Abstract
The spirochete bacterium Borrelia burgdorferi sensu lato is the causative agent of Lyme disease, the most common vector-borne disease in Europe and the United States. The spirochetes can be transmitted to humans via ticks, and then spread to different tissues, leading to arthritis, carditis and neuroborreliosis. Although antibiotics have commonly been used to treat infected individuals, some treated patients do not respond to antibiotics and experience persistent, long-term arthritis. Thus, there is a need to investigate alternative therapeutics against Lyme disease. The spirochete bacterium colonization is partly attributed to the binding of the bacterial outer-surface proteins to the glycosaminoglycan (GAG) chains of host proteoglycans. Blocking the binding of these proteins to GAGs is a potential strategy to prevent infection. In this review, we have summarized the recent reports of B. burgdorferi sensu lato GAG-binding proteins and discussed the potential use of synthetic and semi-synthetic compounds, including GAG analogues, to block pathogen interaction with GAGs. Such information should motivate the discovery and development of novel GAG analogues as new therapeutics for Lyme disease. New therapeutic approaches should eventually reduce the burden of Lyme disease and improve human health.
Keywords: Lyme disease, glycosaminoglycan, proteoglycan, adhesin, heparin, Borrelia burgdorferi
Introduction to Lyme disease
Lyme disease, which is caused by the spirochete Borrelia burgdorferi sensu lato and transmitted by Ixodes ticks, is the most common vector-borne disease in North America and Europe [1]. Three prominent species, B. burgdorferi, B. afzelii and B. garinii, are the causative agents of Lyme disease in Europe, whereas B. burgdorferi sensu stricto is the major species causing Lyme disease in North America [2]. Approximately 30 000 new Lyme disease cases are reported in the United States each year (mainly in the northeastern or midwestern United States), justifying the classification of Lyme disease borreliae as an ‘emerging pathogen’ [1]. Following the tick bite, the spirochete establishes infection by colonizing the bite site in the skin, resulting in the erythema migrans skin rash that is characteristic of the early acute phase of infection [1, 3]. If left untreated, Lyme disease borreliae is able to spread through the bloodstream to different tissues and organs, including the joints, heart and nervous system, leading to multiple disease manifestations, including arthritis, carditis and neuroborreliosis in the late chronic phase of infection [1].
Unfortunately, no effective prophylactic agents to protect humans from Lyme disease are currently available [1]. Whereas antibiotics are commonly used to treat Lyme disease patients in the early acute and late stages of infection [4], some antibiotic-treated individuals continue to demonstrate joint swelling and longstanding arthritis, known as antibiotic-refractory arthritis [5]. Therefore, there is a critical need to investigate other approaches as complementary methods for limiting disease progression and persistence. Lyme disease borreliae requires glycosaminoglycan (GAG)-binding activity to colonize and disseminate to tissues [6, 7]. GAG analogues may thus represent potential therapeutics to block Lyme disease infection. In fact, several kinds of such compounds (e.g. fucoidan, suramin and heparosan) have demonstrated the ability to inhibit the attachment of other pathogens to mammalian cells and reduce infectivity [8–12]. In addition, various GAG-based inhibitors have been examined for their safety in humans and are currently used in patients for different diseases [13], and this could potentially reduce the time required to develop such drugs as treatment for human Lyme disease. In this review, we have summarized the current findings on B. burgdorferi GAG-binding proteins and their ability to facilitate Lyme disease infection. We have also discussed the potential for developing GAG analogues as new therapeutic agents for the treatment of Lyme disease Borreliae to stop the progression of infection-induced manifestations.
Proteoglycans and glycosminoglycans, and the ability of Lyme disease borreliae to bind to these ligands
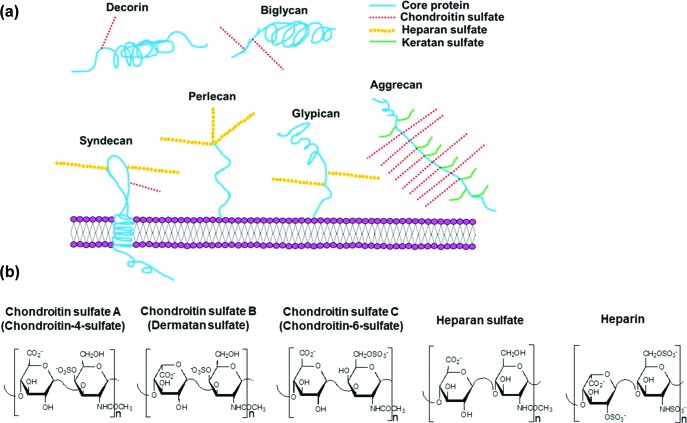
The capacity of Lyme disease borreliae to cause disease manifestations is correlated with its ability to colonize tissues or organs [14]. Tissue colonization is partly attributable to bacterial binding to the extracellular matrix (ECM) on the host cell surface [14]. Proteoglycans (PGs) are ECM molecules composed of a core protein, from which extend long, linear, and negatively charged polysaccharide chains called GAGs [15]. These PGs, including decorin, biglycan, aggrecan, syndecan, glypican and perlecan, are either inserted into the cell membrane or localized to the cell surface ECM (Fig. 1a) [15]. These GAGs are classified based on the structure and composition of their disaccharide repeating units [15]. For example, within the chondroitin sulfate family (indicated by the red dashed lines) of GAGs, there are multiple members with different disaccharide repeating units (Fig. 1b). The most common units are chondroitin-4-sulfate (type-A), dermatan sulfate (type-B) and chondroitin-6-sulfate (type-C) (Fig. 1b) [15]. Heparin, while not found on the cell surface, is structurally similar to other GAGs (Fig. 1b) and has been used as an important model compound for in vitro studies examining the GAG-binding activity of Lyme disease borreliae and their proteins [16–23].
Fig. 1.
Various proteoglycans (PG) produced on mammalian cell surfaces and the structure of disaccharide units of GAG proved to bind to Lyme disease borreliae. (a) Schematic diagram showing the composition of decorin, biglycan, aggrecan, the syndecan family, the glypican family, and perlecan PGs localized on mammalian cell surfaces. Note that there are different types of syndecans and glypicans, and these PGs differ in the numbers and types of GAG chains they carry. The syndecan and glypican depicted in the figure are representative structures. (b) The structure of the major disaccharide units of chondroitin sulfate A (chondoritin-4-sulfate), chondroitin sulfate B (dermatan sulfate, similar to chondroitin sulfate A except that it has an iduronic acid in place of the glucuronic acid residue), chondroitin sulfate C (chondroitin-6-sulfate), heparin, and heparan sulfate [while the major disaccharide of heparan sulfate primarily contains glucuronic acid acid (in place of the iduronic acid prominent in heparin) and is non-sulfated, this GAG can contain many minor sulfated disaccharides with sulfo groups occupying all the same sites found within heparin]. All of these GAGs have structural variability (in molecular weight and the positioning of sulfo groups) and are documented to bind to Lyme disease borreliae.
Lyme disease borreliae bind to different PGs, including decorin, biglycan and aggrecan (Fig. 1a) [24–26]. Decorin-deficient mice are more resistant to spirochete colonization in different tissues, suggesting that decorin-mediated spirochete binding promotes tissue colonization during Lyme infection [27]. B. burgdorferi binds less efficiently to the vascular endothelial cells in the absence of biglycan, suggesting that the biglycan-binding activity of spirochetes promotes the bacterial attachment to this cell type [25]. B. burgdorferi binding to aggrecan results in the degradation of aggrecan and other ECM components, which suggests that the aggrecan-mediated binding may promote the detachment of spirochetes from the initial infection site to disseminate to distal tissues [26, 28].
These PG-mediated binding activities of B. burgdorferi are probably associated with the ability of spirochetes to interact specifically with the GAG chains on these PGs. B. burgdorferi binds to different GAGs, including chondroitin sulfate, dermatan sulfate and heparan sulfate (Fig. 1b) [17, 29]. This binding activity of GAGs such as heparan sulfate, dermatan sulfate, and chondroitin sulfate mediates spirochete attachment to mammalian cells (e.g. epithelial Vero cells) [17]. Further, the ability of these GAGs to promote spirochete binding to cells correlates positively with the length of these GAG chains [16]. This finding suggests that the charge–charge interactions contribute to GAG-binding by B. burgdorferi.
GAG- and PG-binding of Lyme disease borreliae: DbpA and DbpB
Several outer-surface proteins have been shown to confer spirochete binding to GAGs or PGs (see the summary in Table 1). DbpA and DbpB are the first two PG-binding proteins that have been identified as binding to decorin and biglycan [25, 30]. These proteins were later found to bind to the core protein of decorin with higher affinity and the dermatan sulfate of this PG with slightly lower affinity, suggesting that both core protein and dermatan sulfate modulate the decorin-binding activity of DbpA and DbpB [20, 24]. DbpA and DbpB promote spirochete attachment to different mammalian cell types [21, 25, 31, 32] and are required for spirochete colonization during Lyme infection [33–35]. Whereas DbpB is highly conserved, DbpA is polymorphic, with less than 58 % amino acid identity between Lyme disease borreliae variants [36]. Consistent with this polymorphism, DbpA variants promote different allelic ability for binding to decorin, biglycan, dermatan sulfate and mammalian cells under either static or flow conditions [25, 31, 32]. Similarly, these variants also confer the distinct tissue tropism associated with Lyme infection [6]. The strain to strain variations of the in vitro and in vivo phenotypes promoted by DbpA have been attributed to the decorin- and dermatan sulfate-binding activity of this protein. DbpA mutant proteins that are specifically defective in binding to these ligands are incapable of promoting spirochete attachment to mammalian cells and the colonization of mouse tissues [6, 31, 37].
Table 1. The GAG- or PG-binding proteins of Lyme disease borreliae and their reported functions.
| GAG- or PG-binding protein | Species/strain | The demonstrated functions of GAG- or PG-binding proteins | Reference | |||||
|---|---|---|---|---|---|---|---|---|
| PG or GAG ligands | Cell binding | Vascular interaction via i.v. infection* | Tissue interaction via i.v. infection | Tissue colonization via s.c. infection† | ||||
| Static condition | Flow condition | |||||||
| DbpA | B. burgdorferi/B31, N40, N40-D10/E9, 297 | DC‡, BG§, DS|| | +¶ (GOF#, LOF**) | + (GOF) | ND†† | nd | + (LOF) | [6, 20, 21, 25, 30–35, 37] |
| B. garinii/PBr, SBK40 | DC, BG, DS | + (GOF, LOF) | + (GOF) | nd | nd | + (LOF) | [6, 25, 31, 32] | |
| B. afzelii/VS461, A91 | DC, BG, DS | + (GOF,LOF) | + (GOF) | nd | nd | + (LOF) | [6, 25, 31, 32] | |
| DbpB | B. burgdorferi/B31, N40, N40-D10/E9 | DC, BG, DS | + (GOF) | + (GOF) | nd | nd | + (LOF) | [25, 32, 34] |
| B. garinii/SBK40 | DC, BG | + (GOF) | + (GOF) | nd | nd | nd | [25, 32] | |
| B. afzelii/A91 | DC, BG | + (GOF) | + (GOF) | nd | nd | nd | [25, 32] | |
| BBK32 | B. burgdorferi/B31 | DS | + (GOF, LOF) | + (GOF, LOF) | + (GOF, LOF) | + (GOF) | + (LOF) | [7, 22, 39, 40, 42, 43, 71] |
| OspF family proteins | ||||||||
| ErpG (OspG) | B. burgdorferi/B31 | HS‡‡ | + (GOF) | nd | nd | nd | nd | [23] |
| ErpK | B. burgdorferi/B31 | HS | nd | nd | nd | nd | nd | [23] |
| ErpL | B. burgdorferi/B31 | HS | nd | nd | nd | nd | nd | [23] |
| ErpY | B. burgdorferi/B31 | HS | nd | nd | nd | nd | nd | [23] |
| OspF | B. burgdorferi/297 | HS | nd | nd | nd | nd | nd | [23] |
| Erp25 | B. burgdorferi/N40-D10/E9 | HS | nd | nd | nd | nd | nd | [23] |
| Erp27 | B. burgdorferi/N40-D10/E9 | HS | nd | nd | nd | nd | nd | [23] |
| Lmp1 | B. burgdorferi/B31 | C6S§§ | + (LOF) | nd | nd | nd | + (LOF) | [49–51] |
| BbHtrA | B. burgdorferi/B31 | AG|||| | nd | nd | nd | nd | + (LOF) | [26, 28, 56] |
*i.v., intravenous.
†s.c., subcutaneous.
‡ DC, decorin.
§BG, biglycan.
||DS, dermatan sulfate.
¶+, a positive result shown when particular proteins are produced in the respective strain background (gain of function or loss of function strain).
#GOF, gain of function strains. Because genetic manipulation is not available in species other than B. burgdorferi, the function tested using gain of function strains was performed in the background of a high-passage and non-adherent B. burgdorferi strain, B313, B314 or B31A.
**LOF, loss of function strains. Because genetic manipulation is not available in the species other than B. burgdorferi, the function tested using loss of function strains was performed in the background of B. burgdorferi strains B31 or 297.
††nd, not determined, which indicates that particular proteins have not been examined for specific activities.
‡‡HS, heparan sulfate.
§§C6S, chondroitin-6-sulfate.
||||AG, aggrecan.
BBK32
The B. burgdorferi outer-surface protein BBK32 was initially reported to bind to an ECM protein fibronectin and was later identified to also bind to dermatan sulfate [22, 38]. Inoculating mice with a low dose of a bbk32-deficient B. burgdorferi results in reduced colonization at the inoculation site (skin) and joints at early stages of infection, indicating the essential role of BBK32 in promoting optimal infectivity [39, 40]. In addition, ectopically producing BBK32 in a non-infectious and non-adherent B. burgdorferi (gain-of-function strain) leads to spirochete attachment to mammalian cells in vitro and localization at joints in vivo during short-term intravenous inoculation [7, 22]. Using intra-vital microscopy, this BBK32-producing strain has also been demonstrated to attach to vasculature by promoting transient interaction, including tethering and dragging of spirochetes [41, 42]. A bbk32-deficient B. burgdorferi displays decreased levels of binding, specifically to joint vasculature, indicating that BBK32 is a vascular adhesin [43].
The dermatan sulfate- and fibronectin-binding activities of BBK32 have been localized at amino acids 45–68 and 158–182, respectively [7]. The gain-of-function B. burgdorferi producing BBK32 with internal deletion at amino acids 45–68 (BBK32Δ45–68) or 158–182 (BBK32Δ158–182) is incapable of binding to mammalian cells, but the cell types that each of these strains are unable to bind vary [7]. Compared to the strain producing BBK32Δ158–182, the gain-of-function strain producing BBK32Δ45–68 displays reduced dragging interactions with the vasculature, indicating that BBK32-mediated GAG binding contributes to vascular interaction [43]. In addition, the BBK32Δ45–68 producing strain binds to joints less efficiently than a wild-type BBK32-producing strain during short-term intravenous inoculation [7]. Consistent with this observation, a bbk32-deficient B. burgdorferi producing BBK32Δ45–68 colonizes mouse joints less than the gain-of-function strain producing wild-type BBK32 [7]. These results indicate that dermatan sulfate binding of BBK32 confers spirochete localization and colonization specifically at joints.
OspF family proteins
By inoculating mice with phages producing peptides derived from different B. burgdorferi proteins (known as in vivo phage display), ErpK, ErpL, ErpG and BB2.10 have been identified as adhesins that promote the phages binding to the joints, bladders and hearts of mice [44]. These proteins are in the OspF protein family, a sub-family of the Erp proteins (OspEF-related proteins) [45–47]. All OspF family proteins bind to heparan sulfate [23]. ErpG promotes spirochete binding to C6 glial cells, but not other cell types [23], and an ErpG mutant that is defective in heparan sulfate-binding activity is incapable of conferring spirochete attachment to C6 glial cells [23]. B. burgdorferi mutant strains with transposons inserted in erpK display a survival disadvantage in colonizing mouse ears, hearts, joints and inoculation sites [48]. These results suggest that OspF family proteins contribute to mammalian cell attachment and spirochete colonization during infection, likely by their heparan sulfate-binding activity.
Lmp1
In vivo phage display also reveals B. burgdorferi outer-surface protein Lmp1 as an adhesin [44]. Consistent with this finding, an lmp1-deficient spirochete binds to chondroitin-6-sulfate and mammalian cells at decreased levels compared to the wild-type parental strain [49]. The cell- and chondroitin-6-sulfate-binding activities are specifically promoted by the middle region of Lmp1 (Lmp1M), which houses unique repeating sequences of 54 amino acids and folds to an α-helix rich structure [49–51]. In addition, spirochetes that lack Lmp1 production are non-infectious in mice via subcutaneous needle infection [50]. Producing Lmp1M in the lmp1-deficient strain background restores the colonization defects, raising the possibility that the chondroitin-6-sulfate-binding activity of this protein confers infectivity [49].
BbHtrA
B. burgdorferi BbHtrA belongs to the family of high-temperature-requiring proteases, acting as a protease or chaperone to stabilize proteins and regulate signalling processes [52, 53]. This protein was first identified as an aggrecan-binding protein [26] and was then demonstrated to be capable of digesting B. burgdorferi surface proteins, as well as host aggrecan and other ECM molecules, including fibronectin, decorin, and biglycan [28, 54, 55]. The finding that BbHtrA degrades ECM molecules raises the possibility that this protein, by digesting the B. burgdorferi-ECM interactions, facilitates spirochete detachment from the initial infection tissues and dissemination during Lyme infection. Consistent with this, BbHtrA-deficient B. burgdorferi is unable to colonize the inoculation site, heart and bladder during murine infection [56]. However, the protease activity of BbHtrA also targets other adhesion-irrelevant spirochete proteins that are required for bacterial survival [26, 56]. The relevance of the ability of this protein to digest ECM components and contribute to spirochete dissemination still needs to be determined.
As described above, B. burgdorferi produces numerous GAG- and PG-binding proteins that are essential for host colonization and tissue dissemination during infection. The production of these proteins at different stages of infection may reflect the requirement for multiple GAG-binding proteins in B. burgdorferi. Therefore, developing structurally similar molecules (e.g. GAG analogues) as inhibitors and inoculating these inhibitors during infection to block the spirochete attachment to GAGs or PGs of host cells may represent an effective strategy to treat infection with Lyme disease borreliae.
The potential for using synthetic or semisynthetic GAGs as new treatments against Lyme disease
Numerous pathogens, including Lyme disease borreliae, bind to GAG via surface proteins, which promote colonization and dissemination to tissues and organs [57, 58]. Blocking GAG–pathogen interactions has thus been considered to be an efficient mean of eliminating such infections. One strategy to prevent pathogen–GAG interactions is to identify the GAG-binding proteins of pathogens and then design small-molecule analogues to mimic the motif directly contributing to the GAG-binding activity of these proteins. Surfen (bis-2-methyl-4-amino-quinolyl-6-carbamide) was initially developed as an excipient during insulin production. The ability of this small molecule to neutralize the function of heparin motivated further research that identified surfen as an inhibitor for heparan sulfate-mediated cell attachment of herpes simplex virus [59, 60].
The other strategy to block pathogen–GAG interactions is to use GAG analogues, which bind to GAG-binding proteins on the pathogens to prevent their attachment to and colonization of host cells. Several heparin and heparan sulfate-analogues (as known as heparinoids) have been shown to reduce microbial infections, likely due to their ability to block the pathogen–heparan sulfate interaction. The heparinoid ‘fucoidan’, extracted from brown macroalgae, has been demonstrated to decrease infections caused by various viruses, parasites and bacteria [8, 9]. The documented ability of the synthetic heparinoid ‘suramin’ to inhibit viral and parasite infection resulted in the use of this compound as a treatment for African trypanosomiasis and dengue fever [10]. Another heparinoid, ‘heparosan’, derived from the capsule of some pathogenic bacteria, inhibits bacterial attachment to mammalian cells [11, 12]. These findings raise the possibility of developing GAG analogues as a therapeutic agent to treat Lyme disease infection by blocking the GAG-binding protein-mediated spirochete attachment.
GAGs, including heparin, mediate multiple host functions such as anticoagulation, signal transduction of organ development, host inflammatory response and cell migration [61]. Therefore, one of the documented side-effects of GAG-based therapeutic agents is internal bleeding or thrombocytopenia, caused by the potent anticoagulant activity of these agents [62]. However, periodate oxidation has been applied to these heparin-based compounds to cut through the glucuronic acid in the active site causing the anticoagulant ability while retaining the other biological activities of heparin, generating non-anticoagulant heparins [62, 63]. In addition, unfractionated heparin is usually isolated from porcine skin or bovine lung, with an average molecular weight of approximately 14 000 Da. The heterogeneity and high molecular weight of these molecules make their efficient absorption difficult in humans [64]. The recent development of chemoenzymatic synthesis has been utilized to prepare more specific low and ultra-low molecular weight heparins with improved bioavailability and pharmacodynamics [65, 66] A low-molecular-weight non-anticoagulant heparin (NACH) has been synthesized with low toxicity in vivo and enhanced efficacy for inhibiting tumour metastasis [67–70]. Thus, NACH may have the potential to be developed as a therapeutic against Lyme disease-causing bacteria. Testing of the efficacy of this compound as an anti-B. burgdorferi prophylactic agent is currently ongoing in our laboratory. Further, a previous observation that B. burgdorferi treated with Dalteparin (a low-molecular-weight heparin) displays a >75 % reduction of vascular interaction compared to untreated spirochetes [42]. This result suggests the possibility of employing GAG analogues as new prophylaxes and treatments for Lyme disease by blocking the haematogenous dissemination and tissue colonization of Lyme disease borreliae.
Conclusion and future work
GAG-binding activity has been demonstrated to mediate the colonization and dissemination of Lyme disease borreliae. Thus, blocking spirochete attachment to host cells may inhibit disease progression and eventually eradicate these bacteria from humans. GAGs and GAG analogues have been examined for their ability to inhibit the attachment of other pathogens to mammalian cells or tissues. Some of these compounds also display a robust capacity for eliminating pathogen infections. These observations suggest the potential use of GAG analogues as therapeutic agents to treat Lyme disease. Several spirochete GAG-binding proteins have been identified as promoting disease manifestations, which may further facilitate the development of drugs acting against Lyme disease by targeting the binding of these proteins to GAGs. In this review, we have discussed and summarized previous findings concerning spirochete proteins mediating the GAG-binding activity of Lyme disease-causing bacteria, as well as the development of GAG analogues as therapeutics. Such information will provide new directions for the use of GAG analogues as treatments for Lyme disease patients to improve the health of people suffering from Lyme infection.
Funding information
This work was supported by a New York State Department of Health Wadsworth Center Start-Up grant (to Y. L.), as well as the following grants: NIH R01HL094463 and NIH R21HL136271 (to R. J. L.), and NIH R01 HL125371 (to R. J. L. and F. Z.).
Acknowledgements
We thank Rhodaba Ebady (University of Toronto), Anna Boczula (University of Toronto) and Ashley Marcinkiewicz (New York State Department of Health) for critical reading of the manuscript.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: AG, aggrecan; BG, biglycan; C6S, chondroitin-6-sulfate; DC, decorin; DS, dermatan sulfate; ECM, extracellular matrix; GAG, glycosminoglycan; GOF, gain of function; HS, heparan sulfate; Lmp1M, middle region of Lmp1; LOF, loss of function; NACH, non-anticoagulant heparin; ND, not determined; PG, proteoglycan.
References
- 1.Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, et al. Lyme borreliosis. Nat Rev Dis Primers. 2016;2:16090. doi: 10.1038/nrdp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tilly K, Rosa PA, Stewart PE. Biology of infection with Borrelia burgdorferi. Infect Dis Clin North Am. 2008;22:217–234. doi: 10.1016/j.idc.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radolf JD, Caimano MJ, Stevenson B, Hu LT, Lt H. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanchez E, Vannier E, Wormser GP, Hu LT. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA. 2016;315:1767–1777. doi: 10.1001/jama.2016.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steere AC, Angelis SM. Therapy for Lyme arthritis: strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum. 2006;54:3079–3086. doi: 10.1002/art.22131. [DOI] [PubMed] [Google Scholar]
- 6.Lin YP, Benoit V, Yang X, Martínez-Herranz R, Pal U, et al. Strain-specific variation of the decorin-binding adhesin DbpA influences the tissue tropism of the lyme disease spirochete. PLoS Pathog. 2014;10:e1004238. doi: 10.1371/journal.ppat.1004238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin YP, Chen Q, Ritchie JA, Dufour NP, Fischer JR, et al. Glycosaminoglycan binding by Borrelia burgdorferi adhesin BBK32 specifically and uniquely promotes joint colonization. Cell Microbiol. 2015;17:860–875. doi: 10.1111/cmi.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berteau O, Mulloy B. Sulfated fucans, fresh perspectives: structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology. 2003;13:29R–40. doi: 10.1093/glycob/cwg058. [DOI] [PubMed] [Google Scholar]
- 9.Fitton JH. Therapies from fucoidan; multifunctional marine polymers. Mar Drugs. 2011;9:1731–1760. doi: 10.3390/md9101731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mcgeary RP, Bennett AJ, Tran QB, Cosgrove KL, Ross BP. Suramin: clinical uses and structure-activity relationships. Mini Rev Med Chem. 2008;8:1384–1394. doi: 10.2174/138955708786369573. [DOI] [PubMed] [Google Scholar]
- 11.Li P, Sheng J, Liu Y, Li J, Liu J, et al. Heparosan-derived heparan sulfate/heparin-like compounds: one kind of potential therapeutic agents. Med Res Rev. 2013;33:665–692. doi: 10.1002/med.21263. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Ling P, Duan R, Zhang T. Effects of heparosan and heparin on the adhesion and biofilm formation of several bacteria in vitro. Carbohydr Polym. 2012;88:1288–1292. [Google Scholar]
- 13.Volpi N. Therapeutic applications of glycosaminoglycans. Curr Med Chem. 2006;13:1799–1810. doi: 10.2174/092986706777452470. [DOI] [PubMed] [Google Scholar]
- 14.Coburn J, Leong J, Chaconas G. Illuminating the roles of the Borrelia burgdorferi adhesins. Trends Microbiol. 2013;21:372–379. doi: 10.1016/j.tim.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Ly M, Linhardt RJ. Proteoglycan sequence. Mol Biosyst. 2012;8:1613–1625. doi: 10.1039/c2mb25021g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leong JM, Robbins D, Rosenfeld L, Lahiri B, Parveen N. Structural requirements for glycosaminoglycan recognition by the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1998;66:6045–6048. doi: 10.1128/iai.66.12.6045-6048.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leong JM, Wang H, Magoun L, Field JA, Morrissey PE, et al. Different classes of proteoglycans contribute to the attachment of Borrelia burgdorferi to cultured endothelial and brain cells. Infect Immun. 1998;66:994–999. doi: 10.1128/iai.66.3.994-999.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parveen N, Robbins D, Leong JM. Strain variation in glycosaminoglycan recognition influences cell-type-specific binding by lyme disease spirochetes. Infect Immun. 1999;67:1743–1749. doi: 10.1128/iai.67.4.1743-1749.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parveen N, Leong JM. Identification of a candidate glycosaminoglycan-binding adhesin of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:1220–1234. doi: 10.1046/j.1365-2958.2000.01792.x. [DOI] [PubMed] [Google Scholar]
- 20.Parveen N, Caimano M, Radolf JD, Leong JM. Adaptation of the Lyme disease spirochaete to the mammalian host environment results in enhanced glycosaminoglycan and host cell binding. Mol Microbiol. 2003;47:1433–1444. doi: 10.1046/j.1365-2958.2003.03388.x. [DOI] [PubMed] [Google Scholar]
- 21.Fischer JR, Parveen N, Magoun L, Leong JM. Decorin-binding proteins A and B confer distinct mammalian cell type-specific attachment by Borrelia burgdorferi, the Lyme disease spirochete. Proc Natl Acad Sci USA. 2003;100:7307–7312. doi: 10.1073/pnas.1231043100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer JR, LeBlanc KT, Leong JM. Fibronectin binding protein BBK32 of the Lyme disease spirochete promotes bacterial attachment to glycosaminoglycans. Infect Immun. 2006;74:435–441. doi: 10.1128/IAI.74.1.435-441.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin YP, Bhowmick R, Coburn J, Leong JM. Host cell heparan sulfate glycosaminoglycans are ligands for OspF-related proteins of the Lyme disease spirochete. Cell Microbiol. 2015;17:1464–1476. doi: 10.1111/cmi.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo BP, Norris SJ, Rosenberg LC, Höök M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salo J, Pietikäinen A, Söderström M, Auvinen K, Salmi M, et al. Flow-tolerant adhesion of a bacterial pathogen to human endothelial cells through interaction with biglycan. J Infect Dis. 2016;213:1623–1631. doi: 10.1093/infdis/jiw003. [DOI] [PubMed] [Google Scholar]
- 26.Russell TM, Johnson BJ. Lyme disease spirochaetes possess an aggrecan-binding protease with aggrecanase activity. Mol Microbiol. 2013;90:228–240. doi: 10.1111/mmi.12276. [DOI] [PubMed] [Google Scholar]
- 27.Brown EL, Wooten RM, Johnson BJ, Iozzo RV, Smith A, et al. Resistance to Lyme disease in decorin-deficient mice. J Clin Invest. 2001;107:845–852. doi: 10.1172/JCI11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell TM, Delorey MJ, Johnson BJ. Borrelia burgdorferi BbHtrA degrades host ECM proteins and stimulates release of inflammatory cytokines in vitro. Mol Microbiol. 2013;90:241–251. doi: 10.1111/mmi.12377. [DOI] [PubMed] [Google Scholar]
- 29.Isaacs RD. Borrelia burgdorferi bind to epithelial cell proteoglycans. J Clin Invest. 1994;93:809–819. doi: 10.1172/JCI117035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo BP, Brown EL, Dorward DW, Rosenberg LC, Höök M. Decorin-binding adhesins from Borrelia burgdorferi. Mol Microbiol. 1998;30:711–723. doi: 10.1046/j.1365-2958.1998.01103.x. [DOI] [PubMed] [Google Scholar]
- 31.Benoit VM, Fischer JR, Lin YP, Parveen N, Leong JM. Allelic variation of the Lyme disease spirochete adhesin DbpA influences spirochetal binding to decorin, dermatan sulfate, and mammalian cells. Infect Immun. 2011;79:3501–3509. doi: 10.1128/IAI.00163-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salo J, Loimaranta V, Lahdenne P, Viljanen MK, Hytönen J. Decorin binding by DbpA and B of Borrelia garinii, Borrelia afzelii, and Borrelia burgdorferi sensu Stricto. J Infect Dis. 2011;204:65–73. doi: 10.1093/infdis/jir207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weening EH, Parveen N, Trzeciakowski JP, Leong JM, Höök M, et al. Borrelia burgdorferi lacking DbpBA exhibits an early survival defect during experimental infection. Infect Immun. 2008;76:5694–5705. doi: 10.1128/IAI.00690-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Y, Xu Q, McShan K, Liang FT. Both decorin-binding proteins A and B are critical for the overall virulence of Borrelia burgdorferi. Infect Immun. 2008;76:1239–1246. doi: 10.1128/IAI.00897-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blevins JS, Hagman KE, Norgard MV. Assessment of decorin-binding protein A to the infectivity of Borrelia burgdorferi in the murine models of needle and tick infection. BMC Microbiol. 2008;8:82. doi: 10.1186/1471-2180-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts WC, Mullikin BA, Lathigra R, Hanson MS. Molecular analysis of sequence heterogeneity among genes encoding decorin binding proteins A and B of Borrelia burgdorferi sensu lato. Infect Immun. 1998;66:5275–5285. doi: 10.1128/iai.66.11.5275-5285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fortune DE, Lin YP, Deka RK, Groshong AM, Moore BP, et al. Identification of lysine residues in the Borrelia burgdorferi DbpA adhesin required for murine infection. Infect Immun. 2014;82:3186–3198. doi: 10.1128/IAI.02036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Probert WS, Johnson BJ. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol Microbiol. 1998;30:1003–1015. doi: 10.1046/j.1365-2958.1998.01127.x. [DOI] [PubMed] [Google Scholar]
- 39.Seshu J, Esteve-Gassent MD, Labandeira-Rey M, Kim JH, Trzeciakowski JP, et al. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol Microbiol. 2006;59:1591–1601. doi: 10.1111/j.1365-2958.2005.05042.x. [DOI] [PubMed] [Google Scholar]
- 40.Hyde JA, Weening EH, Chang M, Trzeciakowski JP, Höök M, et al. Bioluminescent imaging of Borrelia burgdorferi in vivo demonstrates that the fibronectin-binding protein BBK32 is required for optimal infectivity. Mol Microbiol. 2011;82:99–113. doi: 10.1111/j.1365-2958.2011.07801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moriarty TJ, Norman MU, Colarusso P, Bankhead T, Kubes P, et al. Real-time high resolution 3D imaging of the lyme disease spirochete adhering to and escaping from the vasculature of a living host. PLoS Pathog. 2008;4:e1000090. doi: 10.1371/journal.ppat.1000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norman MU, Moriarty TJ, Dresser AR, Millen B, Kubes P, et al. Molecular mechanisms involved in vascular interactions of the Lyme disease pathogen in a living host. PLoS Pathog. 2008;4:e1000169. doi: 10.1371/journal.ppat.1000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moriarty TJ, Shi M, Lin YP, Ebady R, Zhou H, et al. Vascular binding of a pathogen under shear force through mechanistically distinct sequential interactions with host macromolecules. Mol Microbiol. 2012;86:1116–1131. doi: 10.1111/mmi.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antonara S, Chafel RM, LaFrance M, Coburn J. Borrelia burgdorferi adhesins identified using in vivo phage display. Mol Microbiol. 2007;66:262–276. doi: 10.1111/j.1365-2958.2007.05924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akins DR, Caimano MJ, Yang X, Cerna F, Norgard MV, et al. Molecular and evolutionary analysis of Borrelia burgdorferi 297 circular plasmid-encoded lipoproteins with OspE- and OspF-like leader peptides. Infect Immun. 1999;67:1526–1532. doi: 10.1128/iai.67.3.1526-1532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caimano MJ, Yang X, Popova TG, Clawson ML, Akins DR, et al. Molecular and evolutionary characterization of the cp32/18 family of supercoiled plasmids in Borrelia burgdorferi 297. Infect Immun. 2000;68:1574–1586. doi: 10.1128/IAI.68.3.1574-1586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brissette CA, Cooley AE, Burns LH, Riley SP, Verma A, et al. Lyme borreliosis spirochete Erp proteins, their known host ligands, and potential roles in mammalian infection. Int J Med Microbiol. 2008;298 Suppl 1:257–267. doi: 10.1016/j.ijmm.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin T, Gao L, Zhang C, Odeh E, Jacobs MB, et al. Analysis of an ordered, comprehensive STM mutant library in infectious Borrelia burgdorferi: insights into the genes required for mouse infectivity. PLoS One. 2012;7:e47532. doi: 10.1371/journal.pone.0047532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang X, Lin YP, Heselpoth RD, Buyuktanir O, Qin J, et al. Middle region of the Borrelia burgdorferi surface-located protein 1 (Lmp1) interacts with host chondroitin-6-sulfate and independently facilitates infection. Cell Microbiol. 2016;18:97–110. doi: 10.1111/cmi.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang X, Coleman AS, Anguita J, Pal U. A chromosomally encoded virulence factor protects the Lyme disease pathogen against host-adaptive immunity. PLoS Pathog. 2009;5:e1000326. doi: 10.1371/journal.ppat.1000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang X, Lenhart TR, Kariu T, Anguita J, Akins DR, et al. Characterization of unique regions of Borrelia burgdorferi surface-located membrane protein 1. Infect Immun. 2010;78:4477–4487. doi: 10.1128/IAI.00501-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clausen T, Southan C, Ehrmann M. The HtrA family of proteases: implications for protein composition and cell fate. Mol Cell. 2002;10:443–455. doi: 10.1016/s1097-2765(02)00658-5. [DOI] [PubMed] [Google Scholar]
- 53.Clausen T, Kaiser M, Huber R, Ehrmann M. HTRA proteases: regulated proteolysis in protein quality control. Nat Rev Mol Cell Biol. 2011;12:152–162. doi: 10.1038/nrm3065. [DOI] [PubMed] [Google Scholar]
- 54.Coleman JL, Toledo A, Benach JL. Borrelia burgdorferi HtrA: evidence for twofold proteolysis of outer membrane protein p66. Mol Microbiol. 2016;99:135–150. doi: 10.1111/mmi.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coleman JL, Crowley JT, Toledo AM, Benach JL. The HtrA protease of Borrelia burgdorferi degrades outer membrane protein BmpD and chemotaxis phosphatase CheX. Mol Microbiol. 2013;88:619–633. doi: 10.1111/mmi.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ye M, Sharma K, Thakur M, Smith AA, Buyuktanir O, et al. HtrA, a Temperature- and stationary phase-activated protease involved in maturation of a key microbial virulence determinant, facilitates borrelia burgdorferi infection in mammalian hosts. Infect Immun. 2016;84:2372–2381. doi: 10.1128/IAI.00360-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.García B, Merayo-Lloves J, Martin C, Alcalde I, Quirós LM, et al. Surface proteoglycans as mediators in bacterial pathogens infections. Front Microbiol. 2016;7:220. doi: 10.3389/fmicb.2016.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamhi E, Joo EJ, Dordick JS, Linhardt RJ. Glycosaminoglycans in infectious disease. Biol Rev Camb Philos Soc. 2013;88:928–943. doi: 10.1111/brv.12034. [DOI] [PubMed] [Google Scholar]
- 59.Hunter FR. Evidence for facilitated diffusion of anion in erythrocytes. Nature. 1967;213:816–817. doi: 10.1038/213816a0. [DOI] [PubMed] [Google Scholar]
- 60.Schuksz M, Fuster MM, Brown JR, Crawford BE, Ditto DP, et al. Surfen, a small molecule antagonist of heparan sulfate. Proc Natl Acad Sci USA. 2008;105:13075–13080. doi: 10.1073/pnas.0805862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Linhardt RJ, Toida T. Role of glycosaminoglycans in cellular communication. Acc Chem Res. 2004;37:431–438. doi: 10.1021/ar030138x. [DOI] [PubMed] [Google Scholar]
- 62.Islam T, Butler M, Sikkander SA, Toida T, Linhardt RJ. Further evidence that periodate cleavage of heparin occurs primarily through the antithrombin binding site. Carbohydr Res. 2002;337:2239–2243. doi: 10.1016/S0008-6215(02)00229-X. [DOI] [PubMed] [Google Scholar]
- 63.Casu B, Diamantini G, Fedeli G, Mantovani M, Oreste P, et al. Retention of antilipemic activity by periodate-oxidized non-anticoagulant heparins. Arzneimittelforschung. 1986;36:637–642. [PubMed] [Google Scholar]
- 64.Mousa SA, Linhardt R, Francis JL, Amirkhosravi A. Anti-metastatic effect of a non-anticoagulant low-molecular-weight heparin versus the standard low-molecular-weight heparin, enoxaparin. Thromb Haemost. 2006;96:816–821. doi: 10.1160/TH06-05-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu Y, Masuko S, Takieddin M, Xu H, Liu R, et al. Chemoenzymatic synthesis of homogeneous ultralow molecular weight heparins. Science. 2011;334:498–501. doi: 10.1126/science.1207478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu Y, Chandarajoti K, Zhang X, Pagadala V, Dou W, et al. Synthetic oligosaccharides can replace animal-sourced low-molecular weight heparins. Sci Transl Med. 2017;9:eaan5954. doi: 10.1126/scitranslmed.aan5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sudha T, Phillips P, Kanaan C, Linhardt RJ, Borsig L, et al. Inhibitory effect of non-anticoagulant heparin (S-NACH) on pancreatic cancer cell adhesion and metastasis in human umbilical cord vessel segment and in mouse model. Clin Exp Metastasis. 2012;29:431–439. doi: 10.1007/s10585-012-9461-9. [DOI] [PubMed] [Google Scholar]
- 68.Sudha T, Yalcin M, Lin HY, Elmetwally AM, Nazeer T, et al. Suppression of pancreatic cancer by sulfated non-anticoagulant low molecular weight heparin. Cancer Lett. 2014;350:25–33. doi: 10.1016/j.canlet.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alshaiban A, Muralidharan-Chari V, Nepo A, Mousa SA. Modulation of sickle red blood cell adhesion and its associated changes in biomarkers by sulfated nonanticoagulant heparin derivative. Clin Appl Thromb Hemost. 2016;22:230–238. doi: 10.1177/1076029614565880. [DOI] [PubMed] [Google Scholar]
- 70.Alyahya R, Sudha T, Racz M, Stain SC, Mousa SA. Anti-metastasis efficacy and safety of non-anticoagulant heparin derivative versus low molecular weight heparin in surgical pancreatic cancer models. Int J Oncol. 2015;46:1225–1231. doi: 10.3892/ijo.2014.2803. [DOI] [PubMed] [Google Scholar]
- 71.Ebady R, Niddam AF, Boczula AE, Kim YR, Gupta N, et al. Biomechanics of Borrelia burgdorferi vascular interactions. Cell Rep. 2016;16:2593–2604. doi: 10.1016/j.celrep.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]