Abstract
Background
Clinical trial evidence shows minimal survival gains and higher complication rates from prostatectomy (RP) versus watchful waiting (WW) for elderly men with localized prostate cancer. It is believed that these patients are overtreated. The current analyses aim to explore patient-level heterogeneity in survival effects, examine matching of patients to treatments in practice, and identify patient characteristics driving heterogeneous effects, in order to present more comprehensive evidence about the concerns of overtreatment.
Methods
11-year all-cause and prostate cancer-specific survival among SEER-Medicare patient diagnosed during 1996 to 2002 were analyzed using local instrumental variable approaches.
Results
8,462 (77%) out of 11,036 patients received RP. The average effects of RP over WW on 11-year overall and cancer-specific survival were 1.1 months (95%CI: −25, 28; p = 0.94) and 1.7 months (95%CI: −25, 29; p = 0.90) respectively; effects did not differ significantly according to age, race, grade and stage. Fewer than 1% of patients had significant cancer-specific survival benefit from RP at the 10% level; 6% were expected to gain over 15 months from RP. However, patients with larger expected survival gains from RP were much more likely to receive RP in practice. Such positive self-selection was driven by prostate cancer-specific survival than overall survival. Several comorbidities may play a critical role in predicting who could benefit from RP.
Conclusions
Our analyses corroborate concerns about prostate cancer overtreatment. A small fraction of screen-detected prostate cancer patients derive survival benefits from RP. Prediction tools should account for patient comorbidities to accurately predict survival benefits of RP over WW.
Introduction
Elderly men (65+ years) account for over 60% of patients diagnosed with prostate cancer (PCa); 80% of those diagnoses are for clinically localized cancer and a majority of patients receive aggressive treatments such as surgery or radiation therapy.1 Herein, we focus on the comparative effectiveness evidence underlying a comparison of surgery with radical prostatectomy (RP) versus watchful waiting (WW) within six months of diagnosis of localized PCa among elderly patients and extensively explores the heterogeneity of these effects across patient characteristics in order to assess concerns that patients with prostate cancer are overtreated during the first six months after diagnosis.2,3
Several randomized controlled trials and observational studies have compared surgery with observation for PCa. Prior to the era of prevalent prostate-specific antigen (PSA) screening, an 18-year long Scandinavian trial found no significant difference between RP and WW in overall survival among the few elderly patients it had enrolled.4 The 12 year-long PIVOT trial, which enrolled localized PCa patients detected during the early era of PSA screening in the US, found that RP was associated with a non-significant reduction in all-cause and PCa-specific mortality compared with observation among elderly men.5 Hadley and colleagues studied the comparative effectiveness of RP versus WW in elderly patients using SEER-Medicare data with an instrumental variable approach and found results similar to the PIVOT trial.6 Xia, et al. used micro-simulation to demonstrate a 1.8-month increase in life expectancy for men treated with RP compared with active surveillance.7
The summaries from these studies often distill comparisons down to a single number that represents the average incremental benefit or harm, and this simplified term is often presented or popularized in the media.8 Despite evidence that substantial individual-level variability in PCa treatment effects may exist,1 variability in survival effects remain underexplored. This variability is central to identifying patients at risk for overtreatment, which is defined primarily by the use of the aggressive therapies in the absence of survival benefits.10,11 In clinical trials, post-hoc analyses of broad subgroups were conducted; however, these analyses were age-adjusted, but they were rarely age-stratified.4,5
We employ a recently developed econometric methodology using instrumental variables to address selection biases in observational studies and establish person-centered treatment (PeT) effects.12 PeT effects estimate an average treatment effect for each person in the data, conditioning on their levels of risk factors and accounting for the individualized distribution of unobserved heterogeneity. Consequently, such individualized effects can help study a variety of distributional questions on effectiveness such as examining the benefits and harms of RP versus WW.
METHODS
Data
Data for patients diagnosed with PCa between 1996 and 2002 were extracted from the SEER-Medicare linked dataset that includes data from 1995-200713,14 along with patient zip codes of patients’ residences. Everyone in the sample had at least 6 years follow-up and many had follow-up through 12 years after diagnosis, although we capped follow-up at 11 years due to sample size issues. Various exclusions—in line with previous studies6—were applied as detailed in Online-only Appendix Table A1. We focused our analysis on the group of eligible patients aged 66-79 years at diagnosis.
Outcomes assessed included 11-year all-cause survival and 11-year PCa-specific survival. Date and cause of death data were obtained from SEER files. Comparison was made between RP without any form of radiation or hormone therapy in the first six months of diagnosis versus WW, defined as no use of surgery, hormone therapy or radiation in the first six months of diagnosis along with at least two PSA tests within the first year after diagnosis.
An indicator of surgery is likely to be endogenous for two reasons: true severity of cancer is unobserved as we only have data on the cross-sectional characteristics of the tumor at diagnosis, but not how the tumor is growing or any associated change in PSA. Higher severity may be positively correlated with receipt of surgery and negatively correlated with survival. These correlations render the naïve effects on surgery to be biased downward. Second, general frailties of the patients are unobserved, but would be negatively correlated with both receipt of surgery and survival, thereby generating an upward bias on the naïve effect. Thus the net direction of bias remains ambiguous.
Since the treatment indicator was subject to selection biases, we used an instrumental variable (IV, denoted as Z) that was the hospital referral region (HRR)15-specific rates of WW among PCa patients in the year prior to the diagnosis of an index patient. In addition, we adjusted for independent risk factors (X) that included clinical PCa stage and grade,1 patient demographic characteristics, an indicator for metropolitan area, Elixhauser comorbidity indices based on hospitalization(s) in the year preceding diagnosis, year and state fixed effects, and zip-code level area characteristics on racial makeup, population density, and education levels. We adjusted for other HRR-level characteristics using logged versions of population size, and supply of hospital beds, physicians, specialists, and urologists per 100,000 patients.
Target parameters to explore heterogeneous treatment effects
In the presence of heterogeneity, a long line of statistical work has shown that traditional IV regressions tend to estimate a local average treatment effect parameter that is often not interpretable.10,16-18 Instead, we defined our primary target parameters to be the person-centered treatment (PeT) effects.10 For each person in the sample, a PeT effect is estimated, which is the treatment effect conditional on their observed risk factors and averaged over a distribution of unobserved confounders that is specific to this person as determined by his observed treatment choice. That is, if 80-year old patients, on average, are less likely to choose surgery over WW and yet we observe an 80 year old patient to choose surgery, it tells us something about this patient's unobserved risk factor levels. Thus exploiting a choice model and a continuous instrumental variable, we are able to precisely identify a PeT effect for each individual in our sample. A brief description of this parameter is provided here. More intuitive explanation of this treatment effect parameter is provided in the Online-only Appendix.
In order to overcome the problems of interpreting traditional IV estimators, the concept of the marginal treatment effect (MTE) was developed.14-16 MTE identifies an effect for an individual who is at the margin of choice and is defined to be conditional on observed levels of X and also a specific level of the normed unobserved confounders (U) that makes a person indifferent to choosing between two treatments.14-16 A local instrumental variable (LIV) estimator for MTE is given by the partial derivative of the outcomes, Y, with respect to the estimated propensity to receive treatment P̂(X,Z).
| (1) |
PeT effects can then be computed by aggregating MTE(x,u) over respective distribution of U that corresponds to an individual's treatment choice given everything else.10 The Pet effects provide truly individualized estimates of treatment effects for identifiable individuals in our data. Pet effects can be easily aggregated to form meaningful treatment effect parameters, such as the average treatment effect. An extensive discussion on the definition and identification of these effects are provided in the Online-only Appendix.
Statistical Analysis
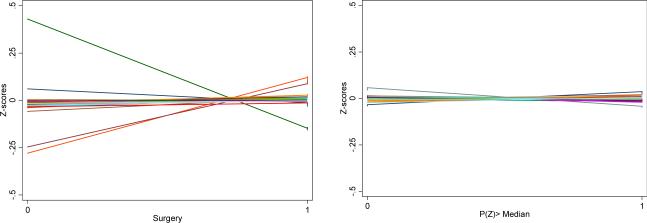
To explore the validity of the IV, we ran a logistic model for RP with only the IV as a regressor. We then compared the imbalance in patient-level independent risk factors across treatment categories with their imbalance across the median of the IV-only predicted propensity to choose RP. A valid IV would necessarily reduce such imbalances. In order to illustrate the imbalances for all covariates across treatment choices and across IV median in the same figure, we standardized each covariate by de-meaning and dividing by its standard deviation to form a Z-score. Levels of these Z-scores are then compared across treatment choices and across median of the IV. Finally, we assessed the strength of the IV in a logistic model for RP along with all other independent risk factors.
A local instrumental variable estimator for survival outcomes
An LIV estimator was implemented using a discrete-time hazard formulation where each observation represented a person-month and a binary indicator, Dk, took on a value of zero in each month (k) through the end of follow-up unless the end of follow-up was when the person died and the indicator value turned to one. Such a model does not enforce a proportional hazard assumption. By construction, E(Dk) = hk = hazard of death in month k and was modeled as:
| (2) |
where g() is a control function that is specified using main effects of P̂, X and k interactions of P̂ with X and k and polynomial of P̂ up to a degree that shows statistical significance using likelihood ratio tests. Extensive sensitivity analyses were performed on the functional form of the LIV estimand. The methods to compute the marginal treatment effects on cumulative survival are shown in the Online-only Appendix. PeT estimates are then derived based on conditional aggregation of the MTE estimates.10
One thousand clustered bootstrap replicates of the data were used to estimate standard errors for mean treatment effects and also individual-level PeT effects.
We evaluated the average counterfactual survival curves (i.e. the average survival curve if all patients had chosen a treatment) under RP and WW by adding or subtracting the average estimated PeT effects on month-specific cumulative survival probabilities to the observed survival probabilities depending on treatment choices. We also calculated the expected incremental survival in months by adding the PeT effects on month-specific cumulative survival probabilities over all 132 months. We studied the average effects by treatment receipt and a variety of subgroups including age, race, tumor grade and tumor stage subgroups. We examined the correlation between the individual effects on overall and PCa-specific survival. We also tested the hypothesis of “passive personalization”, which states that providers in practice may already personalize treatments to patients on specific outcome dimensions and thus patients receiving surgery may, on average, have higher incremental survival benefits than a random person drawn from this population.19,20 We aim to establish a dose-response relationship where patients expected to get larger survival benefits from surgery are more likely to receive surgery.
Finally, since PeT effects are derived on the basis of a non-linear control function that allows for complex interactions across all risk factors, we develop a simple predictive index using the estimated PeT effects as outcomes and studying whether certain combinations of patient characteristics may be predictive of individualized effects favoring RP over WW. We randomly split the estimated PeT's 80:20, trained an index to predict patients who were expected to gain at least a certain number of months of PCa-specific survival with RP over WW, and validated the index in the 20% holdout sample. Note that this process helps in preventing overfitting of the predictive index to the data, but does not provide true external validation of the predictions.
RESULTS
Characteristics of the sample patients
Our final analytic sample consists of 11,036 patients, of whom 8,462 (77%) underwent RP. RP patients were significantly younger and had a higher proportion of clinical T1 PCa and high-grade tumors (Table 1). Both groups of patients had the same average number of comorbidities identified during the year prior to diagnosis (84% had at least one comorbidity; on average, each patient had 2.2 comorbidities). However, the composition of comorbidities varied – RP patients had significantly lower rates of congestive heart failure, peripheral vascular disease, and diabetes, but higher rates of chronic lung disease, obesity, fluid and electrolyte disorders, and deficiency anemias. The IV was found to be strongly predictive of receipt of RP conditional on other factors (F-stat: 14.2, p<0.0001). The Z-scores show larger spread across treatment choices than across IV median implying that IV is able to reduce imbalance in these covariates (Figure 1). The outcomes models (i.e. control functions) satisfied all residual-base goodness-of-fit tests. The results presented below were robust to alternative formulations of LIV estimands.
Table 1.
Characteristics of patients receiving Watchful waiting versus Prostatectomy.
| Covariates | Watchful waiting N = 2,574 (23%) | Prostatectomy N = 8,462 (77%) | |
|---|---|---|---|
| DEMOGRAPHICS | |||
| Age (in years): mean (sd) | 73.5 (3.7) | 70.9 (3.5) | <0.001 |
| T1-stage (Ref: T2) | 36% | 50% | <0.001 |
| Grade | |||
| Well (Ref: Undetermined) | 11% | 08% | |
| Moderate | 72% | 71% | |
| Poor | 9% | 19% | <0.001 |
| Race | |||
| White (Ref: Other) | 86% | 87% | |
| Black | 10% | 8% | |
| Hispanic | 2% | 2% | 0.02 |
| Metropolitan area of residence | 87% | 84% | 0.001 |
| ILLNESS SEVERITY | |||
| No. of hospitalizations in last year | |||
| 1 (Ref: No hosp) | 9% | 10% | |
| 2 | 3% | 3% | |
| >2 | 2% | 2% | 0.79 |
| Congestive heart failure | 13% | 11% | 0.006 |
| Valvular disease | 13% | 12% | 0.17 |
| Peripheral vascular disease | 16% | 13% | <0.001 |
| Paralysis | 3% | 3% | 0.13 |
| Other neurological disorders | 6% | 6% | 0.25 |
| Chronic Lung Disease | 24% | 26% | 0.03 |
| Diabetes | 23% | 21% | 0.02 |
| Diabetes with chronic complications | 6% | 6% | 0.30 |
| Hypothyroidism | 12% | 11% | 0.16 |
| Obesity | 1% | 3% | <0.001 |
| Fluid and electrolyte disorders | 9% | 13% | <0.001 |
| Deficiency Anemias | 21% | 25% | 0.001 |
| Alcohol abuse | 1% | 2% | 0.09 |
| Depression | 5% | 5% | 0.90 |
| Hypertension with complications | 64% | 65% | 0.67 |
Figure 1.
Covariate imbalance across treatments versus across instrumental variable. Each covariate is standardized by de-meaning and dividing by its standard deviation to form a Z-score. Levels of these Z-scores are then compared across treatment choices and across median of the IV. The Z-scores show larger spread across treatment choices than across IV median implying that IV is able to reduce imbalance in these covariates.
All-cause mortality
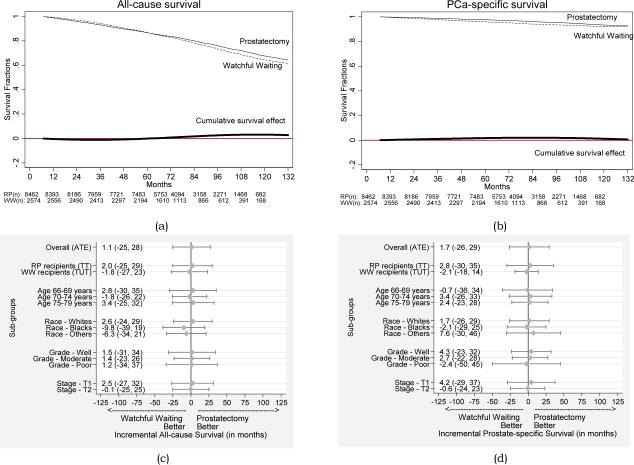
By the end of 2007, 2687 (24%) men had died, including 1930 (23%) men who underwent RP and 757 (29%) men on WW. PeT analyses reveal that the average incremental effect of RP over WW on 11-year overall survival is 1.1 months (95% CI: −25, 28; p = 0.94). The average counterfactual survival months over 11 years is 104.9 months (95% CI: 92.3, 117.5) had all patients received RP and 103.8 months (95% CI: 81.9, 125.7) had all patients undergone WW. The average absolute increase in survival with RP is not significant at any time interval but shows slight improvement after 6 years (Figure 2a; 2.7% increase; 95% CI: −25%, 45%; p = 0.88).
Figure 2.
Predicted average survival functions under radical prostatectomy and watchful waiting for (a) all-cause survival and (b) prostate cancer-specific survival. Here RP(n) and WW(n) represent the at-risk sample size at beginning of each time period in our data. Average incremental effects (in months) and 95% confidence intervals of radical prostatectomy over watchful waiting on 11-year survival, overall and by subgroups for (c) all-cause survival and (d) prostate cancer-specific survival.
Prostate cancer-specific mortality
Death attributed to PCa occurred in 408 men (3.7%). After RP, 301 of 8462 men (3.6%) died from PCa, compared with 107 of 2574 (4.2%) men on WW. Based on the PeT analyses, we find that that the average incremental effect of RP over WW on 11-year PCa-specific survival is 1.7 months (95% CI: −25, 29; p = 0.90). The absolute estimated survival with RP was identical to that with WW throughout the 11-year study period (Figure 2b). After 11 years, RP was associated with a non-significant average absolute increase in survival of 0.6% (95%CI:−30.4, 31.6; p = 0.96).
Heterogeneity and sub-group analyses
The all-cause or PCa-specific survival effects did not differ significantly according to age, race, grade or stage (Figure 2c-d). However, each of these subgroups explained only a small fraction of the total variation in individual level treatment effects (0 - 9%).
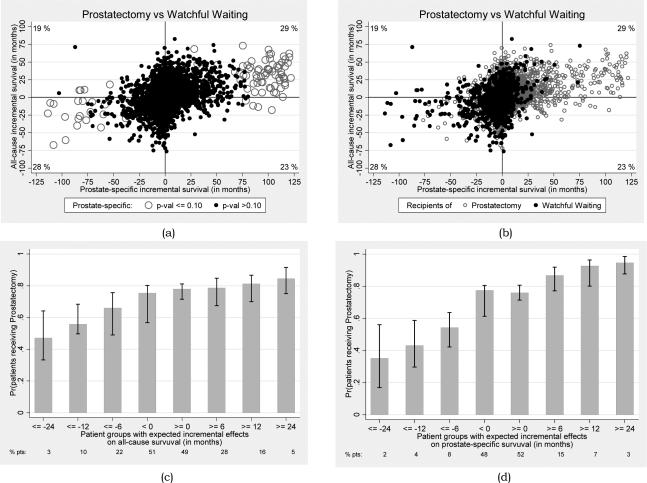
Figure 3a illustrates the full distribution of individual treatment effects across the survival outcomes. Each dot in this figure is a patient, corresponding to his PeT effect on PCa-survival on the X-axis and his overall survival on the Y-axis. There was a strong positive correlation between the all-cause and PCa-specific survival effects (Corr coeff: 0.33; 95% CI: 0.03, 0.53). Only 0.67% of patients had significant positive PCa-specific survival effects of RP over WW at the 10% level. Among these patients, the 11-year PCa-specific survival effects ranged from 15 to 122 months indicating that there were some patients who could presumably get substantial survival benefits from RP but they constituted a small fraction of the PCa population. Overall, about 6% of patients were expected to get at least 15 months of PCa-specific benefits from RP over WW.
Figure 3.
(a) Person-centered treatment (PeT) effects (in months) for each patient in the sample on 11-year all-cause (Y-axis) and prostate cancer-specific survivals (X-axis) and their significances (b) identifying patient who received radical prostatectomy or watchful waiting and their corresponding expected PeT effects. Percentages at the corners represents proportion of the patients who falls in each quadrant. (c) Rate of use of radical prostatectomy among groups of patients with different expected person-centered incremental benefits on all cause-survival and on (d) prostate cancer-specific survival.
Selection in practice
The average survival effects among those who received RP were larger than those who received WW. For all-cause survival, the average effect among those who received RP was 2.0 months (95% CI: −26, 30) and for WW recipients was −1.8 months (95% CI: − 24, 21); the difference of 3.8 months was not significant (95% CI: −3.0, 17.0). For PCa-specific survival, the average effect among those who received RP was 2.8 months (95% CI: −28, 34) and for WW recipients was −2.1 months (95% CI: − 22, 18); the difference of 4.9 months was not significant (95% CI: −6.0, 42.0). However, in both cases, those who received RP were likely to benefit more from RP than those who received WW.
This positive self-selection is further illustrated in Figure 3b - patients who received RP were less likely to get negative survival effects from RP compared with those who received WW. Therefore, patients undergoing RP exhibited greater clustering in the right upper quadrant of Figure 3b, while those receiving WW clustered in the left lower quadrant. Figure 3c-d illustrates the rate of RP use among patients with different levels of expected all-cause (Figure 3c) and PCa-specific (Figure 3d) survival effects of RP over WW. Overall, it is observed that groups of patients with higher expected survival benefits from RP over WW are more likely to get RP. Compared with all-cause survival, the selection of RP in practice appears to be driven mostly by PCa-specific survival. Patients expected to attain 15 months of PCa-specific survival gains from RP over WW almost always received RP. In comparison, the rate of RP use among those who were significantly hurt by RP was much lower.
Identifying patients likely to benefit from radical prostatectomy
The characteristics strongly associated (p-value <0.001) with PCa-specific survival benefits of at least 15 months (~6%) with RP over WW are displayed in Table 2. The factors identified have strong face validity based on the conceptual expectations and associations established in the literature.21-23 Our final predictive index achieved high ROC characteristics in the split-sample analysis (Out-of-sample AUC: 0.89 (95% CI: 0.88 – 0.91).
Table 2.
Patient characteristics associated with significant increase or decrease in the likelihood of obtaining substantial benefits in prostate cancer-specific survival (≥ 12 months) due to prostatectomy over watchful waiting.
| Characteristics associated with significant increase in the likelihood of benefits in prostate cancer-specific survival (≥ 15 months) due to prostatectomy over watchful waiting. | Characteristics associated with significant decrease in the likelihood of benefits in prostate cancer-specific survival (≥ 15 months) due to prostatectomy over watchful waiting. |
|---|---|
| Age at diagnosis ≥ 70 years (Ref: < 70 years) | Race (Ref: Race Other) |
| White | |
| Black | |
| Grade (Ref: Grade Undetermined) | |
| Well | Any hospitalizations in last year (Ref: 0 hosp. last year) |
| Poor | |
| Comorbidities at diagnosis (Ref: No Elixhauser comorbidity) | Comorbidities at diagnosis (Ref: No Elixhauser comorbidity) |
| Paralysis | Congestive Heart Failure |
| Other Neurological disorders | Valvular heart disease |
| Diabetes with complications | Diabetes with no complications |
| Deficiency Anemia | Hypothyroidism |
| Alcohol abuse | Obese |
| Hypertension with complications | Depression |
Note: Reference group is highly less likely to obtain prostate cancer specific survival benefits from prostatectomy versus watchful waiting.
DISCUSSION
We employed novel IV analysis in order to address unmeasured confounders in observational data and also to estimate PeT effects and understand the individual variability in the survival benefit of surgery versus watchful waiting for men with clinically localized PCa. Given the study time frame, the length of patient follow-up, and the difficulty identifying active surveillance with claims data, our cohort and study results compare favorably with PIVOT,24 where the non-operative patients were not managed with active surveillance; rather, PIVOT patients in the observation arm were managed expectantly, without protocol prostate needle biopsies to evaluate for reclassification of their cancers. The SEER-Medicare patients in our study were diagnosed between1996-2002 compared with 1994-2002 for PIVOT. Unlike PIVOT, however, our cases derive from population-based data, and may be more generalizable.
Our study corroborated several recent analyses that—in a screen-detected population—aggressive intervention for localized prostate cancer has minimal benefit on survival compared with conservative management with watchful waiting. In addition, while exploring individual-level heterogeneity in effects, we found that although some patients could presumably get significant survival benefits from RP over WW, they constitute a small fraction of the population, thereby supporting concerns that the contemporary PCa population may be overtreated. Individual characteristics that were associated with benefit from watchful waiting rather than aggressive intervention with RP included a history of recent hospitalization and specific comorbid conditions such as congestive heart failure and valvular heart disease, diabetes, obesity, and depression. Because these individual characteristics are often clustered, our next steps include characterizing projected survival benefits of prostate cancer treatment based on clustered profiles of patient characteristics such as combinations of linked comorbidities, which would align with the movement toward patient-centered outcomes research.25 This clustering of comorbid conditions may explain counterintuitive findings such as projected benefit to RP for patients with complicated diabetes, but projected benefit to WW for patients with uncomplicated diabetes. Our results corroborate findings from Daskivich, et al., that men with greater comorbidity are often treated despite minimal expected benefit from aggressive intervention for their prostate cancers.26
In practice, patients who are more likely to benefit appear to be triaged into aggressive treatment; however, the selection by providers is not accurate. Figure 3 supports that PCa-specific survival drives selection for treatment rather than overall survival. Thus, providers may focus on cancer-specific risk factors such as Gleason score and PSA, and be less inclusive of factors that may belie patient risk for competing causes of mortality into their treatment decision-making. This further supports development of tools such as a predictive personalized calculator, which would aid clinicians in understanding which patients are likely to benefit from consideration of aggressive treatment. For example, a recent published nomogram is a step in the right direction,27 but only classifies patients by cancer characteristics and not their varying comorbid disease burden.
Our study has several limitations. SEER-Medicare data is observational in nature and therefore observed treatment choices are always subject to selection biases, which we have addressed through instrumental variable analysis. We analyzed cancer-specific and overall survival outcomes for a cohort of men diagnosed with prostate cancer over ten years ago. However, this allowed ascertainment of long-term survival outcomes that are critical in conducting comparative effectiveness research in PCa care, given the indolence of even high-risk prostate cancer.28 The SEER data did not include PSA, limiting our ability to accurately project PCa risk category. Evaluation of more contemporary data, as that data matures and long-term outcomes are available, would allow for more specificity in assignment of risk of cancer progression and cancer-specific mortality at the time of PCa diagnosis.
Moreover, we wanted to focus on the comparative question of RP versus WW in line with the clinical trial evidence on this question. However, this means that we have dropped patients who received radiation therapy in practice, in line with the previous analyses.6 This implies that the estimated average effects between RP and WW only extend to the population of patient who are at a point of equipoise between RP and WW.
The PeT effects estimated using LIV methods is a powerful way to explore treatment effect heterogeneity. The typical assumptions of an IV analyses apply to such estimation. In addition, one must have at least one continuous IV that is strong enough to produce a near full support in the estimated propensity scores (in our case 0.12 to 0.98) in order to explore the marginal treatment effects across all the margins of choices. Lastly, proper care should be devoted in developing the control function so that the relationship between outcomes and the estimated propensity scores are not misspecified. We explore a variety of goodness-of-fit tests to detect such misspecifications.
Despite these challenges, we demonstrated that the majority of individuals in this screened population derive minimal long-term survival benefit from RP over WW, confirming concerns of PCa overtreatment. We identified a set of patient characteristics that could help identify the small fraction who benefit from RP, which might inform a personalized decision support calculator. Implementation of such a tool in practice might increase consideration of observational management strategies such as active surveillance and decrease overtreatment of localized PCa.
Supplementary Material
Funding and Acknowledgement
The author acknowledges support from the National Institute of Health Research Grants RC4CA155809 and R01CA155329.
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
References
- 1.Meltzer DO, Egleston B, Abdalla I. Patterns of prostate cancer treatment by clinical stage and age in the United States. American Journal of Public Health. 2001;91(1):126–128. doi: 10.2105/ajph.91.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klotz L. Prostate cancer overdiagnosis and overtreatment. Curr Opin Endocrinol Diabetes Obes. 2013;20(3):204–9. doi: 10.1097/MED.0b013e328360332a. [DOI] [PubMed] [Google Scholar]
- 3.Esserman LJ, Thompson IM, Reid B. Overdiagnosis and Overtreatment in Cancer: An Opportunity for Improvement. JAMA. 2013 doi: 10.1001/jama.2013.108415. Published online July 29th, 2013 - See more at: http://pubweb.fccc.edu/cancerconversations/2013/08/prevent-overtreatmentfor-prostate-cancer/#sthash.P4TNJcyg.dpuf. [DOI] [PubMed]
- 4.Bill-Axelon A, Holmberg L, Filen F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandanavian prostate cancer group 4 randomized trial. Journal of the National Cancer Institute. 2009;100:1144–1154. doi: 10.1093/jnci/djn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilt TJ, Brawar MK, Jones KM, et al. Radical Prostatectomy versus observation for localized prostate cancer. The New England Journal of Medicine. 2012;367(3):203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hadley J, Yabroff KR, Barrett MJ, et al. Comparative effectiveness of prostate cancer treatments: Evaluating statistical adjustments for confounding in observational data. Journal of the National Cancer Institute. 2010;102:1–4. doi: 10.1093/jnci/djq393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia J, Trock BJ, Cooperberg MR, et al. Prostate cancer mortality following active surveillance versus immediate radical prostatectomy. Clin Cancer Res. 2012 Oct 1;18(19):5471–5478. doi: 10.1158/1078-0432.CCR-12-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emery G. Prostate cancer surgery fails to cut deaths in study. Reuters. 2012 [Google Scholar]
- 9.Vickers A, Bennette C, Steineck G, et al. Individualized estimation of the benefit of radical prostatectomy from the Scandinavian Prostate Cancer Group randomized trial. Urology. 2012 doi: 10.1016/j.eururo.2012.04.024. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klotz L. Prostate cancer overdiagnosis and overtreatment. Current Opinion in Endocrinology, Diabetes and Obesity. 2013;20(3):204–9. doi: 10.1097/MED.0b013e328360332a. [DOI] [PubMed] [Google Scholar]
- 11.Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and overtreatment of prostate cancer. European Urology. 2014;65(6):1046–55. doi: 10.1016/j.eururo.2013.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basu A. Person-Centered Treatment (PeT) effects using instrumental variables. Journal of Applied Econometrics. 2014;29(4):671–691. doi: 10.1002/jae.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper GS, Viring B, Klabunde CN, et al. Use of SEER-Medicare data for measuring cancer surgery. Medical Care. 2002;40(Suppl):IV–43 – IV-48. doi: 10.1097/00005650-200208001-00006. [DOI] [PubMed] [Google Scholar]
- 14.Viring BA, Warren JL, Cooper GS, et al. Studying radiation therapy using SEER-Medicare linked data. Medical Care. 2002;40(Suppl):IV–49 – IV-54. doi: 10.1097/00005650-200208001-00007. [DOI] [PubMed] [Google Scholar]
- 15. http://www.dartmouthatlas.org/data/region/
- 16.Heckman JJ, Vytlacil EJ. Local instrumental variables and latent variable models for identifying and bounding treatment effects. Proceedings of the National Academy of Sciences. 1999;96(8):4730–34. doi: 10.1073/pnas.96.8.4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heckman JJ, Vytlacil E. Local instrumental variables. In: Hsiao C, Morimue K, Powell JL, editors. Nonlinear Statistical Modeling: Proceedings of the Thirteenth International Symposium in Economic Theory and Econometrics: Essays in the Honor of Takeshi Amemiya. Cambridge University Press; New York: 2001. pp. 1–46. [Google Scholar]
- 18.Heckman JJ, Vytlacil E. Structural equations, treatment effects and econometric policy evaluation. Econometrica. 2005;73(3):669–738. [Google Scholar]
- 19.Basu A. Personalized Medicine in the Context of Comparative Effectiveness Research. Forum for Health Economics and Policy. 2013;16(2):107–120. doi: 10.1515/fhep-2013-0009. [DOI] [PubMed] [Google Scholar]
- 20.Basu A, Jena AB, Goldman DP, et al. Heterogeneity in action: Role of passive personalization in comparative effectiveness research. Health Economics. 2014;23(3) doi: 10.1002/hec.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong Z, Kristal AR, Schenk JM, et al. Alcohol consumption, finasteride, and prostate cancer risk. Cancer. 2009;115(16):3661–3669. doi: 10.1002/cncr.24423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daskivich TM, Fan K-H, Koyama T, Albertsen PC, et al. Effect of Age, Tumor Risk, and Comorbidity on Competing Risks for Survival in a U.S. Population–Based Cohort of Men With Prostate Cancer. Annals of Internal Medicine. 2013;158(10):709–717. doi: 10.7326/0003-4819-158-10-201305210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Häggström C, Stocks T, Ulmert D, et al. Prospective study on metabolic factors and risk of prostate cancer. Cancer. 2012;118:6199–6206. doi: 10.1002/cncr.27677. [DOI] [PubMed] [Google Scholar]
- 24.Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012 Jul 19;367(3):203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Washington AE, Lipstein SH. The Patient-Centered Outcomes Research Institute--promoting better information, decisions, and health. N Engl J Med. 2011 Oct 13;365(15):e31. doi: 10.1056/NEJMp1109407. [DOI] [PubMed] [Google Scholar]
- 26.Daskivich TJ, Chamie K, Kwan L, et al. Comorbidity and competing risks for mortality in men with prostate cancer. Cancer. 2011 Oct 15;117(20):4642–50. doi: 10.1002/cncr.26104. [DOI] [PubMed] [Google Scholar]
- 27.Gulati R, Inoue LY, Gore JL, et al. Individualized estimates of overdiagnosis in screen-detected prostate cancer. J Nat Cancer Ins. 2014;106(2) doi: 10.1093/jnci/djt367. first published online January 7, 2014 doi:10.1093/jnci/djt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albertsen PC, Hanley JA, Gleason DF, et al. Competing risk analysis of men aged 55 to 74 years at diagnosis managed conservatively for clinically localized prostate cancer. JAMA. 1998;280:975–980. doi: 10.1001/jama.280.11.975. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.