Abstract
A number of past studies have used mismatch negativity (MMN) to identify auditory processing deficits in individuals with autism spectrum disorder (ASD). Our meta-analysis compared MMN responses for individuals with ASD and typically developing controls (TD). We analyzed 67 experiments across 22 publications that employed passive, auditory-based MMN paradigms with ASD and TD participants. Most studies lacked design characteristics that would lead to an accurate description of the MMN. Variability between experiments measuring MMN amplitude was smaller when limited to studies that counterbalanced stimuli. Reduced MMN amplitude was found among young children with ASD compared to controls and in experiments that used nonspeech sounds. Still, few studies included adolescents or those with below-average verbal IQ. Most studies suffered from small sample sizes, and aggregating these data did not reveal significant group differences. This analysis points to a need for research focused specifically on understudied ASD samples using carefully designed MMN experiments. Study of individual differences in MMN may provide further insights into distinct subgroups within the heterogeneous ASD population.
Keywords: Autism, Mismatch negativity, Mismatch field, Oddball, MMN, MMF, Auditory processing, Event-related potential, Meta-analysis, Systematic review
Introduction
Autism Spectrum Disorder (ASD) is characterized by impairments in social communication and interaction as well as by the presence of repetitive and restricted behaviors or interests, including atypical responses to sensory stimuli like sounds (American Psychiatric Association, 2013). Language impairments, while not core symptoms in ASD, often co-occur (Tager-Flusberg et al., 2005). Atypical responses to auditory stimuli and difficulty in learning spoken language are linked to disruptions of auditory filtering, acoustic feature discrimination, sound source identification, and auditory working memory (Anderson & Kraus, 2010; Foss-Feig, Stone, & Wallace, 2012; Näätänen et al., 2012; O’Connor, 2012). Given that these processes are vital components of auditory processing, several researchers have hypothesized that in ASD, there is a common disruption in neural networks that govern basic auditory processing (Bomba & Pang, 2004; Marco, Hinkley, Hill, & Nagarajan, 2011). To pinpoint the underlying bases of atypical auditory processing in brain-based disorders, researchers often turn to measures like electroencephalography (EEG) and magnetoencephalography (MEG). These neuroelectric imaging approaches have the temporal resolution necessary to track neural activity associated with specific auditory events, thereby providing a window into auditory processing not afforded by other noninvasive neural measures2. Here, a meta-analysis was undertaken to determine the extent to which neural response that reflect acoustic feature discrimination and auditory working memory in early auditory processing differs in ASD relative to typical development (TD).
We focused on one common approach that can capture such features of early auditory processing: the mismatch negativity (MMN) paradigm (Näätänen et al., 2012; Näätänen, Paavilainen, Rinne, Alho, 2007). The MMN measures an individual’s ability to detect changes in auditory patterns by presenting a regularly occurring, “standard” pattern that is interrupted at random with rare, “deviant” stimuli (Naatanen, Gaillard, & Mäntysalo, 1978). Deviant stimuli usually differ perceptually from standards on a single acoustic feature, such as intensity, pitch, or phoneme. Typically, the unexpected, rare sounds elicit neural responses not present when that same sound is expected. The size of those neural responses indexes the degree to which a listener has built up a memory trace of an ongoing auditory pattern and detected a deviation from that trace (Kujala & Näätänen, 2010). It has been argued that this neural response is driven by NMDA receptor activity in the bilateral auditory and frontal cortices (Näätänen et al., 2012). MMN components can be well detected on the scalp’s frontal-central midline using EEG and can be quantified as a negative component that occurs 100 to 250 ms following a deviant stimulus onset (Haesen et al., 2011). In source space, the mismatch field arises from frontal and supratemporal generators during a similar time window (Giard et al., 1990; Novak et al., 1990).
The MMN component itself is calculated from the difference between the response evoked by the same event when it is a standard and when it is a deviant. By directly comparing responses to identical stimuli when they are expected versus when they are deviants, the MMN in a baseline-corrected measure, revealing neural activity driven by hearing an unexpected event. Response latency of the MMN is determined based on the timing of the negative peak in the difference waveform. Response amplitude can be computed by taking the average response in a window centered on this negative peak. However, the analysis window used to determine MMN amplitude and latency varies across studies (e.g., it can be based on each individual subject’s waveform, based on the average waveform of each subject group, or based on the average from all participants). Both MMN amplitude and latency metrics signify rapid discrimination that is driven by both bottom-up automatic and top-down attentive processes at early stages of cortical processing (Näätänen et al., 2012; Roberts et al., 2011).
The MMN response can be elicited both during active tasks, where the subject makes an overt response upon detecting the deviant stimulus, and in settings when the subject listens passively, with no overt response required. As such, the MMN is one of the few established neural measures of auditory processing that does not require a high degree of instruction, overt attention, or active participation from the research participant (Bishop, 2007; Näätänen et al., 2012). This makes the MMN attractive to researchers studying individuals with ASD, whose verbal and cognitive abilities range across a wide spectrum; for paradigms measured in an active setting that require subjects to follow instructions, pay attention to stimuli, or perform a behavioral task, variations in subjects’ abilities undoubtedly affect the measured response. To make meaningful cross-group comparisons from experiments that include subjects with and without verbal and cognitive deficits, it is important to use a paradigm for which performance is not significantly influenced by attention or other higher-level cognitive processes.
Many passive MMN experiments have been conducted on the ASD population, but there is no consensus across studies as to whether or not people with ASD exhibit a different MMN response to auditory deviants. Some publications have reported heightened and/or earlier MMN responses to acoustic deviants in ASD, suggesting greater auditory sensitivity to changes in acoustic stimuli (Gomot et al., 2011; Lepistö, Niemin-von Wendt, von Wendt, Näätänen, & Kujala, 2007). Other publications have reported suppressed and/or delayed MMN responses to acoustic deviants in ASD, indicating a weaker sensitivity (Andersson, Posserud, & Lundervold, 2013; Yu et al., 2015). Still others have reported mixed results, such that some deviant stimuli elicit group differences while others do not (Lepistö et al., 2005; Lepistö et al., 2008). While several past reviews have described these conflicting findings (Foss-Feig et al., 2012; Haesen et al., 2011; Kujala et al., 2013; McFadden & Rojas, 2013; Näätänen & Kujala, 2011; O’Connor, 2012; Orekhova & Stroganova, 2014), none have critically evaluated which factors may account for similarities and discrepancies across studies.
This lack of consensus prompted us to conduct a meta-analysis exploring whether there are methodological or stimulus differences that explain apparent inconsistencies across studies. We compared MMN response amplitude and MMN response latency between individuals with ASD and age-matched TD controls. We compiled the results from all experiments that met our inclusion criteria into a comprehensive statistical framework, treating each experiment or statistic as a single data point in our analysis. Given the complexities of collecting EEG and MEG data from individuals with ASD, sample sizes in individual studies tended to be fairly small and lacked strong power on their own. Our meta-analysis synthesized results across studies, thereby increasing the statistical power when testing for group differences.
We began by analyzing all published experiments that measured group differences between ASD and TD participants using either MMN amplitude or latency in a passive, auditory-based MMN paradigm. We then narrowed our analysis to include only those experiments that controlled for general variation in event-related potential or event-related field (ERP/ERF) responses to different stimulus tokens. Specifically, we only included studies in which the MMN was calculated by comparing responses to identical stimuli presented in two different contexts – one in which they were unexpected deviants and the other in which they were expected standards. Without counterbalancing stimuli in this way, any difference in signal morphology between the response to deviants and standards might be due to differences in the unrelated neural responses to the specific stimuli presented, such as a loud sound producing a larger ERP/ERF than a soft sound (Duncan et al., 2009; Kujala et al., 2007). We followed up with analyses examining how stimulus characteristics (speech versus nonspeech sounds) impacted group-difference effect size and whether participant characteristics (age and verbal reasoning) influenced the findings.
2. Methods
2.1 Literature Search and Screening Criteria
Our meta-analysis and systematic review followed PRISMA guidelines (Moher, Liberati, Tetzlaff, & Altman, 2009). We began with a comprehensive literature search to identify publications reporting experiments that measured auditory MMN components in individuals with ASD, using the key terms “MMN,” “MMF”, “mismatch negativity,” “mismatch field,” “oddball,” “autism,” and “ASD” on PubMed, ScienceDirect, and Google Scholar. We used the following inclusion criteria:
The publication had to include an experiment that used a paradigm in which standard stimuli were more prevalent than the interspersed deviant(s).
The publication had to include an experiment that collected data with EEG or MEG.
The publication had to include a passive listening experiment; specifically, participants must have received no instructions to listen and must not have been required to provide a behavioral response (such as a hand raise or lever press) to detected deviant stimuli. This requirement reduces any influence of top-down modulation of neural responses, allowing for a fair comparison of neural responses from TD listeners and the more heterogeneous ASD sample, which included listeners with cognitive deficits.
2.2 Inclusion Criteria for Meta-analysis
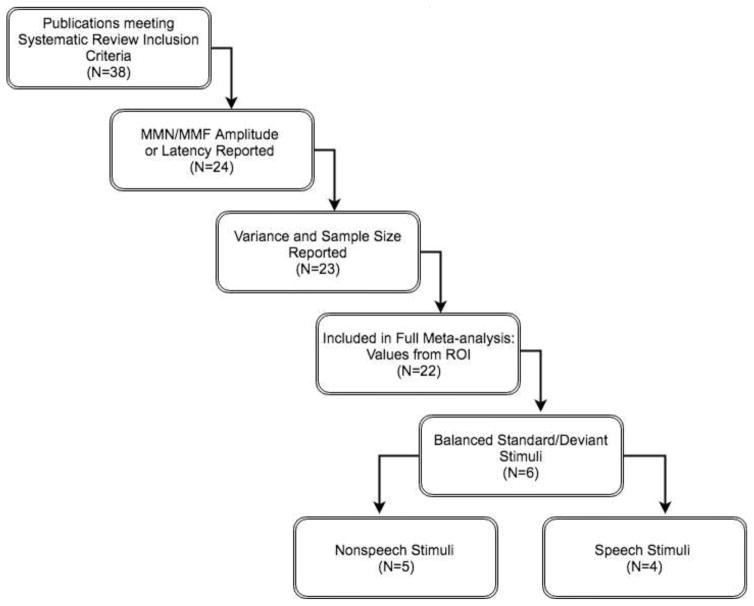
Following our initial screening of publications, we established additional criteria for the inclusion of publications in our meta-analysis (Figure 1). The publication had to include an experiment that reported means, variation of the mean (i.e., standard error or standard deviation of the mean), and sample sizes of either MMN amplitude or latency for both an ASD and a TD comparison group. These descriptive statistics were necessary to calculate effect sizes for the meta-analysis. If any of this information was missing from the publication, we contacted authors of studies published between 2011–20173 and invited them to provide us with that information. Experimental statistics that compared participants with ASD to participants with other neurodevelopmental disorders (e.g., attention deficit hyperactivity disorder, receptive developmental language disorder, tuberous sclerosis, dyslexia) were not included. EEG results had to be reported for mid-frontal electrodes (Fz, or if not available, an average of left and right midfrontal channels); we also included any MEG results that localized source activity attributable to supratemporal generators, such as the superior temporal gyrus (which would appear in mid-frontal electrodes in EEG measurements). To investigate early, automatic processes of mismatch detection, only statistics from latency windows between 50 and 400 ms were used in the meta-analysis. When statistics were available for specific age groups, we used these data to analyze the influence of age on MMN differences between ASD and TD groups.
Figure 1. Flow Chart of Selection for Meta-analysis.
N values represent total number of publications in which experiments were reported. Values were considered as from regions of interest (ROI) when EEG data was collected from mid-frontal electrodes and MEG data was source localized to supratemporal generators.
2.3 Meta-Analysis
We performed all meta-analyses with the ‘meta’ package in R Version 3.1 (CRAN, 2015; Schwarzer, Carpenter, & Rücker, 2015). Meta-analyses operated on effect sizes, which were derived from the results of each experiment. Effect sizes were calculated based on the magnitude of the difference in MMN amplitude or response latency between ASD and TD participants, taking into account the variance of the difference and the sample sizes of each group. We determined Cohen’s d effect size using Hedges’ g, the bias-corrected standardized mean difference estimation (SMD) (Cohen, 1988). Positive SMD values reflected a smaller value in the ASD group, while negative SMD values corresponded to a larger value in the ASD group. Pooled deviation was computed based on parameters of standard deviation and sample size; pooled deviation was then used to calculate the 95% confidence intervals for the effect size.
Statistical variability between experiments, referred to as “between-experiment heterogeneity,” was assessed first across all experiments, then across experiments that counterbalanced stimuli, and finally separately for speech and nonspeech experiments. To assess between-experiment heterogeneity, we used the Cochran’s Q statistic (Cochran, 1954), where a nonsignificant value (above an alpha threshold of 0.05) indicated no significant variance (Schwarzer et al., 2015). In particular, we used Cochran’s Q statistic to determine whether or not the effect size differed within data sets (Qw) and between data sets (Qb) (Schwarzer et al., 2015). If there was no significant between-experiment heterogeneity within a single set of data (Qw), it would suggest that there was a consensus across that set of experiments. Similarly, if there was no significant heterogeneity between two sets of data (Qb) (e.g., data from speech and nonspeech experiments), it would suggest that the sets of results showed similar effects. These methods used DerSimonian and Laird (1986)’s estimator for tau and an inverse variance method for calculations. When heterogeneity between experiments was significant, we computed the effect size using a random effects model; when it was not significant, we computed it with a fixed effects model.
To test for publication bias across reports, we used Egger’s weighted linear regression intercept test with significance threshold set to alpha=0.05 (Egger, Davey Smith, Schneider, & Minder, 1997). This allowed us to determine whether what has been published accurately represents all completed research in the field, and, in turn, whether or not the data we were analyzing was inherently biased. For example, a significant publication bias may be suspected if group differences, like reductions in MMN in ASD, were only published in studies with small sample sizes, or null results were only published in studies with large sample sizes.
2.4 Subject Characteristics
To investigate the effects of age on MMN group differences, we collected mean and variance values for each age in years for ASD groups.
In addition, we quantified MMN effect sizes as a function of verbal reasoning ability. Verbal reasoning skills are highly variable in ASD. Across the studies included in this meta-analysis, there was no single measure used to assess verbal reasoning ability. To quantify the role of verbal reasoning on group effect size, we used verbal intelligence quotients (VIQ), when reported. Publications that reported VIQ scores for the ASD group and were included in our analysis of VIQ used the Wechsler Intelligence Scales and the Stanford Binet (WAIS-R, Wechsler, 1981; WISC-III, Wechsler, 1991; WISC-IV, Wechsler, 2003; WPPSI-R, Wechsler, 1990; SB-IV, Thorndike et al., 1986). While not identical, strong positive correlations have been found between Weschler tests (Ross & Morledge, 1967; Shahim, 1992), as well as between the Weschler tests and the Stanford Binet, Fourth Edition (Frandsen & Higginson, 1951), suggesting that the measures capture similar constructs.
3. Results
3.1 Full Meta-Analysis
Our systematic literature review identified a total of 38 publications published between 1980 and 2017 that had at least one experiment that measured EEG or MEG using a passive auditory mismatch negativity paradigm (Table 1a and Table 1b). Twenty-two of these publications included experiments that met all our criteria, yielding a total of 67 separate experiments; each of these experiments explored group differences between ASD and TD listeners based on MMN amplitude and/or latency (Table 1a). From these experiments, data from a total of 857 ASD and 831 TD subjects were included in this analysis.
Table 1a.
Summary of 22 publication sources used in meta-analysis.
| ID | Source | Stimuli | n | Age (years) | Mean (SD or Range) | VIQ Measure | MMN Analysis Window (ms) | Std-Dev Controlled | ||
|---|---|---|---|---|---|---|---|---|---|---|
| TD | AUT | AUT AGE | AUT VIQ | |||||||
| 1 | Andersson et al. 2013 | C | 12 (0F) | 11 (0F) | ~16 | 16.0 (0.8) | 92 (5) | WISC-III | 140–220 | No |
| 2 | Dunn et al. 2008 (passive) | P | 34 (14F) | 34 (9F) | 6–13 | 9.3 (2) | 84 (23) | SB-IV | 163–213 | Yes |
| 3 | Fan et al. 2014 | CS | 20 (1F) | 20 (1F) | 18–29 | 21.5 (3.8) | NR | WAIS-IV | 150–250 | No |
| 4 | Ferri et al. 2003 | P | 10 (0F) | 10 (0F) | 6–19 | 12.3 (4.9) | NR | NR | NR, Approx 109–147 |
No |
| 5 | Gomot et al. 2002 | P | 15 (3F) | 15 (3F) | 5–9 | 6.8 (1.3) | 50 (27*) | BL-R, EDEI-R | 140–230 | No |
| 6 | Gomot et al. 2011 | P | 27 (6F) | 27 (6F) | 5–11 | 8.3 (1.7) | 43 (14*) | BL-R, EDEI-R | 120–250 | No |
| 7 | Jansson-Verkasalo et al. 2003 | PS | 11 (4F) | 10 (4F) | 7–12 | 9.1 (1.5) | NR | NR | P: 150– 320, S: 200–380 | No |
| 8 | Jansson-Verkasalo et al. 2005 | P | 18 (9F) | 19 (5F) | 7–14 | 10.6 (0.9) | NR | NR | 1: 85–140 2: 140– 220 |
No |
| 9 | Korpilahti et al. 2007 | S | 13 (0F) | 14 (0F) | 9–13 | 11.2 (NR) | 107 (NR) | WISC-III | 150–350 | No |
| 10 | Kujala et al. 2005 | S | 8 (4F) | 8 (4F) | 22–43 | 33 (NR) | NR | WAIS-R | 116–225 | No |
| 11 | Kujala et al. 2007 | C | 10 (2F) | 8 (2F) | 12–42 | 27 (5.6) | 103 | NR | 100–250 | No |
| 12 | Kujala et al. 2010 | S | 13 (2F) | 15 (4F) | 8–12 | 10.8 (0.9) | 112 (19) | WISC-III | 200–320 | No |
| 13 | Lepistö et al. 2005 | CS | 15 (2F) | 15 (2F) | 7–12 | 9.4 (NR) | 59 (40– 90) | WISC-III, WPPSI-R | 100–400 | Yes |
| 14 | Lepistö et al. 2006 | CS | 10 | (2F)10 (2F) | 7–10 | 8.11 (NR) | 108 (86– 129) | WISC-III | 100–400 | Yes |
| 15 | Lepistö et al. 2007 | CS | 9 (1F) | 9 (2F) | 20–41 | 27 (NR) | 104 (90– 126) | WAIS-R | 100–400 | Yes |
| 16 | Lepisto et al. 2008 (constant- feature) | S | 16 (1F) | 10 (1F) | 6–11 | 9.1 (NR) | 54 (41– 70) | WISC-III | 100–400 | No |
| 17 | Lepistö et al. 2009 (oddball) | P | 14 (2F) | 16 (3F) | 7–10 | 8.1 (NR) | 113 (90– 145) | WISC-III | 100–300 | No |
| 18 | Ludlow et al. 2014 | S | 11 (0F) | 11 (0F) | 11–16 | 13.0 (1.1) | 101 (10) | NR | NR | No |
| 19 | Roberts et al., 2011 | P | 27 (15F) | 51 (2F) | 6–15 | 9.36 (2.11) | Approx. 40–120 |
CELF-IV | 150–350 | Yes |
| 20 | Seri et al. 1999 | P | 7 (NR) | 7 (NR) | 7–10 | 8.3 (0.69) | NR | NR | 130–250 | No |
| 21 | Weismüller et al. 2015 | PS | 15 (0F) | 18 (0F) | 6–15 | 9.4 (2.4) | NR | WISC-IV | 120–300 | S: Yes P: No |
| 22 | Yu et al. 2015 | PCS | 1: 16 (3F) 2: 18 (6F) |
1: 18 (2F) 2: 16 (1F) |
6–13 | 1: 9.3 (1.8) 2: 9.6 (1.3) |
NR | NR | 100–250 | No |
P=Pure tone; C=Complex tone; S=Speech; (SD)* = SD calculated from SD=S.E.M.
√N; NR=Not reported;
NR-NWNL=Not reported but indication of not-within normal limits; NR-WNL=Not reported but indication of typical range of scores (within normal limit); Italicized IQs indicate values are Developmental Quotients rather than Intelligence Quotients. Asterisks are placed by the VIQ measures used in our statistical analysis. VIQ or Language Measures: CELF-III=Clinical Evaluation of Language Fundamentals—Third Edition (Semel et al., 1995); EDEI-R=Échelles Différentielles d’Efficiences Intellectuelles—Revised (Perron-Borelli, 1978); PPVT-III=Peabody Picture Vocabulary Test—Third Edition (Dunn & Dunn, 1997); SB-IV=Stanford Binet, Fourth Edition (Thorndike et al., 1986); WAIS-R=Wechsler Adult Intelligence Scale—Revised (Wechsler, 1981); WISC-R=Wechsler Intelligence Scale for Children—Revised (Wechsler, 1974); WISC-III=Wechsler Intelligence Scale for Children—Third Edition (Wechsler, 1991); WISC-IV=Wechsler Intelligence Scale for Children—Fourth Edition (Wechsler, 2003); WPPSI-R=Wechsler Preschool and Primary Intelligence Scales—Revised (Wechsler, 1990).
Table 1b.
Summarized information of additional 16 publications considered in initial systematic review.
| ID | Source | n | AUT | Age (years) | Mean (SD or Range) | VIQ Measure | MMN Analysis Window (ms) | Std-Dev Controlled | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Stimuli | TD | AUT AGE | AUT VIQ | |||||||
| 23 | Abdeltawwab et al. 2015 | P | 30 (12F) | 31 (7F) | 6–17 | 11.3 (2.8) | NR-NWNL* | NR | ~150– 200 | No |
| 24 | Bruneau et al. 2014 | P | NR | NR | 6–14 | NR | NR | NR | NR | No |
| 25 | Ceponiene et al. 2003 | PCS | 10 (1F) | 9 (1F) | 6–13 | 8.9 (2) | NR-NWNL* | RDLS | 176–248 | No |
| 26 | Courchesne et al. 1984 (passive) | S | 7 (NR) | 7 (NR) | 13–21 | NR | 71 (NR) | PPVT | NR | No |
| 27 | Donkers et al. 2013 | P | 39 (8F) | 28 (6F) | 4–12 | 7.5 (2.2) | NR | NR | NR | No |
| 28 | Edelson et al. 1999 | P | 0 (0F) | 5 (NR) | 4–39 | 11.6 (NR) | NR | NR | NR | No |
| 29 | Kasai et al. 2005 | PS | 19 (6F) | 9 (3F) | 15–38 | 27.2 (7.7) | NR-NWNL* | WAIS-R | 100–250 | No |
| 30 | Kemner et al. 1995 (passive) | S | 20 (4F) | 20 (4F) | 6–13 | 9.8 (1.5) | 80 (19) | WISC-RN | 150–325 | No |
| 31 | Kuhl et al. 2005 | S | 15 (2F) | 29 (3F) | 2–5 | 3.8 (NR) | NR | MSEL | 250–400 | No |
| 32 | Lincoln et al. 1993; 1995 (passive) | P | 10 (NR) | 10 (NR) | 8–14 | NR | 58 (12) | WISC-R | NR | Yes |
| 33 | Niwa et al. 1983 (passive) | P | 5 (4F) | 4 (0F) | 11–22 | 14.9 | NR | WAIS, WISC | NR | No |
| 34 | Novick et al. 1980 (passive missing stimulus paradigm) | P | 5 (NR) | 5 (NR) | Adolescent | NR | NR | NR | NR | No |
| 35 | Oades et al. 1988 | P | 9 (NR) | 7 (NR) | 5–18 | 11.3 (4.0) | NR | BAS | NR | No |
| 36 | Oram Cardy et al. 2005 | PC | 9 (4F) | 7 (0F) | 8–17 | 11.9 (3.1) | 81 (16) | WISC-3, WAIS-3, CELF-4 | 80–150 | Yes |
| 37 | Tecchio et al. 2003 | P | 10 (2F) | 14 (3F) | 8–32 | 16 (9) | NR | NR | 100–250 | No |
| 38 | Whitehouse et al. 2008 | CS | 15 (0F) | 15 (0F) | 7–15 | 10.4 (NR) | 94 (70– 113) | Verb Test for Rec of Gram | NR | No |
P=Pure tone; C=Complex tone; S=Speech; (SD)* = SD calculated from SD=S.E.M.
√N; NR=Not reported;
NR-NWNL=Not reported but indication of not-within normal limits. NR-WNL=Not reported but indication of typical range of scores (within normal limit). Italicized IQs indicate values are Developmental Quotients rather than Intelligence Quotients. VIQ or Language Measures: BAS=British Ability Scales (Eliot, 1983); MSEL=Mullen Scales of Early Learning (Mullen, 1984); PPVT=Peabody Picture Vocabulary Test (Dunn et al., 1965); RDLS-2=Reynell Developmental Language Scales—Second Revision (Reynell & Huntley, 1985); VTRG=Test for Reception of Grammar (Bishop, 2005); WISC=Wechsler Intelligence Scale for Children (Wechsler, 1949); WISC-RN=Wechsler Intelligence Scale for Children, Version—Revised, Dutch Version (Van Haasen et al., 1986); WAIS=Wechsler Adult Intelligence Scale (Wechsler, 1955).
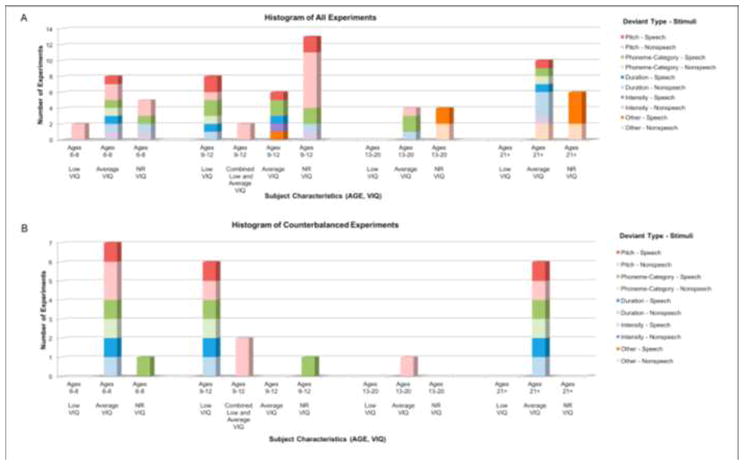
Figure 2a summarizes how the selected 67 experiments characterized deviance detection in different subject groups. We first classified experiments by the type of auditory stimuli used (either natural speech or nonspeech). Speech stimuli included naturally spoken consonants and/or vowels (e.g., “a” and “o” or “ba” and “wa”), or words (e.g., “pie” and “bye”). Nonspeech stimuli included pure tones (i.e., pure sinusoids) or complex tones (i.e., periodic stimuli made up of multiple harmonics). Often, the frequency content of the complex tones was shaped to mimic the formant structure of a certain vowel, so that they simulated detection of phonemic changes; however, such stimuli did not contain the natural spectrotemporal structure of typical speech so we classified these spectrally shaped tones as nonspeech. We further classified experiments by the type of deviant presented. Most commonly, deviants differed in phoneme, pitch (i.e., fundamental frequency), or duration. Less commonly, deviants differed in affective prosody, intensity, rhythm, or spatial location.
Figure 2. Histogram of (A) all 67 experiments included in this meta-analysis and (B) 24 counterbalanced experiments.
Experiments are classified by average age and verbal intelligence standard score of ASD participants. Average or above-average verbal intelligence (“Average VIQ”) is defined by average standard scores of 90 or above. Below average verbal IQ (“Low VIQ”) is defined by average standard scores below 80. Samples that fall between average standard scores of 80 and 90, around the cutoff score for disability (85) are considered as “Combined Low and Average” VIQ samples. Experiments are also classified based on the auditory feature which is deviating (“Deviant Type”) and the nature of the stimuli (“Speech” or “Nonspeech”). The “Other” category includes experiments that deviated stimuli based on “emotional content” (e.g., cheerful, angry, commanding, or sad), gap, or location.
From the distribution in Figure 2a, it is clear that experiments on children included samples with below-average verbal skills (mean verbal IQ below 80), samples with average or above-average verbal skills (mean verbal IQ above 90), and samples with a mixture of low and average or above-average verbal skills abilities (mean VIQ between 80 and 90). In contrast, studies focused on adolescents and adults included only individuals with average or above-average verbal skills. In addition, nonspeech stimuli were more commonly used in experiments conducted on participants with below-average verbal IQ, whereas studies on participants with average or above-average verbal IQ included both speech and nonspeech stimuli.
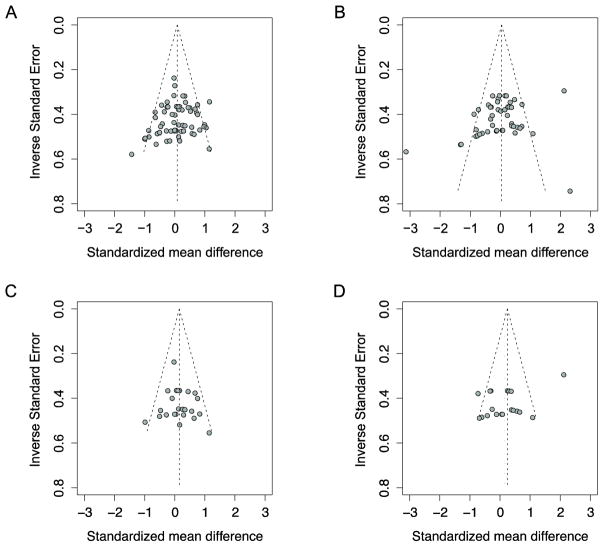
A summary of our meta-analysis across all appropriate experiments in the 22 publications (67 experiments) reporting MMN amplitude and latency appears in Table 2. There were no overall significant group differences in amplitude or latency. However, effect sizes were not consistent, with significant between-experiment variability. Across the studies from 22 publications, effect sizes for reported amplitude differences formed a Gaussian distribution, indicating that studies selected for publication were representative of all studies conducted on this topic (Figure 3a). The available data on latency differences from a total of 17 publications showed evidence of negatively-skewed publication bias; that is, published studies with negative results were over-represented in small-scale studies compared to large-scale studies (Figure 3b).
Table 2.
Effect Size and Tests of heterogeneity between experiments comparing MMN amplitude and MMN latency between ASD and TD groups.Results are computed for all experiments in the meta-analysis and experiments that counterbalanced standards and deviants. Positive SMD values correspond to the ASD group’s overall reduction in that metric, while negative values represent an increase.
| Test of Heterogeneity | Effect Size | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Sample | Q | df | p value | T2 | H | I2 [95% CI] | SMD [95% CI] | z-score | p value |
| Amplitude Measures: All | 98.48 | 63 | <0.01 | 0.09 | 1.25 | 36.0 [13–53] | 0.071 [−0.06–0.20] | 1.1 | 0.27 |
| Amplitude Measures: Counterbalanced | 24.07 | 23 | 0.40 | 0.01 | 1.02 | 4.4 [0–35] | 0.15 [−0.02–0.32] | 1.78 | 0.07 |
| Amplitude Measures: Speech | 11.27 | 10 | 0.34 | 0.02 | 1.06 | 11.2 [0–51] | 0.01 [−0.25–0.27] | 0.07 | 0.94 |
| Amplitude Measures: Nonspeech | 10.87 | 12 | 0.54 | 0.00 | 1.00 | 0.0 [0–52] | 0.25 [0.03–0.46] | 2.26 | 0.02 |
| Latency Measures: All | 154.32 | 49 | <0.01 | 0.36 | 1.79 | 68.9 [58–77] | −0.02 [−0.22–0.19] | −0.14 | 0.89 |
| Latency Measures: Counterbalanced | 67.03 | 18 | <0.01 | 0.47 | 1.93 | 22.9 [0–56] | 0.16 [−0.20–0.53] | 0.89 | 0.37 |
Figure 3. Symmetrical funnel plots suggest no evidence of publication bias based on amplitude effect sizes from (A) the full sample and (C) the counterbalanced sample. In contrast, some evidence of publication bias based on latency effect sizes from (B) the full sample and (D) the counterbalanced sample.
Egger’s regression tests: A. Intercept = −1.23 [95% standard error confidence interval: −2.88–0.41], t = −1.47, p>0.05; B. Intercept: −3.16 [95% standard error confidence interval: −5.90– −0.42], t = −2.26, p=0.02. C: Intercept: 0.57 [95% standard error confidence interval: −1.55–2.68], t=0.53, p>0.05. D: Intercept: −6.03 [95% standard error confidence interval: −11.29– −0.76], t=−2.24, p=0.04
3.2 Deviant-Standard Counterbalanced Experiments
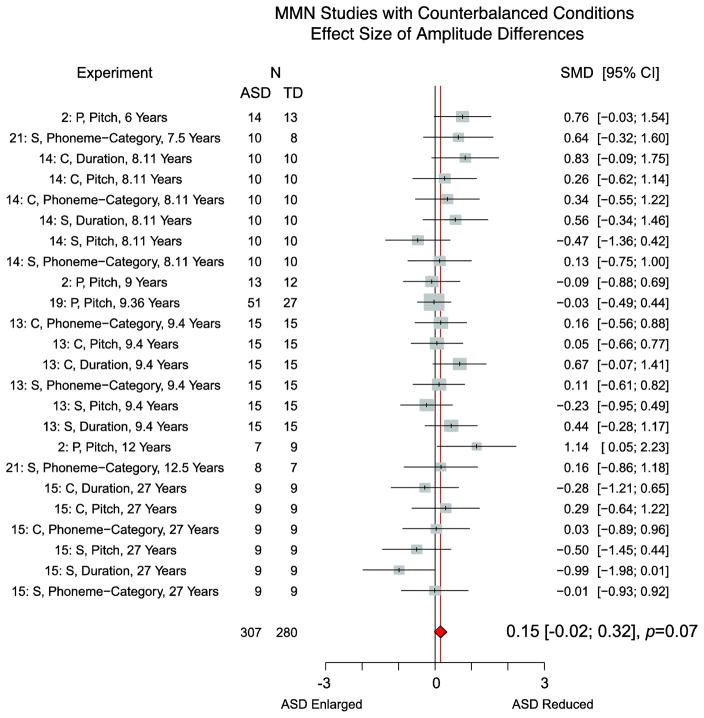
The next stage of our analysis included only those experiments that computed an MMN by taking differences in responses to physically identical stimuli when they were presented as both a deviant and a standard, which resulted in 24 experiments measuring MMN amplitude and 19 experiments measuring MMN latency across 6 publications. A total of 307 ASD and 280 TD subjects were included in the analysis of MMN amplitude and a total of 255 ASD and 231 TD subjects were included in the analysis of MMN latency. Collectively, these experiments included participants between the ages of 6 and 15 and adults over 21; notably, none of these studies focused on adolescents (Figure 2b). In addition, the majority of participants in these studies had average or above-average verbal IQs.
A meta-analysis on this data set revealed a standardized mean difference between ASD and TD groups in their MMN amplitude of 0.15 [−0.01–0.32], p=0.07 (Figure 4; Table 2). Although the group difference in MMN response did not reach statistical significance, there was a trend for children and adults with ASD, collectively, to show smaller MMN amplitudes than their TD peers. Tests of heterogeneity confirmed that the experiments measuring MMN amplitude produced consistent results. Just as in the full sample, there was no evidence of publication bias for MMN amplitude (Figure 3c). A meta-analysis of latency values revealed that there were no significant group differences. Furthermore, there was still considerable within-sample variability and evidence of publication bias (Figure 3d). Therefore, we discontinued our analysis of latency differences.
Figure 4. Meta-analysis of MMN amplitude differences in experiments that counterbalanced deviant and standard stimuli, organized by mean age of the ASD group.
Effect size is governed by standardized mean difference value (SMD). Experiment indicated by “Publication Number, as indicated in Table 1: Stimulus type, Deviant Type, (Mean ASD Age)”. Stimulus Type: P=Pure Tone, S=Speech, C=Complex Tone.
3.3 Effects of Stimuli and Subject Characteristics on Amplitude Differences
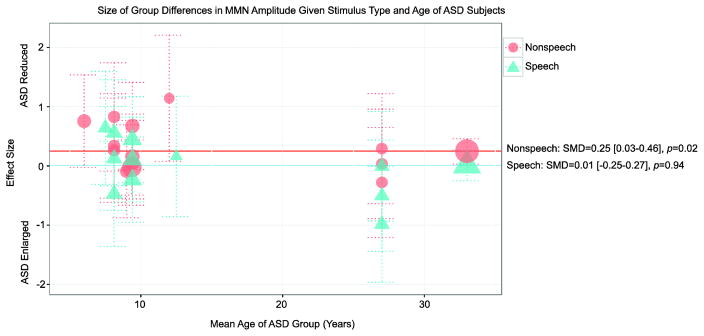
To explore whether stimulus characteristics influenced our findings on MMN amplitude, we separately analyzed counterbalanced experiments using nonspeech and experiments using speech stimuli (Figure 5; Table 2). For nonspeech stimuli, MMN responses were significantly smaller for individuals with ASD than for TD controls (SMD effect size=0.25, p=0.02). In contrast, there were no significant group differences for experiments investigating responses to speech stimuli (SMD effect size=0.009, p>0.05). However, there was no significant difference between the distribution of effect sizes resulting from speech and nonspeech experiments when the two were directly compared (Qb(1)=1.93, p=0.16).
Figure 5. Size of group differences in MMN amplitude given stimulus type and mean age of ASD group.
Data point sizes are weighted based on the experiment’s full sample size.
To examine whether or not the mean age of the ASD participants influenced results, we ran a linear regression in R Version 3.1 (CRAN, 2015; for similar example, see Erickson, Ruffle, & Gold, 2015). Mean age of the ASD group accounted for 25% of the variance in MMN amplitude effect size across experiments (R2=0.25, F(1,22)=7.28, p=0.01) and age significantly predicted effect size (Beta=−0.03, p=0.01). Visual inspection of effect size organized by mean age of the ASD group revealed that the youngest cohorts of ASD subjects had MMN amplitudes that were smaller than TD listeners, while adult cohorts of ASD subjects had MMN amplitudes equal to or larger than those of their TD peers (Figure 4).
A similar linear regression analysis on the influence of verbal IQ explained only 3% of the effect size variance (R2=0.03, F(1,19)=0.60, p=0.45) and did not predict effect size values (Beta=−0.004, p=0.45). In addition, when verbal IQ was included as a covariate in a linear model that measured the degree to which age predicted effect size, the model accounted for 24.7% of the effect size variance but was not statistically significant (R2=0.247, F(2,18)=2.95, p=0.08). In this model, mean age still significantly predicted effect size (Beta=−0.03, p=0.03). These results suggest that effect size differences across age cannot be explained solely by differences in verbal IQ.
4. Discussion
4.1 Summary
Although a fair number of past publications have investigated the MMN in ASD, individual studies have come to different conclusions. We undertook our meta-analysis to try to resolve these differences. Instead, our analysis revealed several important limitations of these studies. First, the majority of published studies included fewer than 20 participants per group. Such small sample sizes lead to problems when aggregating the data on group differences in MMN latency, since our analysis suggests that negative or null effect size results are reported more often in published small studies than in large studies. Furthermore, results from underpowered, small-sample studies are inherently less reliable and noisier than results from larger studies, which limits the power of our meta-analysis. Second, only 24 of the originally identified 67 studies measured MMNs with a rigorous design in which physically identical auditory stimuli were presented as standards and as deviants in different experimental blocks. Third, certain ages and verbal profiles, particularly adolescents and individuals with below-average verbal profiles, were not well represented in the pool of subjects tested. These issues may help explain some of the apparent variability across findings. Once we restricted our meta-analysis to include only appropriately counterbalanced experiments, we found amplitude effect sizes to be more consistent. Within this counterbalanced sample, there was no significant difference between groups in either MMN amplitude or MMN latency. However, there was a trend for ASD subjects to show smaller MMN amplitudes in response to deviant sounds than their TD peers, a finding warranting further investigation. When we divided our analyses by stimulus type, subjects with ASD had a significantly reduced MMN amplitude response to nonspeech, but not speech, deviants. In addition, younger children with ASD tended to exhibit reduced amplitude responses (i.e., greater effect sizes) while adults tended to show equal or larger amplitude responses than TDs (i.e., lower effect sizes). Moreover, no significant effects could be attributed to differences in verbal reasoning.
4.2 Importance of Counterbalanced Experiments
When we considered effect size of group differences across all 67 experiments that met our initial inclusion criteria, no consistent pattern emerged. This was perhaps due to major inconsistencies in MMN measurement; in particular, many of those experiments used stimuli that were not counterbalanced. Differences in the physical nature of standard and deviant stimuli can give rise to different evoked EEG responses, a confound for studies evaluating only the contextual effects of the stimuli. Specifically, the N1 response – which occurs only slightly before the MMN onset – can vary significantly with the acoustic properties of a stimulus (Duncan et al., 2009; Kujala et al., 2007). Different acoustic features in standard and deviant stimuli can contaminate the standard-deviant difference waveform that is computed to quantify the MMN (Kujala et al., 2007). As a result, the “MMN” produced by subtracting these unmatched standard and deviant responses do not solely contain response components that reflect effects of context and detection of an unexpected stimulus.
For example, in this analysis, of the 11 experiments that measured detection of pitch change and did not counterbalance, 8 used deviants that were higher in pitch than their relative standards. In these experiments, the “MMN” might be partially attributable to the fact that the N1 response is larger for high-pitched than low-pitched sounds. Such an effect could be especially significant for those participants with ASD who are particularly sensitive to high pitches (Bonnel et al., 2003; Bonnel et al., 2010). Similarly, in three of the four experiments that measured duration deviance and did not counterbalance stimuli, the duration of the deviant was shorter than the standard. Under such conditions, the N1 offset to the deviant occurs prior to the N1 offset to the standard due to nonlinear effects in the auditory periphery (Kujala et al., 2007). This difference wave in N1 response could either be misinterpreted as an MMN or obscure the real presence of an MMN (Kujala et al., 2007). Although counterbalancing significantly lengthens the duration of an experiment, we recommend that future experiments compute MMNs by using identical stimuli as “standards” and “deviants” in different blocks. Taking this approach, the MMN will capture stimulus change detection and ensure response components are not due to differences in responses to different sounds.
4.3 Variations in Stimulus Features
Prior reports have suggested that group differences between ASD and TDs are specific to either speech or nonspeech stimuli (Fan & Cheng, 2014; Jansson-Verkhasalo et al., 2003; Lepistö et al., 2007; Weismüller et al., 2015; Yu et al. 2015). Of the experiments using nonspeech stimuli, the majority employed complex tones, not pure tones. In our meta-analysis of nonspeech experiments, ASD subjects had smaller MMN responses than TD subjects. These results suggest that complex tone deviants lead to smaller MMN responses in people with ASD. This finding corroborates prior work concluding that individuals with ASD have weak neural and behavioral responses to changes in complex nonspeech stimuli, perhaps due to a weak encoding of spectrally and temporally complex, dynamic information (Samson, Mottron, Jemel, Belin, & Ciocca, 2005). Further, our results support prior work arguing that deficits in nonspeech auditory processing are present in ASD (Foss-Feig et al., 2012). Thus, people with ASD, especially children, may be less efficient in their pre-attentive and automatic processing of auditory regularities in nonspeech stimuli, which is measured by the MMN.
In contrast, our meta-analysis showed no significant MMN group differences in response to speech-based stimuli. Features of early auditory discrimination that arise from deviations in low-level features such as pitch, duration, or intensity deviants should cause similar effects whether the stimuli are nonspeech or speech. However, speech-based stimuli were often used when measuring sensitivity to phonetic deviants, a process which may rely on later stages of cortical processing. Of note, the few studies included in our meta-analysis and systematic review that analyzed results in later latency windows (minimum of window starting at 200 ms) measured speech-elicited neural responses. Processing of speech-feature change may be reflected in later ERP/ERF components like the P3 (Cui, Wang, Liu, & Zhang, 2016; Haeson et al., 2011), rather than relatively early responses like the MMN, which we analyzed. Further investigation comparing late ERP/ERF components in ASD and TD populations should be undertaken to examine whether later neural processing stages for speech stimuli differ between these populations.
4.4 Variations in Subject Characteristics
In addition to our primary meta-analysis, we characterized how group differences in MMN amplitude change across development. This choice was motivated by evidence that the MMN changes over the course of typical developmental and the fact that ASD is a developmental disorder (Martin, Shafer, Morr, Kreuzer, & Kurtzberg, 2003; Shafer, Morr, Kreuzer, & Kurtzberg, 2000). We found that age accounted for 25% of the variability in MMN amplitude. Studies on young children tended to produce the greatest effect sizes, representative of reduced amplitude response in ASD. Studies on adults tended to produce the most negative effect sizes, representative of equal or larger amplitude responses in ASD. However, there were no studies that counterbalanced stimuli and focused on adolescents with ASD. This gap prevented us from fully characterizing age differences in MMN amplitude in ASD compared to TD. Further, the available data was based solely on cross-sectional data. Future studies using a longitudinal approach may uncover whether young children with absent or reduced MMNs develop mature MMN responses with age.
While the conclusions we can make about MMN across age are constrained by limited data, the results of our analysis complement parent-reported data on children with ASD, which suggest that 1) atypical auditory processing in ASD decreases with age (Kern et al., 2006) and 2) sensory modulation symptoms, including abnormal sensitivity to sound, are greatest in middle childhood between the ages of 6 and 9, decreasing thereafter (Ben-Sasson et al., 2009). The parallels between these reports and our findings point to a potential link between neural response to sounds and an overt sensitivity to sounds that should be explored in future studies.
Given the postulated link between auditory processing and language development in ASD, we also considered the verbal reasoning abilities of subjects across studies (Kujala, 2007). Our findings first and foremost demonstrate that the MMN response in individuals with below-average verbal reasoning abilities is still highly understudied. The set of available data was skewed, with the majority of children between the ages of 6 to 8, as well as adults, displaying average or above-average verbal reasoning and the majority of children between the ages 9 to 12 displaying below-average verbal reasoning. Still, verbal IQ, a measure of verbal reasoning abilities, did not significantly predict individual experiment group differences or account for differences in effect size already predicted by age, which reduces concern that differences in the distribution of verbal reasoning in different age groups biased our findings.
Of the 67 MMN studies identified by our meta-analysis, only 15 included individuals with below-average verbal IQ (standardized mean scores of less than 80), the majority of whom were younger than 12. Researchers need to be aware that there are few studies that include individuals with low verbal ability and work to fill this gap, e.g., using a passive auditory MMN paradigm (Bishop, 2007; Näätänen et al., 2012).
4.5 Consideration of Latency
While there was a trend towards significant differences in amplitude between ASD and TD groups, MMN latency did not differ across groups. However, only 19 counterbalanced experiments across 4 publications examined MMN latency, sampling a total of 486 participants (255 ASD and 231 TD). Significant variability was evident within both the whole sample and the counterbalanced-only sample. This variability was driven considerably by one large study that reported large positive results; all other studies included in this analysis had small effect sizes clustered around zero. Moreover, while significant publication bias was identified, its impact cannot be fully isolated from other findings reflected in the large variability across studies (Peters et al., 2010). Meta-analyses of the MMN in populations with other clinical conditions (e.g., attention deficit hyperactivity disorder, specific language impairment and dyslexia, and schizophrenia) have not reported on latency group differences, perhaps because of a similar paucity of such studies (Cheng, Chan, Hsieh, & Chen, 2016; Bishop, 2007; Erickson et al, 2015; Umbricht & Krljes, 2005). It is also likely that few studies report on latency because it is a relatively unreliable way to quantify noisy ERP/ERF data, especially when there may not be definitive, sharp component peaks (Luck, 2005; Bishop, 2007). These issues with noise become especially relevant when analyzing ERPs/ERFs from young children and individuals with neurodevelopmental disorders. The sole MEG study that we found matching our inclusion criteria had large, positive findings. It may be that MEG data are more sensitive to latency differences in STG sources than are EEG data from fronto-central scalp channels; however, with only one study matching our criteria, it is not known if this difference is replicable. Future refinements in EEG/MEG analysis techniques are needed to make it possible to calculate latency with greater precision.
4.5 Future Directions
While we initially identified a large number of published studies describing the MMN component in ASD, only a handful computed the MMN by comparing standard and deviant responses for acoustically identical stimuli, and even fewer contributed data that could help us identify differences between groups influenced by age or verbal reasoning abilities. Among those that measured the MMN, many had small sample sizes, leading to relatively low statistical power. For future work to identify individuals with ASD who are most susceptible to auditory processing deficits, counterbalanced paradigms need to be administered. These paradigms should be applied to large sets of subjects, from young children through adults, that display a range of severity in their clinical ASD features and language abilities. Based on MMN studies in typically developing adults, there are recommendations for procedures, stimulus design, recording, and analysis techniques that elicit a robust MMN (Duncan et al., 2009; Pakarinen, Takegata, Rinne, Huotilainen, & Näätänen, 2007). Such recommendations should be followed when testing individuals with ASD to produce robust, consistent results that can be compared across studies.
Most research has compared average response magnitudes between ASD and TD. Looking across these studies, we found no major group differences; we hypothesize that this is in part due to the heterogeneous nature of ASD. While group-level analysis is important, future research should also consider whether individual differences can be measured reliably in MMN responses. For instance, researchers can investigate metrics such as the percentage of each group that showed a reliable MMN (Bishop & Hardiman, 2010; Dunn, Gomes, & Gavel, 2008). Subjects in the ASD population who do not demonstrate a reliable MMN response may also tend to share a common phenotypic or clinical feature; this kind of result could allow MMN measures to help identify distinct subgroups within the heterogeneous ASD population.
A few of the studies that we reviewed did look at the relationship between MMN response magnitude and phenotypic characteristics other than verbal reasoning (Andersson et al., 2013; Gomot et al., 2011; Kuhl, Coffey-Corina, Padden, & Dawson, 2005), but because similar measures were not readily available across studies, we could not combine results in a meaningful way in our meta-analysis. Studies on other brain-based disorders such as schizophrenia have demonstrated associations between MMN amplitude and clinical characteristics such as symptom severity and duration of symptoms (Daltrozzo, Wioland, Mutschler, & Kotchoubey, 2007; Erickson et al., 2015; Light & Braff, 2005; Umbricht & Krljes, 2005). Given these successes, it seems promising to investigate relationships between established and commonly used measures of ASD severity (e.g., Autism Diagnostic Observation Schedule Calibrated Severity Score; Gotham, Pickles, & Lord, 2009), language (e.g., Peabody Picture Vocabulary Test, Version 4; Dunn & Dunn, 2007), and MMN in future studies.
Considerable work is necessary to determine whether reduced MMNs in young children translate to poorer outcomes in language or other cognitive domains (Friederich, Weber, & Friederici, 2004; Leppänen et al., 2002). Still, the MMN is detectable in infants as young as 8 weeks old (Friederici, Friedrich, & Weber, 2002; Schall, 2015; Shafer et al., 2011; Trainor et al., 2003) and subject-specific analysis may allow clinicians to use MMN responses to identify children who have atypical cortical processing and who thus might be prone to developing auditory processing deficits.
4.6 Conclusion
To our knowledge, our meta-analysis is the first to empirically evaluate the results from a large set of previously published studies reporting MMN responses in ASD. Through this analysis, we found that most studies on this topic were not designed with counterbalanced stimuli and did not produce consistent results. When our analysis was confined to studies that used physically identical stimuli in standard and deviant contexts, we found that there were still no major group differences for MMN amplitude or latency, but that group differences in amplitude became more consistent and changed as a factor of age. Still, these findings were derived from an unrepresentative sample of individuals with ASD and underpowered studies using a small number of participants. Given the heterogeneity of characteristics in ASD and variability we find, studies considering only between-group effects may be overlooking critical information about individual differences in MMN response within the ASD group. These limitations expose major gaps in the current literature on sound change detection in ASD that future work will need to address.
Highlights.
Meta-analyses assessed the auditory MMN response in ASD across 22 publications
Most studies did not counterbalance stimuli
Studies that counterbalanced stimuli showed a trend toward weaker MMN in ASD
Weaker MMN response in ASD was most evident in young children
Weak responses were more evident in nonspeech-based paradigms
MMN response in adolescents and those with below-average verbal IQ is understudied
Lack of group differences may be due to the heterogeneity of ASD
Targeted study of within-group variability may reveal MMN response anomalies
Acknowledgments
Funding: This work was supported by the National Institutes of Health [P50 DC013027]; the National Science Foundation, Arlington, VA [SMA-0835976], and the Autism Speaks Foundation, Princeton, NJ [10085].
Footnotes
For reviews of prior research utilizing neural measures to investigate atypical auditory processing in brain-based disorders like ASD, Specific Language Impairment, Dyslexia, Learning impairment, Schizophrenia, Attention-Deficit/Hyperactivity Disorder, Bipolar Disorder, and Aphasia, see: Aaltonen, Tuomainen, Laine, & Niemi, 1993; Barry, Clarke, & Johnstone, 2003; Bishop, 2007; Chitty, Lagopoulos, Lee, Hickie, & Hermens, 2013; Erickson, Ruffle, & Gold, 2015; Kraus et al., 1996; Kujala et al. 2013; Näätänen & Kahkonen, 2009; O’Connor, 2012; Umbricht & Krlijes, 2005.
Individual correspondence with authors was needed in two instances to receive unpublished, additional information.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for pub lication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aaltonen O, Tuomainen J, Laine M, Niemi P. Cortical Differences in Tonal versus Vowel Processing as Revealed by an ERP Component Called Mismatch Negativity (MMN) Brain and Language. 1993;44(2):139–152. doi: 10.1006/brln.1993.1009. [DOI] [PubMed] [Google Scholar]
- 2.Abdeltawwab MM, Baz H. Automatic Pre-Attentive Auditory Responses: MMN to Tone Burst Frequency Changes in Autistic School-Age Children. The Journal of International Advanced Otology. 2015;11(1):36–41. doi: 10.5152/iao.2014.438. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®) American Psychiatric Pub; 2013. [Google Scholar]
- 4.Andersson S, Posserud MB, Lundervold AJ. Early and late auditory event-related potentials in cognitively high functioning male adolescents with autism spectrum disorder. Research in Autism Spectrum Disorders. 2013;7(7):815–823. doi: 10.1016/j.rasd.2013.03.007. [DOI] [Google Scholar]
- 5.Barry RJ, Clarke AR, Johnstone SJ. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clinical Neurophysiology. 2003;114(2):171–183. doi: 10.1016/S1388-2457(02)00362-0. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Sasson A, Hen L, Fluss R, Cermak SA, Engel-Yeger B, Gal E. A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(1):1–11. doi: 10.1007/s10803-008-0593-3. [DOI] [PubMed] [Google Scholar]
- 7.Bishop DVM. Using mismatch negativity to study central auditory processing in developmental language and literacy impairments: where are we, and where should we be going? Psychological Bulletin. 2007;133(4):651–672. doi: 10.1037/0033-2909.133.4.651. [DOI] [PubMed] [Google Scholar]
- 8.Bishop DVM. Test for reception of grammar—electronic. London: Psychological Corporation; 2005. [Google Scholar]
- 9.Bishop DVM, Hardiman MJ. Measurement of mismatch negativity in individuals: A study using single-trial analysis. Psychophysiology. 2010;47(4):697–705. doi: 10.1111/j.1469-8986.2009.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bomba MD, Pang EW. Cortical auditory evoked potentials in autism: A review. International Journal of Psychophysiology. 2004;53(3):161–169. doi: 10.1016/j.ijpsycho.2004.04.001. http://doi.org/10.1016/j.ijpsycho.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Bonnel A, McAdams S, Smith B, Berthiaume C, Bertone A, Ciocca V, … Mottron L. Enhanced pure-tone pitch discrimination among persons with autism but not Asperger syndrome. Neuropsychologia. 2010;48(9):2465–2475. doi: 10.1016/j.neuropsychologia.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 12.Bonnel A, Mottron L, Peretz I, Trudel M, Gallun E, Bonnel AM. Enhanced pitch sensitivity in individuals with autism: a signal detection analysis. Journal of Cognitive Neuroscience. 2003 doi: 10.1162/089892903321208169. [DOI] [PubMed] [Google Scholar]
- 13.Bruneau N, Cléry H, Malvy J, Barthélémy C, Bonnet-Brilhault F, Gomot M. Hypersensitivity to change in children with autism spectrum disorder: convergent evidence from visual and auditory MMN studies. International Journal of Psychophysiology. 2014;94(2):156. doi: 10.1016/j.ijpsycho.2014.08.693. [DOI] [Google Scholar]
- 14.Ceponiene R, Lepistö T, Shestakova A, Vanhala R, Alku P, Näätänen R, Yaguchi K. Speech-sound-selective auditory impairment in children with autism: they can perceive but do not attend. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(9):5567–72. doi: 10.1073/pnas.0835631100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng CH, Chan PYS, Hsieh YW, Chen KF. A meta-analysis of mismatch negativity in children with attention deficit-hyperactivity disorders. Neuroscience Letters. 2016;612:132–137. doi: 10.1016/j.neulet.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 16.Chitty KM, Lagopoulos J, Lee RSC, Hickie IB, Hermens DF. A systematic review and meta-analysis of proton magnetic resonance spectroscopy and mismatch negativity in bipolar disorder. European Neuropsychopharmacology. 2013;23(11):1348–1363. doi: 10.1016/j.euroneuro.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Cochran WG. The Combination of Estimates from Different Experiments. Biometrics. 1954;10(1):101–129. doi: 10.2307/3001666. [DOI] [Google Scholar]
- 18.Courchesne E, Kilman B, Galambos R, Lincoln A. Autism: Processing of novel auditory information assessed by event-related brain potentials. Electroencephalography and Clinical Neurophysiology. 1984;59:238–248. doi: 10.1016/0168-5597(84)90063-7. [DOI] [PubMed] [Google Scholar]
- 19.Cui T, Wang PP, Liu S, Zhang X. P300 amplitude and latency in autism spectrum disorder: a meta-analysis. European Child and Adolescent Psychiatry. 2017;26(2):177–190. doi: 10.1007/s00787-016-0880-z. http://doi.org/10.1007/s00787-016-0880-z. [DOI] [PubMed] [Google Scholar]
- 20.Daltrozzo J, Wioland N, Mutschler V, Kotchoubey B. Predicting coma and other low responsive patients outcome using event-related brain potentials: A meta-analysis. Clinical Neurophysiology. 2007;118(3):606–614. doi: 10.1016/j.clinph.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Donkers FCL, Schipul SE, Baranek GT, Cleary KM, Willoughby MT, Evans AM, … Belger A. Attenuated auditory event-related potentials and associations with atypical sensory response patterns in children with autism. Journal of Autism and Developmental Disorders. 2015;45(2):506–23. doi: 10.1007/s10803-013-1948-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan CC, Barry RJ, Connolly JF, Fischer C, Michie PT, Näätänen R, … Van Petten C. Event-related potentials in clinical research: guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clinical Neurophysiology. 2009;120(11):1883–1908. doi: 10.1016/j.clinph.2009.07.045. [DOI] [PubMed] [Google Scholar]
- 24.Dunn LM, Dunn DM. Peabody picture vocabulary test—Fourth Edition. Bloomington, MN: Pearson Assessments; 2007. [Google Scholar]
- 25.Dunn LM, Dunn LM. Peabody picture vocabulary test. 3. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- 26.Dunn LM, Dunn LM, Bulheller S, Häcker H. Peabody picture vocabulary test. Circle Pines, MN: American Guidance Service; 1965. [Google Scholar]
- 27.Dunn MA, Gomes H, Gravel J. Mismatch negativity in children with autism and typical development. Journal of Autism and Developmental Disorders. 2008;38:52–71. doi: 10.1007/s10803-007-0359-3. [DOI] [PubMed] [Google Scholar]
- 28.Edelson SM, Arin D, Bauman M, Lukas SE, Rudy JH, Sholar M, Rimland B. Auditory integration training: a double-blind study of behavioral and electrophysiological effects in people with autism. Focus on Autism and Other Developmental Disabilities. 1999;14(2):73–81. doi: 10.1177/108835769901400202. [DOI] [Google Scholar]
- 29.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research Ed) 1997;315(7109):629–34. doi: 10.1136/bmj.316.7129.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliott CD, Murray DJ, Pearson LS. British Ability Scales. Windsor, U.K: Nfer-nelson Publishing Company; 1983. [Google Scholar]
- 31.Erickson MA, Ruffle A, Gold JM. A meta-analysis of mismatch negativity in schizophrenia: from clinical risk to disease specificity and progression. Biological Psychiatry. 2015:1–8. doi: 10.1016/j.biopsych.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan YT, Cheng Y. Atypical mismatch negativity in response to emotional voices in people with autism spectrum conditions. PLoS ONE. 2014;9(7):1–10. doi: 10.1371/journal.pone.0102471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferri R, Elia M, Agarwal N, Lanuzza B, Musumeci Sa, Pennisi G. The mismatch negativity and the P3a components of the auditory event-related potentials in autistic low-functioning subjects. Clinical Neurophysiology. 2003;114(9):1671–1680. doi: 10.1016/S1388-2457(03)00153-6. [DOI] [PubMed] [Google Scholar]
- 34.Foss-Feig JH, Stone WL, Wallace MT. Processing of non-speech auditory stimuli in individuals with autism spectrum disorders: the impact of stimulus characteristics. International Review of Research in Developmental Disabilities. 2012;43:87–145. [Google Scholar]
- 35.Frandsen AN, Higginson JB. The stanford-binet and the wechsler intelligence scale for children. Journal of Consulting Psychology. 1951;15(3):236–238. doi: 10.1037/h0059816. [DOI] [PubMed] [Google Scholar]
- 36.Friederici AD, Friedrich M, Weber C. Neural manifestation of cognitive and precognitive mismatch detection in early infancy. Neuroreport. 2002;13(10):1251–1254. doi: 10.1097/00001756-200207190-00006. [DOI] [PubMed] [Google Scholar]
- 37.Friedrich M, Weber C, Friederici AD. Electrophysiological evidence for delayed mismatch response in infants at-risk for specific language impairment. Psychophysiology. 2004;41(5):772–782. doi: 10.1111/j.1469-8986.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 38.Gomot M, Blanc R, Clery H, Roux S, Barthelemy C, Bruneau N. Candidate electrophysiological endophenotypes of hyper-reactivity to change in autism. Journal of Autism and Developmental Disorders. 2011;41:705–714. doi: 10.1007/s10803-010-1091-y. [DOI] [PubMed] [Google Scholar]
- 39.Gomot M, Giard M, Adrien J, Barthelemy C, Bruneau N. Hypersensitivity to acoustic change in children with autism: electrophysiological evidence of left frontal cortex dysfunctioning. Psychophysiology. 2002 Oct;392002:577–584. doi: 10.1017.S0048577202394058. doi:10.1017.S0048577202394058. [DOI] [PubMed] [Google Scholar]
- 40.Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(5):693–705. doi: 10.1007/s10803-008-0674-3. http://doi.org/10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haesen B, Boets B, Wagemans J. A review of behavioural and electrophysiological studies on auditory processing and speech perception in autism spectrum disorders. Research in Autism Spectrum Disorders. 2011;5:701–714. doi: 10.1016/j.rasd.2010.11.006. [DOI] [Google Scholar]
- 42.Jansson-Verkasalo E, Ceponiene R, Kielinen M, Suominen K, Jäntti V, Linna SL, … Naatänen R. Deficient auditory processing in children with Asperger syndrome, as indexed by event-related potentials. Neuroscience Letters. 2003;338(3):197–200. doi: 10.1016/S0304-3940(02)01405-2. [DOI] [PubMed] [Google Scholar]
- 43.Jansson-Verkasalo E, Kujala T, Jussila K, Mattila ML, Moilanen I, Naatanen R, … Korpilahti P. Similarities in the phenotype of the auditory neural substrate in children with Asperger syndrome and their parents. European Journal of Neuroscience. 2005 Sep;222005:986–990. doi: 10.1111/j.1460-9568.2005.04216.x. [DOI] [PubMed] [Google Scholar]
- 44.Jones CRG, Happé F, Baird G, Simonoff E, Marsden AJS, Tregay J, Phillips R, Goswami U, Thomson J, Charman T. Auditory discrimination and auditory sensory behaviours in autism spectrum disorders. Neuropsychologia. 2009;47(13):2850–2858. doi: 10.1016/j.neuropsychologia.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 45.Kasai K, Hashimoto O, Kawakubo Y, Yumoto M, Kamio S, Itoh K, … Kato N. Delayed automatic detection of change in speech sounds in adults with autism: A magnetoencephalographic study. Clinical Neurophysiology. 2005;116:1655–1664. doi: 10.1016/j.clinph.2005.03.007. http://doi.org/10.1016/j.clinph.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Kemner C, Verbaten MN, Cuperus JM, Camfferman G, van Engeland H. Auditory event-related brain potentials in autistic children and three different control groups. Biological Psychiatry. 1995;38(94):150–165. doi: 10.1016/0006-3223(94)00247-Z. [DOI] [PubMed] [Google Scholar]
- 47.Kern JK, Trivedi MH, Garver CR, Grannemann BD, Andrews AA, Savla JS, Johnson DG, Mehta JA, Schroeder JL. The pattern of sensory processing abnormalities in autism. Autism. 2006;10(5):480–494. doi: 10.1177/1362361306066564. [DOI] [PubMed] [Google Scholar]
- 48.Korpilahti P, Jansson-Verkasalo E, Mattila ML, Kuusikko S, Suominen K, Rytky S, … Moilanen I. Processing of affective speech prosody is impaired in Asperger syndrome. Journal of Autism and Developmental Disorders. 2007;37:1539–1549. doi: 10.1007/s10803-006-0271-2. [DOI] [PubMed] [Google Scholar]
- 49.Kraus N, McGee TJ, Carrell TD, Zecker SG, Nicol TG, Koch DB. Auditory neurophysiologic responses and discrimination deficits in children with learning problems. Science. 1996;273(5277):971–973. doi: 10.1126/science.273.5277.971. [DOI] [PubMed] [Google Scholar]
- 50.Kuhl PK, Coffey-Corina S, Padden D, Dawson G. Links between social and linguistic processing of speech in preschool children with autism: behavioral and electrophysiological measures. Developmental Science. 2005;8(1):F1–F12. doi: 10.1111/j.1467-7687.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- 51.Kujala T. The role of early auditory discrimination deficits in language disorders. Journal of Psychophysiology. 2007;21(3–4):239–250. doi: 10.1027/0269-8803.21.34.239. [DOI] [Google Scholar]
- 52.Kujala T, Aho E, Lepistö T, Jansson-Verkasalo E, Nieminen-von Wendt T, von Wendt L, Näätänen R. Atypical pattern of discriminating sound features in adults with Asperger syndrome as reflected by the mismatch negativity. Biological Psychology. 2007;75:109–114. doi: 10.1016/j.biopsycho.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 53.Kujala T, Kuuluvainen S, Saalasti S, Jansson-Verkasalo E, Von Wendt L, Lepistö T. Speech-feature discrimination in children with Asperger syndrome as determined with the multi-feature mismatch negativity paradigm. Clinical Neurophysiology. 2010;121(9):1410–1419. doi: 10.1016/j.clinph.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 54.Kujala T, Lepistö T, Näätänen R. The neural basis of aberrant speech and audition in autism spectrum disorders. Neuroscience and Biobehavioral Reviews. 2013;37(4):697–704. doi: 10.1016/j.neubiorev.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 55.Kujala T, Lepistö T, Nieminen-Von Wendt T, Näätänen P, Näätänen R. Neurophysiological evidence for cortical discrimination impairment of prosody in Asperger syndrome. Neuroscience Letters. 2005;383:260–265. doi: 10.1016/j.neulet.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 56.Lepistö T, Kuitunen a, Sussman E, Saalasti S, Jansson-Verkasalo E, Nieminen-von Wendt T, Kujala T. Auditory stream segregation in children with Asperger syndrome. Biological Psychology. 2009;82(3):301–7. doi: 10.1016/j.biopsycho.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lepistö T, Kajander M, Vanhala R, Alku P, Huotilainen M, Näätänen R, Kujala T. The perception of invariant speech features in children with autism. Biological Psychology. 2008;77:25–31. doi: 10.1016/j.biopsycho.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 58.Lepistö T, Nieminen-von Wendt T, von Wendt L, Näätänen R, Kujala T. Auditory cortical change detection in adults with Asperger syndrome. Neuroscience Letters. 2007;414:136–140. doi: 10.1016/j.neulet.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 59.Lepistö T, Silokallio S, Nieminen-von Wendt T, Alku P, Näätänen R, Kujala T. Auditory perception and attention as reflected by the brain event-related potentials in children with Asperger syndrome. Clinical Neurophysiology. 2006;117:2161–2171. doi: 10.1016/j.clinph.2006.06.709. [DOI] [PubMed] [Google Scholar]
- 60.Lepistö T, Kujala T, Vanhala R, Alku P, Huotilainen M, Näätänen R. The discrimination of and orienting to speech and nonspeech sounds in children with autism. Brain Research. 2005;1066:147–157. doi: 10.1016/j.brainres.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 61.Leppänen PHT, Richardson U, Pihko E, Eklund KM, Guttorm TK, Aro M, Lyytinen H. Brain responses to changes in speech sound durations differ between infants with and without familial risk for dyslexia. Developmental Neuropsychology. 2002;22(1):407–422. doi: 10.1207/S15326942dn2201_4. [DOI] [PubMed] [Google Scholar]
- 62.Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Archives of General Psychiatry. 2005;62(2):127. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- 63.Lincoln A, Courchesne E, Harms L, Allen M. Contextual probability evaluation in autistic, receptive developmental language disorder, and control children: event-related brain potential evidence. Journal of Autism and Developmental Disorders. 1993;23(1):37–58. doi: 10.1007/BF01066417. [DOI] [PubMed] [Google Scholar]
- 64.Lincoln J, Courchesne E, Harms L, Allen M. Sensory modulation of auditory stimuli in children with autism and receptive developmental language disorder: Event-related brain potential evidence. Journal of Autism and Developmental Disorders. 1995;25(5):521–539. doi: 10.1007/BF02178298. [DOI] [PubMed] [Google Scholar]
- 65.Luck SJ. An introduction to the event-related potential technique. Cambridge, MA: MIT press; 2005. [Google Scholar]
- 66.Ludlow A, Mohr B, Whitmore A, Garagnani M, Pulvermüller F, Gutierrez R. Auditory processing and sensory behaviours in children with autism spectrum disorders as revealed by mismatch negativity. Brain and Cognition. 2014;86:55–63. doi: 10.1016/j.bandc.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 67.Marco EJ, Hinkley LBN, Hill SS, Nagarajan S. Sensory processing in autism: A review of neuropsychologic findings. Pediatric Research. 2011;69(5):48–54. doi: 10.1203/PDR.0b013e3182130c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin Ba, Shafer VL, Morr ML, Kreuzer Ja, Kurtzberg D. Maturation of mismatch negativity: a scalp current density analysis. Ear and Hearing. 2003;24(6):463–71. doi: 10.1097/01.AUD.0000100306.20188.0E. [DOI] [PubMed] [Google Scholar]
- 69.Mcfadden KL, Rojas DC. Electrophysiology of autism. In: Fitzgerald M, editor. Recent advances in autism spectrum disorders. II. Rijeka, Croatia: InTech; 2013. pp. 73–101. [DOI] [Google Scholar]
- 70.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 71.Mullen EM. Mullen scales of early learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- 72.Näätänen R, Gaillard AWK, Mäntysalo S. Early selective-attention effect on evoked potential reinterpreted. Acta Psychologica. 1978;42(4):313–329. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- 73.Näätänen R, Kähkönen S. Central auditory dysfunction in schizophrenia as revealed by the mismatch negativity (MMN) and its magnetic equivalent MMNm: a review. The International Journal of Neuropsychopharmacology. 2009;12(1):125. doi: 10.1017/S1461145708009322. [DOI] [PubMed] [Google Scholar]
- 74.Näätänen R, Kujala T, Escera C, Baldeweg T, Kreegipuu K, Carlson S, Ponton C. The mismatch negativity (MMN) - A unique window to disturbed central auditory processing in ageing and different clinical conditions. Clinical Neurophysiology. 2012;123(3):424–458. doi: 10.1016/j.clinph.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 75.Näätänen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clinical Neurophysiology. 2007;118:2544–2590. doi: 10.1016/j.clinph.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 76.Näätänen R, Kujala T. The mismatch negativity and its magnetic equivalent: An index of language impairment or more general cognitive decline in autism? Biological Psychiatry. 2011;70(3):212–213. doi: 10.1016/j.biopsych.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 77.Niwa S, Ohta M, Yamazaki K. P300 and stimulus evaluation process in autistic subjects. Journal of Autism and Developmental Disorders. 1983;13(1):33–42. doi: 10.1007/BF01531357. [DOI] [PubMed] [Google Scholar]
- 78.Novick B, Vaughan G. An electrophysiologic indication of auditory processing defects in autism. 1980;3(1):107–114. doi: 10.1016/0165-1781(80)90052-9. [DOI] [PubMed] [Google Scholar]
- 79.O’Connor K. Auditory processing in autism spectrum disorder: A review. Neuroscience and Biobehavioral Reviews. 2012;36(2):836–854. doi: 10.1016/j.neubiorev.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 80.Oades RD, Walker MK, Geffen LB, Stern LM. Event-related potentials in autistic and healthy children on an auditory choice reaction time task. International Journal of Psychophysiology. 1988;6(1):25–37. doi: 10.1016/0167-8760(88)90032-3. [DOI] [PubMed] [Google Scholar]
- 81.Oram Cardy JE, Flagg EJ, Roberts W, Roberts TPL. Delayed mismatch field for speech and non-speech sounds in children with autism. Neuroreport. 2005;16(5):521–525. doi: 10.1097/00001756-200504040-00021. http://doi.org/10.1097/00001756-200504040-00021. [DOI] [PubMed] [Google Scholar]
- 82.Orekhova EV, Stroganova TA. Arousal and attention re-orienting in autism spectrum disorders: evidence from auditory event-related potentials. Frontiers in Human Neuroscience. 2014;8(34) doi: 10.3389/fnhum.2014.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pakarinen S, Takegata R, Rinne T, Huotilainen M, Näätänen R. Measurement of extensive auditory discrimination profiles using the mismatch negativity (MMN) of the auditory event-related potential (ERP) Clinical Neurophysiology. 2007;118(1):177–185. doi: 10.1016/j.clinph.2006.09.001. http://doi.org/10.1016/j.clinph.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 84.Perron-Borelli M. Les echelles differentielles d’efficiences intellectuelles EDEI, Manuel. Paris: Editions Scientifiques et Psychotechniques; 1978. [Google Scholar]
- 85.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L, Moreno SG. Assessing publication bias in meta-analyses in the presence of between-study heterogeneity. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2010;173(3):575–591. http://doi.org/10.1111/j.1467-985X.2009.00629.x. [Google Scholar]
- 86.Port RG, Anwar AR, Ku M, Carlson GC, Siegel SJ, Roberts TPL. Prospective meg biomarkers in ASD: pre-clinical evidence and clinical promise of electrophysiological signatures. The Yale Journal of Biology and Medicine. 2015;88:25–36. [PMC free article] [PubMed] [Google Scholar]
- 87.Reynell JK, Huntley M. Reynell Developmental Language Scales, Second Revision. Windsor, U.K: Nfer-nelson Publishing Company; 1985. [Google Scholar]
- 88.Roberts TPL, Cannon KM, Tavabi K, Blaskey L, Khan SY, Monroe JF, … Edgar JC. Auditory magnetic mismatch field latency: A biomarker for language impairment in autism. Biological Psychiatry. 2011 Mar;70:263–269. doi: 10.1016/j.biopsych.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ross RT, Morledge J. Comparison of the WISC and WAIS at chronological age sixteen. Journal of Consulting Psychology. 1967;31(3):331–331. doi: 10.1037/h0021004. [DOI] [PubMed] [Google Scholar]
- 90.Samson F, Mottron L, Jemel B, Belin P, Ciocca V. Can spectro-temporal complexity explain the autistic pattern of performance on auditory tasks? Journal of Autism and Developmental Disorders. 2006;36(1):65–76. doi: 10.1007/s10803-005-0043-4. [DOI] [PubMed] [Google Scholar]
- 91.Schall U. Is it time to move mismatch negativity into the clinic? Biological Psychology. 2015;116:41–46. doi: 10.1016/j.biopsycho.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 92.Schwarzer G, Carpenter JR, Rücker G. Meta-Analysis with R. 2015. [Google Scholar]
- 93.Semel EM, Wiig EH, Secord W. Clinical Evaluation of Language Fundamentals. 3. San Antonio, TX: The Psychological Corporation; 1995. [Google Scholar]
- 94.Seri S, Cerquiglini A, Pisani F, Curatolo P. Autism in tuberous sclerosis: Evoked potential evidence for a deficit in auditory sensory processing. Clinical Neurophysiology. 1999;110:1825–1830. doi: 10.1016/S1388-2457(99)00137-6. [DOI] [PubMed] [Google Scholar]
- 95.Shafer VL, Morr ML, Kreuzer JA, Kurtzberg D. Maturation of mismatch negativity in school-age children. Ear and Hearing. 2000;21(3):242–251. doi: 10.1097/00003446-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 96.Shafer VL, Yu YH, Datta H. The development of English vowel perception in monolingual and bilingual infants: Neurophysiological correlates. Journal of Phonetics. 2011;39(4):527–545. doi: 10.1016/j.wocn.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shahim S. Correlations for Wechsler Intelligence Scale for Children—Revised and the Wechsler Preschool and Primary Scale of Intelligence for Iranian Children. Psychological Reports. 1992;70(1):27–30. doi: 10.2466/pr0.1992.70.1.27. [DOI] [PubMed] [Google Scholar]
- 98.Tager-Flusberg H, Paul R, Lord C. Language and communication in autism. Handbook of autism and pervasive developmental disorders. 2005;1:335–364. [Google Scholar]
- 99.Tecchio F, Benassi F, Zappasodi F, Gialloreti LE, Palermo M, Seri S, Rossini PM. Auditory sensory processing in autism: A magnetoencephalographic study. Biological Psychiatry. 2003;54(3):647–654. doi: 10.1016/s0006-3223(03)00295-6. http://doi.org/10.1016/S0006-3223(03)00295-6. [DOI] [PubMed] [Google Scholar]
- 100.The Comprehensive R Archive Network (CRAN) 2015 Retrieved from http://cran.r-project.org/
- 101.Thorndike RL, Hagen EP, Sattler JM. Stanford-Binet intelligence scale. Riverside Publishing Company; 1986. [Google Scholar]
- 102.Trainor L, McFadden M, Hodgson L, Darragh L, Barlow J, Matsos L, Sonnadara R. Changes in auditory cortex and the development of mismatch negativity between 2 and 6 months of age. International Journal of Psychophysiology. 2003;51(1):5–15. doi: 10.1016/S0167-8760(03)00148-X. [DOI] [PubMed] [Google Scholar]
- 103.Umbricht D, Krljesb S. Mismatch negativity in schizophrenia: A meta-analysis. Schizophrenia Research. 2005;76(1):1–23. doi: 10.1016/j.schres.2004.12.002. http://doi.org/10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 104.Van Haasen PP, De Bruyn EEJ, Pijl YJ, Poortinga YH, Lutje-Spelberg HC, Vander Steene G, … Stinissen J. Wechsler intelligence scale for children-revised, Dutch Version. Lisse, the Netherlands: Swets & Zetlinger; 1986. [Google Scholar]
- 105.Wechsler D. Wechsler Intelligence Scale for Children. San Antonio, TX: Psychological Corporation; 1949. [Google Scholar]
- 106.Wechsler D. Wechsler Intelligence Scale for Children—Revised. New York: Psychological Corporation; 1974. [Google Scholar]
- 107.Wechsler D. Wechsler Intelligence Scale for Children—Third edition. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- 108.Wechsler D. Wechsler Intelligence Scale for Children Fourth—edition. London: Pearson; 2003. [Google Scholar]
- 109.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence—Revised. San Antonio, TX: Psychological Corporation; 1989. [Google Scholar]
- 110.Wechsler D. Wechsler Adult Intelligence Scale—Revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- 111.Weismüller B, Thienel R, Youlden AM, Fulham R, Koch M, Schall U. Psychophysiological Correlates of Developmental Changes in Healthy and Autistic Boys. Journal of Autism and Developmental Disorders. 2015;45:2168–2175. doi: 10.1007/s10803-015-2385-x. [DOI] [PubMed] [Google Scholar]
- 112.Whitehouse AJO, Bishop DVM. Do children with autism “switch off” to speech sounds? An investigation using event-related potentials. Developmental Science. 2008;11(4):516–524. doi: 10.1111/j.1467-7687.2008.00697.x. [DOI] [PubMed] [Google Scholar]
- 113.Yu L, Fan Y, Deng Z, Huang D, Wang S, Zhang Y. Pitch processing in tonal-language-speaking children with autism: an event-related potential study. Journal of Autism and Developmental Disorders. 2015;45(11):3656–67. doi: 10.1007/s10803-015-2510-x. [DOI] [PubMed] [Google Scholar]