Abstract
Purpose
Most children with Sturge-Weber syndrome (SWS) develop seizures that may contribute to neurocognitive status. In this study, we tested the hypothesis that very early seizure onset has a particularly detrimental effect on the cognitive and/or motor outcome of children with unilateral SWS. We also tested whether side of SWS brain involvement modulates the effect of seizure variables on the pattern of cognitive abnormalities.
Methods
Thirty-four children (22 girls; mean age 6.1 years) with unilateral SWS and history of epilepsy in a longitudinal cohort underwent neurological and cognitive evaluation. Global IQ, verbal IQ, non-verbal IQ, and motor function were correlated with epilepsy variables, side and extent of brain involvement on MRI.
Results
Mean age at seizure onset was 1.3 years (0.1–6 years) and mean IQ at follow-up was 86 (45–118). Age at seizure onset showed a logarithmic association with IQ, with maximum impact of seizures starting before age 1 year, both in uni- and multivariate regression analyses. In the left SWS group (N=20), age at seizure onset was a strong predictor of non-verbal IQ (p=0.001); while early seizure onset in the right-hemispheric group had a more global effect on cognitive functions (p=0.02). High seizure frequency and long epilepsy duration also contributed to poor outcome IQ independently in multivariate correlations. Children with motor involvement started to have seizures at/before 7 months of age, while frontal lobe involvement was the strongest predictor of motor deficit in a multivariate analysis (p=0.017).
Conclusion
These findings suggest that seizure onset prior to age 1 year has a profound effect on severity of cognitive and motor dysfunction in children with SWS; however, the effect of seizures on the type of cognitive deficit is influenced by laterality of brain involvement.
Keywords: Sturge-Weber syndrome, epilepsy, seizure onset, cognitive function, outcome
1. INTRODUCTION
Sturge-Weber syndrome (SWS) is a rare neurocutaneous syndrome associated with facial port-wine birthmark, leptomeningeal angiomatosis (LMA) and glaucoma. Facial port-wine birthmark can be diagnosed shortly after birth, before any neurological manifestations appear. Involvement of the upper eyelid and forehead area indicate greater risk of neurologic manifestations [1]. One recent study found that the size of the facial port-wine birthmark correlated positively with ipsilateral severity of brain involvement seen on magnetic resonance imaging (MRI) [2]. Seizures are common in SWS occurring in about 80% of unilateral SWS (representing about 85% of SWS cases) and 95% of those with bilateral SWS [3] with the majority presenting in the first year of life [4]. The clinical outcome of children with SWS is highly variable, and several prognostic factors have been implicated in cognitive outcome, including early seizure onset and electroencephalography (EEG) abnormalities [5], high seizure frequency [6] and bilateral brain involvement [7]. Among these factors, seizure onset would be a potentially modifiable risk factor if onset of the first clinical seizures could be aborted or delayed, for example, with prophylactic anti-seizure medication.
SWS and tuberous sclerosis complex (TSC) provide a unique opportunity to test the effect of preventive anti-seizure medication on neurocognitive outcome, since both of these neurocutaneous disorders are associated with high risk of seizures and the diagnosis can be made by the presence pre-symptomatic skin manifestations in infants. Two previous studies have investigated the potential effect of pre-symptomatic anti-seizure medication treatment in these conditions [8,9]. Ville et al. retrospectively compared the evolution of epilepsy and psychomotor development in SWS between children who received prophylactic phenobarbital treatment and those who did not [8]; reduced likelihood of seizures and better cognitive outcome were noted in the treatment group. In a study of children with TSC, the treatment group had lower proportion of subjects with mental retardation, drug-resistant epilepsy with polytherapy and higher ratio of seizure-free children [9]. However, neither of these two studies involved random assignment to group and thus do not provide definitive evidence regarding the effectiveness of prophylactic epilepsy treatment in these patient groups; nor did either of the studies control for lesion extent or location on outcomes.
In the present study, we estimated the impact of age at seizure onset on the cognitive and motor outcome of children with unilateral SWS. Specifically, we tested if early seizure onset (particularly before 1 year of age) has a detrimental effect on cognitive outcome. Furthermore, the effect of other clinical seizure variables, side of brain involvement, and presence of frontal lobe involvement on severity and pattern of cognitive deficit as well as motor outcome was determined.
2. METHODS
2.1. Subjects
The subjects were enrolled in our prospective longitudinal, clinical and neuroimaging study at Children’s Hospital of Michigan, Detroit between July 1, 2003 and December 31, 2016. The diagnosis of SWS was made by the identification of the characteristic facial port-wine birthmark and the typical MRI findings. Inclusion criteria were: (a) unilateral SWS by MRI, defined as the presence of unilateral LMA and/or enlarged deep medullary veins with or without enlarged choroid plexus on contrast enhanced T1-weighted MRI, (b) history of epilepsy, (c) age of <13 years, (d) age at neurocognitive testing or assessment of >2.5 years, and (e) at least one year follow-up. Exclusion criteria were bilateral SWS on MRI and epilepsy surgery, as we had no sufficient number of patients with cognitive outcome data to include them in the present analysis. Also, patients with bilateral SWS and those requiring epilepsy surgery represent subgroups with substantially worse baseline seizure characteristics that would have confounded our analyses of non-surgical unilateral SWS cases.
Clinical data collected included age at seizure onset, seizure frequency, duration of epilepsy, and physical examination findings, including gross motor involvement. Cognitive function was assessed by determining the intelligent quotient (IQ), as described below.
The study was approved by the Human Investigation Committee at Wayne State University, and written informed consent of the parents or legal guardian and verbal assent (for children 7 years and above) was obtained.
2.2. MRI acquisition and assessment
All children underwent MRI scanning at the time of the first visit and, in most cases, at follow-up visit(s). MRI typically included axial T1, fluid-attenuated inversion recovery (FLAIR), T2-weighted turbo spin-echo acquisition, susceptibility-weighted imaging (SWI), contrast-enhanced MR perfusion-weighted imaging followed by a post-gadolinium axial T1 acquisition. Additional sequences (such as diffusion tensor imaging, MR venography, MR spectroscopy) were acquired in selected cases using a research protocol. Gadolinium-diethylenetriamine pentaacetic acid (Magnevist, Berlex) was injected in a bolus via a peripheral vein at a dose of 0.1mmol/kg body weight. The extent of MRI involvement was determined on the first available MRI (at a mean age of 3.3 years) by identification of the number of lobes in the affected hemisphere involved (ranging from 1 to 4) that showed LMA, atrophy, enlarged deep transmedullary veins and/or calcifications. Presence or absence of frontal lobe involvement was also determined.
2.3. Seizure frequency assessment
Seizure frequency was assessed by parental interview using seizure diaries and review of medical records. A scoring system as described in our previous publication [5] was used: 0 – no seizure in the last one year; 1 – one to 11 seizures per year; 2 – one to 4 seizures per month; and 3 – >4 seizures per month. Seizure frequency scores at the last follow-up (when outcome IQ was also evaluated) were used for analysis.
2.4. Neurocognitive assessment
A licensed neuropsychologist performed or supervised the neuropsychological testing on all subjects. To determine the subjects’ IQ, Wechsler Pre-primary and Preschool Scale of Intelligence-III (WPPSI-III), third edition, and Wechsler Intelligence Scales for Children-III (WISC-III) were used for children between 30–87 months of age and above 87 months respectively. Both tests provide global intelligent quotient (GIQ), verbal IQ, and non-verbal or performance IQ (PIQ).
2.5. Motor assessment
The patients’ motor function was assessed based on neurological examination (gross motor functioning) and age appropriate measure of fine motor dexterity (Purdue Pegboard for children <5 years; Grooved Pegboard for children ≥ 5 years) using the following 3 categories: 0 – no gross or fine motor weakness; 1 – presence of fine motor impairment only (defined as performance on Pegboard >2 SDs below normative mean) without gross motor weakness; 2 – presence of gross motor weakness contralateral to the brain lesion; (none of the children had severe gross motor weakness, only mild/moderate weakness).
2.6. Statistical analysis
First, univariate Pearson’s correlations were performed to determine the association of outcome IQ with predictors such as age of seizure onset, duration of epilepsy, seizure frequency scores, and extent of MRI involvement. In addition to linear correlations, scatter plots were examined for non-linearity and appropriate non-linear (logarithmic) correlations were applied where indicated. IQ measures in patients with vs. without frontal lobe involvement on MRI were compared using unpaired t-tests. Multivariate regression analysis and logistic regression analyses (with high [≥85] vs. low [<85] IQ as the binary outcome variable) were subsequently performed. To determine the impact of seizure variables and frontal lobe involvement on the motor scores, univariate Spearman’s rank correlation followed by binary logistic regression were used. Group comparisons for testing the effect of gender, lesion side, polytherapy, and frontal lobe involvement were performed using unpaired t-tests. SPSS 24.0 (SPSS Inc. Armonk, NY, U.S.A.) was used for data analysis. P value of <0.05 was considered as statistically significant.
3. RESULTS
3.1. Clinical characteristics
Thirty-four children (22 girls and 12 boys) fulfilled the inclusion and exclusion criteria (Table 1). The majority (n=20) had left-sided SWS. The mean age of seizure onset was 1.3 years (range: 0.1 - 6 years) and age at neurocognitive testing was 6.1 years (range: 2.6 – 12.5 years). The mean duration of epilepsy at that time was 4.8 years (range: 1 – 12 years), while mean seizure frequency score was 0.8 (Table 1). The most common frequency score was 1 (1–11 seizures per year, n=17), and scores 2 and 3 were present in 2 children each (n=4 in total); the remaining 13 children had no clinical seizure in the past 1 year (score 0). The majority of the children (19/34 or 56%) were taking one anti-seizure medication at the time of follow-up. Three children were off of any medication, while the remaining 12 children were on multiple (2–3) anti-seizure medications. Oxcarbazepine was the most frequently prescribed medication, used in 17 (monotherapy in 8 and in combination in 9), followed by levetiracetam (n=9, monotherapy in 3 and in combination in 6), and carbamazepine (n=5, 4 monotherapy and in combination in 1). Three patients each were on lamotrigine, topiramate, valproic acid and zonisamide either alone or in combination. Only two patients were prescribed phenobarbital, and one patient each were taking lacosamide and clonazepam in combination with other medications. The mean GIQ of the whole group was 86 (range: 45–118). There was no statistical gender difference in age, extent of MRI involvement, age at seizure onset, duration of epilepsy duration and IQ. For motor deficit, 12 of the 34 children showed mild/moderate gross motor weakness at follow-up (score 2), 6 had only fine motor involvement in the hand contralateral to the SWS lesions, without weakness, and 16 children had no motor symptoms.
Table 1.
Clinical variables (mean and standard deviation) in the whole patient group, and comparison of these values between the left and right hemispheric SWS subgroups.
| Whole group | Left SWS | Right SWS | ||
|---|---|---|---|---|
| Clinical variables | (n=34) | (n=20) | (n=14) | p value |
| Age at seizure onset (years) | 1.3 [1.4] | 1.1 [1.5] | 1.5 [1.3] | 0.5 |
| Extent of MRI involvement* | 2.5 [1.3] | 2.6 [1.3] | 2.5 [1.3] | 0.9 |
| Age at final follow-up (years) | 6.1 [2.7] | 6.7 [3.0] | 5.2 [2.0] | 0.12 |
| Duration of epilepsy (years) | 4.8 [2.9] | 5.6 [3.4] | 3.8 [1.6] | 0.08 |
| Seizure frequency score at follow-up | 0.8 [0.8] | 0.9 [1.0] | 0.6 [0.5] | 0.37 |
| Motor score at follow-up | 0.9 [0.9] | 1.0 [0.9] | 0.7 [0.8] | 0.46 |
| IQ at follow-up | ||||
| GIQ | 86 [18] | 80 [18] | 95 [15] | 0.014 |
| VIQ | 92[19] | 87 [20] | 99 [16] | 0.07 |
| PIQ | 83 [18] | 77 [16] | 94 [16] | 0.006 |
Number of affected lobes on first MRI, at a mean age of 3.3 years
3.2. Predictors of IQ measures: univariate analyses
In initial univariate correlations, significant clinical predictors of IQ measures in the whole group (N=34) included age at seizure onset, seizure frequency, and duration of epilepsy (Table 2). Polytherapy with anti-seizure drugs was also associated with lower GIQ (mean [SD]: 77±20 vs. 91±16 in those on 0–1 medication, p=0.02), earlier seizure onset (0.6±0.4 years vs. 1.6±1.6 years, p=0.03) and higher seizure frequency scores (1.2±0.7 vs. 0.6±0.8, p=0.045). The scatter plots for age at seizure onset indicated a non-linear relation to IQ; therefore, we fitted a logarithmic regression and this improved the p-values (Figure 1). While extent of MRI involvement showed no significant correlations with IQ (p>0.05), GIQ and VIQ were lower in patients with frontal lobe involvement on MRI as compared to those with an intact frontal lobe (GIQ: 78 vs. 92, p=0.03; VIQ 84 vs. 98, p=0.03; PIQ: 78 vs. 88, p=0.12). Patients with left-sided SWS brain involvement showed lower IQ measures than those with right-sided involvement (mean GIQ: 80 vs. 95, p=0.014; PIQ: 77 vs. 94, p=0.006; VIQ: 87 vs. 99, p=0.075).
Table 2.
Univariate correlations between clinical seizure variables, GIQ, VIQ and PIQ. For age at seizure onset, a logarithmic correlation was used based on the scatter plots (see Figure 1).
| Clinical predictors | Correlations | Whole group (N=34) | Left SWS (N=20) | Right SWS (N=14) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GIQ | VIQ | PIQ | GIQ | VIQ | PIQ | GIQ | VIQ | PIQ | ||
| Age at seizure onset | r (log) | 0.51 | 0.37 | 0.62 | 0.41 | 0.19 | 0.67 | 0.61 | 0.6 | 0.49 |
| p | 0.002 | 0.03 | 0.0001 | 0.074 | 0.42 | 0.001 | 0.02 | 0.023 | 0.075 | |
| Seizure frequency | r (linear) | −0.35 | −0.45 | −0.23 | −0.33 | −0.48 | −0.21 | −0.33 | −0.30 | −0.13 |
| p | 0.040 | 0.007 | 0.19 | 0.16 | 0.033 | 0.39 | 0.235 | 0.30 | 0.67 | |
| Duration of epilepsy | r (linear) | − 0.55 | −0.50 | −0.49 | 0.56 | −0.50 | −0.52 | −0.27 | − 0.27 | −0.15 |
| p | 0.001 | 0.003 | 0.004 | 0.011 | 0.025 | 0.02 | 0.34 | 0.35 | 0.61 | |
Blue: 0.01<p<0.05; red: p<0.01
Figure 1.
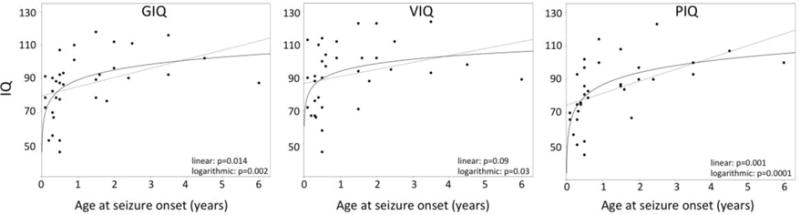
Scatter plots of correlations between age at seizure onset and IQ variables, demonstrating logarithmic correlations. Solid lines represent the logarithmic while dotted lines indicate the linear regression fit. Corresponding p values are also indicated (see also Table 2).
When left- and right hemispheric SWS subgroups were analyzed separately, younger age at seizure onset was positively correlated with PIQ (r=0.67, p=0.001 in the logarithmic correlation) in the left-hemispheric group. VIQ was not impacted by early seizure onset in this subgroup, although it was negatively associated with seizure frequency scores and duration of epilepsy (Table 2). In contrast, in the right-hemispheric SWS subgroup (N=14), younger age at seizure onset was associated with the global IQ (r=0.61, p=0.02) and VIQ (p=0.023), while seizure frequency and epilepsy duration were not related to IQ measures in this subgroup (Table 2).
3.3. Predictors of IQ measures: multivariate correlations
Based on the univariate correlations, all three clinical seizure variables were entered in a multivariate regression for both the whole group and left SWS subgroup, with IQ measures as the outcome (Table 3). For age at seizure onset, a logarithmic term was entered. In the whole group, all three clinical predictors showed a significant contribution to the IQ measures. For PIQ, age at seizure onset was the strongest predictor both in the whole group (partial r=0.62, p<0.001) and in the left SWS subgroup (r=0.71, p=0.001) (Table 3; Figure 2). VIQ was more strongly related to seizure frequency in both the whole group and left SWS subgroup (Table 3). The number of anti-seizure medications did not correlate with IQ when entered in the multivariate regression (p=0.83).
Table 3.
Multivariate correlations between clinical seizure variables as predictors and outcome IQ variables. For age at seizure onset, a logarithmic term [Log[seizure onset]) was used, based on the univariate correlations.
| Clinical predictors | partial correlations | Whole group (N=34) | Left SWS (N=20) | ||||
|---|---|---|---|---|---|---|---|
| GIQ | VIQ | PIQ | GIQ | VIQ | PIQ | ||
| Log[Seizure onset] | r | 0.53 | 0.40 | 0.62 | 0.41 | 0.20 | 0.71 |
| p | 0.002 | 0.023 | <0.001 | 0.09 | 0.43 | 0.001 | |
|
| |||||||
| Seizure frequency | r | −0.47 | −0.52 | −0.37 | −0.47 | −0.54 | −0.53 |
| p | 0.007 | 0.002 | 0.037 | 0.047 | 0.020 | 0.023 | |
|
| |||||||
| Duration of epilepsy | r | −0.42 | −0.37 | −0.32 | −0.42 | −0.43 | −0.28 |
| p | 0.017 | 0.039 | 0.075 | 0.08 | 0.07 | 0.25 | |
Blue: 0.01<p<0.05; red: p<0.01
Figure 2.
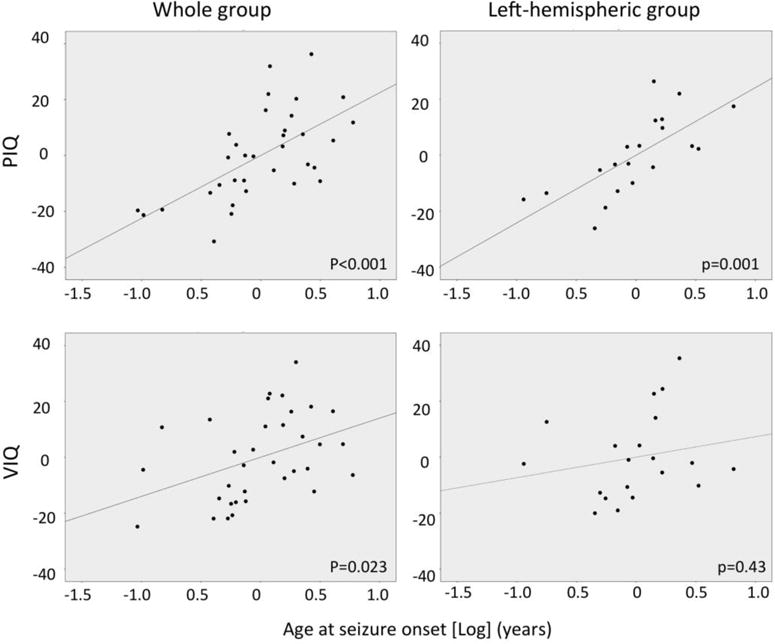
Partial regression plots for Log(seizure onset age) showing the correlation with PIQ and VIQ in the whole group and in children with left hemispheric SWS involvement, from a multivariate regression analysis; the partial plots are corrected for duration of epilepsy and seizure frequency (see also Table 3).
In a subsequent binary logistic regression analysis, with the three seizure variables (see above) and MRI frontal lobe involvement entered as predictors, and GIQ (greater or less than 85) as the binary outcome variable, both log[age at seizure onset] (p=0.03) and seizure frequency (p=0.04) remained a significant IQ predictor, while MRI frontal lobe involvement was not significant (p=0.48).
3.4. Predictors of motor deficit
In univariate Spearman’s rank correlations, predictors of motor function scores included age at seizure onset (rho=−0.64, p=0.00005), and duration of epilepsy (r=0.45, p=0.008), while seizure frequency scores did not correlate with motor scores (r=−0.01, p=0.94). All 12 patients with gross motor involvement started to have seizures at or before 7 months of age (mean age at seizure onset: 4 months). In addition, motor scores were higher in those with frontal lobe involvement on initial MRI (n=15) than in those with intact frontal lobe (mean scores 1.4±0.8 vs. 0.5±0.8, respectively, p=0.002). In a subsequent binary logistic regression analysis, with presence or absence of motor involvement as the outcome variable, the presence of frontal lobe involvement was the only significant predictor (p=0.017), while the contribution of the two seizure variables were not significant (age at seizure onset: p=0.13, duration of epilepsy: p=0.19).
4. DISCUSSION
Consistent with previous studies, here we found that early seizure onset has a significant negative impact on the cognitive outcome in children with SWS. Our data also show that the effect on cognitive outcome is non-linear, with the largest effect in children whose first clinical seizures start before 1year of age, and that the effect of seizure onset on outcome IQ is present regardless of overall extent of MRI involvement or presence of frontal lobe involvement on MRI. Moreover, the effect of early seizures on the pattern of IQ deficit appears to depend on the side of SWS brain involvement: seizures from the left hemisphere affect predominantly non-verbal IQ, while seizures in children with right-hemispheric involvement have a more global effect on IQ. Furthermore, seizures starting at/before 7 months of age predict subsequent motor deficit, which is also associated with frontal lobe involvement, the site of the motor cortex. Altogether, these data demonstrate the profound impact of early seizure onset (particularly below age 1 year) on cognitive outcome and also some negative impact on motor outcome in unilateral SWS. This suggests that early interventions, such as early prophylactic anti-seizure treatment, if successful, could have a positive impact not only on seizures but also on neurocognitive outcome in children with SWS. The finding of the negative impact of early seizure onset on motor outcome may also guide clinicians in the early initiation of physical and occupational therapy in at risk infants with early onset seizures to promote better motor development and outcome.
There is growing body of laboratory [10–12] and clinical [13–17] evidence demonstrating the deleterious influence of early age of seizure onset on cognitive outcome. A study in children with hemiplegic cerebral palsy has shown that very early cerebral damage to either hemisphere, even if extensive, resulted in relatively few and mild neuropsychological deficits if not accompanied by seizure activity [13]. Conversely, if accompanied by seizures, higher incidence and degree of neuropsychological deficits, unrelated to lesion side, have been noted [13]. In children with TSC, early seizure onset, along with high seizure frequency, negatively impacts their neurodevelopmental outcome [15,17]. A similar effect of early seizure onset was observed in children with focal cortical dysplasia [16] and in pediatric temporal lobe epilepsy [14]. Among pediatric epilepsy surgery candidates with seizures related to unilobar brain lesions of various pathologies, seizures in the first 24 months of life is significantly associated with intellectual disability [18]. Taken together, regardless of the etiology, early-life seizures adversely affect neurocognitive development suggesting that seizures are especially deleterious during early stages of brain development.
The inherent vulnerability of the brain during the first year of life to cognitive effects of seizures may be because the neural development during this sensitive period establishes the foundation for the subsequent development of higher-order cognitive function [19]. Early postnatal development is marked by a critical period of enhanced synaptogenesis and neural plasticity [20]. Animal studies suggest that although early life seizures do not always cause neuronal loss, they may result in reduced neurogenesis, altered synaptogenesis, as well as disruption of the normal excitatory/inhibitory balance and network connectivity [12,21]. These changes translate into deficits in spatial learning and memory demonstrated in rodent seizure models [11,22,23], and similar processes may also underlie neuronal damage in the developing human brain exposed to repeated seizures. In SWS, such seizure-induced brain damage may be further aggravated by the potential effect of progressive vascular damage due to chronic impairment of cortical and subcortical blood flow and resulting hypoxia.
Another major novel finding of the present study is that the effect of early seizure onset on verbal vs. non-verbal IQ appears to differ based on the side of SWS brain involvement. Interestingly, younger age at seizure onset predicted lower non-verbal IQ in left-sided SWS, while early seizures in right-sided cases were associated with global IQ. That non-verbal functions are impacted by left-sided lesions is consistent with a “crowding” hypothesis, where verbal functions are prioritized and reorganized to the non-dominant hemisphere, with a concomitant adverse impact on non-verbal functions [24,25]. This process could be well in play in children with left-hemispheric SWS, who showed a 10-point relative decrease of mean non-verbal IQ as compared to VIQ (mean: 77 vs. 87, respectively, see Table 1) in our cohort.
In addition to early seizures, our group has recently shown that interictal epileptiform discharges on EEG were also associated with poor cognitive outcome; this suggested that not only seizures but also interictal epileptiform discharges may be interfering with brain reorganization [5]. In infants with a facial port-wine birthmark, EEG may also be able to detect early brain involvement [26]; such patients may be particularly good candidates for prophylactic anti-seizure medication treatment. Indeed, interictal epileptiform discharges on EEG have been shown to be predictive of the development of epilepsy in children with TSC [27]. No similar data are available in pre-symptomatic SWS infants, and the lack of interictal epileptiform EEG abnormalities in most SWS children (perhaps due to a masking effect of the vascular malformation and/or brain atrophy) [5] may render EEG a less useful biomarker of epilepsy in such children.
With regard to the motor outcome, age at seizure onset and duration of epilepsy were strong clinical predictors in the univariate analysis. All 12 patients with hemiparesis had early seizure onset (at or before 7 months of age). Not surprisingly, those with frontal lobe involvement also had higher motor scores. Hemiparesis in SWS may be due to several factors including venous congestion, stasis and hypoxia leading to brain ischemia and resulting atrophy affecting the motor cortex and its connections. Neuroimaging studies have shown the role of impaired brain perfusion in SWS [28,29] with the degree of hypoperfusion correlating with motor deficits and disability score [28]. Extension of brain damage from the typically involved occipital lobes into the parietal, temporal and frontal cortex is associated with greater psychomotor delay and hemiparesis [30]. Repeated seizure activity in SWS could further impair the already compromised cerebral circulation and cerebral autoregulation in SWS resulting in a mismatch between cerebral metabolic demand and delivery of glucose and oxygen resulting in ischemic injury [31].
Our study is limited by some patient selection bias, as we included only patients who did not undergo epilepsy surgery. Thus, some of the most severely affected patients have been excluded, but the main goal was to evaluate predictors of IQ and motor function without the modifying effect of surgery. In addition, anti-seizure medications could have modified neuropsychological deficits; however, this contribution would be difficult to account for due to the wide variability of such medication across subjects. The majority of the children were on only one, newer generation anti-seizure medication (oxcarbazepine or levetiracetam). Although there have been few controlled trials regarding cognitive side effects of anti-seizure medications, the available data show that newer anti-seizure drugs have a safe cognitive profile at the appropriate dose and when prescribed as monotherapy [32]. Only two patients were on phenobarbital, an anti-seizure medication associated with greater cognitive impairment; however, one of them (with phenobarbital monotherapy) had normal IQ (GIQ 107) at age 7.5 years, while the other child was on three anti-seizure medication with a GIQ of 72 at age 2.6 years. Thus, it is unlikely that phenobarbital treatment had a profound effect on our overall results. It is also possible that the early onset of seizures may be related to specific underlying brain pathology including the presence of cortical developmental malformations that have recently emerged as common pathology associated with SWS [33,34]. A detailed analysis of imaging signs of these pathologies and also resected SWS brain tissue with the seizure and neurocognitive outcome could clarify such a relationship.
Our results are also limited by the wide age range when IQ testing was performed and the lack of longitudinal IQ observation; however, accurate evaluation of baseline IQ in young children is difficult, and the majority of children started to have seizures before 30 months of age. Since longer duration of epilepsy is associated with lower IQ, we entered epilepsy duration as a co-variate in the multivariate regressions to diminish this effect. Finally, the extent of SWS brain involvement on MRI was evaluated at different ages, and we only used a broad measure (number of lobe of SWS brain involvement), while not taking into account the potential effects of various SWS-related brain pathology (such as leptomeningeal malformation, deep medullary veins, atrophy, calcification, etc.). Future studies with detailed MRI analysis may provide imaging biomarkers that could modify the effect of early seizures on neuro-cognitive outcome.
5. CONCLUSION
Our findings show that the effect of seizure onset on cognitive outcome is non-linear, with the largest impact on young children with seizure onset prior to 1 year of age. Importantly, the effect of early seizures on verbal vs. non-verbal IQ appears to be determined by the side of brain involvement, possibly indicating a side-specific crowding effect in the non-SWS-affected hemisphere. Furthermore, seizure onset at or before 7 months of age, along with early frontal lobe involvement, is also a predictor of motor deficit. These findings may have important clinical implications for a more accurate prognostication of the severity and pattern of neurocognitive outcome in young children with unilateral SWS. In addition, our data suggest that even a moderate delay of seizure onset, e.g., by using prophylactic anti-seizure medication in children below 1 year of age, may have a positive impact on their cognitive and motor outcome. Whether prophylactic medication indeed has a disease-modifying effect in infants with SWS, should be addressed by a prospective, controlled trial.
Highlights.
Most children with Sturge-Weber syndrome (SWS) develop seizures
Seizure onset before 1 year of age has a particularly strong impact on IQ in SWS
The pattern of cognitive deficit is also affected by side of brain involvement
Motor deficit is affected by early seizure onset and frontal lobe involvement
Delayed seizure onset may improve cognitive outcome in children with SWS
Acknowledgments
The authors thank Cynthia Burnett, BA, and Cathie Germain, MA for assisting patient recruitment and scheduling; Jane Cornett, RN, and Anne Deboard, RN, for performing sedation for imaging; and Xuan Yang, BS, for assisting with MR imaging data acquisition. We are thankful for Anne Comi, MD (Kennedy Krieger Institute, Johns Hopkins University, Baltimore, MD) and Anna Pinto MD (Boston Childrens Hospital, Boston, MA) for the critical review of the manuscript. We are also grateful to the Sturge-Weber Foundation and the families who participated in these studies.
Funding: This work was supported by the National Institutes of Health (grant number R01 NS041922 to C.J.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Waelchli R, Aylett SE, Robinson K, Chong WK, Martinez AE, Kinsler VA. New vascular classification of port-wine stains: improving prediction of Sturge-Weber risk. Br J Dermatol. 2014;171:861–867. doi: 10.1111/bjd.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dymerska M, Kirkorian AY, Offermann EA, Lin DD, Comi AM, Cohen BA. Size of Facial Port-Wine Birthmark May Predict Neurologic Outcome in Sturge-Weber Syndrome. J Pediatr. 2017;188:205–209. doi: 10.1016/j.jpeds.2017.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachur CD, Comi AM. Sturge-Weber syndrome. Curr Treat Options Neurol. 2013;15:607–617. doi: 10.1007/s11940-013-0253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sujansky E, Conradi S. Sturge-Weber syndrome: age of onset of seizures and glaucoma and the prognosis for affected children. J Child Neurol. 1995;10:49–58. doi: 10.1177/088307389501000113. [DOI] [PubMed] [Google Scholar]
- 5.Bosnyak E, Behen ME, Guy WC, Asano E, Chugani HT, Juhasz C. Predictors of Cognitive Functions in Children With Sturge-Weber Syndrome: A Longitudinal Study. Pediatr Neurol. 2016;61:38–45. doi: 10.1016/j.pediatrneurol.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer U, Kahana E, Shorer Z, Ben-Zeev B. Outcome of infants with unilateral Sturge-Weber syndrome and early onset seizures. Dev Med Child Neurol. 2000;42:756–759. doi: 10.1017/s0012162200001407. [DOI] [PubMed] [Google Scholar]
- 7.Alkonyi B, Chugani HT, Karia S, Behen ME, Juhasz C. Clinical outcomes in bilateral Sturge-Weber syndrome. Pediatr Neurol. 2011;44:443–449. doi: 10.1016/j.pediatrneurol.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ville D, Enjolras O, Chiron C, Dulac O. Prophylactic antiepileptic treatment in Sturge-Weber disease. Seizure. 2002;11:145–150. doi: 10.1053/seiz.2001.0629. [DOI] [PubMed] [Google Scholar]
- 9.Jozwiak S, Kotulska K, Domanska-Pakiela D, et al. Antiepileptic treatment before the onset of seizures reduces epilepsy severity and risk of mental retardation in infants with tuberous sclerosis complex. Eur J Paediatr Neurol. 2011;15:424–431. doi: 10.1016/j.ejpn.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Bo T, Jiang Y, Cao H, Wang J, Wu X. Long-term effects of seizures in neonatal rats on spatial learning ability and N-methyl-D-aspartate receptor expression in the brain. Brain Res Dev Brain Res. 2004;152:137–142. doi: 10.1016/j.devbrainres.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Karnam HB, Zhou JL, Huang LT, Zhao Q, Shatskikh T, Holmes GL. Early life seizures cause long-standing impairment of the hippocampal map. Exp Neurol. 2009;217:378–387. doi: 10.1016/j.expneurol.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmes GL. Effect of Seizures on the Developing Brain and Cognition. Semin Pediatr Neurol. 2016;23:120–126. doi: 10.1016/j.spen.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vargha-Khadem F, Isaacs E, van der Werf S, Robb S, Wilson J. Development of intelligence and memory in children with hemiplegic cerebral palsy. The deleterious consequences of early seizures. Brain. 1992;115:315–329. doi: 10.1093/brain/115.1.315. [DOI] [PubMed] [Google Scholar]
- 14.Cormack F, Cross JH, Isaacs E, et al. The development of intellectual abilities in pediatric temporal lobe epilepsy. Epilepsia. 2007;48:201–204. doi: 10.1111/j.1528-1167.2006.00904.x. [DOI] [PubMed] [Google Scholar]
- 15.Chu-Shore CJ, Major P, Camposano S, Muzykewicz D, Thiele EA. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51:1236–1241. doi: 10.1111/j.1528-1167.2009.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korman B, Krsek P, Duchowny M, Maton B, Pacheco-Jacome E, Rey G. Early seizure onset and dysplastic lesion extent independently disrupt cognitive networks. Neurology. 2013;81(8):745–751. doi: 10.1212/WNL.0b013e3182a1aa2a. [DOI] [PubMed] [Google Scholar]
- 17.Capal JK, Bernardino-Cuesta B, Horn PS, et al. Influence of seizures on early development in tuberous sclerosis complex. Epilepsy Behav. 2017;70:245–252. doi: 10.1016/j.yebeh.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasconcellos E, Wyllie E, Sullivan S, et al. Mental retardation in pediatric candidates for epilepsy surgery: the role of early seizure onset. Epilepsia. 2001;42:268–274. doi: 10.1046/j.1528-1157.2001.12200.x. [DOI] [PubMed] [Google Scholar]
- 19.Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- 20.Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35:147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben-Ari Y, Holmes GL. Effects of seizures on developmental processes in the immature brain. Lancet Neurol. 2006;5:1055–1063. doi: 10.1016/S1474-4422(06)70626-3. [DOI] [PubMed] [Google Scholar]
- 22.Lee CL, Hannay J, Hrachovy R, Rashid S, Antalffy B, Swann JW. Spatial learning deficits without hippocampal neuronal loss in a model of early-onset epilepsy. Neuroscience. 2001;107:71–84. doi: 10.1016/s0306-4522(01)00327-x. [DOI] [PubMed] [Google Scholar]
- 23.Sayin U, Sutula TP, Stafstrom CE. Seizures in the developing brain cause adverse long-term effects on spatial learning and anxiety. Epilepsia. 2004;45:1539–1548. doi: 10.1111/j.0013-9580.2004.54903.x. [DOI] [PubMed] [Google Scholar]
- 24.Strauss E, Satz P, Wada J. An examination of the crowding hypothesis in epileptic patients who have undergone the carotid amytal test. Neuropsychologia. 1990;28:1221–1227. doi: 10.1016/0028-3932(90)90057-u. [DOI] [PubMed] [Google Scholar]
- 25.Gleissner U, Kurthen M, Sassen R, et al. Clinical and neuropsychological characteristics of pediatric epilepsy patients with atypical language dominance. Epilepsy Behav. 2003;4:746–752. doi: 10.1016/j.yebeh.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Ewen JB, Kossoff EH, Crone NE, et al. Use of quantitative EEG in infants with port-wine birthmark to assess for Sturge-Weber brain involvement. Clin Neurophysiol. 2009;120:1433–1440. doi: 10.1016/j.clinph.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu JY, Peters JM, Goyal M, et al. Clinical Electroencephalographic Biomarker for Impending Epilepsy in Asymptomatic Tuberous Sclerosis Complex Infants. Pediatr Neurol. 2016;54:29–34. doi: 10.1016/j.pediatrneurol.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin DD, Barker PB, Hatfield LA, Comi AM. Dynamic MR perfusion and proton MR spectroscopic imaging in Sturge-Weber syndrome: correlation with neurological symptoms. J Magn Reson Imaging. 2006;24:274–281. doi: 10.1002/jmri.20627. [DOI] [PubMed] [Google Scholar]
- 29.Evans AL, Widjaja E, Connolly DJ, Griffiths PD. Cerebral perfusion abnormalities in children with Sturge-Weber syndrome shown by dynamic contrast bolus magnetic resonance perfusion imaging. Pediatrics. 2006;117:2119–2125. doi: 10.1542/peds.2005-1815. [DOI] [PubMed] [Google Scholar]
- 30.Marti-Bonmati L, Menor F, Mulas F. The Sturge-Weber syndrome: correlation between the clinical status and radiological CT and MRI findings. Childs Nerv Syst. 1993;9:107–109. doi: 10.1007/BF00305319. [DOI] [PubMed] [Google Scholar]
- 31.Comi AM. Sturge-Weber syndrome and epilepsy: an argument for aggressive seizure management in these patients. Expert Rev Neurother. 2007;7:951–956. doi: 10.1586/14737175.7.8.951. [DOI] [PubMed] [Google Scholar]
- 32.Lagae L. Cognitive side effects of anti-epileptic drugs. The relevance in childhood epilepsy. Seizure. 2006;15:235–241. doi: 10.1016/j.seizure.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Maton B, Krsek P, Jayakar P, et al. Medically intractable epilepsy in Sturge-Weber syndrome is associated with cortical malformation: implications for surgical therapy. Epilepsia. 2010;51:257–267. doi: 10.1111/j.1528-1167.2009.02304.x. [DOI] [PubMed] [Google Scholar]
- 34.Pinto AL, Chen L, Friedman R, et al. Sturge-Weber syndrome: Brain magnetic resonance imaging and neuropathology findings. Pediatr Neurol. 2016;58:25–30. doi: 10.1016/j.pediatrneurol.2015.11.005. [DOI] [PubMed] [Google Scholar]


