [17–19]. Together these strains have contributed substantially to our understanding of dengue pathogenesis and innate and adaptive immune responses to infection, and to the evaluation of candidate vaccines and antivirals.
We previously reported the development of AG129 mouse models of lethal disseminated infection using a non-mouse adapted DENV-3 strain, C0360/94 [20], and DENV-4 strains 703/4 and TVP376 [21, 22]. To extend the utility of the AG129 mouse for dengue research we describe here the characterization of a model of disseminated lethal DENV-1 disease produced by the non-mouse-adapted DENV-1 strain, West Pacific 74 (WP 74) [23]. This model allows comparative pathogenesis studies and the evaluation of vaccine and antiviral candidates against all four DENV serotypes.
Results
DENV-1 WP 74 produces a lethal infection in AG129 mice
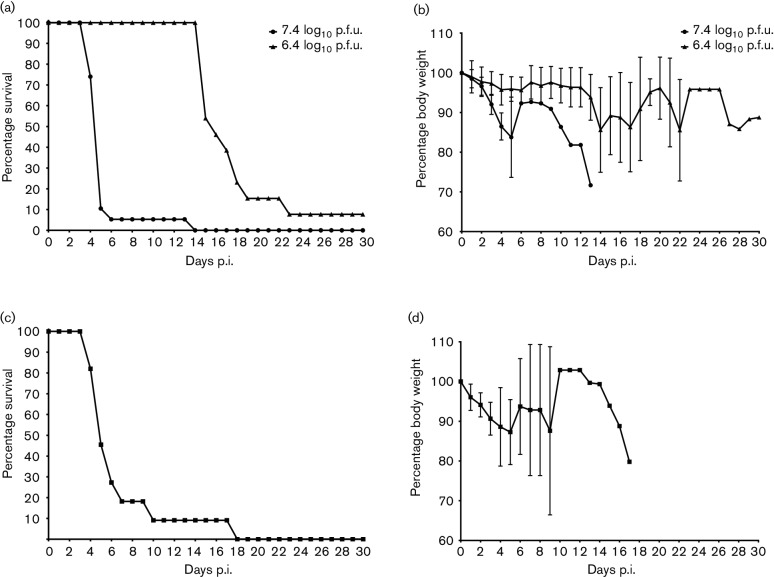
Initial studies investigated the outcome of DENV-1 WP 74 infection in young adult (6–8 week) AG129 mice inoculated by the i.p. route with 7.4 log10 p.f.u. (N=19) or 6.4 log10 p.f.u. (N=13) of the virus. To ensure that the results were consistent, two different virus stocks were prepared and tested independently in vivo. The study outcomes for the two stocks were comparable and the combined results are presented here. Animals were examined daily for morbidity (weight loss and clinical signs) and survival. Administration of the higher dose of virus resulted in an infection characterized by rapid weight loss accompanied by disease progression, including ruffled coat, hunched posture and reduced activity, but with no obvious signs of neurological disease, such as paresis or paralysis. The infection was uniformly lethal, with 18/19 (95 %) of the animals succumbing by day 6 p.i. and a mean day of death (MDD±sd) of 5.3±2.2 (Fig. 1a, b). Animals administered the lower dose of virus did not experience the same rapid progressive disease during this period. There was a mild reduction in weight in some animals, but no other signs of disease. However, beginning approximately 2 weeks after challenge, these animals experienced progressive weight loss and disease signs, resulting in uniform lethality with an MDD 16.8±2.5 (Fig. 1a, b). Interestingly, although the progression of disease in these animals was similar to that of the high-dose group, 4/12 (33 %) animals inoculated with the lower dose developed hind-limb paralysis, indicating that neurological disease was a factor in the later onset disease.
Fig. 1.
DENV-1 WP 74 produces lethal infection in AG129 mice. (a) Survival curves of 6–8-week-old mice following intraperitoneal inoculation with 7.4 log10 p.f.u. (n=19) or 6.4 log10 p.f.u. (n=13) DENV-1 WP 74. Mice were monitored daily for 30 days. (b) Morbidity among these animals as measured by weight loss. Values are percentage change in body weight (mean±sd) relative to weight at challenge. (c) Survival curve for 18-week-old mice inoculated with 7.4 log10 p.f.u. DENV-1 WP 74 (n=11). (d) Morbidity measured by weight loss in the 18-week-old animals.
The susceptibility of older animals (18 weeks old) to infection following inoculation with 7.4 log10 p.f.u. virus (N=11) was also examined. The course of disease and clinical signs in the older animals were very similar to those observed in younger animals, including rapid weight loss preceding the onset of clinical signs and high lethality (Fig. 1c, d). The MDD in older animals was 6.8±4.1, which was similar to that seen in high-dose younger animals. In addition, none of the older animals showed signs of neurological disease throughout the 30-day observation period.
Evaluation of virus dissemination in DENV-1 WP74-infected AG129 mice
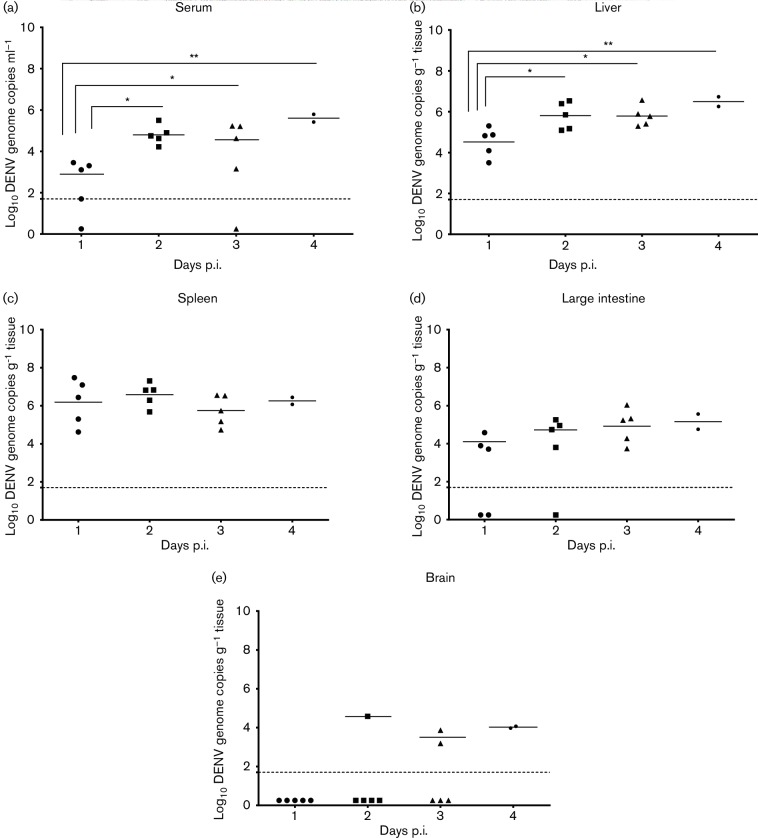
In the next series of studies, 6–8-week-old AG129 mice were inoculated with 7.4 log10 p.f.u. of DENV-1 WP 74 and groups of animals (n=5/day) were allocated for sacrifice on days 1, 2, 3 and 4 p.i. Samples of serum, liver, spleen, large intestine and brain were collected from the animals and examined by QRT-PCR (which is equivalent to approximately 10 genome copies p.f.u.−1) to determine the course of the virus infection (Fig. 2). The majority of animals had viral RNAemia throughout the study. The mean titre on day 1 was 2.8±0.9 log10 genome copies ml−1, which increased significantly on day 2 (4.8±0.5; P<0.05) and remained at the higher level on day 3 (4.6±1.0; P<0.05). By day 4 there was already high lethality and only 2/5 animals survived to be sampled, although the RNAemia in those animals remained high (5.5±0.2; P<0.05) (Fig. 2a). Viral RNA was detected in liver samples from all (17/17) animals and the results closely mirrored those of the viral RNAemia. Specifically, viral RNA levels increased significantly from day 1 p.i. (4.5±0.7 log10 genome copies g−1) to day 2 (5.8±0.7 log10 genome copies g−1; P<0.05), day 3 (5.7±0.5 log10 genome copies g−1; P<0.05) and day 4 (6.5±0.3; P<0.05) (Fig. 2b). Viral RNA was also detected in the spleens of all animals. However, the viral RNA load on day 1 p.i. was higher than in the other tissues (6.2±1.2 log10 genome copies g−1) and did not increase significantly as the infection progressed (6.6±0.6 genome copies g−1 on day 2, 5.7±0.8 genome copies g−1 on day 3 and 6.3±0.3 genome copies g−1 on day 4; Fig. 2c). The frequency of detection of viral RNA in large intestine samples increased as the study progressed (3/5 on day 1, 4/5 on day 2, 5/5 on day 3 and 2/2 on day 4 p.i.). However, this was not accompanied by a significant increase in the virus load as the infection progressed (4.1±0.5 log10 genome copies g−1 on day 1, 4.7±0.6 genome copies g−1 on day 2, 4.9±0.9 genome copies g−1 on day 3 and 5.2±0.6 genome copies g−1 on day 4 p.i.; Fig. 2d). In contrast to the other tissues, viral RNA was detected in brain samples from only 29 % (5/17) of the animals (4.5±0.0 log10 genome copies g−1 on day 2 [n=1/5], 3.5±0.5 on day 3 [n=2/5] and 4.0±0.1 on day 4 [n=2/2]; Fig. 2e).
Fig. 2.
DENV-1 WP 74 inoculation results in sustained viral RNAemia and virus dissemination to multiple organs. AG129 mice (6–8 weeks old) were inoculated with 7.4 log10 p.f.u. DENV-1 WP 74. On days 1, 2 and 3 p.i., groups of five animals were sacrificed and serum and organs harvested. Organs were weighed and homogenized and virus load was determined by QRT-PCR. Results are shown for (a) serum, (b) liver, (c) spleen, (d) large intestine and (e) brain. Each symbol represents one animal. Serum titres are expressed as log10 genome copies ml−1 and organ titres are expressed as log10 genome copies g−1 of tissue. The dashed horizontal line represents the limit of detection of the assay. Symbols represent individual sample titres; short solid horizontal lines represent the mean daily titre.
Effect of DENV-1 WP 74 infection on blood chemistry values and complete blood cell (CBC) counts
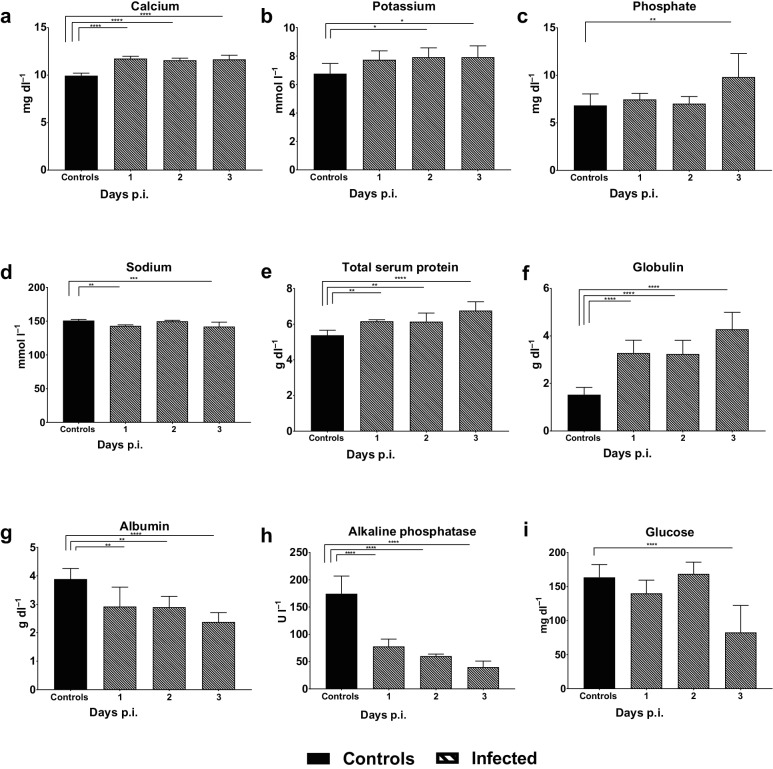
Human dengue infection is accompanied by alterations in blood chemistry values. Therefore, we examined the impact of DENV-1 WP 74 infection on blood chemistry profiles in AG129 mice. Significant changes were found in multiple parameters, with some beginning as early as day 1 p.i. (Fig. 3). The levels of the electrolytes calcium (Fig. 3a), potassium (Fig. 3b) and phosphate (Fig. 3c) all increased in virus-infected animals, with significant elevation of calcium on day 1 p.i. and potassium and phosphate on days 2 and 3 p.i., respectively. In contrast to other electrolytes, sodium was reduced in virus-infected animals on both days 1 and 3 p.i. (Fig. 3d). Further, total serum protein levels increased significantly and progressively throughout infection (Fig. 3e), and there was also an increase in serum globulin (Fig. 3f). However, albumin levels were significantly decreased (Fig. 3g). Serum alkaline phosphatase levels were significantly lower in virus-infected animals than controls on days 1–3 of the study (Fig. 3h). Alanine aminotransferase and blood urea nitrogen levels were also reduced, although the reduction only reached significance on one of the days tested for each (day 2 and day 3 respectively; data not shown). The blood glucose levels in the virus-infected and control animals were comparable to those of the controls on days 1 and 2 p.i., but decreased significantly on day 3 p.i. (Fig. 3i).
Fig. 3.
DENV-1 WP 74 infection alters blood chemistry profiles. Six-week-old AG129 mice were inoculated with 7.4 log10 p.f.u. DENV-1 WP 74 and blood was collected on days 1, 2 and 3 p.i. Samples were analysed using the Vetscan comprehensive diagnostic profile and the results were compared to those from media-inoculated control animals bled over the same period. Bars represent the mean (±sd) daily value. *P<0.05, **P<0.01, ***P<0.005, ****P<0.001 (ANOVA with Dunnett's post test).
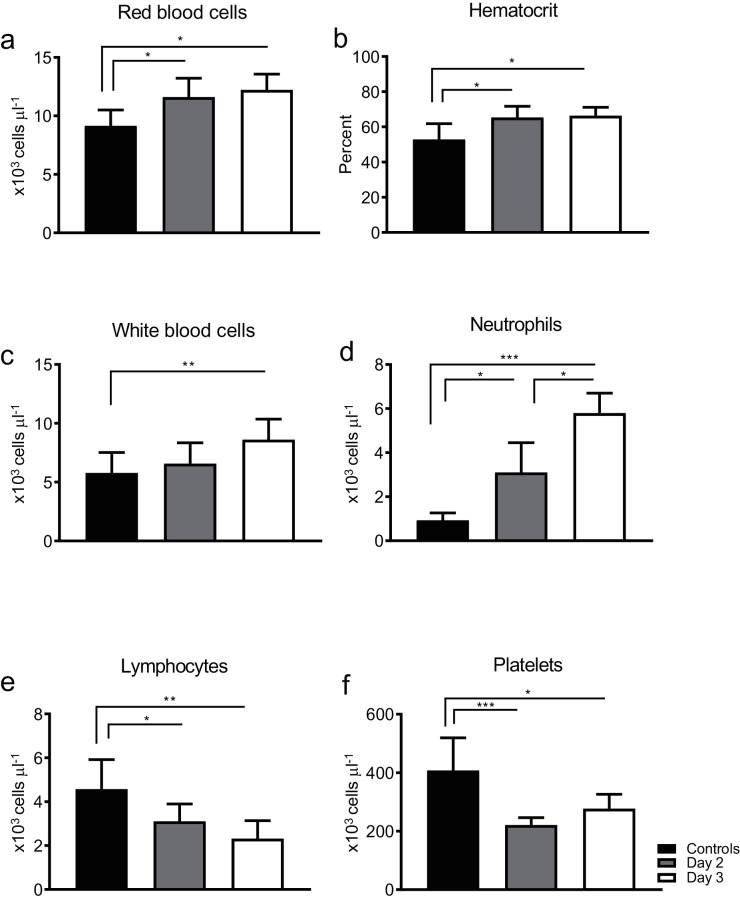
The impact of DENV-1 WP 74 infection on CBC values was also evaluated. Blood was collected from virus-infected animals on days 2 or 3 p.i. (n=7–8/day) and the values were compared to those from uninfected age matched control animals (n=8) bled in parallel; each animal was only bled once during the study. Both the red blood cell numbers and hematocrit values in virus-infected animals increased significantly by day 2 p.i. and remained elevated on day 3 p.i. (Fig. 4a, b). There was also a gradual but significant increase in white blood cell numbers, with a minimal increase on day 2 p.i., followed by a significant increase on day 3 p.i. (Fig. 4c). This increase wass likely largely due to the number of neutrophils, which rose significantly by day 2 p.i. in infected animals and subsequently increased significantly again from day 2 to day 3 p.i. (Fig. 4d). In contrast, there was a significant and continuing decrease in lymphocyte numbers as the infection progressed (Fig. 4e). Virus infection also resulted in a thrombocytopenia in the animals, with significantly reduced platelet numbers on both of the days on which they were tested. (Fig. 4f).
Fig. 4.
DENV-1 WP 74 infection impacts on multiple CBC parameters. Six-to-eight-week-old AG129 mice were inoculated with 7.4 log10 p.f.u. DENV-1 WP 74 or served as uninfected controls (n=8). Blood was collected on days 2 (n=8) and 3 (n=7) p.i. Bars represent the mean (±sd) daily value. *P<0.05, **P<0.01 and ***P<0.001 (ANOVA with Dunnett's post test) compared to controls.
Vascular leakage in the liver and large intestine of DENV-1 WP 74-infected mice
To determine whether DENV-1 WP 74 infection resulted in vascular leakage, we measured Evans blue dye leakage from the vasculature into the liver and large intestine of DENV-1-infected mice on day 3 p.i. in two independent studies. The results showed a modest increase in dye leakage in the virus-infected animals in both tissues (<10 % increase in the liver and ~20 % increase in the intestine). However, the increase did not reach significance for either tissue (data not shown). These data suggest that although vascular leakage occurs following DENV-1 WP 74 infection, it is not a major factor in disease progression.
Histopathology of DENV-1 WP 74 infected tissues
H&E-stained tissue sections from control mice were compared to sections from mice inoculated with 7.4 log10 p.f.u. DENV-1 WP 74. The brain sections of virus-infected animals were indistinguishable from those of mock-infected controls (data not shown). In contrast, sections from the spleen, liver and large intestine of virus-infected animals contained histological changes when compared to those of the controls (Fig. 5). The spleen sections from control animals contained characteristic delineated red and white pulp regions (Fig. 5a). In contrast, comparable sections from virus-infected animals showed diminished red pulp and an overall breakdown in splenic structure (Fig. 5b). Under high magnification, increased leukocyte content and activity were seen in the infected sections (Fig. 5c, d). Liver sections from virus-infected animals contained pleomorphic nuclei, inflammation and areas of focal necrosis that were not seen in those from the controls (Fig. 5e, f). Although histological changes in the spleen and liver were seen beginning on day 1 p.i., the in the large intestine (Fig. 5g, h) occurred more slowly and were only seen on day 3 p.i. when the virus-infected sections showed inflammatory changes, with the most prominent feature being leukocyte migration out of the Peyer’s patches.
Fig. 5.
Histological changes resulting from DENV-1 WP 74 infection; H&E section micrograph images from AG129 mice mock-infected (a, c, e, g) or infected with 7.4 log10 p.f.u. DENV-1 WP 74 (b, d, f, h) show the major changes seen in infected animals. (a, b) Ultrastructural changes in the virus infected spleen, with breakdown of normal architecture in the DENV-1-infected section. (c, d) Higher magnification images showing activated cells throughout the infected section. (e, f) Normal liver architecture in the control sections, but inflammation and focal necrosis in the DENV-1-infected section. (g, h) Areas of cellular expansion outside the Peyer’s patches in the large intestine of DENV-1-infected animals. Magnification: ×4 (a, b, e, f, g, h) or ×10 (c, d)
Impact of DENV-1 WP 74 infection on cytokine and chemokine responses
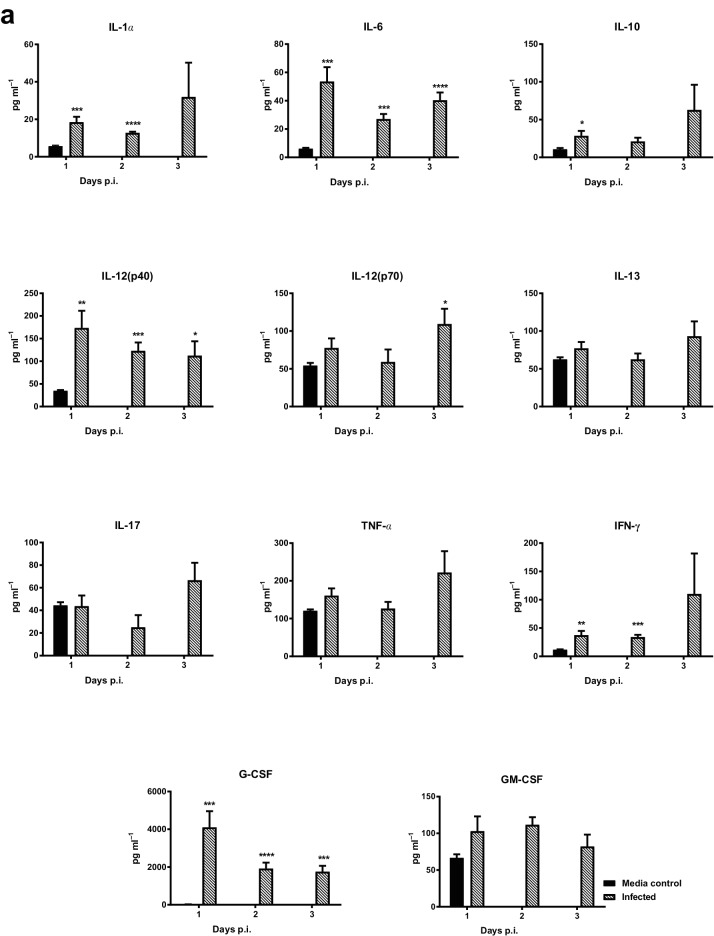
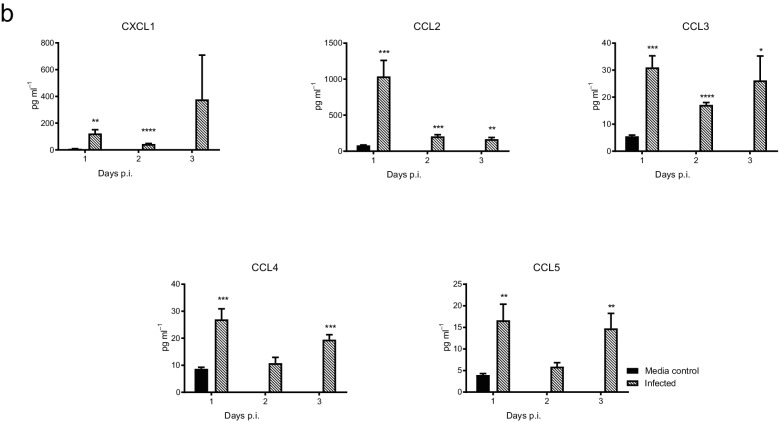
The innate immune response to DENV-1 WP 74 infection was characterized following infection with 7.4 log10 p.f.u. of virus or an equal volume of tissue culture medium. On days 1, 2 and 3 p.i. animals (n=5/group/day) were sacrificed, serum was collected and Bioplex analysis was performed. DENV-1 infection resulted in significantly elevated levels of a number of cytokines and chemokines (Fig. 6). The levels of IL-1α, IL-6, IL-10, IL-12p40, IFN-γ and G-CSF were significantly higher in virus-infected animals compared to mock-infected controls, beginning on day 1 p.i. Although the levels of all of these cytokines remained elevated in infected animals on all three days on which they were examined, the increases were not significant for the entire period for IL-10 (day 1 only) and IL-1α and IFN-γ (days 1 and 2). In contrast, the levels of IL-12 p70 were only significantly elevated compared to the controls on day 3 p.i., while the IL-13, IL-17, TNF-α and GM-CSF levels were not significantly higher than those for the controls on any of the days on which they were tested. The levels of the CXCL1 (KC), CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β) and CCL5 (RANTES) chemokines were all significantly higher than those for the mock-treated controls on at least one of the days on which they were tested.
Fig. 6.
DENV-1 WP 74 infection results in alterations in the levels of cytokines and chemokines in serum. Serum was collected from 6 to 8-week-old AG129 mice (n=5/day) infected with DENV-1 WP 74 or age-matched media-treated controls (n=6). The levels of cytokines (a) and chemokines (b) were quantified from a panel of 23 cytokines using a multiplex bead assay (Bio-Rad, Hercules, CA, USA) following the manufacturer’s protocols. The cytokine abbreviations are as follows: IL, interleukin; KC, keratinocyte chemoattractant; G-CSF, granulocyte colony-stimulating factor; IFNγ, interferon gamma; TNFα, tumor necrosis factor alpha; MCP-1 (CCL2), monocyte chemoattractant protein-1 (CCL3 or 4); MIP, macrophage inflammatory protein; RANTES (CCL5), regulated on activation, normal T cell expressed and secreted; GM-CSF, granulocyte/macrophage colony-stimulating factor. *P<0.01, **P<0.001 (Student's t-test) compared to controls.
Discussion
Johnson and Roehrig first described lethal DENV-2 infection in AG129 mice [15]. Subsequently, AG129 mouse models of disseminated infections that share features with human dengue have been described using a number of other DENV-2 strains and more recently also DENV-3 and DENV-4 strains. This has expanded the utility of the AG129 mouse model so that it has become the mainstay small-animal model for dengue research (reviewed in [13, 14]). However, there has been a dearth of comparable DENV-1 models. Recently, Watanabe et. al. [24] reported a lethal AG129 mouse model of DENV-1 infection using antibody-dependent enhancement (ADE) with animals being administered 50 µg of the monoclonal antibody 4G2 1 day prior to intravenous injection with 7.8 log10 p.f.u. of DENV-1 EDEN1, a low passage non-mouse adapted clinical isolate from Singapore. Under these conditions 100 % of the animals died by day 5 p.i. However, in the absence of ADE, the same inoculum produced a sustained viraemia but no clinical outcome in the animals.
We recently described DENV-3 and DENV-4 models in AG129 mice characterized by a lethal disseminated infection without neurological signs and that do not require challenge under ADE conditions. In developing these models our preferred approach has been to screen low-passage non-mouse-adapted virus strains to identify strains that are able to multiply to high titre in mosquito C6/36 cells for in vivo evaluation in young adult AG129 mice [20–22]. In all of these models, an inoculum of ≥7.0 log10 p.f.u. of virus was required to produce disseminated infection with high lethality during the first 7 days after challenge without neurological signs of disease. However, the models were also characterized by a limited dynamic range, with a 10-fold reduction in the inoculum resulting in a loss of lethality. In the DENV-1 studies reported here we adopted a similar approach, identifying a number of non-mouse-adapted strains, including DENV-1 WP 74, that were able to replicate to high titre in mosquito C6/36 cells, and evaluated them for clinical signs and lethality in young adult AG129 mice following high-inoculum (>7.0 log10 p.f.u.) challenge. DENV-1 WP 74 was the only tested strain that produced a rapidly progressing lethal infection that did not involve neurological signs.
We also evaluated DENV-1 WP 74 for clinical signs and lethality in older adult (18-week-old) AG129 mice and showed that the outcome was similar to that for younger animals, with a mild increase in the time to death (MDD 6.8±4.1 vs 5.3±2.2) in the older animals. As with the younger animals challenged with this virus inoculum, older animals did not show overt clinical signs of neurological disease. These results strongly suggest that, in addition to therapeutics, the model is well suited to testing vaccine candidates.
Studies to further characterize the disease resulting from high-titre DENV-1 WP 74 challenge in young adult animals showed that there were a number of similarities to the infection produced by other DENV serotypes in AG129 mice, in that DENV-1 WP 74 produced a rapidly progressive infection in which the virus disseminated to multiple organs. Viral RNAemia was detected by day 1 p.i., with the serum titres subsequently increasing significantly and being maintained on days 2, 3 and 4 p.i. The virus kinetics in the liver and serum were similar, with the titres increasing significantly between days 1 and 2 p.i., and the increased levels being maintained until day 4. Liver sections from virus infected animals also showed altered histology, including cells with pleomorphic nuclei, areas of inflammation and focal necrosis. High levels of viral RNA were detected in the spleen on all of the days on which they were examined, and the histology showed breakdown in the normal splenic structure, characterized by a reduction in the red pulp content in virus-infected animals. Gut involvement increased as the infection progressed, with both the number of animals in which viral RNA was detected and the amount of RNA increasing from day 1 to 4 p.i. This increase in virus replication at later times corresponded with altered histology in the large intestine, where there were inflammatory changes, with the most prominent feature being leukocyte migration out of the Peyer’s patches. Virus replication in the gut accompanied by histological changes has also been reported in AG129 mouse models of both DENV-2 and DENV-4 infection, although the changes in both the DENV-1- and DENV-4-infected animals were less severe than those found in DENV-2-infected animals [16, 18, 19, 21]. Viral RNA was detected in the brains of infected mice, but the overall incidence across the 4 days on whch they were sampled was only 29 % (5/17), with the rate increasing on days 3 and 4 p.i. This rate is comparable to the rates seen on day 3 p.i. in our previous studies with DENV-3 and DENV-4 (30 and 43 % respectively) [20, 21], and lower than that reported for DENV-2 strain D2S10, where virus was detected in the brains of 100 % (7/7) of mice on day 3 p.i. [16]. Further, no histological changes were seen in the brains of virus-infected animals. Thus, the low incidence of virus isolation, lack of neurological signs and absence of histopathological changes strongly indicate that neurological disease is not an important component of the rapid lethal infection seen following high-dose challenge in this model.
Thrombocytopenia is an important feature of clinical dengue infection and has been suggested to be a predictive marker for disease severity [25, 26]. AG129 mice infected with DENV-1 WP 74 experienced a significant reduction in platelet numbers, a feature shared with our previously described AG129 mouse models of DENV-3 and DENV-4 infection [20–22]. Another common feature with the models was a significant reduction in lymphocytes but an increase in neutrophil numbers. However, in DENV-1 WP 74-infected mice there was an overall increase in white blood cells as the infection progressed, rather than the leukopenia seen in the other models. Interestingly, DENV-1 WP 74-infected animals also experienced both increased red blood cell numbers and hematocrit values. This again differs from the results seen with DENV-3 and DENV-4, but is similar to that reported for DENV-2 strain D2Y98P [18, 19]. These results suggest that the animals experienced hypovolemia with attendant hemoconcentration. Vascular leakage, another feature of clinical dengue infection, could be a cause of such hypovolemia. However, while there was increased vascular leakage in infected animals, it did not reach significance and may be a less important aspect of DENV-1 infection in this model compared to other DENV serotypes [16, 19–21]. In addition to the CBC changes, DENV-1-infected animals experienced dysregulated blood chemistry values, including elevated electrolytes and increased total serum protein levels, both features of dehydration and renal failure. This suggests that the observed haemoconcentration, which occurs in parallel with reduced food and water intake and rapid weight loss, is likely a result of dehydration, possibly accompanied by acute kidney failure, another recognized complication of clinical dengue [27].
Multiple studies have examined cytokine responses during clinical dengue infection, frequently with the aim of identifying predictive biomarkers for severe disease [28, 29]. Despite the considerable number of studies in this area, interpreting them is complex because multiple factors, including age at infection, disease severity, time of sampling and whether it is a first or a repeat infection, can all affect the cytokine responses. However, in general, elevated levels of IFN-γ, TNF-α, IL-6 and IL-10 are believed to be important and to contribute to disease pathogenesis, particularly increased vascular permeability. In AG129 mice, TNF-α was important in disease progression in the DENV-2 D2S10 model [16] and increased TNF-α, IFN-γ and IL-6 levels were also seen after infection with another DENV-2 strain, D2Y98P [18]. In addition, the levels of all four cytokines were increased during severe disease in ADE models of DENV-2 infection [17, 30, 31]. Furthermore, DENV-3 strain C0360/94 and DENV-4 strain 703/4 infection in AG129 mice both resulted in significantly elevated levels of multiple cytokines and chemokines, including the four discussed. In general, the response to DENV-1 strain WP 74 infection was similar, with levels of IFN-γ, IL-6 and IL-10 all being significantly elevated in virus-infected animals. Interestingly, TNFα levels were not significantly elevated in DENV-1-infected animals on any of the days on which they were tested. This difference to the response seen with the other DENV serotypes may contribute to the reduced importance of vascular leakage as a feature of disease in this model.
Another notable difference between this DENV-1 model and the DENV-3 and DENV-4 models described previously was seen in AG129 mice inoculated with a 10-fold lower virus challenge. For both DENV-3 and DENV-4 the lower inoculum produced no lethality or signs of disease [20, 21]. In contrast, low-dose (6.4 log10 p.f.u.) DENV-1-challenged animals showed only transient mild weight loss during the period when the animals challenged with the higher dose of virus developed rapid progressive lethal disease. However, ~2 weeks after virus challenge these animals experienced rapid progressive weight loss, leading to a uniform lethality that was similar to that seen earlier in animals challenged with the higher inoculum. However, in the lower-dose animals, disease was accompanied by neurological signs in 33 % of the animals. Thus, the DENV-1 WP 74 model has a greater dynamic range than the other DENV serotype models.
In summary, we describe a new non-mouse-adapted DENV-1 model of lethal infection in AG129 mice that shares many characteristics with the DENV-3 and DENV-4 strains that we described previously and the original DENV-2 strain D2S10 model. Specifically, high-titre virus challenge produced a sustained viral RNAemia accompanied by rapid dissemination and replication in multiple visceral tissues. Further, the infection shows many virologic, immunologic, and biochemical similarities to human dengue. However, the model also shows differences from these other models, most importantly a delayed lethal infection, with a reduced virus inoculum and increased haemoconcentration as infection progresses, but less significant vascular leakage. We believe that this model is an important addition to the field of dengue research. It means that similar AG129 mouse models are now available for all four DENV serotypes and so provides the opportunity for a spectrum of activity testing for vaccines and therapeutics under comparable conditions, as well as for potential applications in understanding similarities and differences in the pathological basis of the disease caused by different viral serotypes.
Methods
Cells and virus
Vero (monkey kidney) cells were maintained at 37 °C in 5 % CO2 in minimum essential media (MEM) supplemented with 2 mM l-glutamine, 0.1 mM non-essential amino acids, 100 U ml−1 penicillin–100 µg ml−1 streptomycin and 8 % bovine growth serum (BGS). C6/36 mosquito cells were maintained at 28 °C in MEM supplemented with 2 mM l-glutamine, 0.1 mM non-essential amino acids, 100 U ml−1 penicillin–100 µg ml−1 streptomycin, 1 mM sodium pyruvate, tryptose phosphate buffer and 10 % foetal bovine serum.
DENV-1 strain Western Pacific 74 (WP 74) was isolated from a human dengue case on the Pacfic Island of Nauru in 1974 [23]. The virus was obtained from the World Reference Center for Emerging Viruses and Arboviruses at UTMB and passaged three times in C6/36 cells prior to generating stocks to be used for in vivo studies. These stocks were prepared in C6/36 cells, harvested and concentrated using 50K MWCO Amicon filters at 3000 r.p.m. and 4 °C for 20 min, and stored frozen (−80 °C) until it was used. Prior to use, stocks were quantified by plaque titration assays in Vero cells. Briefly, an aliquot of the virus was thawed and Vero cell monolayers were infected with 10-fold virus dilutions for 30 min before overlaying with MEM containing 2 % BGS-1 % agar and incubation for 4 days at 37 °C. Plaques were counted 2 days post-second overlay with MEM agar containing 2 % neutral red.
Infection of AG129 mice
AG129 (interferon α/β- and γ-receptor-deficient) mice were bred and maintained at animal facilities at the University of Texas Medical Branch (UTMB). Six-to-eight or 18-week-old animals were inoculated by i.p. injection with DENV-1 WP 74 in a 0.1 ml volume. Following inoculation, the mice were weighed daily, visually monitored and scored for morbidity as previously described [21]. Mice exhibiting signs of severe disease or with weight loss >20 % of initial body weight were euthanized and counted as dead on the following day of the study for analysis.
Virus quantitation by QRT-PCR
On days 1, 2 and 3, p.i. the mice were euthanized. Blood was collected by cardiac puncture and the serum was separated by centrifugation. In addition, samples of liver, spleen, large intestine and brain tissues were collected into pre-weighed cryotubes. The tissue samples were homogenized using a tissue lyser (Qiagen, Hilden, Germany) and clarified by centrifugation before RNA was extracted using Aurum total RNA isolation kit (Bio-Rad, Hercules, CA, USA). The RNA was converted to cDNA and evaluated using a qPCR assay. Each 25 µl PCR reaction contained 12.5 µl of iQ Sybr Green Supermix (Bio-Rad), 2.0 µl (5 µM) each of the forward and reverse primers DV1.U and DV.L1R [32], 3 µl cDNA and nuclease-free water to volume. The qPCR were conducted using a C1000 thermocycler equipped with a CFX reaction module (Bio-Rad) using the following parameters. Cycle 1: 95 °C, 90 s. Cycle 2: step 1, 95 °C, 30 s; step 2, 64.2 °C, 60 s, repeat 45×. Fluorescent signal data were collected at the end of each annealing/extension step. Starting quantity values were extrapolated from the standard curves of a plasmid harbouring the PCR target generated in parallel for each run.
Blood chemistry and cell count determinations
For the blood chemistry studies, mice were inoculated with 7.4 log10 p.f.u. DENV-1 WP 74 and five animals/day were sacrificed on days 1–3 p.i. Blood was collected to obtain plasma and immediately analysed using a comprehensive diagnostic panel rotor (Vetscan, Abaxis, Union City, CA, USA) according to the manufacturer’s instructions. The results were compared to those from pooled control samples obtained from age-matched mice (n=8) inoculated with medium and sacrificed 1–3 days later. For the cell counts, EDTA was added to prevent clotting and the samples were analysed immediately using a HEMAVET950TS (Drew Scientific, Dallas, TX, USA) according to the manufacturer’s instructions.
Histology
The mice were euthanized and liver, spleen, large intestine and brain samples were harvested and immediately fixed in 10 % neutral-buffered formalin. The tissues were paraffin-embedded, sectioned and stained with hematoxylin and eosin (H&E) at the UTMB Research Histopathology Core Laboratory. Slides from DENV-1 WP 74 infected animals were compared to those from mock-infected and naive controls to determine the changes resulting from virus infection.
Quantification of cytokine and chemokine responses and blood cell counts
The mice were inoculated with 7.4 log10 p.f.u. DENV-1 WP 74 and serum was collected from the animals on days 1–3 p.i. Samples from individual animals (15 µl) were analysed using the Bio-Rad Bio-Plex Pro Mouse Cytokine 23-plex, which was used to quantify the cytokine levels according to the manufacturer’s instructions. Samples from virus-infected animals were compared to samples collected from age-matched animals inoculated with an equivalent volume of virus-free concentrated tissue culture medium.
Statistical analyses
Incidence data for infection and disease outcomes were compared by Fisher’s exact test. ANOVA with an appropriate post-test or Student’s t-test was used for comparisons involving group mean values as appropriate. All of the presented P values are two-tailed, with values <0.05 being considered significant. All statistical analyses were performed using GraphPad Prism 7.0 for PC.
Funding information
This work was supported in part by funds from the Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institute of Health, Department of Health and Human Services under contracts N01 AI 30065 and HHSN272201000040I HHSN27200009 A71. V. V. S. was supported in part by NIAID T32 postdoctoral fellowship AI 07536.
Acknowledgements
We thank Li Li, Summer Gorder and Diana Zavala for excellent technical assistance.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch. The studies were carried out in strict compliance with the recommendations of the Guide for the Care and Use of Laboratory Animals published by the National Research Council.
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shepard DS, Undurraga EA, Halasa YA, Stanaway JD. The global economic burden of dengue: a systematic analysis. Lancet Infect Dis. 2016;16:935–941. doi: 10.1016/S1473-3099(16)00146-8. [DOI] [PubMed] [Google Scholar]
- 3.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6:e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messina JP, Brady OJ, Scott TW, Zou C, Pigott DM, et al. Global spread of dengue virus types: mapping the 70 year history. Trends Microbiol. 2014;22:138–146. doi: 10.1016/j.tim.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4:e08347. doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. 2009. p. 147. p. [PubMed] [Google Scholar]
- 7.Cassetti MC, Halstead SB. Consultation on dengue vaccines: progress in understanding protection, 26–28 June 2013, Rockville, Maryland. Vaccine. 2014;32:3115–3121. doi: 10.1016/j.vaccine.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol. 2013;158:1445–1459. doi: 10.1007/s00705-013-1645-3. [DOI] [PubMed] [Google Scholar]
- 9.Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11:532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- 10.Hadinegoro SR, Arredondo-García JL, Capeding MR, Deseda C, Chotpitayasunondh T, et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med. 2015;373:1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- 11.Villar L, Dayan GH, Arredondo-García JL, Rivera DM, Cunha R, et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med. 2015;372:113–123. doi: 10.1056/NEJMoa1411037. [DOI] [PubMed] [Google Scholar]
- 12.WHO Dengue vaccine: WHO position paper – July 2016. Wkly Epidemiol Rec. 20162016;91:349–364. [PubMed] [Google Scholar]
- 13.Chan KW, Watanabe S, Kavishna R, Alonso S, Vasudevan SG. Animal models for studying dengue pathogenesis and therapy. Antiviral Res. 2015;123:5–14. doi: 10.1016/j.antiviral.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Sarathy VV, Milligan GN, Bourne N, Barrett AD. Mouse models of dengue virus infection for vaccine testing. Vaccine. 2015;33:7051–7060. doi: 10.1016/j.vaccine.2015.09.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson AJ, Roehrig JT. New mouse model for dengue virus vaccine testing. J Virol. 1999;73:783–786. doi: 10.1128/jvi.73.1.783-786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shresta S, Sharar KL, Prigozhin DM, Beatty PR, Harris E. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J Virol. 2006;80:10208–10217. doi: 10.1128/JVI.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zellweger RM, Prestwood TR, Shresta S. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe. 2010;7:128–139. doi: 10.1016/j.chom.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan GK, Ng JK, Trasti SL, Schul W, Yip G, et al. A non mouse-adapted dengue virus strain as a new model of severe dengue infection in AG129 mice. PLoS Negl Trop Dis. 2010;4:e672. doi: 10.1371/journal.pntd.0000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan GK, Ng JK, Lim AH, Yeo KP, Angeli V, et al. Subcutaneous infection with non-mouse adapted Dengue virus D2Y98P strain induces systemic vascular leakage in AG129 mice. Ann Acad Med Singapore. 2011;40:523–532. [PubMed] [Google Scholar]
- 20.Sarathy VV, White M, Li L, Gorder SR, Pyles RB, et al. A lethal murine infection model for dengue virus 3 in AG129 mice deficient in type I and II interferon receptors leads to systemic disease. J Virol. 2015;89:1254–1266. doi: 10.1128/JVI.01320-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milligan GN, Sarathy VV, Infante E, Li L, Campbell GA, et al. A Dengue virus type 4 model of disseminated lethal infection in AG129 mice. PLoS One. 2015;10:e0125476. doi: 10.1371/journal.pone.0125476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarathy VV, Infante E, Li L, Campbell GA, Wang T, et al. Characterization of lethal dengue virus type 4 (DENV-4) TVP-376 infection in mice lacking both IFN-α/β and IFN-γ receptors (AG129) and comparison with the DENV-2 AG129 mouse model. J Gen Virol. 2015;96:3035–3048. doi: 10.1099/jgv.0.000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Repik PM, Dalrymple JM, Brandt WE, Mccown JM, Russell PK. RNA fingerprinting as a method for distinguishing dengue 1 virus strains. Am J Trop Med Hyg. 1983;32:577–589. doi: 10.4269/ajtmh.1983.32.577. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe S, Chan KW, Dow G, Ooi EE, Low JG, et al. Optimizing celgosivir therapy in mouse models of dengue virus infection of serotypes 1 and 2: The search for a window for potential therapeutic efficacy. Antiviral Res. 2016;127:10–19. doi: 10.1016/j.antiviral.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Simmons CP, Farrar JJ, Nguyen VV, Wills B. Dengue. N Engl J Med. 2012;366:1423–1432. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen MT, Ho TN, Nguyen VV, Nguyen TH, Ha MT, et al. An evidence-based algorithm for early prognosis of severe dengue in the outpatient setting. Clin Infect Dis. 2017;64:656–663. doi: 10.1093/cid/ciw863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vakrani GP, Subramanyam NT. Acute renal failure in dengue infection. J Clin Diagn Res. 2017;11:OC10–OC3. doi: 10.7860/JCDR/2017/22800.9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rathakrishnan A, Wang SM, Hu Y, Khan AM, Ponnampalavanar S, et al. Cytokine expression profile of dengue patients at different phases of illness. PLoS One. 2012;7:e52215. doi: 10.1371/journal.pone.0052215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YH, Leong WY, Wilder-Smith A. Markers of dengue severity: a systematic review of cytokines and chemokines. J Gen Virol. 2016;97:3103–3119. doi: 10.1099/jgv.0.000637. [DOI] [PubMed] [Google Scholar]
- 30.Balsitis SJ, Williams KL, Lachica R, Flores D, Kyle JL, et al. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog. 2010;6:e1000790. doi: 10.1371/journal.ppat.1000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orozco S, Schmid MA, Parameswaran P, Lachica R, Henn MR, et al. Characterization of a model of lethal dengue virus 2 infection in C57BL/6 mice deficient in the alpha/beta interferon receptor. J Gen Virol. 2012;93:2152–2157. doi: 10.1099/vir.0.045088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanesa-Thasan N, Sun W, Kim-Ahn G, van Albert S, Putnak JR, et al. Safety and immunogenicity of attenuated dengue virus vaccines (Aventis Pasteur) in human volunteers. Vaccine. 2001;19:3179–3188. doi: 10.1016/S0264-410X(01)00020-2. [DOI] [PubMed] [Google Scholar]