Abstract
MtrAB is a highly conserved two-component system implicated in the regulation of cell division in the Actinobacteria. It coordinates DNA replication with cell division in the unicellular Mycobacterium tuberculosis and links antibiotic production to sporulation in the filamentous Streptomyces venezuelae. Chloramphenicol biosynthesis is directly regulated by MtrA in S. venezuelae and deletion of mtrB constitutively activates MtrA and results in constitutive over-production of chloramphenicol. Here we report that in Streptomyces coelicolor, MtrA binds to sites upstream of developmental genes and the genes encoding ActII-1, ActII-4 and RedZ, which are cluster-situated regulators of the antibiotics actinorhodin (Act) and undecylprodigiosin (Red). Consistent with this, deletion of mtrB switches on the production of Act, Red and streptorubin B, a product of the Red pathway. Thus, we propose that MtrA is a key regulator that links antibiotic production to development and can be used to upregulate antibiotic production in distantly related streptomycetes.
Keywords: Streptomyces, antibiotics, sporulation, cryptic gene clusters
The multicellular filamentous bacteria in the genus Streptomyces have complex life cycles and make numerous specialized metabolites, including more than half of all known antibiotics [1]. Most of these antibiotics were discovered more than 50 years ago, but the alarming rise in antimicrobial resistance over the last five decades has driven a resurgence of interest in Streptomyces natural products in the 21st century. This has largely been driven by genome sequencing, which has revealed that up to 90 % of the specialized metabolites encoded by Streptomyces strains are not produced under laboratory conditions [2]. The environmental signals and signal transduction systems controlling expression of their specialized metabolite biosynthetic gene clusters (BGCs) are poorly understood, which is why most of them remain cryptic. However, antibiotic production has long been known to be linked to the differentiation of actively growing substrate mycelium into aerial mycelium and spores, the equivalent of cell division in unicellular bacteria [1]. Identification and manipulation of the global signalling pathways that control these processes could therefore enable the discovery of new and useful natural products, or be used to make antibiotic overproducing strains for industry. To this end, we recently characterized a two-component system called MtrAB in Streptomyces venezuelae [3]. The MtrAB two-component system is highly conserved in the phylum Actinobacteria and is best characterized in M. tuberculosis, where it coordinates DNA replication with cell division [4, 5]. We reported that MtrA coordinates antibiotic production with sporulation and that deletion of the sensor kinase gene mtrB results in constitutively active MtrA and constitutive high-level production of chloramphenicol, as well as a global shift in the metabolome of S. venezuelae [3].
In this work, we characterized MtrAB in another streptomycete, Streptomyces coelicolor, a model species that makes the pigmented antibiotics actinorhodin (Act) and undecylprodigiosin (Red). The 16S rDNA phylogenetic tree of the family Streptomycetaceae shows that S. venezuelae (clade 40) is highly divergent from S. coelicolor (clade 112), which is why we chose to characterize the system in these distantly related species [6]. Unlike S. venezuelae, S. coelicolor does not sporulate in liquid culture, but grows as a vegetative mycelium, and this further enabled us to examine the role of MtrA during vegetative growth. We previously isolated an in-frame unmarked ∆mtrA mutant in S. coelicolor and reported that expression of the mce operon is reduced in this background [7]. For this study, we made further in-frame deletions in the mtrB and lpqB genes using Redirect PCR targeting and Flp recombinase [8] (Table S1, available in the online Supplementary Material). LpqB is an accessory lipoprotein that interacts with and reduces MtrB activity in M. smegmatis, and deletion of lpqB results in a filamentous strain that is reminiscent of streptomycetes and suggestive of a defect in cell division [9]. In-frame deletion of S. coelicolor lpqB had no visible effect on growth or development, but in-frame deletion of mtrB resulted in a small colony phenotype and a delay in sporulation, as judged by visible late production of the brown WhiE spore pigment in these colonies (Fig. S1). In trans complementation was attempted by introducing the relevant gene into the phiBT1 site on the integrative vector pMS82, under the control of the mtrA operon promoter [10]. This restored the wild-type phenotype to the ∆mtrB mutant (Fig. S1).
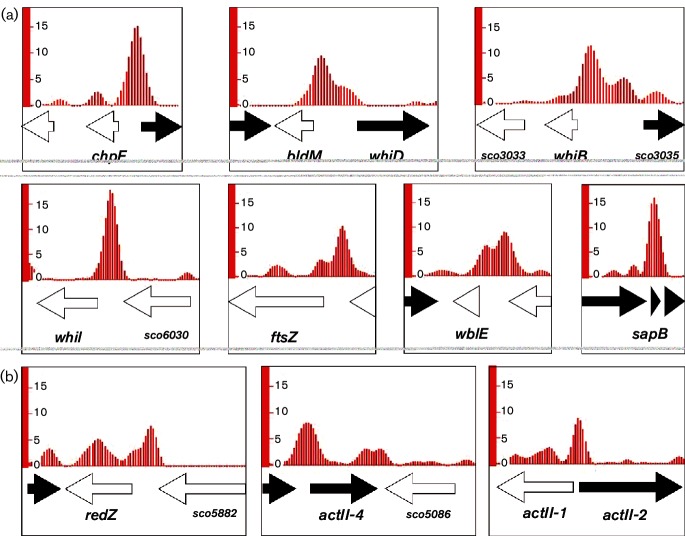
Unlike S. venezuelae, S. coelicolor does not sporulate in liquid culture, but grows as a vegetative mycelium, and this allowed us to use chromatin immunoprecipitation followed by sequencing (ChIP-seq) to identify MtrA targets in vegetatively growing Streptomyces and to compare these targets to those identified in differentiating S. venezuelae. To determine where MtrA binds on the S. coelicolor genome we introduced a construct expressing MtrA-3xFlag under the control of the mtrA promoter into the phiBT1 site of the previously isolated ∆mtrA mutant and performed ChIP-seq on cultures of this strain grown for 16 and 20 h in liquid maltose–yeast extract/malt extract (MYM) medium [11] with the wild-type as a control. ChIP-seq was performed as described previously and Bowtie was used to generate plots that could be visualized using Integrated Genome Browser [12, 13]. A full list of targets for the 16 and 20 h samples are given in Table S2 (NCBI Geo database accession number: GSE84311). The developmental and secondary metabolism genes bound by MtrA in both S. venezuelae NRRL B-65442 and S. coelicolor M145 are listed in Table 1. Many of the developmental genes bound by MtrA in S. venezuelae were not enriched in the S. coelicolor data, most likely because S. coelicolor is growing vegetatively and most specialized metabolite BGCs are not conserved between these species [3]. It is interesting that the promoter of the ectABCD operon is highly enriched in MtrA ChIP-seq experiments in both S. coelicolor and S. venezuelae. In the latter it was the most highly enriched target in the entire dataset [3]. This BGC encodes for the osmolytes ectoine and 5′ hydroxyectoine, but we could not detect either compound in the wild-type or ∆mtrB strains, suggesting that MtrA may repress ectABCD. One of the conserved targets that is particularly worth noting is CdgB, which makes the secondary messenger cyclic di-GMP that controls the activity of the master regulator of differentiation BldD [14]. The bldD gene is an MtrA target in S. coelicolor but not in S. venezuelae, at least under the conditions used for these experiments (Table S2)[3]. Additional conserved targets include WhiB, WhiD and WblE, which are all members of the WhiB-like (Wbl) family of Fe–S-containing transcription factors that are restricted to actinobacteria (Fig. 1a and Table 1). WhiB and WhiD regulate early- and late-stage sporulation, respectively [15, 16]. WblE is essential in S. coelicolor and M. tuberculosis, and although its function is still unknown this suggests that it must play a key role in their life cycles [17].
Table 1. Developmental and secondary metabolism genes bound by MtrA in vegetatively growing Streptomyces coelicolor M145 and differentiating Streptomyces venezuelae NRRL B-65442 [3].
| Gene name | Gene number | Function | Reference |
|---|---|---|---|
| cdgB | sco4281 | Cyclic di-GMP metabolism | [21] |
| bldM | sco4768 | Orphan RR, forms homo- and heterodimers with WhiI to regulate differentiation, encoded divergently from whiD. | [22, 23] |
| chpF | sco2705 | Surfactant required for aerial hyphae formation | [24] |
| sapB | sco6682 | Surfactant required for aerial hyphae formation | [25] |
| filP | sco5396 | Filament forming protein involves in hyphal growth | [26] |
| ftsZ | sco2082 | FtsZ is a tubulin homologue and forms Z rings to mark the sites of cell division | [27] |
| smc | sco5577 | Structural maintenance of chromosomes | [28] |
| wblE | sco5240 | Essential WhiB-like (Wbl) protein and transcription factor | [17] |
| whiB | sco3034 | Wbl protein that regulates early-stage sporulation | [29] |
| whiD | sco4767 | Wbl protein that regulates late sporulation, encoded divergently from bldM | [15] |
| whiI | sco6029 | Orphan RR, forms heterodimers with BldM to regulate differentiation | [23] |
| ectABCD | sco1864 | Biosynthesis of the secondary metabolites ectoine and 5′ hydroxyectoine | [30] |
Fig. 1.
(a) MtrA ChIP peaks upstream of the S. coelicolor M145 developmental genes chpF, the divergent bldM and whiD, whiB, whiI, ftsZ, wblE and sapB. (b). MtrA ChIP peaks upstream of the cluster-situated regulatory genes redZ (undecylprodigiosin), actII-1 and actII-4 (actinorhodin). The y-axis gives the enrichment value relative to the surrounding region of 4000 nucleotides.
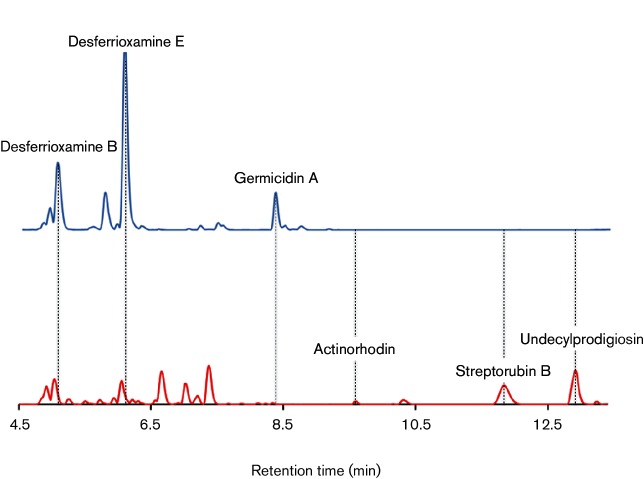
S. coelicolor is the best characterized Streptomyces species in terms of its specialized metabolites and their BGCs [18, 19], and the ChIP-seq data show that genes encoding the cluster-specific regulators for Act and Red are enriched (Fig. 1b). RedZ and ActII-4 are activators and ActII-1 is a repressor. The divergently encoded ActII-2 is a putative transporter for Act. The ∆mtrB mutant produces more pigments than the wild-type, suggesting that MtrA may activate production of Act and/or Red (Figs 2 and S1). To test this, we grew the S. coelicolor wild-type and ∆mtrB mutants in biological triplicates and extracted the whole broth with methanol. The resulting supernatants were analysed by UPLC-HRMS (see the Supplementary Material for the methods used) and the results showed that while the S. coelicolor ∆mtrB mutant produces Act and Red, they are not detectable in the wild-type strain in liquid medium (Fig. 2). Given that MtrA is likely to be constitutively active in the absence of MtrB, it is possible that MtrA directly activates the production of Act and Red. We did not perform expression studies on the ∆mtrA mutant because we could not fully complement the mutation. However, we have confidence in the ChIP-seq data because MtrA-3xFlag rescues an S. venezuelae ∆mtrA mutant and because many of the MtrA targets we identified in S. coelicolor ∆mtrA+MtrA-3xFlag are conserved MtrA targets in S. venezuelae (Table 1) [3]. We also detected significant amounts of streptorubin B in the ∆mtrB cultures, a specialized metabolite encoded by the Red biosynthetic pathway [20]. The production of the siderophores desferrioxamine B and E is reduced in the ∆mtrB mutant and we could not detect germicidin A (Fig. 2), although the BGCs encoding the production of these compounds are not bound by MtrA (Table S2).
Fig. 2.
Representative UPLC-HRMS traces of culture extracts of wild-type S. coelicolor M145 (top) and the isogenic ΔmtrB mutant (bottom) are shown for comparison. The y-axes represent the total ion count and are normalized. The x-axis indicate retention time and refers to both traces. The siderophores desferrioxamines A and B were down-regulated and germicidin A was not detected in the ΔmtrB mutant, while actinorhodin, undecylprodigiosin and streptorubin B were produced in the absence of MtrB but were not detectable in the wild-type extracts.
In conclusion, we have demonstrated that the MtrAB two-component system helps control antibiotic production in the distantly related S. coelicolor and S. venezuelae, and binds to developmental genes in both vegetatively and developmentally growing species. We have also shown that deletion of mtrB or manipulation of MtrA activity can be used to increase antibiotic production in these Streptomyces species. Given the fact that MtrAB is conserved in all Streptomyces species and in other filamentous actinomycetes, we suggest that manipulation of MtrA activity could be a general tool for upregulating antibiotic production in these bacteria.
Funding information
This research was supported by a BBSRC PhD studentship to N. S., a UEA-funded PhD studentship to F. K., a Medical Research Council grant G0801721 to M. I. H. and Natural Environment Research Council responsive mode grants NE/M015033/1 and NE/M014657/1 to M. I. H. and B. W. An earlier version of this work was published as a preprint on bioRxiv [31].
Acknowledgements
We thank Elaine Patrick for excellent technical support and Mark Buttner and Matt Bush for useful discussions.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Supplementary Data
Supplementary Data
Footnotes
Abbreviations: Act, actinorhodin; BGC, biosynthetic gene cluster; ChIP-seq, chromatin immunoprecipitation followed by sequencing; Red, undecylprodigiosin.
ChIP-seq NCBI Geo database accession number =GSE84311.
One supplementary figure and two supplementary tables are available with the online Supplementary Material.
Edited by: S. Gebhard and F. Sargent
References
- 1.van der Meij A, Worsley SF, Hutchings MI, van Wezel GP. Chemical ecology of antibiotic production by actinomycetes. FEMS Microbiol Rev. 2017;41:392–416. doi: 10.1093/femsre/fux005. [DOI] [PubMed] [Google Scholar]
- 2.Devine R, Hutchings MI, Holmes NA. Future directions for the discovery of antibiotics from actinomycete bacteria. Emerg Top Life Sci. 2017;1:1–12. doi: 10.1042/ETLS20160014. [DOI] [PubMed] [Google Scholar]
- 3.Som NF, Heine D, Holmes NA, Munnoch JT, Chandra G, et al. The conserved actinobacterial two-component system MtrAB coordinates chloramphenicol production with sporulation in Streptomyces venezuelae NRRL B-65442. Front Microbiol. 2017;8:1237–11. doi: 10.3389/fmicb.2017.01145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoskisson PA, Hutchings MI. MtrAB–LpqB: a conserved three-component system in actinobacteria? Trends Microbiol. 2006;14:444–449. doi: 10.1016/j.tim.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Purushotham G, Sarva KB, Blaszczyk E, Rajagopalan M, Madiraju MV. Mycobacterium tuberculosis oriC sequestration by MtrA response regulator. Mol Microbiol. 2015;98:586–604. doi: 10.1111/mmi.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labeda DP, Goodfellow M, Brown R, Ward AC, Lanoot B, et al. Phylogenetic study of the species within the family Streptomycetaceae. Antonie van Leeuwenhoek. 2012;101:73–104. doi: 10.1007/s10482-011-9656-0. [DOI] [PubMed] [Google Scholar]
- 7.Clark LC, Seipke RF, Prieto P, Willemse J, van Wezel GP, et al. Mammalian cell entry genes in Streptomyces may provide clues to the evolution of bacterial virulence. Sci Rep. 2013;3:1109. doi: 10.1038/srep01109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gust B, Challis GL, Fowler K, Kieser T, Chater KF. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc Natl Acad Sci USA. 2003;100:1541–1546. doi: 10.1073/pnas.0337542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen HT, Wolff KA, Cartabuke RH, Ogwang S, Nguyen L. A lipoprotein modulates activity of the MtrAB two-component system to provide intrinsic multidrug resistance, cytokinetic control and cell wall homeostasis in Mycobacterium. Mol Microbiol. 2010;76:348–364. doi: 10.1111/j.1365-2958.2010.07110.x. [DOI] [PubMed] [Google Scholar]
- 10.Gregory MA, Till R, Smith MC. Integration site for Streptomyces phage φBT1 and development of site-specific integrating vectors. J Bacteriol. 2003;185:5320–5323. doi: 10.1128/JB.185.17.5320-5323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA. Practical Streptomyces Genetics. John Innes Foundation; Norwich: 2000. [Google Scholar]
- 12.Munnoch JT, Martinez MTP, Svistunenko DA, Crack JC, Le Brun NE, et al. Characterization of a putative NsrR homologue in Streptomyces venezuelae reveals a new member of the Rrf2 superfamily. Sci Rep. 2016;6:29495. doi: 10.1038/srep31597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicol JW, Helt GA, Blanchard SG, Raja A, Loraine AE. The Integrated Genome Browser: free software for distribution and exploration of genome-scale datasets. Bioinformatics. 2009;25:2730–2731. doi: 10.1093/bioinformatics/btp472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tschowri N, Schumacher MA, Schlimpert S, Chinnam NB, Findlay KC, et al. Tetrameric c-di-GMP mediates effective transcription factor dimerization to control Streptomyces development. Cell. 2014;158:1136–1147. doi: 10.1016/j.cell.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molle V, Palframan WJ, Findlay KC, Buttner MJ. WhiD and WhiB, homologous proteins required for different stages of sporulation in Streptomyces coelicolor A3(2) J Bacteriol. 2000;182:1286–1295. doi: 10.1128/JB.182.5.1286-1295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bush MJ, Bibb MJ, Chandra G, Findlay KC, Buttner MJ. Genes required for aerial growth, cell division, and chromosome segregation are targets of WhiA before sporulation in Streptomyces venezuelae. MBio. 2013;4:e00684-13. doi: 10.1128/mBio.00684-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fowler-Goldsworthy K, Gust B, Mouz S, Chandra G, Findlay KC, et al. The actinobacteria-specific gene wblA controls major developmental transitions in Streptomyces coelicolor A3(2) Microbiology. 2011;157:1312–1328. doi: 10.1099/mic.0.047555-0. [DOI] [PubMed] [Google Scholar]
- 18.Challis GL. Exploitation of the Streptomyces coelicolor A3(2) genome sequence for discovery of new natural products and biosynthetic pathways. J Ind Microbiol Biotechnol. 2014;41:219–232. doi: 10.1007/s10295-013-1383-2. [DOI] [PubMed] [Google Scholar]
- 19.Van Keulen G, Dyson PJ. Production of specialized metabolites by Streptomyces coelicolor A3(2) Adv Applied Microbiol. 2014;89:217–266. doi: 10.1016/B978-0-12-800259-9.00006-8. [DOI] [PubMed] [Google Scholar]
- 20.Withall DM, Haynes SW, Challis GL. Stereochemistry and mechanism of undecylprodigiosin oxidative carbocyclization to streptorubin B by the rieske oxygenase RedG. J Am Chem Soc. 2015;137:7889–7897. doi: 10.1021/jacs.5b03994. [DOI] [PubMed] [Google Scholar]
- 21.Tran NT, Den Hengst CD, Gomez-Escribano JP, Buttner MJ. Identification and characterization of CdgB, a diguanylate cyclase involved in developmental processes in Streptomyces coelicolor. J Bacteriol. 2011;193:3100–3108. doi: 10.1128/JB.01460-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molle V, Buttner MJ. Different alleles of the response regulator gene bldM arrest Streptomyces coelicolor development at distinct stages. Mol Microbiol. 2000;36:1265–1278. doi: 10.1046/j.1365-2958.2000.01977.x. [DOI] [PubMed] [Google Scholar]
- 23.Al-Bassam MM, Bibb MJ, Bush MJ, Chandra G, Buttner MJ. Response regulator heterodimer formation controls a key stage in Streptomyces development. PLoS Genet. 2014;10:e1004554. doi: 10.1371/journal.pgen.1004554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elliot MA, Karoonuthaisiri N, Huang J, Bibb MJ, Cohen SN, et al. The chaplins: a family of hydrophobic cell-surface proteins involved in aerial mycelium formation in Streptomyces coelicolor. Genes Dev. 2003;17:1727–1740. doi: 10.1101/gad.264403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capstick DS, Willey JM, Buttner MJ, Elliot MA. SapB and the chaplins: connections between morphogenetic proteins in Streptomyces coelicolor. Mol Microbiol. 2007;64:602–613. doi: 10.1111/j.1365-2958.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- 26.Holmes NA, Walshaw J, Leggett RM, Thibessard A, Dalton KA, et al. Coiled-coil protein Scy is a key component of a multiprotein assembly controlling polarized growth in Streptomyces. Proc Natl Acad Sci USA. 2013;110:E397. doi: 10.1073/pnas.1210657110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwedock J, Mccormick JR, Angert ER, Nodwell JR, Losick R. Assembly of the cell division protein FtsZ into ladder-like structures in the aerial hyphae of Streptomyces coelicolor. 1997;25:847–858. doi: 10.1111/j.1365-2958.1997.mmi507.x. [DOI] [PubMed] [Google Scholar]
- 28.Kois A, Swiatek M, Jakimowicz D, Zakrzewska-Czerwińska J. SMC protein-dependent chromosome condensation during aerial hyphal development in Streptomyces. J Bacteriol. 2009;191:310–319. doi: 10.1128/JB.00513-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis NK, Chater KF. The Streptomyces coelicolor whiB gene encodes a small transcription factor-like protein dispensable for growth but essential for sporulation. Mol Gen Genet. 1992;232:351–358. doi: 10.1007/BF00266237. [DOI] [PubMed] [Google Scholar]
- 30.Bursy J, Kuhlmann AU, Pittelkow M, Hartmann H, Jebbar M, et al. Synthesis and uptake of the compatible solutes ectoine and 5-hydroxyectoine by Streptomyces coelicolor A3(2) in response to salt and heat stresses. Appl Environ Microbiol. 2008;74:7286–7296. doi: 10.1128/AEM.00768-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Som NF, Heine D, Munnoch JT, Holmes NA, Knowles F, et al. MtrA is an essential regulator that coordinates antibiotic production and sporulation in Streptomyces species. bioRxiv. 2016 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.