Abstract
Cognitive decline accompanying the clinically more salient motor symptoms of Huntington's disease (HD) has been widely noted and can precede motor symptoms onset. Less clear is how such decline bears on language functions in everyday life, though a small number of experimental studies have revealed difficulties with the application of rule-based aspects of language in early stages of the disease. Here we aimed to determine whether there is a systematic linguistic profile that characterizes spontaneous narrative speech in both pre-manifest and/or early manifest HD, and how it is related to striatal degeneration and neuropsychological profiles. Twenty-eight early-stage patients (19 manifest and 9 gene-carriers in the pre-manifest stage), matched with 28 controls, participated in a story-telling task. Speech was blindly scored by independent raters according to fine-grained linguistic variables distributed over 5 domains for which composite scores were computed (Quantitative, Fluency, Reference, Connectivity, and Concordance). Voxel-based morphometry (VBM) was used to link specific brain degeneration patterns to loci of linguistic decline. In all of these domains, significant differences were observed between groups. Deficits in Reference and Connectivity were seen in the pre-manifest stage, where no other neuropsychological impairment was detected. Among HD patients, there was a significant positive correlation only between the values in the Quantitative domain and gray matter volume bilaterally in the putamen and pallidum. These results fill the gap of qualitative data of spontaneous narrative speech in HD and reveal that HD is characterized by systematic linguistic impairments leading to dysfluencies and disorganization in core domains of grammatical organization. This includes the referential use of noun phrases and the embedding of clauses, which mediate crucial dimensions of meaning in language in its normal social use. Moreover, such impairment is seen prior to motor symptoms onset and when standardized neuropsychological test profiles are otherwise normal.
Keywords: Huntington's disease, Narrative speech, Grammatical deficits, Voxel-based morphometry, Basal ganglia
1. Introduction
Huntington's disease (HD) is an autosomal dominant genetic neurodegenerative disease that involves cognitive and psychiatric disorders in addition to motor impairment. Cognitive decline can precede motor impairments by several years (Stout et al., 2011, Stout et al., 2016). The earliest cognitive impairments in clinically manifest HD have been found to implicate attention, executive functions, memory, and social cognition (Caine et al., 1977, Foroud et al., 1995, Ho et al., 2003, Papoutsi et al., 2014). Some of these impairments, including deficits in social cognition (Bora, Velakoulis, & Walterfang, 2016), can characterize, in milder forms, the pre-manifest stage.
Since the discovery of the genetic polyglutamine expansion as the cause of the disease (Gusella et al., 1983), an increasing amount of research has focused on detecting biomarkers that would allow tracking disease progression before the onset of the clinical manifestations, which usually start in the late thirties or forties of affected individuals. The potential of language as such a biomarker has barely been explored, though Vogel, Shirbin, Churchyard, and Stout (2012) suggested that markers of speech in the acoustic domain related to speech timing could be potential signals in the early symptomatic and perhaps the prodromal stage. While cognitive dysfunction has been extensively studied in HD, only a handful of studies have investigated the effects of the disease on language function (De Diego-Balaguer et al., 2008, Longworth et al., 2005, Sambin et al., 2012, Teichmann et al., 2005, Teichmann et al., 2006, Teichmann et al., 2008a, Teichmann et al., 2008b, Ullman et al., 1997). This is despite the fact that, cognitive, behavioral and motor dysfunctions are expected to be reflected in the language use of patients and to affect their everyday social interactions. Normal language use requires the interaction and integration of a myriad of cognitive systems, including memory, perception, attention, and the various subsystems of language itself. Furthermore, language is a primary tool used for conveying mental states and determining them in others, and hence may relate to the early impairments in the ability to understand the mental states of others (theory of mind', ToM) noted in HD (Adenzato and Poletti, 2013, Bora et al., 2016, Brüne et al., 2011; Eddy, Sira Mahalingappa, & Rickards, 2012; Saft et al., 2013).
HD involves primary neural death in the striatum extending progressively to widespread cortical areas. The striatum forms part of the cortico-subcortical language network, though its functional role and degree of specificity remain unclear. Available evidence supports its role both in the application of syntactic rules in language (Teichmann et al., 2005) and in the access to lexical aspects of grammatical processing (Friederici and Kotz, 2003, Friederici et al., 1999, Moro et al., 2001). Striatal damage in early manifest HD has been shown to affect the application of structural rules in different aspects of language, while leaving lexical knowledge unaffected (De Diego-Balaguer et al., 2008, Sambin et al., 2012, Teichmann et al., 2008a, Teichmann et al., 2005). In particular, impairments have been reported in sentence comprehension (Sambin et al., 2012, Teichmann et al., 2005) and the perception (Teichmann et al., 2006) and production (Longworth et al., 2005, Ullman et al., 1997) of verbal inflection.
Syntactic structuring and verbal inflection in sentences require temporal processing (Bornkessel-Schlesewsky & Schlesewsky, 2013), just as motor sequencing does. Thus, the linguistic deficits described could derive from a more general role of the basal ganglia shared by different aspects of motor and cognitive functions. As first proposed by Graybiel, 1995a, Graybiel, 1995b, the role of the basal ganglia could be that of a more general ‘pattern generator’, supporting the sequencing of meaningful behavioral repertoires, reiteration, and timing (Kotz and Schwartze, 2010, Kotz et al., 2009, Lieberman, 2007) in both motor sequences and cognitive sequences. This is consistent with findings of impaired control over the timing and duration of speech units in patients with striatal damage (Hertrich and Ackermann, 1994, Ludlow et al., 1987, Vogel et al., 2012). Indeed, temporal processing has been linked to the sequential processing necessary for syntactic structuring (Bornkessel-Schlesewsky & Schlesewsky, 2013) and the motor system appears to sustain this timing function. Thus, given its relation with a variety of cognitive and motor functions, some language changes could serve as an important and sensitive objective behavioral marker of cognitive decline and disease progression in HD, as has been suggested in the case of the schizophrenia prodrome as well (Bedi et al., 2015).
Previous studies on HD have studied language dysfunctions in constrained situations designed to test specific deficits in experimental tasks. Although this effort has helped to pinpoint that HD patients have particular difficulties with different aspects of syntactic processing accompanied by less impairment in lexico-semantic processing, these tasks may not reflect HD speech in more natural situations and in its normal social use. Narrative speech is a more ecologically natural condition, which poses distinctive cognitive challenges. These may in part overlap with, but also add to those of the experimental tasks previously mentioned. Specifically, narrative speech requires introducing story characters and tracking them throughout the story, setting up and developing the story line, and bringing it to a conclusion. Since agents act because of the reasons and intentions that underlie and rationalize their actions, moreover, storytelling depends on representing these mental states. This point is particularly interesting since, as noted, deficits in ToM have been reported in mild to moderate stages of HD (Brüne et al., 2011; Eddy et al., 2012), though not the pre-manifest stage (Saft et al., 2013). In addition, referencing story characters and objects in language and tracking them in a narrative requires specific grammatical devices, such as the functional elements ‘a’ or ‘the’ in front of nouns such as ‘girl’, where the former normally functions so as to introduce a character, and the latter to reference an already introduced one. In comprehension, the use of such devices has already been reported to be deficient in HD (Sambin et al., 2012) in experimental settings. Referencing of mental states exploits specific forms of grammatical complexity as well, such as the use of embedded clauses to report the content of a mental state (e.g., [She thought that [he was her grandmother]).
In this way, narrative represents language use in one of its cognitively most complex forms. Neurocognitive impairments are thus expected to bear on narrative performance. This impact has been demonstrated in the case of autism spectrum disorders (King, Dockrell, & Stuart, 2013), where language decline transpires in narrative tasks even when the clinical group is matched with controls on standardized language scores (Banney et al., 2015, Norbury and Sparks, 2013), and in schizophrenia (Zinken, Blakemore, Zinken, Butler, & Skinner, 2011). The disintegration of narrative competence thus opens a fine-grained window for shedding light on cognitive decline across different neurodegenerative and neurodevelopmental conditions. Given the variety of functions necessary to perform narrative tasks, these are a prime domain to study cognitive and linguistic deficits with a greater level of sensitivity than the tasks previously used for the study of spontaneous speech (e.g., picture description, structured interviews, etc.).
Despite its inherent interest, only a few studies have focused on HD spontaneous speech and narrative. These studies found spontaneous speech to be typically reduced, with fewer words and syntactic structures formed in short, simple sentence constructions and more paraphasic errors (Chenery et al., 2002, Murray and Lenz, 2001, Gordon and Illes, 1987, Podoll et al., 1988). In spontaneous speech answering to open-ended autobiographical questions, Illes' (1989) study found that the reduction of syntactic complexity was a landmark of HD speech as compared to that of patients with Alzheimer's and Parkinson's disease. More recently, using a picture description task, Jensen, Chenery, and Copland (2006) found that patients with HD produced significantly more grammatical errors and less action verbs than both a group of patients with non-thalamic subcortical lesions following stroke and healthy subjects. Finally, there is practically no evidence of altered language patterns in spoken narrative, though Caine, Bamford, Schiffer, Shoulson, and Levy (1986) reported a pattern of defective naming, impaired repetition and decreased language output in written narratives by patients with HD who were very early in the course of their illness.
Overall, these findings indicate language impairment in spontaneous speech of HD converging with the experimental studies to point to syntactic deficits in HD. However, sample sizes of the few existing studies have been all small (typically less than 12 HD patients), and they have mixed patients at different stages of the disease. Furthermore, none of the previous studies included prodromal cases and thus, there is little evidence for how spontaneous speech changes pattern across the different stages of the disease. More importantly, another limitation of this literature is that it did not develop a detailed linguistic classification of deficits and thus it remains unclear whether HD exhibits a systematic and distinctive profile.
The goal of the present study was to determine the spontaneous speech profile in both patients with HD and prodromal identified gene-carriers more systematically, based on a narrative task. This also allowed us to test whether previously reported linguistic deficits, which were based on experimentally controlled setups, could be linked to deficits seen in spontaneous speech. We further investigated whether those deficits were related to specific brain degeneration patterns by using a voxel-based morphometry (VBM) analysis constrained to the brain areas showing gray matter atrophy compared to controls matched in age and education. More specifically, we were interested in observing whether linguistic deficits were related to the neurodegeneration of areas from the cortico-striatal motor network, in order to illuminate whether there is a relationship between motor dysfunction and at least some of the linguistic deficits observed, and whether different patterns of degeneration including cortical areas outside of the motor circuit may relate to the some of the linguistic errors. This functional relation between motor and linguistic deficits was complemented with correlations from the neurological and neuropsychological assessments.
2. Material and methods
2.1. Participants
28 HD gene-carriers and 28 controls matched in age, gender and educational background were tested. Nine of the gene-carriers were at a prodromal stage of the disease (pre-HD), defined as carriers of the genetic mutation with a unified HD diagnostic confidence score (DCS) of less than 4. All patients and controls had Spanish as their native language. Table 1 summarizes the demographic, genetic and clinical data from the participants. None of the gene-carriers and controls reported previous history of traumatic brain injury or neurological disorder other than HD. All participants signed an informed consent to participate in this study that was approved by the ethics committee of the University of Barcelona and the Bellvitge Hospital.
Table 1.
Genetic, clinical and demographic information for controls and Huntington's disease patients, further divided in manifest and pre-HD patients.
| Controls | HD | Manifest HD | Pre-HD | |
|---|---|---|---|---|
| N | 28 | 28 | 19 | 9 |
| Gender (M/F) | 8/20 | 9/19 | 9/10 | 0/9 |
| Age in years | 45 ± 15.2 | 46.8 ± 12.3 | 52.4 ± 9.7 | 35 ± 6.8 |
| Education in years | 13.1 ± 2.8 | 11.7 ± 3.1 | 10.6 ± 2.9 | 14.1 ± 1.7 |
| CAG repeats | – | 43.5 ± 2.9 | 45.2 ± 2.6 | |
| TFC | – | 11.5 ± 1.4 | 12.9 ± 0.3 | |
| Disease Burden score | – | 398 ± 96.6 | 339.1 ± 105 | |
| UHDRS motor score | – | 20.3 ± 10.5 | 2.7 ± 4 | |
| UHDRS cognitive score | – | 193.19 ± 49.9 | 311.6 ± 57.3 |
2.2. General clinical and specific neuropsychological evaluation
All patients were evaluated using the Unified Huntington's Disease Rating Scale (UHDRS; The Huntington Study Group, 1996), which comprises motor, cognitive and behavioral scores and functional capacity. The medical and psychiatric history, HD history and current medications were also collected. The cognitive UHDRS includes the Stroop Test (Golden & Freshwater, 1978) and the verbal letter fluency test (Butters, Wolfe, Granholm, & Martone, 1986) assessing executive functions; the Symbol Digit Test (SDT, Wechsler, 2008) assessing processing speed and the Trail Making Test (Tombaugh, 2004) part A (TMT-A) assessing processing speed and sustained visual attention and part B (TMT-B) and A–B assessing cognitive flexibility. In addition, patients completed the Mattis Dementia Rating Scale (MDRS; Mattis, 1976); the Hopkins Verbal Learning Test revised to evaluate memory (HVLT-R; Rieu, Bachoud-Lévi, Laurent, Jurion, & Dalla Barba, 2006); the Boston Naming Test for word retrieval (Kaplan, Goodglass, & Weintrab, 1983) and the digit span test (forward to assess attention, and backward, to assess working memory; Wechsler, 2008). In addition, we selected from this evaluation the TMT and the digit span test and tested our sample of controls in order to have two cognitive indexes of executive functions and working memory characterizing the specific sample of control participants studied. The clinical evaluation was performed within three months of the narrative task and the MRI acquisition.
2.3. Procedure
Participants were presented with a four-minute long muted video clip that showed a summary of the story of Cinderella. Afterwards, they were presented with a wordless picture book of Cinderella in a computer screen that was kept open as support to remember the content of the story while they were telling it. Participants were instructed to shortly tell the story to the experimenter and they were informed that their speech would be recorded. Each speech sample was transcribed and then analyzed sentence by sentence by two independent raters using CLAN (MacWhinney, 2008). Since a sufficiently comprehensive speech analysis tool for this population is not currently available, we developed such a tool (available upon request), intended to cover all core structural dimensions of language. Prosodic aspects, though crucial to a wider HD speech profile, were not considered. The recordings were anonymized and the transcription and subsequent analysis were blind to the medical condition of the participant (pre-HD, symptomatic or control). Once the analysis of all participants' recordings was finished, both ratings were compared under the supervision of the second author, until an agreement of all three was reached on all of the few cases where disagreement was observed, always keeping blind the medical condition of the participants. No cases of unresolvable dispute remained.
2.4. MRI acquisition
MRI data were acquired through a 3 T whole-body MRI scanner (Siemens Magnetom Trio; Hospital Clínic, Barcelona), using a 32-channel phased array head coil. Structural images comprised a conventional high-resolution 3D T1 image [magnetization-prepared rapid-acquisition gradient echo sequence (MPRAGE), 208 sagittal slices, repetition time (TR) = 1970 msec, echo time (TE) = 2.34 msec, inversion time (IT) = 1050 msec, flip angle = 9°, FOV = 25.6 cm, 1 mm isotropic voxel].
3. Analysis
3.1. Speech error categorization
Narratives of patients and controls were subjected to a fine-grained linguistic analysis (57 variables) focused on the grammatical level. Beyond the scope of the current analysis were the most distal motoric components of speech (dysarthria), which can characterize speech in HD, and anomalous prosodic patterns. Linguistic variables were organized around a single ‘quantitative’ domain and four qualitative ‘error’ domains: 1. Quantitative, comprising the Mean Length of Utterances and number of words per Minute. 2. Fluency, capturing disturbances in the flow of speech due to pauses, word truncations, prolongations, filled pauses (e.g., ehm), repetitions of words or part of words. 3. Connectivity, capturing how clauses are grammatically combined with others. 4. Reference, defined to capture problems in the referential use of language to identify story characters and objects and to establish topics of the discourse. 5. Concordance, which comprised deficits in grammatical agreement. A more detailed description of these five domains and the specific variables included in each one are given under Supplementary Methods.
3.2. Statistical analysis of speech
Once speech variables were classified into the above five domains, a composite score was generated for each one of these. Specifically, additive combinations of the variable values composing each domain were used to characterize error levels in each language domain for each individual. In order to obtain relative frequencies comparable between individuals, these composite scores were derived from the variable values divided by the total number of words in the individuals' speech. Next, to equate the weight of each variable in the composite score, variable values were divided by their standard deviation as calculated from the sample of controls.
Composite scores in the three groups (controls, pre-HD and symptomatic gene-carriers) were compared by means of ANOVA tests, and in those domains where ANOVAs were statistically significant (at a p < .01 level), Tukey's Honest Significant Difference (HSD) post-hoc tests were applied to know which pairs were different. In three of the domains (Sentence connectivity, Reference, and Concordance) logarithmic transformations were previously applied to meet the parametric requirements of the ANOVA test.
Apart from comparing language abnormalities at the domain level, comparisons were also carried out for each one of the individual speech variables. Specifically, variables with less than 50% of null values (zeros) were compared with Kruskal–Wallis tests (non-parametric ANOVA), and variables with more than 50% of zeros were binarized and compared with Fisher's Exact Tests. A False Discovery Rate (FDR) correction (Benjamini & Yekutieli, 2001) was applied to the p-values of all tests to control for the multiple comparisons.
3.3. Correlations of speech domain composite scores with clinical tests
In order to study the relationship between each of the speech domains with different aspects of cognition, a correlation analysis was carried out between each composite score and those neuropsychological tests in which there were statistically significant differences between patients and controls, that is, TMT-A, TMT-B, digit span forward and digit span backward. A FDR correction was applied to account for multiple comparisons in this analysis. Furthermore, a correlation analysis was also performed between each composite score and two important clinical scores, the cognitive UHDRS and the motor UHDRS total scores. In order to have a more specific analysis of the relation of the linguistic domains with the specific motor dysfunctions that may affect more directly language production, we derived an additional subscore including the items associated with dysarthria, protrusion and Luria sequencing.
3.4. VBM analysis
Morphometric analysis was carried out using the vbm8 toolbox (http://dbm.neuro.uni-jena.de/vbm/) in the SPM8 software package (Welcome Department of Imaging Neuroscience Group, London, UK) running on MATLAB (v12.b, Mathworks, Natick, MA). Specifically, unified segmentation (Ashburner & Friston, 2005) was applied to the structural T1-weighted images of each subject to estimate tissue probability maps (Gray matter or GM maps). During this segmentation step, spatial regularization (regularization: .02, discrete cosine transform warp frequency cutoff of 22) was adapted to account for striatal neurodegeneration and ventricle dilatation. The resulting GM maps were then imported and fed into DARTEL to achieve spatial normalization into MNI space. DARTEL normalization alternates between computing an average template of the GM and white matter (WM) segmentations from all subjects and warping all subject's GM and WM segmentations into a better alignment with the template created (Ashburner, 2009). GM normalized images were modulated by their Jacobian determinants in order to identify regional differences in the volume or amount of GM (Mechelli, Price, Friston, & Ashburner, 2005). These normalized and modulated images were smoothed by using an isotropic spatial filter (FHWN = 8 mm) to reduce residual inter-individual variability. Finally, these images were visually inspected to ensure good quality in the normalization step.
The individual smoothed GM volume images for both the HD patients and controls were entered into a second-level analysis using a two-sample t-test, including intracranial volume (ICV) as a covariate to create an explicit mask to differentiate between HD patients and controls. This mask was used in the following correlation analysis to restrict the analysis to the areas with significant atrophy in the HD group, as described in the next subsection. The estimation for the ICV was taken as the value calculated by FreeSurfer (v. 5.1).
3.5. Correlation analysis
After defining those regions with tissue differences between HD and the control group, we investigated potential GM individual differences among the HD group, which may relate to the language deficits observed. A regression analysis within a linear model was applied individually for each language-related domains (i.e., Quantitative, Fluency, Concordance, Connectivity and Reference), including the GM volume images and the corresponding domain as covariates. ICV was also included as a nuisance variable in the model in order to correct for global differences in GM volume (Buckner et al., 2004).
We report two statistical thresholds: p < .05 with FDR correction for multiple comparisons at cluster level, and an exploratory threshold of p < .001 uncorrected and taken at a minimal cluster size of 20 voxels. The maxima of suprathreshold regions were localized by rendering them onto T1 structural template-image on the MNI reference brain.
4. Results
4.1. Spontaneous speech errors
The ANOVAs for the five composite scores were all significant, indicating that, at least, one of the groups differed from the others [Quantitative: F(2,53) = 11.88, p < .001; Fluency: F(2,53) = 12.61 p < .001; Connectivity: F(2,53) = 15.71, p < .001; Reference: F(2,53) = 22.09, p < .001; Concordance: F(2,53) = 8.423, p < .001].
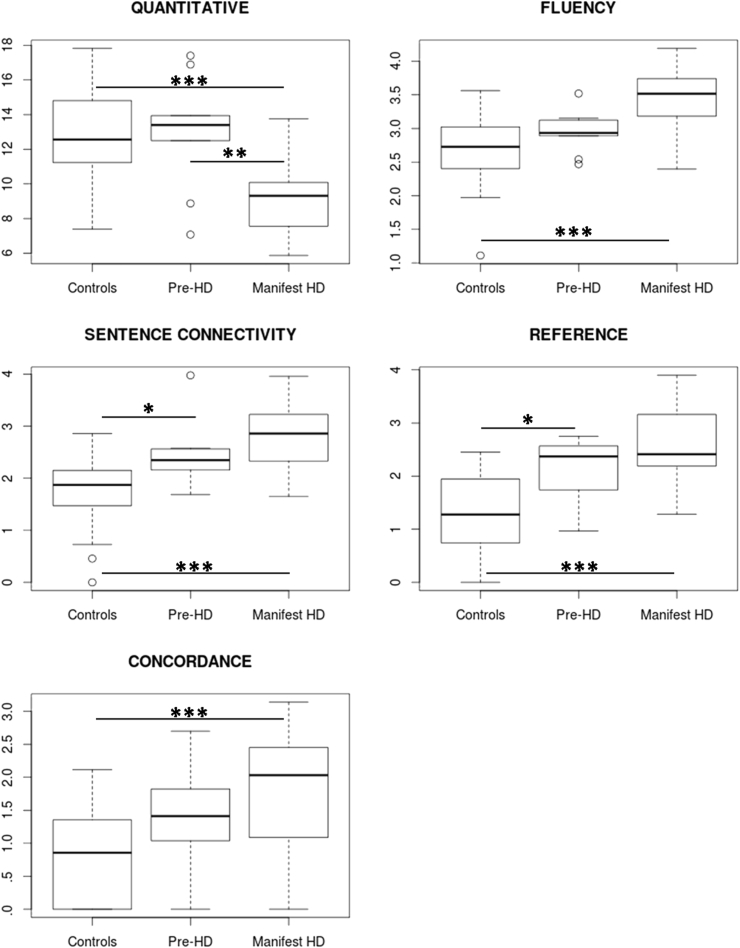
Fig. 1 contains box plots for the five domains, including results from the Tukey's HSD post hoc tests. As expected from the ANOVA results, the two most dissimilar groups (controls and manifest patients) differed significantly in all domains. The general Quantitative composite score showed no hint of decline in the pre-HD group but is clearly diminished in the symptomatic group. In the other remaining domains a gradual pattern of impairment is observed. For two of the domains (Connectivity and Reference) significant alterations were already observed in the pre-HD group. Since the subgroup of pre-HD and manifest patients differed from controls in terms of gender distribution, we conducted the same analysis with a subset of controls matched in gender in addition to age and educational background to each subgroup. The pattern of results was the same. Both sentence connectivity (p = .021) and reference (p = .019) were the only domains altered in pre-HD compared to controls. All domains were affected in manifest compared to controls (all p < .001).
Fig. 1.
Boxplots for the composite scores (y axis) of the five defined language domains in the three groups. Units in the y axis correspond to added values of variables of each domain after being divided by the total number of words in the individuals' speech and by their standard deviation calculated from the control sample. Significant differences between pairs of groups as given by the Tukey's HSD test are also shown (*: p < .05, **: p < .01, ***p < .001). Pre-HD: pre-symptomatic patients.
In the whole group comparisons, comparisons directly carried out on the individual speech variables led to a subset of significant variables after FDR correction for multiple comparisons (see Supplementary Table S1). The same comparisons comparing each subgroup of patients (pre-HD and manifest) to their gender, education and age matched control subgroups showed that these results were carried by the manifest group since none of these variables were significantly different between pre-HD and controls after FDR correction.
4.2. General clinical and neuropsychological assessment
The pre-HD group showed comparable scores to the control group in all neuropsychological assessments. The manifest HD group showed moderate impairment in immediate memory recall (HVLT-R total recall, see Table 2) and mild impairment in delayed recall (HVLT-R delayed recall) and speed processing (symbol digit code) in the general clinical assessment. In the more specific assessment of executive function and working memory a significant impairment in all subtests except one (digit span forward-backward) was observed only in manifest HD group when compared with matched controls (see Table 3).
Table 2.
Results of the neuropsychological evaluation in the pre-HD and manifest HD groups including the Hopkins Verbal Learning Test (HVLT-R), the Mattis Dementia Rating Scale (MDRS), the subtests of the UHDRS-Cognitive score and the Boston Naming Test (BNT).
| Manifest HD | Pre-HD | |
|---|---|---|
| MDRS | 132.5 ± 7.4§ | 141.2 ± 4.6 |
| HVLT-R total recall | 15.4 ± 4.8a§ | 28 ± 5.9 |
| HVLT-R delayed recall | 4.3 ± 2.5ǂ§ | 10 ± 1.4 |
| HVLT-R percentage retained | 66.9 ± 25.5§ | 99.4 ± 23.4 |
| HVLT-R discrimination index | 8.1 ± 4.2§ | 11,6 ± 0,5 |
| Letter fluency (FAS) | 24.4 ± 9.2§ | 43 ± 14.5 |
| Stroop interference | 2.9 ± 4.4§ | 13.8 ± 11.5 |
| Symbol digit code | 28.4 ± 11.2ǂ§ | 51 ± 9.4 |
| BNT | 50.1 ± 3.9§ | 56.7 ± 2.2 |
Moderate impairment compared to standardized scores in published norms of the tests (standard score < 30). ǂMild impairment compared to standardized scores (standard score 30–34). § ignificantly different from pre-HD (in pairwise comparisons, p < .05). MDRS = Mattis Dementia Rating Scale; HVLT-R = Hopkins Verbal Learning Test-Revised; BNT = Boston Naming Test.
Table 3.
Results of the subtests of the neuropsychological evaluation selected for the assessment of executive (TMT) and working memory functions (digit span) in the control sample and in the HD group.
| Controls | Manifest HD | Pre-HD | |
|---|---|---|---|
| TMT A (sec) | 35 ± 10 | 63.7 ± 24.3a | 32.4 ± 11.2 |
| TMT B (sec) | 82.9 ± 41.8 | 258.7 ± 206.5a | 65.9 ± 39.3 |
| TMT B–A (sec) | 47.9 ± 40.9 | 194.9 ± 184.4a | 33.4 ± 33 |
| Digit span forward | 6.6 ± 1.1 | 4.32 ± 1.3a | 5.7 ± 1.1 |
| Digit span backward | 4.9 ± 1.1 | 3 ± 0.7a | 4.6 ± 1.1 |
| Digit span forward–backward | 1.6 ± 1.3 | 1.6 ± 0.6 | 1.1 ± 0.9 |
Significant differences between manifest patients and controls (p < .05). Values are given in means ± SD; TMT = Trail Making Test.
The Quantitative domain correlated significantly with the working memory score (digit span backwards: r = .60, p = .012) and, in the general clinical assessment with the total cognitive (r = .59, p = .001) UHDRS score (Table 2). The Fluency and Reference domains correlated with executive function (TMT B: r = .54, p = .025 and r = .63, p = .009, respectively) and, in the general clinical assessment, with the cognitive UHDRS (r = −.40, p = .035 and r = −.380, p = .046, respectively) (Table 2, Table 3). No significant correlation was observed between the Connectivity and Concordance domains and any of the neuropsychological or clinical assessments.
Focusing more narrowly on the relation with the motor disabilities, we correlated the domains with the UHDRS motor score. Overall UHDRS motor score correlated only with the Quantitative domain (r = −.49, p = .008). We also created a more specific subscore including the items of the UHDRS affecting mouth movement (dysarthria, tongue protrusion) and Luria sequencing to focus on those items that could more specifically tap into motor aspects more associated to language production. This subscore in the UHDRS was again only significantly correlated with the Quantitative domain (r = −.38, p = .043).
4.3. Neuroimaging results
4.3.1. Group comparison
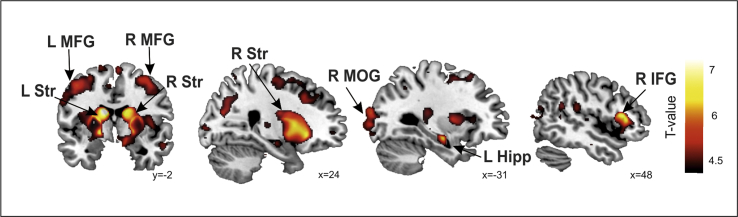
When comparing HD patients with controls, regional decrease of GM volume indicative of atrophy was predominantly observed in the basal ganglia, including the dorsal and ventral regions. Additionally, evidenced atrophy was also shown in different cortical regions mainly involved in motor, executive and fronto-parietal networks (see Table 4, Fig. 2). More specifically, regional decrease of GM volume indicative of the atrophy in HD compared to controls was predominantly observed in the bilateral basal ganglia (caudate, putamen, pallidum) but also in different bilateral cortical regions in the temporal (middle temporal gyrus [MTG], superior TG [STG], hippocampus), frontal (Supplementary Motor Area [SMA], Middle Frontal Gyrus [MFG], Inferior Frontal Gyrus [IFG], Insula), Parietal (Supramarginal Gyrus [SMG]) and Occipital Cortices.
Table 4.
Peak coordinates of the voxel-wise comparison between the HD and the Control groups. IFG: Inferior Frontal Gyrus, SMA: Supplementary Motor Area, MFG: Middle Frontal Gyrus, ITG: Inferior Temporal Gyrus, IPG: Inferior Parietal Gyrus, INS: Insula, STG: Superior Temporal Gyrus (p < .001, FDR < .05 corrected at cluster level).
| Brain regions | Cluster extent | Coordinates |
p-Value | |||
|---|---|---|---|---|---|---|
| x | y | z | T-value | |||
| L Caudate | 43,631 | −10 | 0 | 21 | 8.54 | .001 |
| L Putamen/Pallidum | −14 | 3 | −2 | 7.61 | ||
| R Pallidum/Putamen | 14 | 4 | −5 | 7.61 | ||
| R Caudate | 10 | 0 | 19 | 7.01 | ||
| L Hippocampus | −31 | −10 | −15 | 6.5 | ||
| LIFG | −40 | 31 | 1 | 4.85 | ||
| R INS | 39 | 1 | 6 | 4.67 | ||
| R STG | −54 | −19 | 9 | 4.48 | ||
| L STG | −46 | −27 | 10 | 4.19 | ||
| R Hippocampus | 24 | −12 | −13 | 4.19 | ||
| R IFG | −45 | 24 | 9 | 3.96 | ||
| L MOG | 24,470 | 33 | −87 | 6 | 6.37 | .001 |
| R MOG | −38 | −87 | 12 | 5.91 | .001 | |
| R MFG | 17,436 | 38 | 0 | 57 | 5.85 | .001 |
| R Precentral G | 39 | −19 | 57 | 4.11 | ||
| SMA | 1 | 0 | 46 | 3.45 | ||
| L MFG | −39 | 19 | 58 | 5.67 | .001 | |
| L Precentral G | −36 | −27 | 60 | 4.65 | ||
| R ITG | 659 | 62 | −43 | −9 | 5.06 | .037 |
| R MTG | 944 | 56 | −1 | −20 | 4.35 | .014 |
| R MTG | 869 | −60 | −19 | −20 | 4.35 | .016 |
| L IPG | 1018 | −56 | −52 | 40 | 4.04 | .012 |
Fig. 2.
Regional differences between controls and HD patients. Regions with significantly reduced gray matter volume in HD patients compared with controls were rendered onto sagittal and axial views with MNI coordinates at the bottom right of each slice. Statistical maps are thresholded at a p < .005, uncorrected threshold, for illustrative purposes. IFG: Inferior Frontal Gyrus, MFG: Middle Frontal Gyrus, Str: Striatum, MOG: Middle Occipital Gyrus, Hipp: Hippocampus, R: Right, L: Left.
4.3.2. Language-related correlations within the HD group
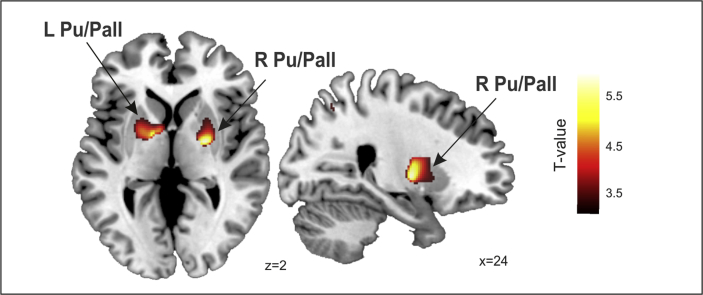
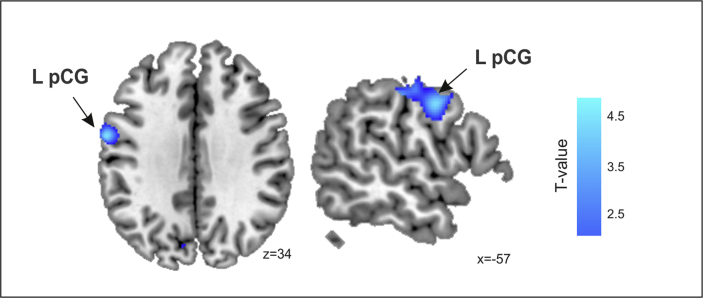
Among the HD patients, there was a significant positive correlation between the Quantitative domain and the GM volume bilaterally in the dorsal basal ganglia (Putamen/Pallidum) (Table 5, Fig. 3). In contrast, for the other language related domains (Concordance, Connectivity and Reference), no significant structural correlations were observed, except for a correlation tendency in the post central gyrus (i.e., somatosensory cortex) when a more permissive statistical threshold (p < .001, uncorrected) was used, which revealed a decrease in the gray matter volume related to the Fluency scores (Table 5, Fig. 4).
Table 5.
Peak coordinates of the main contrast of interest masked by the Controls > HD contrast for the Quantitative domain (*whole brain level p < .001, FDR < .05 corrected at cluster level) and for the Lack of Fluency domain (+whole brain level p < .001, uncorrected).
| Brain regions | Cluster extent | Coordinates |
p-Value | |||
|---|---|---|---|---|---|---|
| x | y | z | T-value | |||
| Quantitative domain | ||||||
| R Putamen/Pallidum | 1468 | 23 | −6 | 1 | 5.42 | .004* |
| L Putamen/Pallidum | 846 | −14 | 0 | 0 | 4.92 | .022* |
| Fluency domain | ||||||
| L postcentral gyrus | 23 | −62 | −3 | 39 | 3.64 | .001+ |
Fig. 3.
Quantitative domain correlated with gray matter volume. Correlations were rendered onto sagittal and coronal views with MNI coordinates at the bottom right of each slice. Statistical maps are thresholded at a p < .005, uncorrected threshold, for illustrative purposes. Pu: Putamen, Pall: Globus Pallidum, R: Right, L: Left.
Fig. 4.
Fluency correlated with gray matter volume. Correlations were rendered onto sagittal and coronal views with MNI coordinates at the bottom right of each slice. Statistical maps are thresholded at a p < .005, uncorrected threshold, for illustrative purposes. L pCG: Left postcentral Gyrus.
5. Discussion
This study sought to define the spontaneous speech profile of HD manifest patients as compared with prodromal gene-carriers and controls, based on a story-telling task that requires a good coordination of several cognitive and linguistic capacities. Linguistic variables were grouped into five domains defined by linguistic criteria, one ‘quantitative’ and four qualitative ‘error’ domains, comprising core dimensions of the grammatical organization of language, leaving out peripheral aspects relating to articulation, as well as prosody. Results show that in all five domains, language changes take place in the HD group as compared with controls. Importantly, these are not restricted to purely quantitative measures (Mean Length of Utterances, the Number of Words, and Words per Minute), but also affect the flow of speech and its structuring into meaningful units as indexed by anomalous pausing, truncations, and repetitions (Fluency). They further affect core aspects of grammatical organization that are central to normal linguistic functions in communication and discourse, as captured in the remaining three domains, which we will discuss in more detail below. In all four error domains, the language level in pre-manifest gene-carriers is between that of controls and manifest HD. Two of these domains, however, namely Connectivity and Reference, stand out insofar as even the pre-manifest group differs significantly in relation to controls, in the absence of neurocognitive decline as measured with standardized tests, while in the Quantitative domain our measures showed no hint of decline prior to the manifest phase.
These findings entail that HD, as a motor disease, not only presents with linguistic symptoms, but actually starts out linguistically before motor symptoms are detected. Moreover, cognitive decline widely noted to affect the pre-symptomatic phase may be first detectable in the domain of linguistic cognition. In short, at a stage when motor and cognitive functions present no detectable deficit behaviorally or neuro-psychologically yet, there is linguistic impairment affecting core higher-order linguistic functions (Reference and Connectivity). This suggests the clinical significance of language as an early disease marker of HD, and motivates the development of more fine-grained clinical linguistic tests of language function, which could be applied to pre-HD in particular. However, the clinical diagnostic utility of such tests at an individual level needs to be demonstrated, given the high variability of clinical presentations along a number of dimensions, which interact with language at the individual level.
Reference is a primary function of all ordinary language use. Language cannot function normally without speakers using noun phrases such as the girl, some food, everybody, the car crash, etc., to pick out particular objects, persons, or events about which they wish to provide some information. Reference in this sense is a complex phenomenon that integrates a number of linguistic sub-domains including the lexicon, the grouping of words into phrases, and grammatical relations between them. Moreover, in the context of normal discourse and especially storytelling, the referential function of language is not confined to merely picking particular objects out. It also relates these objects to others, and to events in which they play a role. It further allows tracking them through a narrative and update our knowledge about them. Such reference tracking is a primary function of the word the in English, which is standardly used to refer to objects that have been introduced before. Storytelling derails when it is not clear to the hearer which of a number of possible referents is intended by the speaker using a phrase like the man, or what topic a new utterance is providing further information about. Two of the individual variables in the Reference domain that turned out to be significant, Ambivalence and Vague or missing topic (see Supplementary Methods, Table S1) directly reflect this difficulty in the HD group of using referential devices such as noun phrases to establish a coherent narrative.
Clausal Connectivity, affected from the pre-symptomatic stage of HD as well, also captures a crucial, high-level element of grammatical organization on which the meaning of ordinary discourse depends. Within this domain, our variables particularly relate to the use of mental state verbs or verbs of communication such as think or say, whose function is to represent in discourse the content of what someone thinks, feels or says. Such content are represented through clauses such as she is happy, which feature as the subordinated complements of the verbs in question, as in He thinks she is happy. Utterances involving these verbs therefore have a meta-representational function: they represent what someone else mentally represents. If subordination is misused or underused, this meta-representation function fails. In particular, connecting clauses with coordinating conjunctions (e.g., and) cannot have this function: The man came and she was unhappy only states two facts. Individual variables in the domain of connectivity that were significantly different between patients and controls (see Supplementary Methods, Table S1) included use (quantity) of subordinations and both correct and incorrect coordinations. The HD group used less subordinations in total with significantly less correct subordination (i.e., SUB RIGHT, see Table S1). This is in contrast with coordination, which was significantly higher in both right and wrong instances of it (i.e., CRD RIGHT, CRD WRONG, see Table S1). This result strongly suggests that coordination was replacing subordination in HD patients, which would entail a loss in meta-representational capacity. A difficulty with clausal connectivity predicts poor story-telling, since rationalizing people's actions depends on representing what they think, say, or desire. This could relate to the noted ‘theory of mind’ (ToM) deficits in patients with HD (Adenzato and Poletti, 2013, Bora et al., 2016, Brüne et al., 2011, Saft et al., 2013). More direct testing of language-ToM correlations in this population are therefore called for. A link between performance on sentences with embedded clauses and performance on false belief tasks has been widely demonstrated, both in preschool typically developing children (De Villiers, 2007) and in older children with Autism Spectrum Disorders (Lind & Bowler, 2009).
With respect to earlier studies on HD spontaneous speech, the present study significantly fine-grains results from studies documenting a reduction in grammatical complexity in HD speech (Chenery et al., 2002, Gordon and Illes, 1987, Illes, 1989, Murray and Lenz, 2001, Podoll et al., 1988), by showing that error patterns can be meaningfully grouped into linguistically coherent domains made up of linguistically highly specific variables. Specifically, our findings in the domain of Reference provide important information with respect to earlier experimental studies manipulating specific aspects of grammatical complexity. In particular, Sambin et al. (2012) showed, controlling for working memory and executive functioning deficits, that early-stage HD patients face difficulties in grammatical principles governing the comprehension of the referential use of noun phrases such as proper names and pronouns. Here we show that errors in this domain are highly manifest in spontaneous speech as well, and concern the referential use of language more generally as based on grammar. This is clear from linguistic variables included in the Reference domain, none of which concern lexical-level errors such as word-finding difficulties, word approximations, or neologisms. Instead they concern grammatical-level anomalies, such as the setting (e.g., vagueness or lack) of topics, the use of determiner phrases, grammatical agreement between determiner and noun, missing referents for pronouns, or incorrect or inappropriate referents. Therefore, while error patterns recorded here do not suggest that errors are due to a defect in any highly specific syntactic principle as suggested in Sambin et al. (2012), they do clearly indicate that they concern the role of grammar in reference.
In line with previous VBM studies in HD patients (Kassubek et al., 2004, Kassubek et al., 2005) reduced GM was detected in the whole striatum and in widespread cortical areas including the parietal, temporal and frontal cortices. However, despite the clearly deviant patterns of spontaneous speech in HD and the prominent striatal and cortical degeneration observed, neurodegeneration measures showed only significant correlations with the Quantitative domain. Specifically, patients with poorer speech in terms of length of utterances, number of words per sentence, etc. had greater neurodegeneration in the bilateral putamen and pallidum. These striatal structures are part of the motor loop involving the SMA, premotor and somatosensory areas. The deficits observed within this domain may partially be explained by underlying motor alterations in this circuit. This is consistent also with the correlations between the Quantitative and the UHDRS motor scores and with the working memory scores that also have a motor component for the articulatory rehearsal of phonological information to be maintained for the task. The Quantitative score was also correlated with a subscore derived from the UHDRS items measuring dysarthria, tongue protrusion and Luria sequencing indicating that part of the variability in this domain could only derive from altered mouth movements and sequencing dysfunction. However, the Quantitative factor also correlated with the UHDRS cognitive score suggesting that these errors derive not only from a motor component.
In the domains of Reference and Connectivity, linguistic impairment occurs pre-symptomatically in the absence of neurocognitive impairment detectable at least through the standardized clinical and neuropsychological tests used in regular clinical assessment of HD. The Fluency and Reference domains did correlate both with executive function measures (TMT B) and, in the general clinical assessment, with the general cognitive UHDRS score (r = −.40, p = .035 and r = −.380, p = .046, respectively) (Table 2, Table 3). These facts, and the absence of any correlations with clinical or neuropsychological measures in the domains of Connectivity and Concordance, suggest the importance of measuring cognitive decline linguistically in HD and the linguistic specificity of these deficits.
The above VBM results raise the question of why no correlations with specific brain areas of neurodegeneration were found in any of the other four error domains. One possibility is that the deficits may be only detectable for a purely quantitative analysis of speech whereas the association between brain degeneration and decline in the more subtle quality of speech organization cannot be observed before more advanced stages of the disease. Indeed, it is noteworthy that, as mentioned above, it is only in the Quantitative domain that language levels in pre-HD are essentially at the same level as in controls. Neuronal dysfunction precedes cell death (Levine et al., 2004, Tobin and Signer, 2000) and psychiatric, cognitive, and motor symptoms often appear alongside cellular and synaptic alterations in the absence of neuronal loss (Vonsattel & DiFiglia, 1998). These qualitative deficits in speech errors may be more sensitive to individual differences in brain dysfunction and not sensitive to brain atrophy measures.
The lack of correlations with neurodegeneration may also be explainable from the fact that all the five domains are aspects of language that require the confluence of multiple linguistic and cognitive mechanisms involved in ordinary language use and functioning. Therefore a widespread neuronal network may be necessary. For example, reference is a high-level, integrative linguistic function in the sense that it comprises multiple systems interacting coherently, including different linguistic subcomponents (lexical organization, phrase structure, agreement, and grammatical relations) and interfacing working-memory, and executive functions. It is in line with this that the Fluency and Reference domains did correlate both with executive function measures (TMT B) and with the general cognitive UHDRS scores (Table 2, Table 3). Despite the fact that distributed networks are likely to be involved, each of our error domains comprised linguistically highly specific variables. We avoided linguistically non-specific variables (e.g., ‘coherence in discourse’). This specificity of the variables is further attested by the fact that within error domains, several of these variables dissociated from others in being significant in the individual (non-domain based) analysis (see Table S1).
Reference is one possible domain where specific brain regions may be expected to be associated with the errors observed, despite its relation with multiple language levels of processing and the engagement of different functions. Several studies have recently identified a critical recruitment of the parietal lobe (SMG and angular gyrus) in the processing of reference (Brodbeck and Pylkkänen, 2017, Egorova et al., 2016; Peeters, Snijders, Hagoort, & Özyürek, 2017). Although we did observe decreased GM volume in the inferior parietal lobe (including SMG), no significant correlation was obtained with neurodegeneration in this brain area and the score of the reference domain. As noted, this domain was correlated with executive function, which is related to a widespread brain network, varying depending on the specific executive function studied (e.g., dorsolateral prefrontal cortex, anterior cingulate, parietal lobe). It remains to be seen whether the relation between reference deficits and inferior parietal lobe function would be visible with functional responses in this brain area instead of structural measures that may only be more sensitive with greater progression of the disease.
6. Conclusions
In sum, our analysis of spontaneous narrative speech comparing controls and pre- and symptomatic HD patients reveals that cognitive impairment in HD shows in primary abnormalities in core domains of linguistic organization and function. Moreover, it does so prior to motor impairment and before cognitive decline is detectable at least through the standardized clinical and neuropsychological tests used here. This supports the value of using grammatical measures to track disease progression, which appear to show greater sensitivity than earlier work focusing on the acoustic parameters of speech (Vogel et al., 2012).
Funding
This research was supported by the Ministerio de Economía y Competitividad (MINECO, Spanish Government), grant FFI2013-40526P to WH and PSI2011-23624 to RDB, by an ERC-StG, Grant agreement 313841 TuningLang from the European Commission to RDB and by the Instituto de Salud Carlos III, which is an agency of the MINECO, co-funded by the European Regional Development Fund ‘A way of building Europe’ (CP13/00225 and PI14/00834, both to EC).
Acknowledgments
We thank the physicians and neuropsychologists Núria Caballol, Matilde Calopa, Jaime Kulisevsky, Celia Mareca, Saül Martínez-Horta, Esteban Muñoz, Jesús Pérez, Nadia Rodriguez-Dechichá, Jesús M. Ruiz, Pilar Santacruz, Susana Subirá and Irene Vaquer who provided the clinical evaluations of the patients. We are grateful to the patients and their families for their participation.
Reviewed 28 May 2017
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.cortex.2017.07.022.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Adenzato M., Poletti M. Theory of mind abilities in neurodegenerative diseases: An update and a call to introduce mentalizing tasks in standard neuropsychological assessments. Clinical Neuropsychiatry. 2013;10(5):226–234. [Google Scholar]
- Ashburner J. Computational anatomy with the SPM software. Magnetic Resonance Imaging. 2009;27(8):1163–1174. doi: 10.1016/j.mri.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Banney R.M., Harper-Hill K., Arnott W.L. The autism diagnostic observation schedule and narrative assessment: Evidence for specific narrative impairments in autism spectrum disorders. International Journal of Speech-Language Pathology. 2015;17(2):159–171. doi: 10.3109/17549507.2014.977348. [DOI] [PubMed] [Google Scholar]
- Bedi G., Carrillo F., Cecchi G.A., Slezak D.F., Sigman M., Mota N.B. Automated analysis of free speech predicts psychosis onset in high-risk youths. Npj Schizophrenia. 2015;1:1–7. doi: 10.1038/npjschz.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Annals of Statistics. 2001:1165–1188. [Google Scholar]
- Bora E., Velakoulis D., Walterfang M. Social cognition in Huntington's disease: A meta-analysis. Behavioural Brain Research. 2016;297:131–140. doi: 10.1016/j.bbr.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Bornkessel-Schlesewsky I., Schlesewsky M. Reconciling time, space and function: A new dorsal-ventral stream model of sentence comprehension. Brain and Language. 2013;125(1):60–76. doi: 10.1016/j.bandl.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Brodbeck C., Pylkkänen L. Language in context: Characterizing the comprehension of referential expressions with MEG. NeuroImage. 2017;147:447–460. doi: 10.1016/j.neuroimage.2016.12.006. (December 2016) [DOI] [PubMed] [Google Scholar]
- Brüne M., Blank K., Witthaus H., Saft C. “Theory of mind” is impaired in Huntington's disease. Movement Disorders. 2011;26(4):671–678. doi: 10.1002/mds.23494. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Head D., Parker J., Fotenos A.F., Marcus D., Morris J.C. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Butters N., Wolfe J., Granholm E., Martone M. An assessment of verbal recall, recognition and fluency abilities in patients with Huntington's disease. Cortex. 1986;22(1):11–32. doi: 10.1016/s0010-9452(86)80030-2. [DOI] [PubMed] [Google Scholar]
- Caine E.D., Bamford K.A., Schiffer R.B., Shoulson I., Levy S. A controlled neuropsychological comparison of Huntington's disease and multiple sclerosis. Archives of Neurology. 1986;43(3):249–254. doi: 10.1001/archneur.1986.00520030039009. [DOI] [PubMed] [Google Scholar]
- Caine E.D., Ebert M.H., Weingartner H. An outline for the analysis of dementia. The memory disorder of Huntington's disease. Neurology. 1977;27(11):1087–1092. doi: 10.1212/wnl.27.11.1087. [DOI] [PubMed] [Google Scholar]
- Chenery H.J., Copland D.A., Murdoch B.E. Complex language functions and subcortical mechanisms: Evidence from Huntington's disease and patients with non-thalamic subcortical lesions. International Journal of Language & Communication Disorders. 2002;37(4):459–474. doi: 10.1080/1368282021000007730. [DOI] [PubMed] [Google Scholar]
- De Diego-Balaguer R., Couette M., Dolbeau G., Dürr A., Youssov K., Bachoud-Lévi A.C. Striatal degeneration impairs language learning: Evidence from Huntington's disease. Brain. 2008;131(11):2870–2881. doi: 10.1093/brain/awn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Villiers J. The interface of language and theory of mind. Lingua. International Review of General Linguistics. Revue Internationale de Linguistique Generale. 2007;117(11):1858–1878. doi: 10.1016/j.lingua.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy C.M., Sira Mahalingappa S., Rickards H.E. Is Huntington's disease associated with deficits in theory of mind? Acta Neurologica Scandinavica. 2012;126:376–383. doi: 10.1111/j.1600-0404.2012.01659.x. [DOI] [PubMed] [Google Scholar]
- Egorova N., Shtyrov Y., Pulvermüller F. Brain basis of communicative actions in language. NeuroImage. 2016;125:857–867. doi: 10.1016/j.neuroimage.2015.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroud T., Siemers E., Kleindorder D., Bill D.J., Hodes M.E., Norton J.A. Cognitive scores in carriers of Huntington's disease gene compared to noncarriers. Annals of Neurology. 1995;37(5):657–664. doi: 10.1002/ana.410370516. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Kotz S.A. The brain basis of syntactic processes: Functional imaging and lesion studies. NeuroImage. 2003;20:S8–S17. doi: 10.1016/j.neuroimage.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Steinhauer K., Frisch S. Lexical integration: Sequential effects of syntactic and semantic information. Memory & Cognition. 1999;27(3):438–453. doi: 10.3758/bf03211539. [DOI] [PubMed] [Google Scholar]
- Golden C.J., Freshwater S.M. Stoelting; Wood Dale, IL: 1978. Stroop color and word test. [Google Scholar]
- Gordon W.P., Illes J. Neurolinguistic characteristics of language production in Huntington's disease: A preliminary report. Brain and Language. 1987;31(1):1–10. doi: 10.1016/0093-934x(87)90056-3. [DOI] [PubMed] [Google Scholar]
- Graybiel A.M. Building action repertoires: Memory and learning functions of the basal Ganglia. Current Opinion in Neurobiology. 1995;5:733–741. doi: 10.1016/0959-4388(95)80100-6. [DOI] [PubMed] [Google Scholar]
- Graybiel A.M. The basal Ganglia. Trends in Neurosciences. 1995;18:60–62. [PubMed] [Google Scholar]
- Gusella J.F., Wexler N.S., Conneally P.M., Naylor S.L., Anderson M.A., Tanzi R.E. A polymorphic DNA marker genetically linked to Huntington's disease. Nature. 1983;306(5940):234–238. doi: 10.1038/306234a0. [DOI] [PubMed] [Google Scholar]
- Hertrich I., Ackermann H. Acoustic analysis of speech timing in Huntington' s disease. Brain and Language. 1994;47(2):182–196. doi: 10.1006/brln.1994.1048. [DOI] [PubMed] [Google Scholar]
- Ho A.K., Sahakian B.J., Brown R.G., Barker R.A., Hodges J.R., Ané M.N. Profile of cognitive progression in early Huntington's disease. Neurology. 2003;61(12):1702–1706. doi: 10.1212/01.wnl.0000098878.47789.bd. [DOI] [PubMed] [Google Scholar]
- Illes J. Neurolinguistic features of spontaneous language production dissociate three forms of neurodegenerative disease: Alzheimer's, Huntington's, and Parkinson's. Brain and Language. 1989;37(4):628–642. doi: 10.1016/0093-934x(89)90116-8. [DOI] [PubMed] [Google Scholar]
- Jensen A.M., Chenery H.J., Copland D.A. A comparison of picture description abilities in individuals with vascular subcortical lesions and Huntington's disease. Journal of Communication Disorders. 2006;39(1):62–77. doi: 10.1016/j.jcomdis.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Kaplan E., Goodglass H., Weintrab S. Lea & Febiger; Philadelphia: 1983. The Boston naming test. [Google Scholar]
- Kassubek J., Juengling F.D., Kioschies T., Henkel K., Karitzky J., Kramer B., Landwehrmeyer G.B. Topography of cerebral atrophy in early Huntington's disease: A voxel based morphometric MRI study. Journal of Neurology, Neurosurgery, and Psychiatry. 2004;75(2):213–220. http://jnnp.bmj.com/cgi/content/long/75/2/213 Retrieved from. [PMC free article] [PubMed] [Google Scholar]
- Kassubek J., Unrath A., Huppertz H.J., Lulé D., Ethofer T., Sperfeld A.D. Global brain atrophy and corticospinal tract alterations in ALS, as investigated by voxel-based morphometry of 3-D MRI. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders. 2005;6:213–220. doi: 10.1080/14660820510038538. [DOI] [PubMed] [Google Scholar]
- King D., Dockrell J.E., Stuart M. Event narratives in 11–14 year olds with autistic spectrum disorder. International Journal of Language & Communication Disorders. 2013;48:522–533. doi: 10.1111/1460-6984.12025. [DOI] [PubMed] [Google Scholar]
- Kotz S.A., Schwartze M. Cortical speech processing unplugged: A timely subcortico-cortical framework. Trends in Cognitive Science. 2010;14(9):392–399. doi: 10.1016/j.tics.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Kotz S.A., Schwartze M., Schmidt-Kassow M. Non-motor basal ganglia functions: A review and proposal for a model of sensory predictability in auditory language perception. Cortex. 2009;45(8):982–990. doi: 10.1016/j.cortex.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Levine M.S., Cepeda C., Hickey M.A., Fleming S.M., Chesselet M.-F. Genetic mouse models of Huntington's and Parkinson's diseases: Illuminating but imperfect. Trends in Neurosciences. 2004;27(11):691–697. doi: 10.1016/j.tins.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Lieberman P. The evolution of human speech; its Anatomical and neural bases. Current Anthropology. 2007;48:39–66. [Google Scholar]
- Lind S.E., Bowler D.M. Language and theory of mind in autism spectrum disorder: The relationship between complement syntax and false belief task performance. Journal of Autism and Developmental Disorders. 2009;39(6):929–937. doi: 10.1007/s10803-009-0702-y. [DOI] [PubMed] [Google Scholar]
- Longworth C.E., Keenan S.E., Barker R.A., Marslen-Wilson W.D., Tyler L.K. The basal ganglia and rule-governed language use: Evidence from vascular and degenerative conditions. Brain. 2005;128(3):584–596. doi: 10.1093/brain/awh387. [DOI] [PubMed] [Google Scholar]
- Ludlow C.L., Connor N.P., Bassich C.J. Speech timing in Parkinson's and Huntington's disease. Brain and Language. 1987;32:195–214. doi: 10.1016/0093-934x(87)90124-6. [DOI] [PubMed] [Google Scholar]
- MacWhinney B. Enriching CHILDES for morphosyntactic analysis. In: Behrens H., editor. Trends in corpus research: Finding structure in data. Benjamins; Amsterdam: 2008. [Google Scholar]
- Mattis S. Mental status examination for organic mental syndrome in elderly patients. In: Bellak L., Karasu T.B., editors. Geriatric psychiatry. NY: Grune & Stratton; New York: 1976. pp. 71–121. [Google Scholar]
- Mechelli A., Price C.J., Friston K.J., Ashburner J. Voxel-based morphometry of the human brain: Methods and applications. Current Medical Imaging Reviews. 2005;1(2):105–113. [Google Scholar]
- Moro A., Tettamanti M., Perani D., Donati C., Cappa S.F., Fazio F. Syntax and the brain: Disentangling grammar by selective anomalies. NeuroImage. 2001;13(1):110–118. doi: 10.1006/nimg.2000.0668. [DOI] [PubMed] [Google Scholar]
- Murray L.L., Lenz L.P. Productive syntax abilities in Huntington's and Parkinson's diseases. Brain and Cognition. 2001;46(1):213–219. doi: 10.1016/s0278-2626(01)80069-5. [DOI] [PubMed] [Google Scholar]
- Norbury C.F., Sparks A. Difference of disorder? Cultural issues in understanding neurodevelopmental disorders. Developmental Psychology. 2013;49(1):45–58. doi: 10.1037/a0027446. [DOI] [PubMed] [Google Scholar]
- Papoutsi M., Labuschagne I., Tabrizi S.J., Stout J.C. The cognitive burden in Huntington's disease: Pathology, phenotype, and mechanisms of compensation. Movement Disorders. 2014;29(5):673–683. doi: 10.1002/mds.25864. [DOI] [PubMed] [Google Scholar]
- Podoll K., Caspary P., Lange H.W., Noth J. Language functions in Huntington's disease. Brain. 1988;111(6):1475–1503. doi: 10.1093/brain/111.6.1475. [DOI] [PubMed] [Google Scholar]
- Peeters D., Snijders T.M., Hagoort P., Özyürek A. Linking language to the visual world: Neural correlates of comprehending verbal reference to objects through pointing and visual cues. Neuropsychologia. 2017;95:21–29. doi: 10.1016/j.neuropsychologia.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Rieu D., Bachoud-Lévi A.C., Laurent A., Jurion E., Dalla Barba G. Adaptation française du «Hopkins verbal learning test». Revue Neurologique. 2006;162(6):721–728. doi: 10.1016/s0035-3787(06)75069-x. [DOI] [PubMed] [Google Scholar]
- Saft C., Lissek S., Hoffmann R., Nicolas V., Tegenthoff M., Juckel G. Mentalizing in preclinical Huntington's disease: An fMRI study using cartoon picture stories. Brain Imaging and Behavior. 2013;7(2):154–162. doi: 10.1007/s11682-012-9209-9. [DOI] [PubMed] [Google Scholar]
- Sambin S., Teichmann M., de Diego Balaguer R., Giavazzi M., Sportiche D., Schlenker P. The role of the striatum in sentence processing: Disentangling syntax from working memory in Huntington's disease. Neuropsychologia. 2012;50(11):2625–2635. doi: 10.1016/j.neuropsychologia.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Stout J.C., Glikmann-Johnston Y., Andrews S.C. Cognitive assessment strategies in Huntington's disease research. Journal of Neuroscience Methods. 2016;265:19–24. doi: 10.1016/j.jneumeth.2015.12.007. [DOI] [PubMed] [Google Scholar]
- Stout J.C., Paulsen J.S., Queller S., Solomon A.C., Whitlock K.B., Campbell J.C. Neurocognitive signs in prodromal Huntington disease. Neuropsychology. 2011;25(1):1–14. doi: 10.1037/a0020937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann M., Dupoux E., Cesaro P., Bachoud-Lévi A.C. The role of the striatum in sentence processing: Evidence from a priming study in early stages of Huntington's disease. Neuropsychologia. 2008;46(1):174–185. doi: 10.1016/j.neuropsychologia.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Teichmann M., Dupoux E., Kouider S., Bachoud-Lévi A.C. The role of the striatum in processing language rules: Evidence from word perception in Huntington's disease. Journal of Cognitive Neuroscience. 2006;18(9):1555–1569. doi: 10.1162/jocn.2006.18.9.1555. [DOI] [PubMed] [Google Scholar]
- Teichmann M., Dupoux E., Kouider S., Brugières P., Boissé M.F., Baudic S. The role of the striatum in rule application: The model of Huntington's disease at early stage. Brain. 2005;128(5):1155–1167. doi: 10.1093/brain/awh472. [DOI] [PubMed] [Google Scholar]
- Teichmann M., Gaura V., Démonet J.F., Supiot F., Delliaux M., Verny C. Language processing within the striatum: Evidence from a PET correlation study in Huntington's disease. Brain. 2008;131(4):1046–1056. doi: 10.1093/brain/awn036. [DOI] [PubMed] [Google Scholar]
- Tobin A.J., Signer E.R. Huntington's disease: The challenge for cell biologists. Trends in Cell Biology. 2000;10(12):531–536. doi: 10.1016/s0962-8924(00)01853-5. [DOI] [PubMed] [Google Scholar]
- Tombaugh T.N. Trail making test A and B: Normative data stratified by age and education. Archives of Clinical Neuropsychology. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Ullman M.T., Corkin S., Coppola M., Hickok G., Growdon J.H., Koroshetz W.J. A neural dissociation within language: Evidence that the mental dictionary is part of declarative memory, and that grammatical rules are processed by the procedural system. Journal of Cognitive Neuroscience. 1997;9:266–276. doi: 10.1162/jocn.1997.9.2.266. [DOI] [PubMed] [Google Scholar]
- Vogel A.P., Shirbin C., Churchyard A.J., Stout J.C. Speech acoustic markers of early stage and prodromal Huntington's disease: A marker of disease onset? Neuropsychologia. 2012;50(14):3273–3278. doi: 10.1016/j.neuropsychologia.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Vonsattel J.P.G., DiFiglia M. Huntington disease. Journal of Neuropathology & Experimental Neurology. 1998;57(5):369–384. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Wechsler D. NCS Pearson; San Antonio, TX: 2008. Wechsler adult intelligence scale – fourth edition (WAIS-IV) [Google Scholar]
- Zinken J., Blakemore C., Zinken K., Butler L., Skinner T.C. Narrating psychological distress: Associations between cross-clausal integration and mental health difficulties. Applied Psycholinguistics. 2011;32(2):263–274. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.