Abstract
Mycobacterium abscessus is a fast-growing environmental organism and an important emerging pathogen. It is highly resistant to many antibiotics and undergoes a smooth to rough colony morphology change that appears to be important for pathogenesis. Smooth environmental strains have a glycopeptidolipid (GPL) on the surface, while certain types of clinical strains are often rough and lack this GPL, due to mutations in biosynthetic genes or the mmpL4b transporter gene. We report here the development and evaluation of an allelic exchange system for unmarked alleles in M. abscessus ATCC19977, using a suicide vector bearing the E. coli galK gene and 2-deoxygalactose counterselection. We describe here two variant galK suicide vectors, and demonstrate their utility in constructing a variety of mutants with deletion alleles of the mmpL4b GPL transporter gene, the mbtH GPL biosynthesis gene, the known β-lactamase gene MAB_2875 and a putative β-lactamase gene, MAB_2833. We also show that a novel allele of the E. coli aacC4 gene, conferring apramycin resistance (aacC41), can be used as a selectable marker in M. abscessus ATCC19977 at single copy.
Keywords: mutagenesis, mycobacteria, Mycobacterium abscessus, allelic exchange
Introduction
Mycobacterium abscessus is an emerging pathogen within the rapidly growing, non-tubercular mycobacterial (NTM) species [1]. The organism can cause a plethora of infections, ranging from wound and subcutaneous infections to serious pulmonary infections, the latter of which are often seen in elderly, immunocompetent patients with underlying lung disease, or tall, elderly woman with low body mass [1–3]. M. abscessus is also an important pathogen in patients with cystic fibrosis and in patients receiving anti-tumour necrosis factor alpha therapy [4–7]. Environmental isolates typically form smooth colonies on solid media and isolates from deep tissue infections have a rough colony morphotype [8–10]. This type switch can be due to loss of a glycopeptidolipid (GPL) that is present in the outermost part of the cell envelope of the smooth morphotype. Loss of the GPL and development of the rough colony phenotype occurs by mutation of genes in the biosynthetic gene cluster or mmpL4b, which encodes a membrane transporter that is important for transfer of the GPL from the cytoplasm [9, 11]. M. abscessus is understudied, compared to other mycobacterial pathogens such as M. tuberculosis or M. marinum, partly due to a dearth of genetic tools. M. abscesssus is highly resistant to many antibiotics, which limits the use of some tools [12], and while standard E. coli-mycobacterial shuttle vectors can replicate in M. abscessus, there is a paucity of robust systems for allelic exchange.
There are three standard tools for introducing mutations into mycobacterial genomes: a temperature-sensitive mycobacteriophage for specialized transduction, electroporation of double-stranded DNA (with or without recombineering) and a suicide vector approach using some sort of counterselectable marker [13–15]. Unfortunately, M. abscessus is refractory to allelic exchange using the TM4 mycobacteriophage-based specialized transduction system [16]. Electroporation of dsDNA can work, either without [17] or with the expression of recombineering proteins [16, 18], but the efficiency is low [16]. In addition, the specialized transduction or dsDNA electroporation methods cannot easily be used for allelic exchange of unmarked mutations such as point mutations, although they can be used with mutations marked with resolvable resistance markers [13, 19]. Hence, the preferred method is a suicide vector system with a counterselectable marker, since it can be used for both marked and unmarked mutant alleles, either in-frame deletions or point mutations. A commonly used counterselectable marker in mycobacteria, sacB, which confers susceptibility to sucrose, does not work in M. abscessus for allelic exchange [16]. A recent report described the use of isoniazid counterselection for allelic exchange in M. abscessus [20].
In this article, we describe a method for the allelic exchange of unmarked mutations in M. abscessus using suicide vectors with the counterselectable marker galK, encoding galactokinase, which confers susceptibility to 2-deoxygalactose (2-DOG) [21]. We demonstrate its reproducibility in making several single and double deletion mutants and show that the characteristics and efficiency of mutant construction in M. abscessus using counterselectable suicide plasmids mirror those seen in other mycobacteria such as Mycobacterium tuberculosis and Mycobacterium smegmatis.
Methods
Bacterial strains and growth conditions
Strains are listed in Table 1. The E. coli strain DH10B was used for all routine plasmid construction, while strain BW25113 was used as the source for the galK gene. E. coli cultures were grown at 37 °C in Luria–Bertani (LB) broth (Difco, BD Biosciences, San Jose, CA) or on LB agar. Mycobacterial cultures for electroporation were grown at 37 °C in LBT broth (LB broth with 0.05 % Tween-80) or on LB agar. All other mycobacterial cultures were grown at 37 °C in Middlebrook 7H9 broth and were plated on Middlebrook 7H10 agar (Difco, BD Biosciences). The Middlebrook 7H10 medium was supplemented with 0.2 % glycerol and ADS (0.5 % bovine serum albumin fraction V, 0.2 % dextrose, and 0.85 % NaCl), while the 7H9 medium was supplemented with the same and 0.05 % Tween-80. 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) was used at a concentration of 80 µg ml−1, while 2-deoxygalactose (2-DOG) was used at a final concentration of 0.2 % made from a 10 % (w/v) filter-sterilized aqueous stock solution. Antibiotics were used at the following concentrations: kanamycin, 50 µg ml−1 for E. coli and 25 µg ml−1 for M. abscessus; apramycin, 50 µg ml−1 for E. coli and 25 µg ml−1 for M. abscessus; ampicillin, 100 µg ml−1 for M. abscessus. Kanamycin stocks were made using the active amount of antibiotic per milligram as reported by the supplier. Bovine serum albumin fraction V was obtained from Roche Applied Science (Indianapolis, IN); all other antibiotics and additives were obtained from Sigma-Aldrich Chemical (St. Louis, MO).
Table 1. Bacterial strains used in this study.
| Strain | Description | Source or Reference |
|---|---|---|
| E. coli | ||
| DH10B | F- mcrA Δ(mcrBC-hsdRMS-mrr) [φ80dΔlacZΔM15] ΔlacX74
deoR recA1
endA1 araD139 Δ(ara, leu)7697 galU galK λ- rpsL nupG |
Invitrogen |
| BW25113 | ∆(araD-araB)567 ∆lacZ4787(:: rrnB-3) lambda- rph-1∆(rhaD-rhaB)568 hsdR514 | CGSC |
| M. smegmatis | ||
| mc2155 | ept-1 | [36] |
| M. abscessus | ||
| ATCC19977 | Clinical isolate, type strain, smooth | ATCC |
| PM3044 | Smooth colony clone of ATCC19977 | This work |
| PM3363 | PM3044 :: pMP1265 (ΔmmpL4b Primary recombinant) | This work |
| PM3365 | PM3363 mmpL4b mutant (Secondary recombinant) | This work |
| PM3366 | PM3363 mmpL4b mutant (Secondary recombinant) | This work |
| PM3386 | PM3044 ΔMAB_2875 | This work |
| PM3389 | PM3044 ΔMAB_2833 :: aacC41 | This work |
| PM3395 | PM3386 ΔMAB_2833 :: aacC41 (ΔMAB_2875 ΔMAB_2833 :: aacC41) | This work |
| PM3404 | PM3386/pMV261 (ΔMAB_2875/VC) | This work |
| PM3405 | PM3386/pMP1270 (ΔMAB_2875/MAB_2875+) | This work |
| PM3492 | PM3044 ΔmbtH | This work |
| PM3498 | PM3492/pMP1294 (ΔmbtH/mbtH+) | This work |
DNA manipulation
Molecular biology techniques for plasmid cloning were performed as previously described [22]. Genomic DNA from E. coli was prepared using a guanidinium thiocyanate method [14], while that from M. abscessus was prepared using a cetyltrimethylammonium bromide protocol [23]. Restriction and DNA modification enzymes were obtained from Fermentas (Hanover, MD) or New England Biolabs (Beverly, MA). DNA fragments were isolated using agarose electrophoresis and were purified using GENECLEAN (MP Biomedicals, Santa Ana, CA). Oligonucleotides were synthesized by Eurofins (Huntsville, Al), while DNA sequencing was performed by Genewiz (South Plainfield, NJ). Plasmids used for recombination were prepared using Qiagen columns (Qiagen, Valencia, CA) and, when required, the DNA was UV treated by irradiating 15 µl of a ~0.5 mg ml−1 DNA preparation on a sterile, lidless polystyrene Petri dish in a UV Stratalinker 2400 (Agilent Technologies, Santa Clara, CA) on the default setting for timed exposure. One microlitre of each irradiated suicide vector was used for each electroporation, under conditions previously described for M. smegmatis [23]. Southern blotting was done with genomic DNA using the Amersham ECL DIRECT Nucleic acid labelling and detection system (GE Healthcare Bio-Sciences, Pittsburgh, PA).
Construction of plasmids
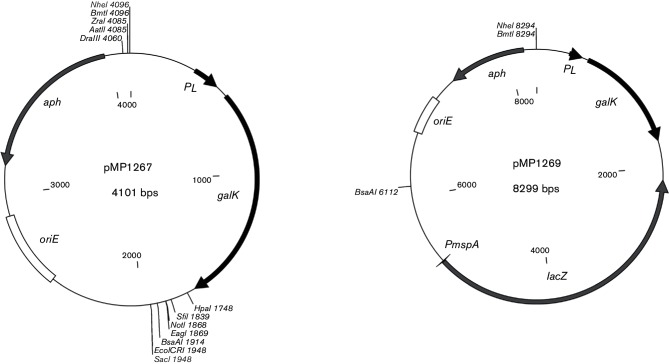
Table 2 describes all the plasmids used in this study. Detailed descriptions of plasmid construction can be obtained from the corresponding author. Brief summaries of the construction of the suicide plasmids are below. Two replicating galK tester plasmids were constructed, both based upon the shuttle vector pMV261. The first plasmid, pMP1240, has galK downstream of the mycobacterial groEL promoter (PgroEL), while the second, pMP1262, has galK expressed from the mycobacteriophage L5 major left promoter (PL). The first successful galK suicide plasmid, pMP1265, was made by replacing the pAL500 mycobacterial origin of replication in pMP1262 with an unmarked, in-frame deletion allele of the M. abscessus mmpL4b gene to generate pMP1265. Once we showed that this plasmid worked, we then removed the ΔmmpL4b insert from pMP1265 to yield the first-generation galK suicide vector, pMP1267. To make the second-generation galK vector (pMP1269), containing the lacZ gene, we inserted a BsaAI-XbaI (blunted) fragment from the plasmid pMP731, containing the E. coli lacZ gene expressed from the M. smegmatis mspA promoter (PmspA), into the BsaAI site of pMP1267.
Table 2. Plasmids used in this study.
| Plasmid | Description | Source or Reference |
|---|---|---|
| pMV261 | KmR E. coli–Mycobacterium shuttle vector, contains the groEL promoter, ColE1 origin, pAL500 origin | [37] |
| pMP349 | pMV261 with aacC41 replacing aphA-1 ApR | [25] |
| pMP1205 | pMV261.MAB_2875 | This work |
| pMP1240 | pMV261.PgroEL-galK | This work |
| pMP1262 | pMV261.PL-galK | This work |
| pMP1265 | pMP1262(ΔpAL500 origin). ΔmmpL4b | This work |
| pMP1267 | KmR mycobacterial suicide vector, ColE1, PL-galK | This work |
| pMP1269 | KmR mycobacterial suicide vector, ColE1, PL-galK, PmspA-lacZ | This work |
| pMP1270 | pMP1269.ΔMAB_2875 | This work |
| pMP1271 | pMP1269.ΔMAB_2833::aacC41 | This work |
| pMP1294 | pMV261.mbtH | This work |
| pMP1296 | pMP1267.ΔmbtH | This work |
ATCC, American Type Culture Collection; CGCS, E. coli Genetic Stock Center.
All deletion alleles for mmpL4b, mbtH, MAB_2875 and MAB_2833 were made by PCR amplification of each gene, with ~1 kb of DNA flanking each side, from WT M. abscessus genomic DNA [24], using iProof High-Fidelity DNA Polymerase from Bio Rad (Hercules, CA). All amplicons were cloned into plasmids, which were then used for inverse PCR reactions to create in-frame deletions. The deletion alleles mmpL4b, mbtH and MAB_2875 were left unmarked, while that of MAB_2833 was marked by insertion of the E. coli aacC41 gene, which confers apramycin resistance [25]. All alleles were then inserted into the galK suicide plasmids. The ΔmmpL4b and ΔmbtH suicide plasmids have the first-generation galK vector backbone, while the ΔMAB_2875 and ΔMAB_2833 :: aacC41 suicide plasmids have the second-generation galK lacZ vector backbone. Mutant alleles were confirmed by PCR and sequence analysis. Analysis of mutants following allelic exchange was done using standard iProof PCR with genomic DNA templates prepared from mutant strains, with diagnostic oligonucleotide primers specific for sequences approximately 500 bp flanking the sequences initially amplified for construction of each deletion allele. The exception was the mmpL4b region, in which the 5′ diagnostic primer was within the original flanking region. The mmpL4b mutants were also analysed by Southern blotting.
Susceptibility testing
For minimal inhibitory concentration (MIC) determination of ampicillin or cefoxitin, we used a microdilution method with the vital dye Alamar Blue [26]. Overnight broth cultures (0.5–1.0 OD600) in 7H9 media were adjusted to ~0.5 at OD600 and diluted 100-fold in 7H9 medium with or without the β-lactamase inhibitors sulbactam, tazobactam or clavulanic acid, each at 4 µg ml−1. Antibiotics were serially diluted in a 96-well format, and the wells inoculated with 50 µl of the diluted cell suspension and incubated stationary at 37 °C for 48 h. Each well then received 50 µl of a freshly prepared 1 : 1 mixture of Alamar Blue:10 % (v/v) Tween-80, and incubation continued for another 24 h at 37 °C. The concentration of antibiotic that prevented a colour change from blue to pink was recorded as the MIC. Two independent cultures for each strain were assayed in duplicate and the measurements did not vary for each strain, so no statistical analysis was required.
Results and discussion
Allelic exchange using a suicide vector that is unable to replicate in the species of interest, coupled with a counterselectable marker, is an extremely flexible way to introduce a variety of mutations into a bacterial chromosome. We sought to develop an efficient suicide vector system for allelic exchange in M. abscessus. The key to this is a suitable counterselectable marker, such as sacB (conferring sucrose sensitivity), rpsL (conferring streptomycin susceptibility in a streptomycin-resistant background) or galK (conferring 2-deoxygalactose susceptibility) [14, 27–29]. Others have shown that sacB does not work in M. abscessus, and the rpsL system requires a pre-existing streptomycin resistance mutation in the choromosomal copy of rpsL, which complicates strain construction [16, 23, 29]. Additionally, in preliminary experiments (not shown), we found it difficult to isolate streptomycin-resistant mutants that could be used with our previously developed rpsL suicide vectors. Thus we turned to galK, an E. coli gene that has previously been shown to work in M. smegmatis and M. tuberculosis [27]. The GalK enzyme converts galactose to galactose-1-phosphate but can also catalyse the conversion of 2-deoxygalactose (2-DOG) to 2-deoxygalactose-1-phosphate, which is toxic [21].
We first tested galK in M. abscessus by transforming strain PM3044 (a smooth clone of the wild-type strain ATCC19977) with plasmid pMP1240, which has the E. coli galK gene expressed from the mycobacterial groEL promoter. We observed a 1000-fold decrease in viability of PM3044/pMP1240 on media containing 0.2 % 2-DOG compared to the vector control strain PM3044/pMV261 (data not shown). A similar decrease in viability was seen comparing the growth of PM3044/pMP1240 on media with 0.2 % 2-DOG versus media lacking the compound (data not shown). Subsequent attempts to use the PgroEL-galK at single copy in the chromosome of M. abscessus revealed that the expression was too low to confer susceptibility to 2-DOG, and so we made and tested another replicating plasmid, pMP1262, in which galK was expressed from the strong major left promoter (PL) of mycobacteriophage L5 [23]. This plasmid turned out to be somewhat toxic to M. abscessus, resulting in reduced colony size even in the absence of 2-DOG, suggesting that the galK gene was overexpressed relative to the expression from the pMP1240 plasmid. Hence, the PL-galK cassette was used for the development of the exchange vector. We built two suicide vector backbones, using pMP1262 as the starting material. Both vectors have the ColE1 origin for replication in E. coli, and a kanamycin resistance gene (aphA-1) for selection in E. coli and M. abscessus. The first-generation vector is pMP1267 (Fig. 1) while the second-generation vector, pMP1269, was made from pMP1267 but has the E. coli lacZ gene, expressed from the M. smegmatis major porin promoter, PmspA (Fig. 1).
Fig. 1.
galK suicide vectors. Maps of the first-generation vector, pMP1267, and the second-generation vector, pMP1269. The unique cloning sites for each are shown, along with the following genes or regions: aph (aphA-1 aminoglycoside phosphotransferase), PL (mycobacteriophage L5 major left promoter), galK (E. coli galactokinase), lacZ (E. coliβ-galactosidase), PmspA (M. smegmatis MpsA porin promoter) and oriE (ColE1 origin of replication).
In this method the suicide plasmid, bearing the mutant allele with ~1 kb of flanking DNA, is introduced into the bacteria by electroporation and the population plated onto media containing kanamycin to select for cells that have integrated the suicide plasmid into the chromosome at the wild-type gene locus via homologous recombination. Since the plasmid cannot replicate, the only way kanamycin-resistant clones can arise is by recombination of the plasmid into the chromosome; with other mycobacterial species, this generally occurs at a frequency of 10−4–10−5 relative to the electroporation efficiency of the cells, as determined by a control electroporation with a replicating plasmid [14]. The primary recombination event is a so-called ‘single crossover’ wherein the entire plasmid integrates into the chromosome, causing a duplication of the cloned DNA. These ‘primary recombinants’ are resistant to kanamycin and sensitive to 2-DOG. A primary recombinant clone is then subcultured into liquid medium lacking kanamycin and grown to saturation. During that time, the duplicated regions at the integration site can undergo a secondary recombination that results in the looping out of the plasmid. Depending upon the location of the secondary recombination, either the wild-type or the mutant allele will be removed with the excised plasmid, leaving the other allele behind. Since the plasmid cannot replicate, it will eventually be lost in the progeny. The appearance of these ‘secondary recombinants’ is relatively rare, occurring at a frequency of 10−3 to 10−5 in the population [14, 23]. Since these secondary recombinants have lost the plasmid, they can be selected for by taking advantage of the conditionally lethal nature of the counterselectable marker. Cells that have not undergone a secondary recombination event will still have the counterselectable marker and will be killed on the 2-DOG medium, while those clones that have recombined a second time (losing that marker in the process) will survive. It is then a matter of screening the secondary recombinants for the mutant cells. In practical usage, there are three confounding factors in the procedure: low electroporation frequencies, background antibiotic resistance in the primary recombinant pool and inactivation of the counterselectable marker in the secondary recombinant pool [14]. Because of the latter two problems, it is important to screen for other markers carried on the plasmid during mutant construction.
Allelic exchange of ΔmmpL4b andΔmbtH
We decided to test our system by deleting the mmpL4b gene, encoding the transporter required to export the GPL to the outer surface of the cell envelope and confer the smooth phenotype to M. abscessus colonies. We chose this gene because mmpL4b mutations are tolerated and the smooth to rough colony morphotypes are easily discernible on agar plates [9]. For these experiments we typically electroporated two control plasmids in parallel into the cells: pMV261, a replicating plasmid to obtain the electroporation efficiency of the cells, and pMP1240, the PgroEL-galK tester plasmid as a positive control for 2-DOG sensitivity. One of the limiting factors in allelic exchange in mycobacteria is delivery of DNA into the cells. Usually, the best mycobacterial electroporation efficiencies are 105–106 transformants per electroporation, achieved with saturating amounts of DNA [14, 23]. Since integration of suicide vectors occurs at a frequency of approximately 10−5, mycobacterial suicide vector methods are often balanced on the edge of success, and thus we perform multiple electroporations (two to four) to increase the chances of obtaining primary recombinants. For allelic exchange of ΔmmpL4b we used the suicide vector pMP1265, which has an in-frame deletion of the mmpL4b gene, flanked with approximately 1 kb of DNA on each side.
We typically obtained approximately 10–20 colonies per electroporation with the suicide vector pMP1265, which was similar to the number of colonies obtained from control electroporations lacking DNA and likely due to spontaneous resistance to kanamycin in the population. Increasing the concentration of kanamycin in the media did not improve matters. Our electroporation efficiencies with M. abscessus were always in the range of 105 kanamycin-resistant clones per electroporation with the replicating control plasmid, similar to what we have obtained with M. smegmatis and M. tuberculosis, the latter under optimal conditions [14]. In our first set of electroporations with the suicide plasmid, we screened 64 KmR clones and found two that were also susceptible to 2-DOG. The presence of the PL-galK cassette at single copy did not affect colony size of these two clones in the absence of 2-DOG. One of the clones, PM3363, was grown overnight to saturation in media lacking kanamycin and plated for viable counts on media with or without 0.2 % 2-DOG. We obtained 2-DOG resistant clones at a frequency of 6×10−5, similar to what we have observed with suicide vector allelic exchange in other mycobacterial species. Within the 2-DOGR population, we found that ~90 % were kanamycin susceptible and therefore true secondary recombinants. Inactivation of counterselectable markers in mycobacteria can range from 10 % or less for rpsL, to higher, and variable, numbers for sacB [14, 23, 27]. The galK marker does not seem to be inactivated as highly as sacB, although this phenomenon typically varies between locations in the genome. Within the secondary recombinant population we noted a mutant frequency of 21 %, as evident by colony morphology. We did not see any rough mutants lacking the expected phenotype of 2-DOGR, KmS. We repeated the outgrowth experiments and 2-DOG selection with the primary recombinant PM3363 using either lower concentrations of 2-DOG or Middlebrook media and LBT in parallel. We found that decreasing the 2-DOG concentration from 0.2 to 0.1 % still afforded selection, but it was not as clean as 0.2 % and so we continued to use the higher concentration. There was no appreciable difference in the frequency of 2-DOG resistance (10−4) or mutant generation (8, 20 %) using Middlebrook compared to LBT, respectively.
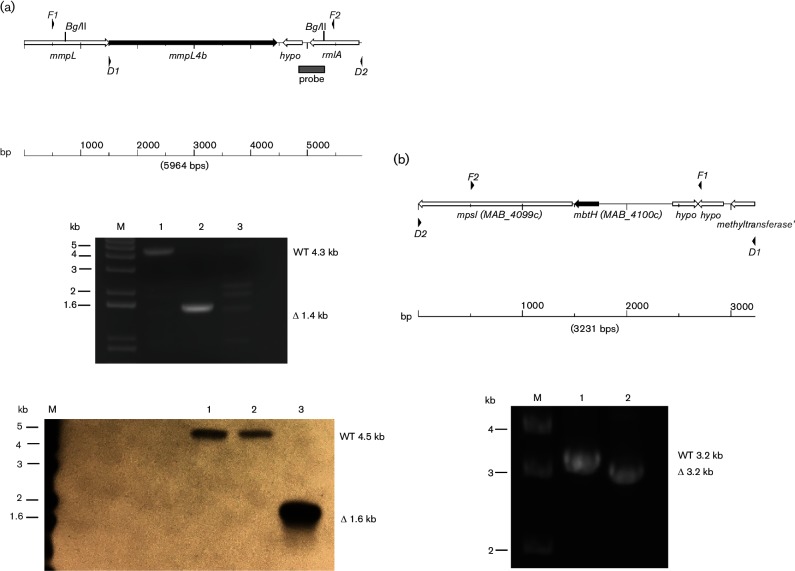
We sought to confirm allelic exchange in one ΔmmpL4b mutant, strain PM3366, by PCR. We typically do this by using diagnostic primers homologous to sequences approximately 500 bp beyond the regions flanking the sites for the initial PCR to amplify the region for construction of a deletion allele (see Fig. 2a). However, we had considerable problems with the PCR reactions with multiple products (data not shown). After performing several primer swapping experiments, we found that the problems were due to non-specific binding of the 5′ diagnostic primer, which was homologous to the gene upstream of mmpL4b, which is another mmpL4-like gene with 73 % nucleotide identity to the 3′ half of mmpL4b. We tried several versions of 5′ diagnostic primers but were only able to get unambiguous PCR reaction products with PM3366 using a 5′ diagnostic primer homologous to the intergenic region (primer D1 in Fig. 1a, top), which lies within the cloned regions. We were able to confirm the ΔmmpL4b allele in PM3366 (Fig. 2a, middle, Lane 2) using this primer. However, we performed the same PCR reactions using genomic DNA from another rough, secondary recombinant from the same experiment, strain PM3365, and obtained ambiguous results (Fig. 2a, middle, Lane 3). We then performed a Southern blot on BglII-digested DNA from wild-type and the two mmp4Lb mutants, using a probe that encompassed a region downstream of the mmpL4b gene, but within the region used for allelic exchange (see the map in Fig. 2a, top). Surprisingly, the PM3366 mutant is the same as the wild-type strain (Fig. 2a, bottom, Lane 2), while PM3365 has the deletion allele (Fig. 2a, bottom, Lane 3). This blot was repeated with new DNA preps, which yielded the same results (data not shown). The BglII-digested genomic DNA samples used for Southern blotting were also used as templates in PCR reactions (data not shown) and yielded the same results as in the initial PCR reactions shown in Fig. 2. We found that the PM3366 strain could be complemented back to the smooth wild-type morphotype with mmpL4b+on a replicating plasmid, but that PM3365 could only be partially complemented (data not shown). We surmise that the problems encountered with allelic exchange at the mmpL4b locus may be due to aberrant recombination resulting from the proximity of the upstream mmpL4-like gene.
Fig. 2.
Analysis of mmpL4b and mbtH allelic exchange mutants. (a), top: map of genomic region for mmpL4b. F1 and F2 are locations for primers used to amplify region for the construction of the deletion alleles, while D1 and D2 are the locations for primers used for diagnostic PCR. The grey box indicates the probe fragment used for the Southern blot. (a), middle: ethidium bromide-stained gel of PCR reactions using D1 and D2 diagnostic primers for mmpL4b and DNA prepared from the parental strain and two mutants: Lane 1 (PM3044, mmpL4b+ parental strain), Lane 2 (PM3366, mmpL4b mutant), Lane 3 (PM3365, mmpL4b mutant). (a), bottom: Southern blot of genomic DNA digested with BglII and probed with the DNA fragment shown in the map in (a), top. Lane 1 (PM3044, mmpL4b+ parental strain), Lane 2 (PM3366, mmpL4b mutant), Lane 3 (PM3365, mmpL4b mutant). (b), top: map of genomic region for mbtH. F1 and F2 are locations for primers used to amplify region for the construction of the deletion alleles, while D1 and D2 are the locations for primers used for diagnostic PCR. (b), bottom: ethidium bromide stained gel of PCR reactions using D1 and D2 diagnostic primers for mbtH and DNA prepared from the parental strain and the mbtH mutant: Lane 1 (PM3044, mbtH+ parental strain), Lane 2 (PM3492, ΔmbtH mutant). M is the 1 kb DNA ladder marker lane.
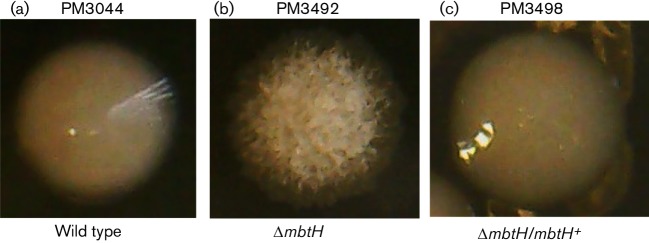
The results with the mmpL4b mutants were surprising so we decided to generate another rough mutant by deletion of mbtH, the first gene in the GPL peptide biosynthesis operon [11, 30]. We performed allelic exchange with the wild-type PM3044 and the ΔmbtH suicide plasmid pMP1296. We obtained primary and secondary recombinants at similar frequencies as seen for the mmpL4b allelic exchange experiments, but inactivation of galK was high (~30 %) in the secondary recombinant pool and we obtained rough mutants at a frequency of 13 %. We chose one mutant, PM3492, for analysis and confirmed deletion of mtbH by PCR using diagnostic primers specific to regions flanking the cloned region (Fig. 2b). Strain PM3492 could be fully complemented back to a smooth and glistening wild-type morphology with pMP1294, a multi-copy plasmid bearing the wild-type mbtH gene [Fig. 3(c), strain PM3498, compared to (b) and (a)].
Fig. 3.
Colony morphology of the wild-type parent PM3044 (a), the ΔmbtH mutant strain PM3492 (b) and ΔmbtH/mbtH+ complemented strain PM3498 (c).
Allelic exchange with the second-generation galK vector
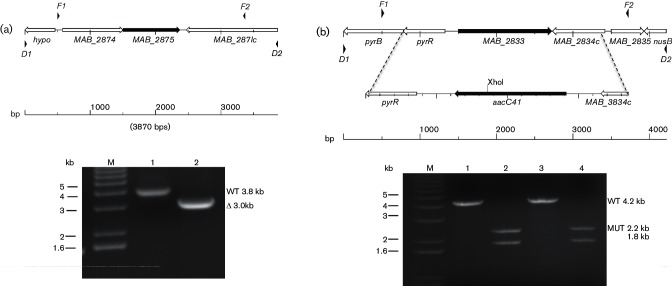
While allelic exchange in M. abscessus is similar to that seen with other mycobacteria, the level of spontaneous kanamycin resistance is problematic since it obscures the kanamycin-resistant primary recombinants. Although the latter clones can be identified by subsequent screening for 2-DOG sensitivity, we sought to improve the first-generation vector by adding another marker that could be used to screen colonies on the initial primary selection media, along with kanamycin selection. We initially incorporated a mycobacterial codon-optimized GFP gene, which produces bright yellow–green colonies on solid media when carried on a multi-copy plasmid. At single copy however, we could only detect GFP+ clones if they were re-streaked onto plates and analysed using a fluorescent scanner (data not shown). We then decided to insert the E. coli lacZ gene in the next-generation vector and use it with the β-galactosidase substrate, X-gal, incorporated into the primary selection media. We inserted a cassette consisting of the lacZ gene, driven by the M. smegmatis major porin A promoter, into the first-generation galK vector to produce pMP1296, which was used for subsequent experiments. We tested the new vector by deleting the MAB_2875 gene, encoding the known M. abscessusβ-lactamase, as this would be a different locus to target. A marked deletion mutant had previously been made by another group using dsDNA electroporation, and the mutant has an ampicillin hypersusceptibility phenotype that can be screened on plates [18]. The galK lacZ plasmid pMP1270 has an in-frame deletion of MAB_2875, and was used with PM3044 (wild-type). This experiment yielded KmR clones at similar frequencies to those we had previously obtained with the ΔmmpL4b suicide plasmid, pMP1265, but we only obtained clones on 7H10 media lacking X-gal. Direct selection of kanamycin-resistant M. abscessus from electroporation mixtures was not possible on 7H10 media with X-gal. These early experiments also failed to produce primary recombinants. We then repeated the electroporations with plasmid DNA that had been pre-treated with UV radiation, as this has been shown to stimulate recombination [31], and saw that this almost doubled the number of initial KmR clones in an electroporation set with PM3044 and pMP1270 (average of 46 KmR clones per electroporation with UV-treated DNA versus 25 KmR clones per electroporation with untreated DNA, in triplicate for each) on media lacking X-gal. Furthermore, out of the KmR population, we found more true primary recombinants (2-DOGS) in the pool from UV-treated DNA (30 %) versus the pool from untreated DNA (10 %). We were also able to use the lacZ marker as a secondary screen to confirm the 2-DOG phenotype, which positively correlated, although the plates needed to be held for a day or two at room temperature or 4 °C to see a difference between LacZ+ and LacZ- clones. It is not possible to do this at the initial selection after electroporation, as we initially intended, but it can be done during the screening for 2-DOG susceptibility at the primary and secondary recombinant screening stages. From this electroporation set, we isolated the mutant PM3386 (ΔMAB_2875) identified on the basis of being 2-DOGR, KmS, and sensitive to ampicillin on patch plates. The identity of PM3386 was confirmed by PCR (see Fig. 4a, top, for the map, and Panel A, bottom, Lane 2).
Fig. 4.
Analysis of MAB_2875 and MAB_2833 allelic exchange mutants. (a), top: map of genomic region for MAB_2875. F1 and F2 are locations for primers used to amplify region for the construction of the deletion alleles, while D1 and D2 are the locations for primers used for diagnostic PCR. (a), bottom: PCR reactions using diagnostic primers D1 and D2 for MAB_2785 and DNA prepared from: Lane 1 (PM3044 MAB_2875+ parental strain), Lane 2 (PM3386 ΔMAB_2875 mutant). (b), top: map of genomic regions for MAB_2833 with inset showing the replacement of MAB_2833 with the aacC41 apramycin resistance cassette. F1 and F2 are locations for primers used to amplify region for the construction of the deletion alleles, while D1 and D2 are the locations for primers used for diagnostic PCR. Note the unique XhoI restriction endonuclease site in the aacC41 gene inserted into the MAB_2833 deletion. Products from the MAB_2833 PCR reactions were purified and the DNA subjected to XhoI digestion prior to electrophoresis. The mutant ΔMAB_2833::aacC41 amplicon (4.0 kb) will yield two fragments of 2.2 and 1.8 kb after XhoI digestion, while the wild-type amplicon will remain unaffected at 4.2 kb. (b), bottom: ethidum bromide-stained gel of PCR reactions using diagnostic primers D1 and D2 for MAB_2833 and DNA prepared from: Lane 1 (PM3044 MAB_2833+ parental strain), Lane 2 (PM3389 ΔMAB_2833 :: aacC41 mutant), Lane 3 (PM3386 ΔMAB_2875 MAB_2833+ parental) and Lane 4 (PM3395 ΔMAB_2875ΔMAB_2833 :: aacC41 double mutant 2.2 and 1.8 kb). M is the 1 kb DNA ladder marker lane.
In homology searches in the M. abscessus genome using the MAB_2875 protein, we found open reading frame, MAB_2833, which is annotated as a putative β-lactamase, belonging to the same InterPro family (APR012338) as the known β-lactamase MAB_2875. While there is only limited homology between the two proteins (15 %), we decided to explore this putative β-lactamase gene. Attempts to express this gene in E. coli or an M. smegmatis strain lacking β-lactamases were not successful, although we could overexpress MAB_2875 in both species and confer an ampicillin resistance phenotype (data not shown). We decided to construct a suicide vector containing a marked deletion of the gene in order to track the mutation. We chose an allele of the E. coli aacC4 gene, which encodes an aminoglycoside acetyltransferase with activity against both apramycin and kanamycin, as the marker. We had previously generated a mutant allele, aacC41, which does not confer kanamycin resistance but does confer apramycin resistance [25], and inserted the allele in place of MAB_2833 (Fig. 4b, top). We performed 2-DOG-mediated allelic exchange with the plasmid pMP1271 and PM3044 (wild-type) and PM3386 (ΔMAB_2875), and successfully isolated PM3389 (ΔMAB_2833 :: aacC41) and PM3395 (ΔMAB_2875 ΔMAB_2833 :: aacC41), which were confirmed by PCR (Fig. 4b, bottom).
We examined the susceptibility of our strains to ampicillin by MIC analysis (Table 3), and as expected, we found that wild-type is highly resistant, even in the presence of β-lactamase inhibitors such as sulbactam, tazobactam and clavulanic acid, although the MIC shifted from >256 to 256 in the presence of tazobactam and clavulanic acid. In contrast, the ampicillin susceptibility of M. smegmatis, which expresses a β-lactamase, BlaS, that is sensitive to inhibitors [32], shifted 4-, 8- and 16-fold with each of the inhibitors, respectively. The MAB_2875 enzyme has been shown to be sensitive to the novel inhibitor avibactam, but we could not test this as we were repeatedly unable to obtain the compound from the manufacturer [18]. The ampicillin MIC for the MAB_2875 deletion mutants shifted from >256 to 32 µg ml−1, and this could be complemented back to the wild-type phenotype by a multi-copy plasmid bearing the wild-type MAB_2875 gene (see Table 3, strains PM3404 versus PM3405). We also tested the mutants for the cefoxitin MIC, since this antibiotic is used to treat M. abscessus infections [33], and found no substantial change (Table 3).
Table 3. Ampicillin and cefoxitin susceptibility testing (MIC µg ml−1).
| Strain | Genotype | AMP* | AMP+S* | AMP+T* | AMP+C* | CEF |
|---|---|---|---|---|---|---|
| PM3044 | WT M. abscessus | >256 | >256 | 256 | 256 | 32 |
| mc2155 | WT M. smegmatis | 256 | 64 | 32 | 16 | nd |
| PM3386 | ΔMAB_2875 | 32 | nd | nd | nd | 16 |
| PM3389 | ΔMAB_2833 :: aacC41 | >256 | nd | nd | nd | 32 |
| PM3395 | ΔMAB_2875 ΔMAB_2833 :: aacC41 |
32 | nd | nd | nd | 32 |
| PM3404 | ΔMAB_2875/pMV261 | 8 | nd | nd | nd | nd |
| PM3405 | ΔMAB_2875/pMP1205 | >256 | nd | nd | nd | nd |
*Key: AMP, ampicillin; AMP+S, ampicillin with 4 µg ml−1 sulbactam; AMP+T, ampicillin with 4 µg ml−1 tazobactam;, AMP+C, ampicillin with 4 µg ml−1 clavulanic acid; CEF, cefoxitin; nd, not determined.
We then tested the wild-type parental strain PM3044, and the beta-lactamase mutants PM3386 (ΔMAB_2875) and PM3395 (ΔMAB_2875 ΔMAB_2833 :: aacC41) by disc diffusion analysis using the following antibiotics: kanamycin, rifampin, ciprofloxacin, isoniazid, ethambutol, streptomycin, trimethoprim, technoplainin and vancomycin, but saw no changes in susceptibility (data not shown). We also tested the mutants against various cell envelope-interacting agents. However, we found no phenotypic differences (either viable plate counts, colony size or colony morphology) between the wild-type and mutant strains when challenged with the detergent sodium dodecyl sulphate, the dyes malachite green and congo red, or lysozyme (data not shown).
The results of our analyses of the MAB_2875 β-lactamase mutants in this article are in agreement with a previous report that MAB_2875 is the primary β-lactamase in this species, is resistant to standard inhibitors and its loss primarily affects susceptibility to penicillins and some of the cephalosporin antibiotics [18]. We also found that loss of the putative β-lactamase MAB_2833 resulted in no discernable phenotype under the conditions tested. Given that deletion of the MAB_2875 gene results in loss of detectable β-lactamase activity [18], it would seem that the MAB_2833 gene is either not expressed or it does not encode a β-lactamase. Mycobacterial genomes typically encode a number of genes annotated as potential β-lactamase/esterase enzymes [34], and M. abscessus is no exception, with six additional genes besides MAB_2875 and MAB_2833, that are putative β-lactamases [24]. Some of these may peptidoglycan carboxypeptidases, as β-lactamases and carboxypeptidases share a common ancestor [35]. However, we could find no cell wall phenotype in the MAB_2833 mutants, suggesting that the gene may not encode a peptidoglycan carboxypeptidase, that it may be a redundant enzyme that is dispensable or that it plays no role in cell wall biology.
We have demonstrated that suicide plasmid-mediated allelic exchange using galK as a counterselectable marker is possible in M. abscessus, that suicide vector-mediated allelic exchange with this species is as efficient as it is in most other mycobacterial species, and is best augmented by using UV-treated plasmid DNA for the initial recombination event. In addition, our results suggest that using this method for the generation of rough mutants in best done by targeting GPL biosynthesis, rather than targeting the GPL transporter.
Funding information
This work was supported by a University of Rochester Respiratory Pathogen Research Center Innovation Award from the NIAID/NIH/HHS contract No. HHSN272201200005C (M. S. P., J. B.), and NIAID/NIH/HHS grant AI500145 (M. S. P., S. A. G).
Acknowledgements
The authors would like to acknowledge Janice Spence for the construction of pMP731, and Justin Kim for the construction of plasmid pMP1205.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: 2-DOG, 2-deoxy-galactose; ADS, albumin-dextrose-saline; GPL, glycopeptidolipid; LB, Luria-Bertani; MIC, minimum inhibitory concentration.
Edited by: K. M. Dobos and S. V. Gordon
References
- 1.Saleeb P, Olivier KN. Pulmonary nontuberculous mycobacterial disease: new insights into risk factors for susceptibility, epidemiology, and approaches to management in immunocompetent and immunocompromised patients. Curr Infect Dis Rep. 2010;12:198–203. doi: 10.1007/s11908-010-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan ED, Bai X, Kartalija M, Orme IM, Ordway DJ. Host immune response to rapidly growing mycobacteria, an emerging cause of chronic lung disease. Am J Respir Cell Mol Biol. 2010;43:387–393. doi: 10.1165/rcmb.2009-0276TR. [DOI] [PubMed] [Google Scholar]
- 3.Kendall BA, Winthrop KL. Update on the epidemiology of pulmonary nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:87–94. doi: 10.1055/s-0033-1333567. [DOI] [PubMed] [Google Scholar]
- 4.Besada E. Rapid growing mycobacteria and TNF-α blockers: case report of a fatal lung infection with Mycobacterium abscessus in a patient treated with infliximab, and literature review. Clin Exp Rheumatol. 2011;29:705–707. [PubMed] [Google Scholar]
- 5.Jönsson BE, Gilljam M, Lindblad A, Ridell M, Wold AE, et al. Molecular epidemiology of Mycobacterium abscessus, with focus on cystic fibrosis. J Clin Microbiol. 2007;45:1497–1504. doi: 10.1128/JCM.02592-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanguinetti M, Ardito F, Fiscarelli E, La Sorda M, D'Argenio P, et al. Fatal pulmonary infection due to multidrug-resistant Mycobacterium abscessus in a patient with cystic fibrosis. J Clin Microbiol. 2001;39:816–819. doi: 10.1128/JCM.39.2.816-819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winthrop KL, Chang E, Yamashita S, Iademarco MF, Lobue PA. Nontuberculous mycobacteria infections and anti-tumor necrosis factor-α therapy. Emerg Infect Dis. 2009;15:1556–1561. doi: 10.3201/eid1510.090310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malcolm KC, Nichols EM, Caceres SM, Kret JE, Martiniano SL, et al. Mycobacterium abscessus induces a limited pattern of neutrophil activation that promotes pathogen survival. PLoS One. 2013;8:e57402. doi: 10.1371/journal.pone.0057402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nessar R, Reyrat JM, Davidson LB, Byrd TF. Deletion of the mmpL4b gene in the Mycobacterium abscessus glycopeptidolipid biosynthetic pathway results in loss of surface colonization capability, but enhanced ability to replicate in human macrophages and stimulate their innate immune response. Microbiology. 2011;157:1187–1195. doi: 10.1099/mic.0.046557-0. [DOI] [PubMed] [Google Scholar]
- 10.Roux AL, Ray A, Pawlik A, Medjahed H, Etienne G, et al. Overexpression of proinflammatory TLR-2-signalling lipoproteins in hypervirulent mycobacterial variants. Cell Microbiol. 2011;13:692–704. doi: 10.1111/j.1462-5822.2010.01565.x. [DOI] [PubMed] [Google Scholar]
- 11.Pawlik A, Garnier G, Orgeur M, Tong P, Lohan A, et al. Identification and characterization of the genetic changes responsible for the characteristic smooth-to-rough morphotype alterations of clinically persistent Mycobacterium abscessus. Mol Microbiol. 2013;90:612–629. doi: 10.1111/mmi.12387. [DOI] [PubMed] [Google Scholar]
- 12.Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother. 2012;67:810–818. doi: 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- 13.Bardarov S, Bardarov S, Pavelka MS, Sambandamurthy V, Larsen M, et al. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology. 2002;148:3007–3017. doi: 10.1099/00221287-148-10-3007. [DOI] [PubMed] [Google Scholar]
- 14.Pavelka MS, Jacobs WR. Comparison of the construction of unmarked deletion mutations in Mycobacterium smegmatis, Mycobacterium bovis bacillus Calmette-Guérin, and Mycobacterium tuberculosis H37Rv by allelic exchange. J Bacteriol. 1999;181:4780–4789. doi: 10.1128/jb.181.16.4780-4789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Kessel JC, Hatfull GF. Recombineering in Mycobacterium tuberculosis. Nat Methods. 2007;4:147–152. doi: 10.1038/nmeth996. [DOI] [PubMed] [Google Scholar]
- 16.Medjahed H, Reyrat JM. Construction of Mycobacterium abscessus defined glycopeptidolipid mutants: comparison of genetic tools. Appl Environ Microbiol. 2009;75:1331–1338. doi: 10.1128/AEM.01914-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi GE, Shin SJ, Won CJ, Min KN, Oh T, et al. Macrolide treatment for Mycobacterium abscessus and Mycobacterium massiliense infection and inducible resistance. Am J Respir Crit Care Med. 2012;186:917–925. doi: 10.1164/rccm.201111-2005OC. [DOI] [PubMed] [Google Scholar]
- 18.Dubée V, Bernut A, Cortes M, Lesne T, Dorchene D, et al. β-Lactamase inhibition by avibactam in Mycobacterium abscessus. J Antimicrob Chemother. 2015;70:1051–1058. doi: 10.1093/jac/dku510. [DOI] [PubMed] [Google Scholar]
- 19.Martinelli DJ, Pavelka MS. The RipA and RipB peptidoglycan endopeptidases are individually nonessential to Mycobacterium smegmatis. J Bacteriol. 2016;198:1464–1475. doi: 10.1128/JB.00059-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rominski A, Roditscheff A, Selchow P, Böttger EC, Sander P. Intrinsic rifamycin resistance of Mycobacterium abscessus is mediated by ADP-ribosyltransferase MAB_0591. J Antimicrob Chemother. 2017;72:376–384. doi: 10.1093/jac/dkw466. [DOI] [PubMed] [Google Scholar]
- 21.Alper MD, Ames BN. Positive selection of mutants with deletions of the gal-chl region of the Salmonella chromosome as a screening procedure for mutagens that cause deletions. J Bacteriol. 1975;121:259–266. doi: 10.1128/jb.121.1.259-266.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, et al. Current Protocols in Molecular Biology. New York: Greene Publishing Associates and Wiley-Interscience; 1987. [Google Scholar]
- 23.Pavelka MS, Jacobs WR. Biosynthesis of diaminopimelate, the precursor of lysine and a component of peptidoglycan, is an essential function of Mycobacterium smegmatis. J Bacteriol. 1996;178:6496–6507. doi: 10.1128/jb.178.22.6496-6507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ripoll F, Pasek S, Schenowitz C, Dossat C, Barbe V, et al. Non mycobacterial virulence genes in the genome of the emerging pathogen Mycobacterium abscessus. PLoS One. 2009;4:e5660. doi: 10.1371/journal.pone.0005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Consaul SA, Pavelka MS. Use of a novel allele of the Escherichia coli aacC4 aminoglycoside resistance gene as a genetic marker in mycobacteria. FEMS Microbiol Lett. 2004;234:297–301. doi: 10.1111/j.1574-6968.2004.tb09547.x. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Lian LL, Wan L, Zhang J, Zhao X, et al. Antimicrobial susceptibility of standard strains of nontuberculous mycobacteria by microplate alamar blue assay. PLoS One. 2013;8:e84065. doi: 10.1371/journal.pone.0084065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barkan D, Stallings CL, Glickman MS. An improved counterselectable marker system for mycobacterial recombination using galK and 2-deoxy-galactose. Gene. 2011;470:31–36. doi: 10.1016/j.gene.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelicic V, Jackson M, Reyrat JM, Jacobs WR, Gicquel B, et al. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1997;94:10955–10960. doi: 10.1073/pnas.94.20.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sander P, Meier A, Böttger EC. rpsL+: a dominant selectable marker for gene replacement in mycobacteria. Mol Microbiol. 1995;16:991–1000. doi: 10.1111/j.1365-2958.1995.tb02324.x. [DOI] [PubMed] [Google Scholar]
- 30.Ripoll F, Deshayes C, Pasek S, Laval F, Beretti JL, et al. Genomics of glycopeptidolipid biosynthesis in Mycobacterium abscessus and M. chelonae. BMC Genomics. 2007;8:114. doi: 10.1186/1471-2164-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinds J, Mahenthiralingam E, Kempsell KE, Duncan K, Stokes RW, et al. Enhanced gene replacement in mycobacteria. Microbiology. 1999;145:519–527. doi: 10.1099/13500872-145-3-519. [DOI] [PubMed] [Google Scholar]
- 32.Flores AR, Parsons LM, Pavelka MS. Genetic analysis of the beta-lactamases of Mycobacterium tuberculosis and Mycobacterium smegmatis and susceptibility to beta-lactam antibiotics. Microbiology. 2005;151:521–532. doi: 10.1099/mic.0.27629-0. [DOI] [PubMed] [Google Scholar]
- 33.Brown-Elliott BA, Wallace RJ. Rapidly growing myobacteria. In: Schlossberg D, editor. Tuberculosis and Nontuberculous Mycobacterial Infections. Washingron, DC: ASM Press; 2011. pp. 565–577. (editor) [Google Scholar]
- 34.Slayden RA, Jackson M, Zucker J, Ramirez MV, Dawson CC, et al. Updating and curating metabolic pathways of TB. Tuberculosis. 2013;93:47–59. doi: 10.1016/j.tube.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galleni M, Raquet X, Lamotte-Brasseur J, Fonzé E, Amicosante G, et al. DD-peptidases and β-lactamases: catalytic mechanisms and specificities. J Chemother. 1995;7:3–7. doi: 10.1179/joc.1995.7.1.3. [DOI] [PubMed] [Google Scholar]
- 36.Snapper SB, Melton RE, Mustafa S, Kieser T, Jacobs WR. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol. 1990;4:1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 37.Stover CK, de La Cruz VF, Fuerst TR, Burlein JE, Benson LA, et al. New use of BCG for recombinant vaccines. Nature. 1991;351:456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]