Abstract
Renal cell carcinoma is the most common primary renal neoplasm in adults. Although renal cell carcinoma is known to spread to unusual sites, the ampulla of Vater is considered a rare site for metastasis. Here we present a case of renal cell carcinoma metastasized to the ampulla of Vater along with literature review. A 62-year-old Korean male had a history of hypertension and right-sided renal cell carcinoma diagnosed in September 2004, for which he underwent right radical nephrectomy in October 2004. The patient eventually underwent laparoscopic pylorus-preserving total pancreaticoduodenectomy in January 2017. The surgery was successful without postoperative complications. Previous studies have shown that surgical resection for solitary metastases of renal cell carcinoma can provide favorable survival rates. Our case report provides evidence that pancreaticoduodenectomy may be a treatment of choice for suitable patients with solitary renal cell carcinoma ampullary metastasis. A minimally invasive approach may result in early recovery of patient to be suitable for subsequent chemotherapy. Further evidence is needed to address the exact role of minimally invasive pancreaticoduodenectomy in renal cell carcinoma metastasized to the ampulla of Vater.
Keywords: Renal cell carcinoma, Pancreas metastasis, Laparoscopic, Pancreaticoduodenectomy
INTRODUCTION
Renal cell carcinoma is the most common primary renal neoplasm in adults.1 It accounts for approximately 2–4% of all adult malignancies.2,3 Surgery may be curative if the disease is localized. However, many patients eventually experience relapse. The most common metastasis sites for renal cell carcinoma are lungs, bones and liver.4 Although renal cell carcinoma is known to spread to unusual sites, the ampulla of Vater is considered a rare metastasis site. When this occurs, it may be indistinguishable from primary ampullary tumor during initial presentation. Interestingly, renal cell carcinoma is not the only malignancy known to spread to the ampulla of Vater. Melanomas,5,6 breast carcinomas,7,8 squamous cell carcinoma of the larynx,9 and cervical carcinoma10 can also spread to the ampulla of Vater. Despite advances in laparoscopic techniques and experiences, it remains controversial whether laparoscopic pancreaticoduodenectomy should be regarded as a safe and effective surgical approach for selected patients.11 In this case report, we present a patient with renal cell carcinoma metastasized to the ampulla of Vater who was successfully treated with laparoscopic pancreaticoduodenectomy. To the best of our knowledge, this is the first report of laparoscopic pancreaticoduodenectomy for renal cell carcinoma metastasized to the ampulla of Vater. We also provided a brief literature review.
CASE
Patient description
A 62-year-old Korean male patient presented with jaundice and dark urine in December 2016. He had a history of hypertension and right-sided renal cell carcinoma diagnosed in September 2004, for which he underwent right radical nephrectomy in October 2004. Pathological analysis reported a conventional type, Fuhrman nuclear grade 2 tumor with hemorrhage and sclerosis (T1N0M0). He had no known family history of renal malignancy. He did not smoke. He did not excessively drink alcohol either.
Preoperative findings
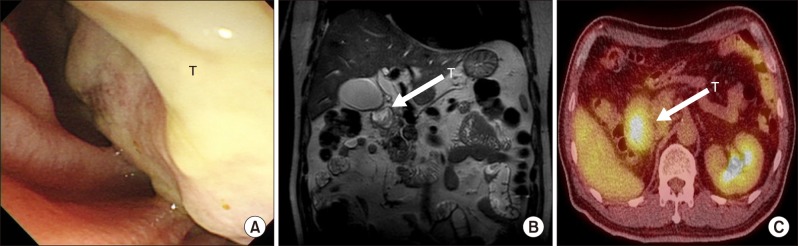
Physical examination did not yield any significant findings. Laboratory results showed the following: aspartate transaminase (AST), 169 U/L; alanine transaminase (ALT), 213 U/L; total bilirubin, 2.4 mg/dl; gamma glutamyl transferase (γ-GT), 992 U/L; lipase, 221 U/L; amylase, 155 U/L; alkaline phosphatase (ALP), 650 U/L; carcinoembryonic antigen (CEA), 4.21 ng/ml; and cancer antigen 19-9 (CA19-9), 10.9 U/ml. Magnetic resonance imaging (MRI) pancreaticobiliary, positron emission tomography-computed tomography (PET-CT), and endoscopic retrograde cholangiopancreatography (ERCP) showed a firm whitish mass from the duodenum superior duodenal angle to the ampulla of Vater (Fig. 1). Endoscopic ultrasound-fine needle aspiration (EUS-FNA) cytology analysis revealed clusters of atypical pancreaticobiliary epithelial cells suspicious for malignancy. The initial impression was ampulla of Vater cancer with duodenal and pancreatic extension. Differential diagnoses of duodenal cancer or pancreatic cancer were also considered.
Fig. 1. (A) Endoscopic view showing whitish firm mass from the superior duodenal angle to the ampulla of Vater. (B) Preoperative MRI pancreaticobiliary T2 weighted image showing a papillary shaped mass in the ampulla of Vater with duodenal and pancreas extension. (C) PET-CT showing increased FDG uptake of the tumor involving the duodenum and pancreatic head. T, tumor.
Operation and pathologic findings
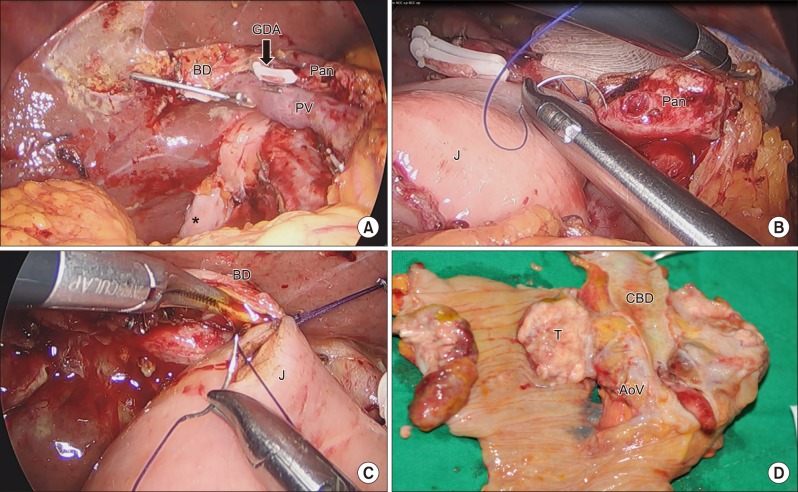
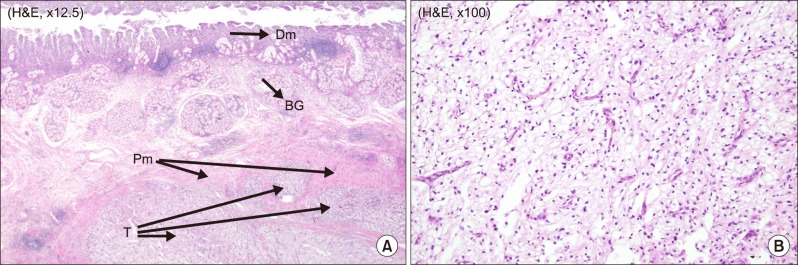
The patient underwent laparoscopic pylorus preserving total pancreaticoduodenectomy in January 2017. As a result of the previous operation, there were severe adhesions at retroperitoneum and previous renal vein ligation sites. The colon and duodenum were rotated due to the absence of the right kidney. It was difficult to dissect the superior mesenteric artery (SMA) lateral margin due to adhesion after the previous retroperitoneal space dissection. After the resection phase, pancreaticojejunostomy (duct-to-mucosa) and hepaticojejunosotmy were done via laparoscopic approach. Duodenojejunostomy was done through a small mini laparotomy around the umbilicus where surgical specimen needed to be removed. Operation time was 510 min and estimated blood loss was 400 ml. Gross findings for the specimen included a protruding mass on the ampulla of Vater (Fig. 2). Pathological examination revealed metastatic clear cell renal carcinoma involving the duodenum, ampulla of Vater, and pancreas (Fig. 3). The tumor size was 5.4 cm in maximum diameter. Lymphovascular invasion was noted without perineural invasion. All resection margins including the common bile duct, pancreatic duct, duodenum, and retroperitoneum were negative for carcinoma cells. Total retrieved lymph nodes were 13 without any positive nodes.
Fig. 2. Operative findings after resection phase. (A) Bile duct was clamped and the gastroduodenal artery (GDA) was ligated. (B) Reconstruction phase of laparoscopic pancreaticojejunostomy (duct-to-mucosa). (C) Reconstruction phase of laparoscopic hepaticojejunostomy. (D) Specimen gross finding showing the protruding mass on the ampulla of Vater. BD, bile duct; GDA, gastroduodenal artery; Pan, pancreas; PV, portal vein; J, jejunum; T, tumor; CBD, common bile duct; AoV, ampulla of Vater.
Fig. 3. Pathologic report. (A) Tumor invading the proper duodenum muscle layer (×12.5). (B) Characteristic appearance of renal clear cell carcinoma with clear cytoplasm arranged in nests (×100). Dm, Duodenum mucosa; BG, Brunner's gland; Pm, Proper muscle layer; T, Tumor.
Postoperative recovery
The length of hospital stay was 12 days postoperatively. During hospital stay, there were no significant postoperative complications. The patient was regularly followed up in Urology and Surgery Outpatient Departments to consider adjuvant chemotherapy.
Literature review
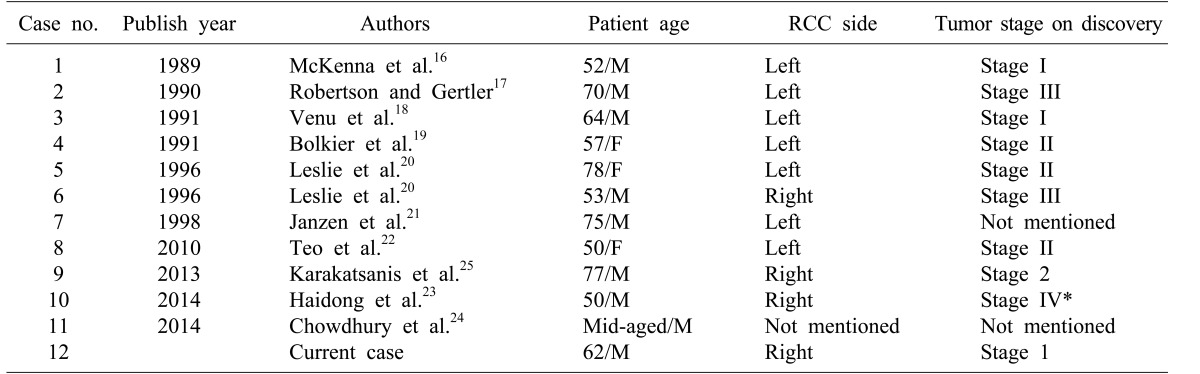
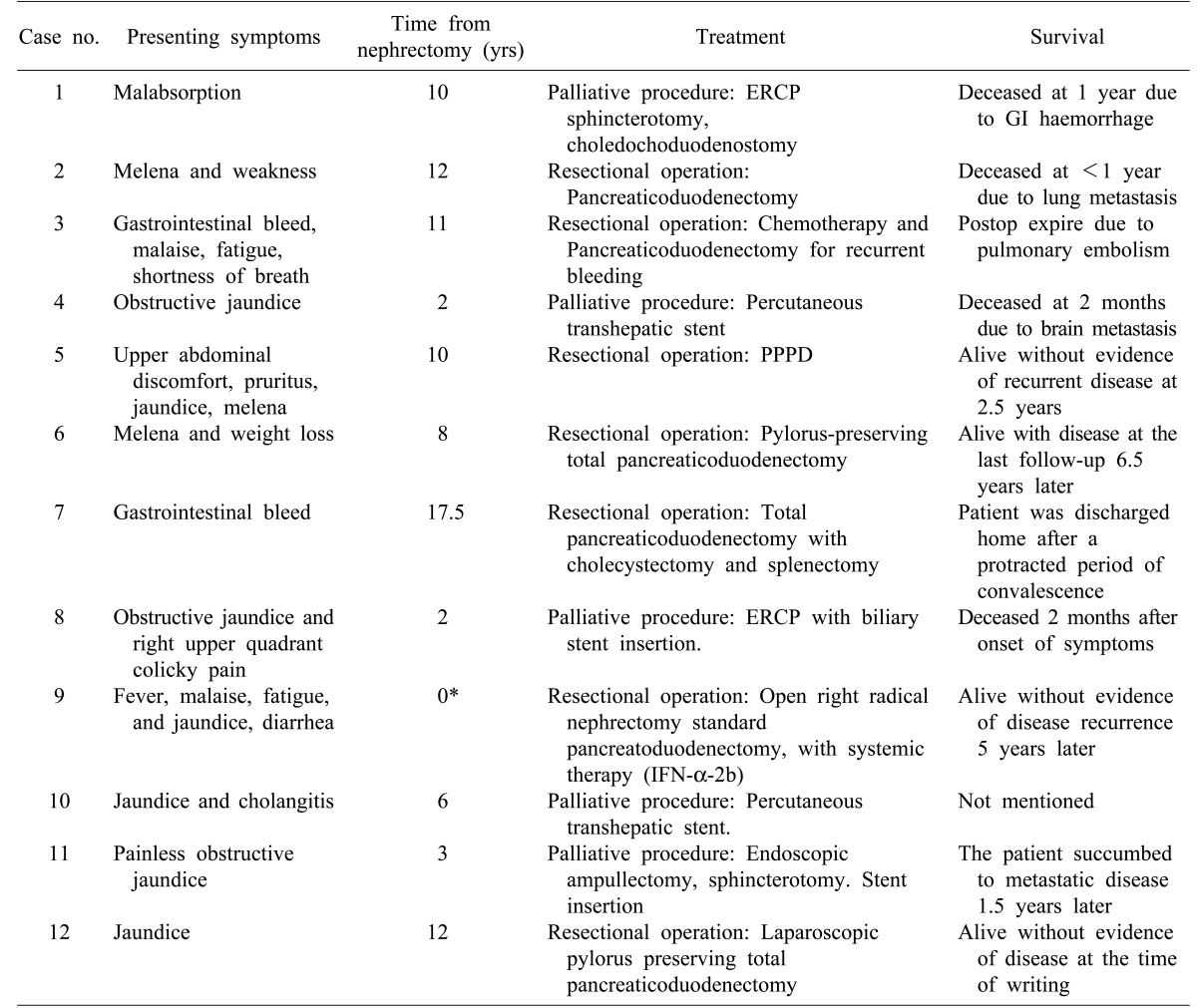
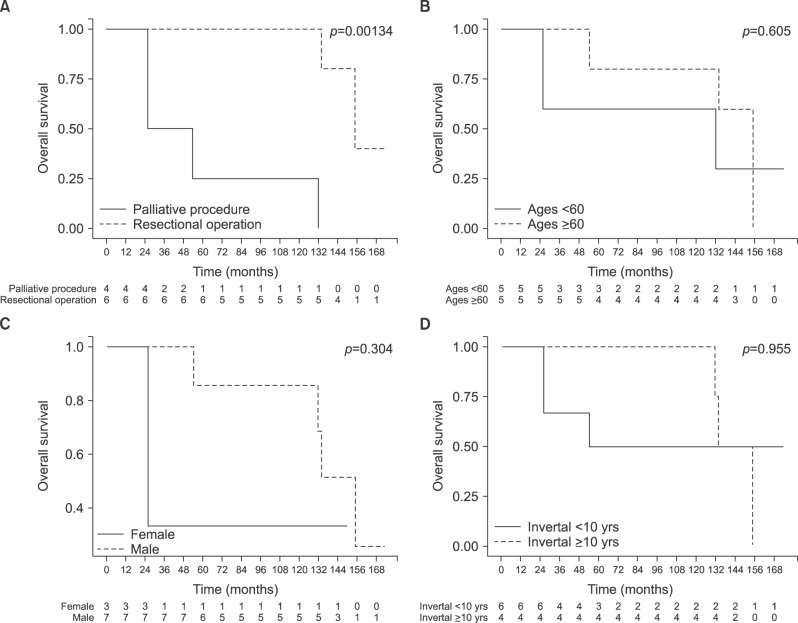
Based on literature review, a total of 11 patients with renal cell carcinoma metastasized to the ampulla of Vater have been reported (Table 1). Nine patients were males and three were females. Their mean age was 62.6±11.6 years. These patients had relatively early stage renal cell carcinoma when initial nephrectomy was performed. Mean time interval from initial nephrectomy to diagnosis of metastatic renal cell carcinoma to the ampulla of Vater was 82.5±64.9 months. The most common symptom was obstructive jaundice. Palliative procedures such as ERCP with sphincterotomy, endoscopic ampullectomy, percutaneous transhepatic biliary drainage with stent insertion, and choledochoduodenostomy were performed in five patients. Pancreaticoduodenectomy was performed in seven patients, including the present case (Table 2). Mean overall survival time for patients who had metastatic renal cell carcinoma to the ampulla of Vater from initial nephrectomy was 106.3±57.9 months (95% CI: 72.6–137.8 months), excluding two cases without mentioning the survival time. Mean survival after a resection for metastatic ampulla of Vater cancer was found to be superior to that with palliative management (137±40.1 months vs. 59.5±50.1 months; p=0.00134). However, there was no statistically significant difference in overall survival between the two groups according to age, gender, or interval time from initial nephrectomy to diagnosis of metastasis to the ampulla of Vater (Fig. 4).
Table 1. Cases of renal cell carcinoma metastasized to the ampulla of Vater.
*Metastatic cancer to the ampulla of Vater was discovered at the same time as RCC
RCC, Renal cell carcinoma
Table 2. Summary of specific symptoms, treatment, interval time to recurrence and overall survival in each case.
*Metastatic cancer to the ampulla of Vater was discovered at the same time as renal cell cancer
ERCP, endoscopic retrograde cholangiopancreatography; PPPD, pylorus-preserving pancreaticoduodenectomy; IFN, interferon
Fig. 4. Literature review-based oncologic outcome analysis of patients with metastatic renal cell carcinoma to the ampulla of Vater. (A) Overall survival between patients who underwent palliative procedures and those who underwent resectional operations. (B) Overall survival between the two groups of patients according to age. (C) Overall survival between the two groups of patients according to gender. (D) Overall survival between the two groups of patients according to the interval time from initial nephrectomy to diagnosis of metastasis.
DISCUSSION
Here we report a patient with right-sided renal cell carcinoma metastasized to the ampulla of Vater presenting with jaundice 12 years after initial treatment for renal malignancy. To the best of our knowledge, this is the first case of renal cell carcinoma metastasized to the ampulla of Vater treated with laparoscopic pylorus-preserving total pancreaticoduodenectomy among a handful of similar cases reported in the literature. Most patients had left-sided renal cell carcinoma. They were middle aged to elderly males. Based on our observation, the most common presentations were obstructive jaundice and gastrointestinal bleeding. Other presentations included malabsorption, fatigue, weight loss, abdominal pain/discomfort and recurrent cholangitis.
The mean time from initial nephrectomy to diagnosis of metastatic ampulla of Vater cancer was 82.5±64.9 months. The longest was 210 months. The mean time from initial nephrectomy to diagnosis of metastatic ampulla of Vater cancer is longer than that for metastases to other regions, most of which is within the first two to three years following nephrectomy.11 It is known that a prolonged interval between nephrectomy and appearance of metastasis is associated with better prognosis.12 Based on literature review, this trend was seen in most cases, but not in all cases. Except for two cases that did not mention the overall survival time, patients who underwent resectional operation for metastatic ampulla of Vater cancer had favorable long-term survival compared to those in the palliative management group. However, we could not find any statistically significant difference in survival according to gender, age, or interval time from initial nephrectomy to diagnosis of metastatic lesions. There was no association between initial stage of primary disease and interval time to recurrence either.
Solitary metastases of renal cell carcinoma have been treated successfully by surgical resection, achieving 5-year survival rates of 24%–60%.11,13,14 In one study of twelve patients who underwent pancreaticoduodenectomy for metastatic ampullary and pancreatic tumors, surgical resection for solitary metastases of renal cell carcinoma provided favorable survival rates.15 Based on literature review, most patients who received surgery for solitary metastasis to the ampulla of Vater had good post-operative survival. Due to the lack of data, we were unable to calculate the exact average survival in number of years. Nonetheless, we believe that pancreaticoduodenectomy is a treatment choice for suitable patients with solitary ampullary metastasis of renal cell carcinoma. Minimally invasive approach may provide patient a potential chance for early recovery to be suitable for subsequent chemotherapy. Further evidence is needed to address the exact role of minimally invasive pancreaticoduodenectomy in renal cell carcinoma metastasized to the ampulla of Vater.
References
- 1.Schlomer B, Figenshau RS, Yan Y, Venkatesh R, Bhayani SB. Pathological features of renal neoplasms classified by size and symptomatology. J Urol. 2006;176:1317–1320. doi: 10.1016/j.juro.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin JK, Lipworth L, Tarone RE. Epidemiologic aspects of renal cell carcinoma. Semin Oncol. 2006;33:527–533. doi: 10.1053/j.seminoncol.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4.Ljungberg B, Alamdari FI, Rasmuson T, Roos G. Follow-up guidelines for nonmetastatic renal cell carcinoma based on the occurrence of metastases after radical nephrectomy. BJU Int. 1999;84:405–411. doi: 10.1046/j.1464-410x.1999.00202.x. [DOI] [PubMed] [Google Scholar]
- 5.Bendic A, Glavina Durdov M, Stipic R, Karaman I. Melanoma in the ampulla of Vater. Hepatobiliary Pancreat Dis Int. 2013;12:106–108. doi: 10.1016/s1499-3872(13)60016-8. [DOI] [PubMed] [Google Scholar]
- 6.Uiterwaal MT, Mooi WJ, Van Weyenberg SJ. Metastatic melanoma of the ampulla of Vater. Dig Liver Dis. 2011;43:e8. doi: 10.1016/j.dld.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Bastos T, Souza TF, Otoch JP, Grecco E, Àvila F, Artifon EL. Metastasis of breast cancer to major duodenal papilla. Rev Gastroenterol Peru. 2014;34:149–150. [PubMed] [Google Scholar]
- 8.Rego RF, Atiq M, Velchala N, Nevin D, McElreath DP, McKnight WD, et al. Ampullary metastasis from breast cancer: an unusual finding. Endoscopy. 2009;41(Suppl 2):E278–E279. doi: 10.1055/s-0029-1215071. [DOI] [PubMed] [Google Scholar]
- 9.Büyükçelik A, Ensari A, Sarioğlu M, Işikdogan A, Içli F. Squamous cell carcinoma of the larynx metastasized to the ampulla of Vater. Report of a case. Tumori. 2003;89:199–201. doi: 10.1177/030089160308900219. [DOI] [PubMed] [Google Scholar]
- 10.Lee TH, Park SH, Lee CK, Lee SH, Chung IK, Kim SJ, et al. Ampulla of Vater metastasis from recurrent uterine cervix carcinoma presenting as groove pancreatitis. Gastrointest Endosc. 2011;73:362–363. doi: 10.1016/j.gie.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Chin AI, Lam JS, Figlin RA, Belldegrun AS. Surveillance strategies for renal cell carcinoma patients following nephrectomy. Rev Urol. 2006;8:1–7. [PMC free article] [PubMed] [Google Scholar]
- 12.Brookman-May SD, May M, Shariat SF, Novara G, Zigeuner R, Cindolo L, et al. Time to recurrence is a significant predictor of cancer-specific survival after recurrence in patients with recurrent renal cell carcinoma--results from a comprehensive multi-centre database (CORONA/SATURN-Project) BJU Int. 2013;112:909–916. doi: 10.1111/bju.12246. [DOI] [PubMed] [Google Scholar]
- 13.Kavolius JP, Mastorakos DP, Pavlovich C, Russo P, Burt ME, Brady MS. Resection of metastatic renal cell carcinoma. J Clin Oncol. 1998;16:2261–2266. doi: 10.1200/JCO.1998.16.6.2261. [DOI] [PubMed] [Google Scholar]
- 14.Thyavihally YB, Mahantshetty U, Chamarajanagar RS, Raibhattanavar SG, Tongaonkar HB. Management of renal cell carcinoma with solitary metastasis. World J Surg Oncol. 2005;3:48. doi: 10.1186/1477-7819-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Borgne J, Partensky C, Glemain P, Dupas B, de Kerviller B. Pancreaticoduodenectomy for metastatic ampullary and pancreatic tumors. Hepatogastroenterology. 2000;47:540–544. [PubMed] [Google Scholar]
- 16.McKenna JI, Kozarek RA. Metastatic hypernephroma to the ampulla of Vater: an unusual cause of malabsorption diagnosed at endoscopic sphincterotomy. Am J Gastroenterol. 1989;84:81–83. [PubMed] [Google Scholar]
- 17.Robertson GS, Gertler SL. Late presentation of metastatic renal cell carcinoma as a bleeding ampullary mass. Gastrointest Endosc. 1990;36:304–306. doi: 10.1016/s0016-5107(90)71032-2. [DOI] [PubMed] [Google Scholar]
- 18.Venu RP, Rolny P, Geenen JE, Hogan WJ, Komorowski RA, Ferstenberg R. Ampullary tumor caused by metastatic renal cell carcinoma. Dig Dis Sci. 1991;36:376–378. doi: 10.1007/BF01318213. [DOI] [PubMed] [Google Scholar]
- 19.Bolkier M, Ginesin Y, Moskovitz B, Munichor M, Levin DR. Obstructive jaundice caused by metastatic renal cell carcinoma. Eur Urol. 1991;19:87–88. doi: 10.1159/000473588. [DOI] [PubMed] [Google Scholar]
- 20.Leslie KA, Tsao JI, Rossi RL, Braasch JW. Metastatic renal cell carcinoma to ampulla of Vater: an unusual lesion amenable to surgical resection. Surgery. 1996;119:349–351. doi: 10.1016/s0039-6060(96)80122-x. [DOI] [PubMed] [Google Scholar]
- 21.Janzen RM, Ramj AS, Flint JD, Scudamore CH, Yoshida EM. Obscure gastrointestinal bleeding from an ampullary tumour in a patient with a remote history of renal cell carcinoma: a diagnostic conundrum. Can J Gastroenterol. 1998;12:75–78. doi: 10.1155/1998/429832. [DOI] [PubMed] [Google Scholar]
- 22.Teo M, Ryan B, Swan N, McDermott RS. A case of metastatic renal cell cancer presenting as jaundice. World J Oncol. 2010;1:218–220. doi: 10.4021/wjon247w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haidong W, Jianwei W, Guizhong L, Ning L, Feng H, Libo M. Ampullary tumor caused by metastatic renal cell carcinoma and literature review. Urol J. 2014;11:1504–1507. [PubMed] [Google Scholar]
- 24.Chowdhury SD, Masih D, Chawla G, Pal S, Kurien RT, Augustine J. Metastasis of renal cell carcinoma to the duodenal papilla. Indian J Gastroenterol. 2014;33:493–494. doi: 10.1007/s12664-013-0398-y. [DOI] [PubMed] [Google Scholar]
- 25.Karakatsanis A, Vezakis A, Fragulidis G, Staikou C, Carvounis EE, Polydorou A. Obstructive jaundice due to ampullary metastasis of renal cell carcinoma. World J Surg Oncol. 2013;11:262. doi: 10.1186/1477-7819-11-262. [DOI] [PMC free article] [PubMed] [Google Scholar]