Abstract
Aberrant cell migration leads to the dispersal of malignant cells. The ubiquitous lipid mediator lysophosphatidic acid (LPA) modulates cell migration and is implicated in tumor progression. Yet, the signaling cascades that regulate LPA’s effect on cell motility remain unclear. Using time-lapse imaging and quantitative analyses, we studied the role of signaling cascades that act downstream of LPA on the motility of MCF10CA1a breast cancer cells. We found that LPA alters cell motility via two major signaling pathways. The Rho / ROCK signaling cascade is the predominant pathway that increases E-Cadherin containing cell-cell adhesions and cortical arrangement of actomyosin to promote slow, directional, spatially coherent and temporally consistent movement. In contrast, Gαi/o- and Gαq/11- dependent signaling cascades lessen directionality and support the independent movement of cells. The net effect of LPA on breast cancer cell migration therefore results from the integrated signaling activity of the Rho / ROCK and Gαi/o- and Gαq/11-dependent pathways, thus allowing for a dynamic migratory response to changes in the cellular or microenvironmental context.
Keywords: Lysophosphatidic acid, cell motility, sheet migration, breast cancer, signal transduction
1. Introduction
Cell migration is crucial for tissue development and repair. It is tightly regulated by microenvironmental cues and cellular signal transduction. In tumors, aberrant cell migration leads to dispersal of malignant cells into the local microenvironment and throughout the body. Interestingly, short range dispersal of tumor cells has also been suggested to change the rate of tumor growth and tumor heterogeneity [1]. Thus, understanding the regulation of tumor cell migration is essential for the understanding of tumor development.
Lysophosphatidic acid (LPA) is a ubiquitous lipid mediator that modulates cell migration and is implicated in tumor progression [2–7]. Remarkably, LPA can either stimulate or reduce cell migration [8–10], and can act as a chemoattractant or chemorepellant [11–13]. These contradictory effects are mediated by the activation of several G-protein coupled receptors (LPA1-6), and downstream signaling cascades that include Rho/Rock-, Akt-, PKC-, PI3K-, and Rac-signaling [4,7,9,13–16]. However, it is unclear if the specific migratory response of cells is the result of activation of a distinct pathway, or of the integrated signaling activity throughout the LPA signaling network.
We hypothesize that specific parameters of cell motility are regulated by different LPA signaling pathways within the same cell and that a cell’s migratory response to LPA is determined by the integrated activity of these pathways. We used an established model, migration of human invasive, lung colony forming breast cancer cells (MCF10CA1a) into a cell-free area [17–19], to determine if and how multiple LPA signaling cascades affect specific parameters of cell morphology and cell motility to contribute to the observed net effect of LPA on MCF10CA1a cells. We found that the LPA-signaling cascades that regulate MCF10CA1a migration fall into two main groups: (i) the predominant LPA1 / Rho / ROCK pathway increases cell-cell junctions and cell contractility to promote the observed phenotype - slow, directional, consistent and coherent movement, and (ii) the Gαq/11- and Gαi/o-dependent signaling cascades support non-directional, individual cell movement. It is thus the integrated activity of different signaling cascades that determines the observed net effect of LPA on the migratory activity of MCF10CA1a cells, and that allows a dynamic migratory response to changes in the microenvironmental and cellular signaling context.
2. Materials and Methods
2.1. Cell culture
Human breast cancer cells (MCF10CA1a"MCF10CA1A”, Karmanos Research Institute) were cultured in DMEM/F12 (Invitrogen) supplemented with 5 % horse serum (Invitrogen) at 5 % CO2 in a humidified atmosphere as described previously [17,18,20,21]. For dot assays, cells were trypsinized and resuspended to 3×106 cell/ml. 10 µl cell suspension were placed in each well of a 12 well glass bottom plate (MatTek) coated with 10 ug/ml collagen IV (BD Biosciences) and allowed to adhere for 30 min to 45 min before non-attached cells were removed, and cell colonies were incubated in full medium overnight [17]. Cells were then serum starved in DMEM / F12 containing 0.1 % horse serum for 3 h before stimulation with LPA (1-oleoyl lysophosphatidic acid, 1µM, Tocris). Inhibitors or vehicle were added 30 min before addition of LPA. Pertussis toxin and blebbistatin were purchased from Sigma. Ki16425, GO6983, GO6976, GSK429286, and H2L5186303 were purchased from Tocris. Mas7 and (2S)-OMPT were purchased from Cayman Chemical. PD98059, SB203580, Y27632, LY294002 and Akt inhibitor VIII were purchased from EMD Millipore. EHT1864 and Gallein were purchased from R&D Systems. 1-myristoyl-LPA was purchased from Avanti Polar Lipids.
Knockdown of E-Cadherin was performed as previously described [19]. RhoA knock-out (RhoA KO) cells were generated using CRISPR /cas9 [22,23]. Briefly, the target sequence 5’ - GAACTATGTGGCAGATATCG - 3’ was cloned into LentiCRISPRv2, and lentiviral particles were generated in HEK293T cells using the pPACKH1 kit (both System Biosciences) per the manufacturer’s instructions. Infected MCF10CA1a cells were selected with puromycin (Invitrogen), cloned, and RhoA protein expression analyzed by Western blotting.
2.2. Western Blotting
Western blotting was performed following established protocols [18,19]. Primary antibodies used were: anti-E-cadherin (1 : 8000, Invitrogen), anti-RhoA (1 : 1000, respectively, Cell Signaling), and anti-actin (1 : 60000, Millipore). HRP-conjugated secondary antibodies (1 : 30000 to 1 : 50000) were obtained from Pierce or Jackson ImmunoResearch. ECL reagents were from Pierce.
2.3. Immunostaining
Cells were fixed with 4 % formaldehyde or ice cold methanol (ROCK1), followed by quenching of autofluorescence with 100 mM glycine, and blocking of unspecific antibody binding with 1 % BSA. Antigens were labeled with primary antibody in 0.1 % BSA (anti-E-Cadherin (1:800, Invitrogen), anti-myosin IIb (1:200, Cell Signaling), anti-ROCK1 (1:300, ab134181, abcam,) at 4 °C overnight, followed by detection with secondary, fluorochrome labeled antibodies (1:250 in 0.1 % BSA, Invitrogen). F-actin was labeled using phalloidin -TRITC (1:200, Invitrogen). Nuclei were labeled with DAPI (Invitrogen). Specimens were imaged using a Zeiss Observer Z1 inverse microscope, a Leica DMi8 confocal microscope, or a Zeiss 880 / airyscan. Image analysis was performed in ImageJ 1.49 / Fiji 1.0 as previously described [17]. Briefly, membranes and cytoplasms of each cell were manually gated and mean signal intensities determined. Ratios of the membrane and the cytoplasmic signal were calculated, and analyzed and plotted using GraphPad Prism 7. Scale bars apply to all images in each panel.
2.4. Time lapse imaging and image analysis
For time lapse imaging, cultures were placed in the CO2- and temperature-controlled incubator chamber (37 °C, 5 % CO2) of a Zeiss Observer 2.1 with automated stage. Based on preliminary experiments, phase contrast images using a 10×objective were taken every 3 min for 12 h to facilitate reliable particle image velocimetry (PIV) and to ensure that cells were not negatively affected by starving conditions [19]. Data were analyzed between frame 10 and 180 (30min to 9h), when the migratory phenotype of cells as well as the focus of the microscope had stabilized. PIV and migration analysis of time-lapse images were performed using MATLAB as described earlier [18,19,24]. Briefly, the leading edge of the migrating cell sheet was found using a custom MATLAB segmentation code. Edge length was found by measuring the cumulative distance between adjacent points on the segmented edge. Speed was calculated as displacement per time. Angular spread was calculated as shown in Equation (1) and ranges from zero (aligned velocity vectors) to (highly uncoordinated vectors).
| (1) |
Finite-time Lyapunov exponent (FTLE) values were calculated based on the trajectories of virtual tracer particles in the PIV flow field, as previously described [19]. Correlations were calculated as shown in Equation (2).
| (2) |
2.5. Statistical Analysis
Data were analyzed using GraphPad Prism 7. Briefly, data were evaluated for normal distribution using D’Agostino Shapiro and Pearson-, Shapiro-Wilk-, and KS normality tests. Data sets that passed all three tests were considered normal distributed.
Statistical tests performed:
-
-
Paired data sets with normal distribution were analyzed by paired t-test or ANOVA / Sidak’s multiple comparison test.
-
-
Paired data sets without normal distribution were analyzed by Wilcoxon matched-pairs sign rank test or Friedman test / Dunn’s multiple comparison test.
-
-
Unpaired datasets were analyzed by Mann-Whitney test (non-parametric), ANOVA / Sidak’s multiple comparison test (parametric) or Kruskal-Wallis / Dunn’s multiple comparison test (non-parametric). Data are presented as mean and 95% confidence interval. Significance levels are indicated as follows: ns p ≥ 0.05; * p < 0.5; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
Migration analyses were summarized using paired t-statistics and clustered using methods similar to those described by Bazellieres et al. [25]. Additional details on the clustering methods are provided in the supplementary materials.
3. Results
3.1. LPA alters the morphological and migratory phenotype of MCF10A1a cells
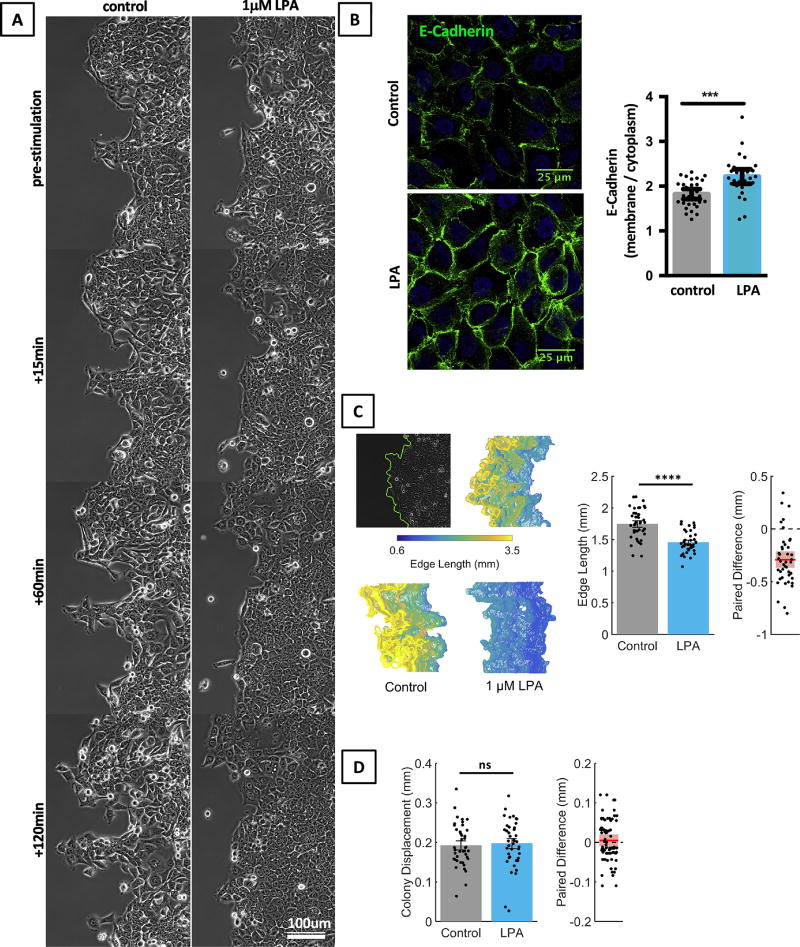
We studied which signaling pathways contribute to LPA-induced morphology and motility changes in MCF10CA1a cells using a two-dimensional, unconstrained migration assay [17,19]. Briefly, cells were plated in circular colonies. Live cell imaging of two opposing edges of the epithelial sheet was performed after stimulation of cells with LPA (1 µM), and cell movement at the sheet edge was analyzed by particle image velocimetry (PIV). We found that unstimulated MCF10CA1a cells were irregular in shape and formed sheets with irregular, occasionally frayed edges (Fig. 1A). In contrast, LPA-stimulated cells rapidly formed smooth-edged epithelial sheets with flat, polygonal cells and smooth sheet edges, indicating a more cohesive and coherent movement of cells (Fig. 1A). Concurrent with these observed morphological changes we found that LPA-stimulated cells had increased membrane localization of the cell adhesion protein E-Cadherin (Fig. 1B).
Fig. 1. LPA changes the morphological appearance of MCF10CA1a cells.
A. LPA (1 µM) causes MF10CA1a cells to flatten and assume a polygonal cell shape; at the same time, the edge length of LPA (1 µM) treated cultures remains smoother than that of control cells. B. LPA increases the membrane staining pattern of E-Cadherin in MCF10CA1a cells as shown by immunofluorescence. For quantification, cytoplasmic and membrane E-Cadherin was gated separately and quantified using ImageJ. MCF10CA1a cells were stimulated with LPA (1 µM) for 30min. Mann Whitney test. n=30 cells per group. Scale bar applies to all images of this panel. C. LPA (1 µM) treated cell colonies have smoother edges than control colonies. Top panel: the colony edge is automatically traced (left), tracks are color coded for length and stacked for each field. Bottom panel: colony edges of a LPA stimulated cell culture and a control culture. Bar diagrams: average edge length for LPA treated and untreated cultures. Paired t-test, n = 42 experiments. D. LPA (1 µM) does not alter the average colony diameter ΔR, or edge displacement, of MCF10CA1a cells sheets. Wilcoxon matched-pairs sign rank test, n = 42 experiments.
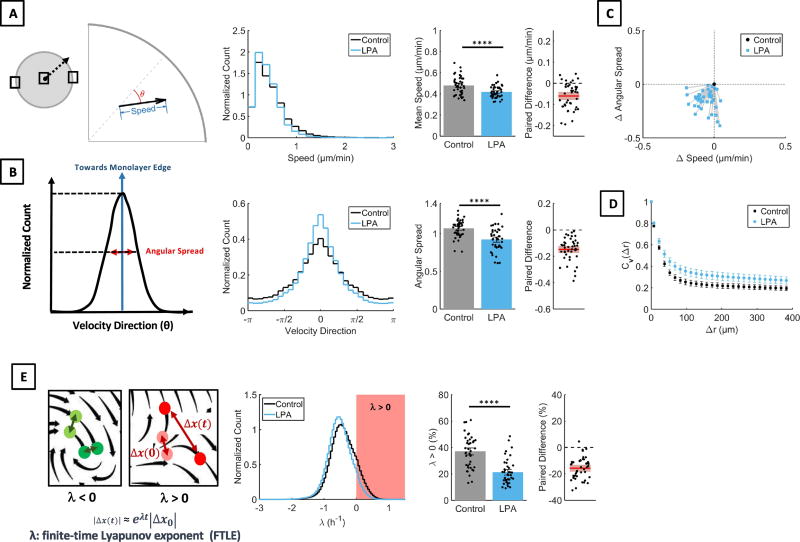
The movement of the cell sheets can be described by separate parameters each of which may be independently regulated by specific signaling pathways. To test this idea, we identified the following six quantifiable parameters that describe LPA-induced changes in MCF10CA1a cells. (1) Edge length. To quantify the effect of LPA on the colony contour, we traced the edge of epithelial sheets and determined its length as a surrogate marker for the smoothness of the epithelial edge (Fig. 1C). Indeed, we found that LPA stimulated sheets had smooth contours and short (small) edge lengths, while controls had frayed sheet contours and longer (larger) edge lengths (Fig. 1C). (2) Edge displacement. The edge displacement was calculated as increase of the colony radius (dR). LPA treatment did not alter edge displacement of MCF10CA1a sheets. (Fig. 1D). (3) Cell speed. Cell speed was calculated as displacement per time. LPA treatment reduced cell speed (Fig. 2A). (4) Directionality. We used the angular spread of velocity vectors to determine changes in directionality (Fig. 2B). High angular spread indicates low directionality, whereas low angular spread indicates high directionality. LPA treatment decreased angular spread and increased directionality of cell sheets. (5) Coherence of cell movement. Velocity autocorrelations, Cv(Δr) (Fig. 2D) determine if neighboring vectors are alike. In the context of sheet migration, Cv(Δr) was used to analyze if neighboring cell move together. High Cv(Δr) over a far distance indicates that large groups of cell move together, while low Cv(Δr) indicates that neighboring cells move independently. Thus, Cv(Δr) determines spatially coherent movement. LPA increased coherent movement (Cv(Δr)) of cell sheets. (6) Consistency of cell movement. The finite-time Lyapunov exponent (FTLE, henceforth λ) assesses the rate of separation of neighboring trajectories in a flow field (Fig. 2E). As particles move towards each other, λ will be negative, while positive values indicate that particles move away from each other. In the context of this manuscript, λ was used to quantify the predictability or consistency of cell movement over time. Positive values of λ are an indication of less predictable or less consistent movement over time, while a decrease in λ signifies an increased predictability or increased consistency of movement over time. LPA decreased the fraction of the cell sheet with λ > 0, indicating consistent movement of the cell sheet. Taken together, these findings show that LPA treatment increases membrane localization of E-Cadherin and induces a slow, directional, coherent and consistent movement of MCF10CA1a cell sheets without changing the net displacement of the sheet edge.
Fig. 2. LPA reduces cell speed, and increases cell directionality and coherent movement of the sheet.
A. Cells were plated in a circular colony and the edges as well as the center of the colony were imaged. Displacement vectors of optical features between frames were established by particle image velocimetry. Speed was calculated as displacement per time. Directionality was analyzed by observing the variability of velocity direction (θ). Treatment of MCF10CA1a cells with 1 µM LPA led to reduction of cell speed. Paired t-test, n = 42 experiments. B. For analysis of directionality the angular spread was analyzed. LPA treatment (1µM) reduced angular spread, i.e. increased directionality, of MCF10CA1a cells. Paired t-test, n = 42 experiments. C. LPA (1 µM) decreased cell speed and increased directionality as shown in a centered 2D scatter plot. n = 42 experiments. D. Similarity of neighboring vectors over a distance Δr was assessed using product velocity autocorrelation, Cv(Δr). LPA (1 µM) increased the distance Δr over which vectors showed good correlation or similarity of motion within the cell sheet. E. The finite time Lyapunov exponent (λ) was used to analyze chaotic movement of MCF10CA1a cells. The FTLE value characterizes the rate of separation of close trajectories. For λ < 0, the trajectories remain close to each other, while the distance between trajectories increases for λ > 0; λ > 0 (red) is associated with chaotic behavior. Stimulation of MCF10CA1a with LPA (1 µM) decreased the fraction of the cell sheet with λ > 0, suggesting decreased chaotic behavior within the cell sheet. Wilcoxon matched-pairs sign rank test, n = 42 experiments.
3.2 The LPA-induced morphological and migratory changes of MCF10CA1a cells require LPA1 and LPA3
Small molecule inhibitors (Fig. 3A) as well as knockdown or knockout of proteins were used to examine which specific pathways regulate individual parameters.
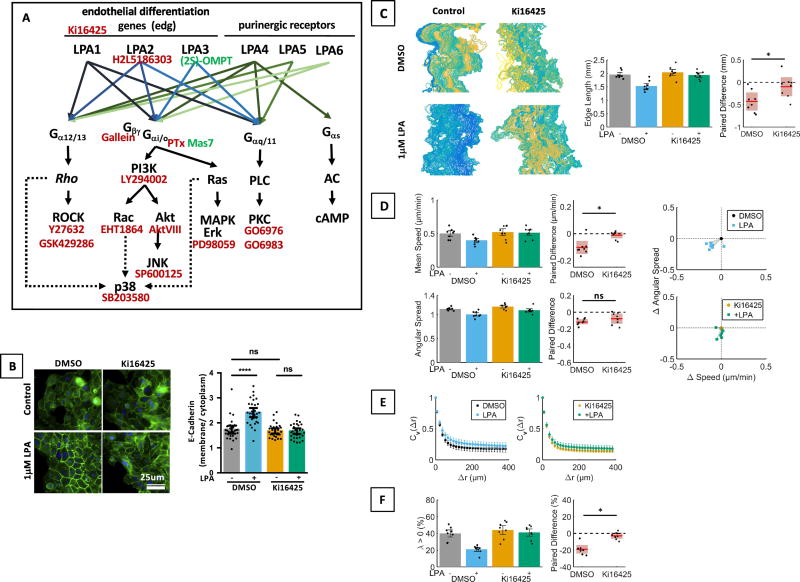
Fig. 3. Effects of LPA on MCF10CA1a cell migration are receptor mediated.
A. Schematic of the LPA signaling network (modified from [4,16]) and inhibitors used in this study. B. LPA mediated E-cadherin localization was reduced by treatment of MCF10CA1a cells with Ki16425 (10µM) as shown by immunofluor bescence. n = 30 cells per group, representative of 4 experiments. Kruskal Wallis test / Dunn’s Multiple comparison. Scale bar applies to all images of this panel. C–F. Inhibition of LPA1,3 with Ki16425 reduced the effect of LPA on edge length (C, paired t-test), cell speed (Wilcoxon matched-pairs sign rank test) and angular spread (albeit not significantly, paired t-test, D), velocity autocorrelations (E), and λ (Wilcoxon matched-pairs sign rank test, F). n = 8 experiments.
Using mRNA microarray, we previously showed that MCF10CA1a cells express LPA1, LPA2, LPA3, LPA4, and LPA5 [18]. In order to assess if the observed effects of LPA on cell migration are receptor mediated and which LPA receptors are involved in the cellular response, we treated cells with established LPA receptor inhibitors and agonists. We first treated cells with Ki16425 (10µM), an inhibitor of LPA1 and LPA3, which may also exert some effect on LPA2 , and found that inhibition of these receptors significantly reduced LPA effects on E-Cadherin membrane localization (Fig. 3B), smoothening of the sheet contour (Fig. 3C), and to a lesser extent on cell speed, (Fig. 3D) and coherent movement (Cv(Δr), Fig. 3F). This indicates that the observed LPA effects are dependent on LPA1, LPA3 or LPA2. We next further narrowed down which of these receptors are involved by using receptor-specific inhibitors and agonists. Inhibition of LPA2 with H2L5186303 (Fig. S1A - D) did not have appreciable effects on sheet morphology or migration. The LPA3 agonist (2S)-OMPT reduced cell speed (Fig. S1F) and smoothened sheet edges (Fig. S1E) in concentrations between 0.5 µM to 3.5 µM. At higher concentrations (2µM to 3.5µM) (2S)-OMPT also increased consistent movement (λ, Fig. S1H), an effect that may be non-specific considering the low EC50 of (2S)-OMPT [26,27]. (2S)-OMPT did not affect directionality or coherent movement (Cv(Δr), Fig. S1 F, G). It therefore appears that LPA exerts most of its effects via LPA1, with LPA3 having some control over cell speed and colony morphology.
3.3. LPA signaling alters the migratory phenotype of MCF10CA1a cells via Rho / ROCK-dependent changes in cell-cell adhesions and cell contractility
We next explored the involvement of the Rho / ROCK signaling cascade (Fig. S2 – S4). The Rho / ROCK signaling cascade is downstream of Gα12/13 and can be activated by LPA1 [4]. We found that Rho / ROCK signaling mediate the LPA-induced formation of tight epithelial sheets that move slowly, directionally, coherently and consistently. Specifically, CRISPR / cas9-mediated knock-out of RhoA (Fig. S2A) reduced LPA-induced membrane localization of E-Cadherin (Fig. S2B), and as a trend lesser effects of LPA on cell motility were observed in RhoA KO as compared to control cells. Inhibition of ROCK, a downstream effector of Rho, by Y27632 (Fig. S3) had stronger effects than RhoA KO and decreased LPA’s effect on E-Cadherin membrane localization (Fig. 4A, Fig. S3A), edge length (Fig. S3B), cell speed (Fig. S3C), and consistent movement (λ, Fig. S3E). Both, RhoA KO and inhibition of ROCK tended to reduce LPA effects on coherent movement (Cv(Δr), Figs. S2E, S3D). The ROCK inhibitor GSK 429286 had qualitatively similar but lesser effects than Y27632 (Fig. S4).
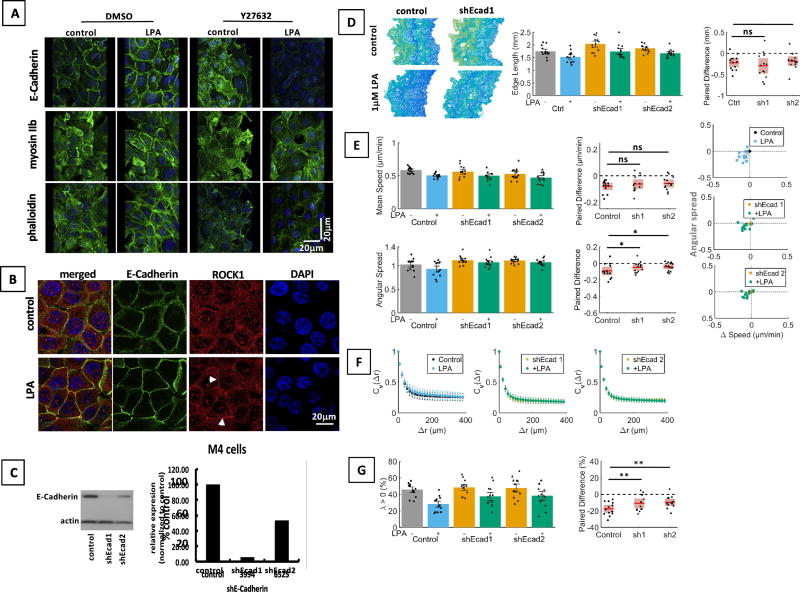
Fig. 4. The Rho/Rock signaling cascade mediates membrane localization of E-Cadherin.
A. Inhibition of ROCK1 with Y27632 (10µM) reduces effects of LPA on E-Cadherin-, F-actin- and myosin IIb staining patterns as shown by immunofluorescence. Cells were serum starved (0.1% horse serum) for 3 h, and then stimulated with 1 µM LPA for 30 min. Images presented are rotated 3D renderings (40°, y-axis) of Z-stacks Green: E-Cadherin or myosin II or phalloidin; blue: nuclei. Scale bar applies to all images of this panel. B. LPA induces localization of ROCK1 to E-Cadherin containing adherens junctions as shown by immunofluorescence. Scale bar applies to all images of this panel. C. shRNA was used to downregulate E-Cadherin protein expression in MCF10CA1a cells as shown by Western blotting. D–G. Low expression of E-Cadherin alters the migratory response of MCF10CA1a cells to LPA. Cells were serum starved for 3 h before stimulation with LPA and time-lapse imaging. Cells with low E-Cadherin expression still respond to LPA with a shortening of the colony edge (ANOVA / Sidak’s multiple comparison test, D) and reduced speed (Friedman / Dunn’s multiple comparison test, E). However, normal E-Cadherin levels are required for effect of LPA on angular spread (ANOVA, Sidak’s multiple comparison test, E), and λ (ANOVA / Sidak’s multiple comparison test, G). n = 13 experiments.
Having shown that LPA induced changes of cell motility and of E-Cadherin membrane localization in a Rho / ROCK-dependent manner, we hypothesized that the Rho / ROCK / E-Cadherin signaling cascade mediates at least some of the LPA effects on cell migration (Fig. 4). Indeed, we found that LPA increased colocalization of ROCK1 and E-Cadherin (Fig.4B) and that reduced expression of E-Cadherin (Fig. 4C) led to reduced LPA effects on cell directionality (Fig. 4E) and consistent movement (λ, Fig. 4G). Another known downstream target of Rho / ROCK signaling is myosin II [28]. We found that LPA induced cortical arrangement of actomyosin in a ROCK-dependent manner (Fig. 4A). The myosin II inhibitor blebbistatin [29] reduced the effects of LPA on the cortical arrangement of F-actin (Fig. S5A) and edge length (Fig. S5B), as well as all of the effects on cell motility (Fig. S5C–E). Thus, LPA-induced Rho / ROCK signaling increases E-Cadherin containing cell-cell adhesions and alters actomyosin contractility to promote slow, directional, coherent and consistent movement of MCF10CA1a cell sheets.
3.4. LPA regulates the migratory phenotype of MCF10CA1a cells via two groups of signaling cascades
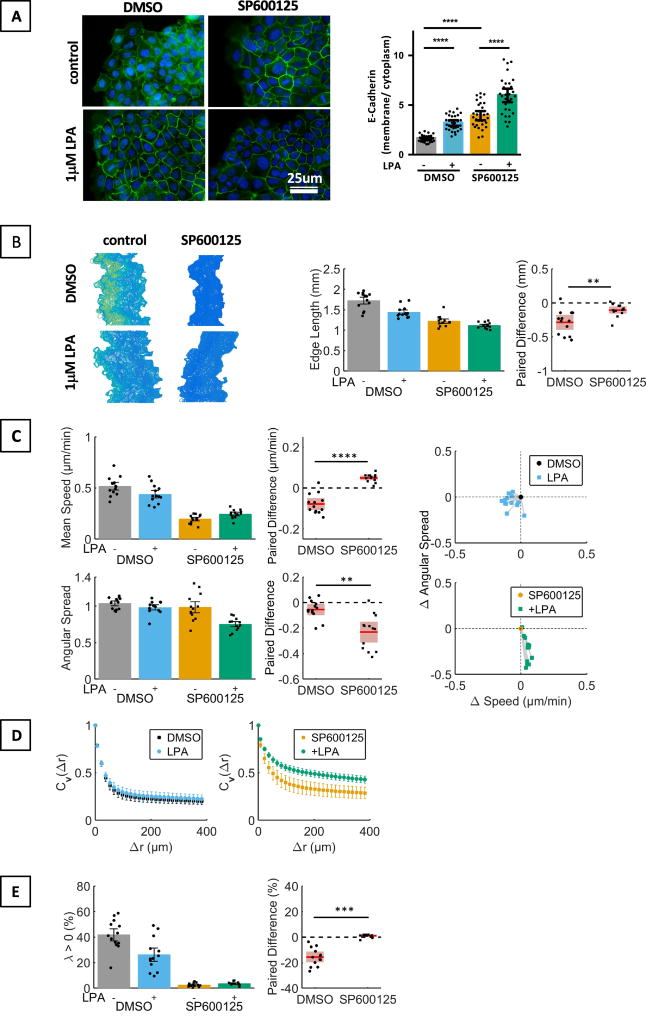
We hypothesized that Gαi/o- and Gαq/11-dependent signaling cascades contribute to LPA mediated changes in cell migration because LPA3, which does not activate Gα12/13-dependent Rho / ROCK signaling [4], mimicked some of the observed LPA effects (Fig. S1). Inhibition of JNK, which is downstream of Gαi/o, by SP600125 increased the membrane localization of E-Cadherin, which, surprisingly, was further increased by LPA stimulation (Fig.5A). Inhibition of JNK, which itself affected edge length, cell speed, coherent and consistent movement, accentuated LPA effects on cell directionality (Fig. 5C) and coherent movement (Cv(Δr), Fig. 5D), while reducing the LPA effect on sheet contour (Fig. 5B), cell speed (Fig. 5C), and consistent movement (λ, Fig. 5E). These findings suggest that JNK signaling curtails LPA-induced changes in E-Cadherin membrane localization, directionality, and coherent movement and thus opposes the Rho / ROCK-mediated LPA effects.
Fig. 5. Effect of inhibition of JNK signaling with SP600125 on LPA-mediated changes of the morphological and migratory phenotype of MCF10CA1a cells.
MCF10CA1a cells were starved in serum reduced medium (0.1 % horse serum) starting 3 h before stimulation with LPA (1 µM). The JNK inhibitor SP600125 (10 µM) was added 30 min before stimulation of cells with LPA. A. Immunostaining of E-Cadherin and quantification of membrane and cytoplasmic signal. ANOVA / Sidak’s multiple comparison test. n = 30 cells per group, representative of 4 experiments. Scale bar applies to all images of this panel. B–E. Effect of SP600125 and LPA on edge length (Wilcoxon matched-pairs sign rank test, B), cell speed (paired t-test) and angular spread (paired t-test) (C), Cv(Δr) (D), and λ (Wilcoxon matched-pairs sign rank test, E). n= 13 experiments.
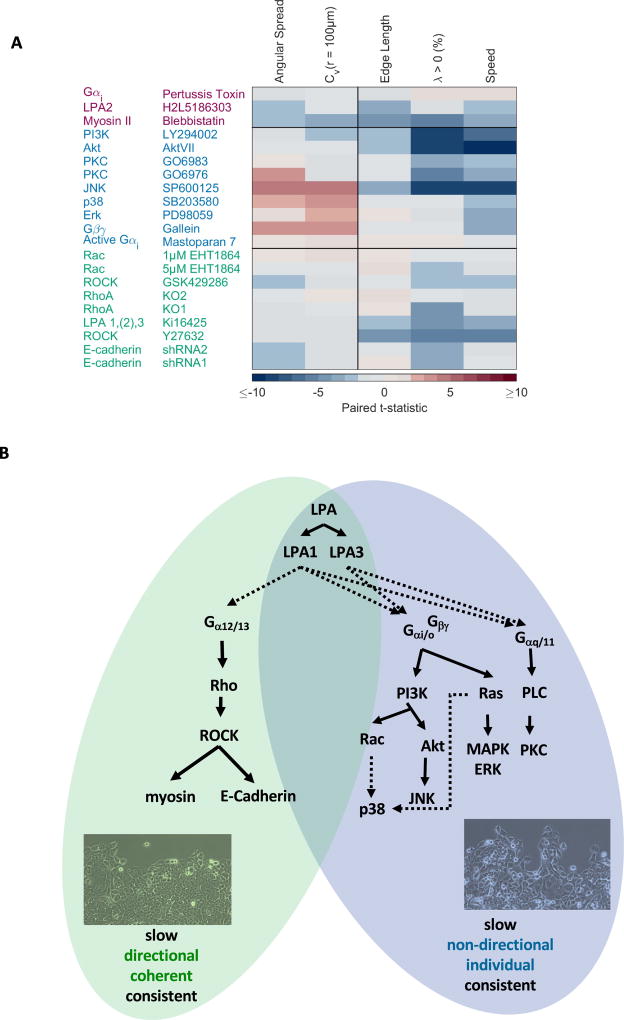
Further analysis of Gαi/o- and Gαq/11-dependent pathway, namely the Gαi-, Gβγ-, PI3K-, Akt-, PKC-, p38-, Erk-, and Rac-signaling pathways (Fig. 3A), revealed that each signaling cascade modulates multiple parameters of cell migration, and that each parameter is regulated by multiple LPA signaling cascades (Figs. S6–15). To objectively group these different pathways regarding their contribution to LPA-induced changes of cell motility, we calculated paired t-statistics and performed unsupervised clustering analysis of the entire data body (Fig. 6A). We found that LPA-induced signaling cascades fall into two main categories: (i) the Rho / ROCK signaling cascade, which generally promotes the observed changes on LPA-induced cell motility and (ii) the Gαi/o and Gαq/11-dependent signaling cascades (Fig. 5, Fig. 6A, Figs. S6 – 15), which generally mediate the observed effects of LPA on cell speed and consistent movement (λ), and can oppose the observed LPA effects on cell directionality, coherent movement (Cv(Δr)), as well as E-Cadherin membrane localization. Closer inspection of Fig. 6A shows that Gαi/o and Gαq/11-dependent signaling cascades fall into two subgroups. Specifically, inhibition of Gβγ-dependent activation of PI3K and Gβγ by Gallein [30] (Fig. S6), Erk by PD98059 (Fig. S7), or p38 by SB203580 (Fig. S8), reduced the LPA effects on cell speed and accentuated the LPA effects on E-Cadherin localization, coherent movement (Cv(Δr)) and cell directionality (except for inhibition of Erk where LPA-induced reduction of cell directionality was observed but did not reach statistical significance) (Fig. S6 – S8). Thus, LPA-induced Gβγ-, Erk-, p38- and JNK-signaling promotes the slow and individual movement of cells. Inhibition of PI3K by LY294002 (Fig. S9), Akt by the Akt inhibitor VIII (Fig. S10), and PKC by GO6976 or GO6983 (Fig. S11–S12), mostly resulted in a reduction of LPA effects on cell speed and consistent movement (λ). Inhibition or activation of Gαi by Pertussis toxin (Fig. S13) or Mas7 (Fig. S14), respectively, had no relevant effects on MCF10CA1a cell migration. Likewise, we found that inhibition of Rac by EHT1864 (Fig. S15) did not result in appreciable changes of any observed parameter. Thus, Gαi- and Rac-signaling do not contribute to the observed LPA-induced migratory phenotype of MCF10CA1a cells although they were, as to be expected, assigned into a cluster during unsupervised clustering. Myosin II, which promotes all of the observed effects of LPA in a ROCK dependent manner (Fig. 4, Fig. S5), did not cluster with either the Rho /ROCK - or the Gαi/o- and Gαq/11-dependent pathway (Fig. 6A) and was assigned into a smaller, third cluster. However, our experimental data strongly suggest that myosin IIb is downstream of Rho / ROCK-signaling (Fig. 4, Fig. S5) and thus is part of the Rho /Rock pathway.
Fig. 6. The effects of LPA on the migratory phenotype of MCF10CA1a cells are regulated by two opposing signaling pathways.
A. Clustering reveals that components of the LPA signaling network that are involved in mediating effects of LPA on MCF10CA1a cells fall into two main categories: (a) the Rho / ROCK/ E-Cadherin cascade and (b) Gαi/o and Gα11/q-dependent signaling cascades. B. Signaling cascades within the LPA signaling network [4,16] differentially contribute to effects of LPA on MCF10CA1a morphology and migration: Rho / ROCK / E-Cadherin / Myosin II signaling is the predominant pathway to mediate observed LPA effects on MCF10CA1a cells. The Gαi/o- and Gαq/11- dependent signaling pathway opposes the Rho / ROCK signaling pathway.
In summary, pathways mediating the LPA-induced migration of MCF10CA1a cells, fall into two main groups: (i) the LPA1 / Rho / ROCK / E-Cadherin / myosin II signaling cascade promotes slow, directional and coherent migration and is the predominant pathway to mediate the observed LPA-induced phenotype; (ii) the Gαi- and Gαq/11- dependent signaling pathway promotes LPA-induced slow, consistent, but individual movement of cells (Fig. 6B). It is therefore the integrated activity of the LPA signaling network that determines the LPA-induced observed phenotype - slow, directional, coherent and consistent movement of MCF10CA1a sheets.
4. Discussion
The ubiquitous lipid mediator lysophosphatidic acid (LPA) is a known modulator of cell migration and has been implicated in tumor progression[2,16]. We now show that LPA regulates MCF10CA1a motility via two major signaling pathways that have opposing effects on cell motility: Rho / ROCK and Gαi/o- and Gα11/q. Each of the signaling cascades described here has previously been implicated in LPA-induced cell motility. Both, LPA1 and LPA3, can be involved in LPA-induced migration and chemotaxis of melanoma, colon, pancreatic, or breast cancer cells [8,9,11,31,32]. Likewise, Rho / ROCK- signaling, PI3K-, Rac-, p38-, JNK-, Erk-, or PKC-signaling have each been implicated in LPA-induced cell migration in different experimental systems including migration of breast cancer cell in wound and Boyden chamber assays [33–41]. Our study reconciles these varying descriptions of LPA’s role in the regulation of cell migration by showing that it is the integrated signaling activity throughout the LPA signaling network that determines the observed effects of LPA on cell motility.
Rho / ROCK affects cell motility via E-Cadherin membrane localization as well as actomyosin rearrangement, both of which are known targets of Rho / ROCK signaling [28,42,43]. ROCK1 associates with E-Cadherin via p120-catenin to stabilize adherens junctions [42], consistent with our observation that LPA treatment leads to the colocalization of ROCK1 and E-Cadherin. E-Cadherin in turn couples to cortical actin filaments to aid actin assembly [44]. Indeed, we found that LPA induced cortical actin rearrangement. In addition, Rho / ROCK signaling can increase myosin II activity by suppressing myosin light chain (MLC) phosphatase activity, thereby increasing phosphorylated MLC, and by phosphorylation of MLC at Ser19 [45]. This, in combination with E-Cadherin-mediated cortical actin rearrangement, is likely the underlying mechanism of the cortical actomyosin reorganization observed in MCF10CA1a cells in response to LPA.
E-Cadherin loss has been described during breast cancer progression [46]. In cells lacking E-Cadherin, LPA cannot increase adherens junction formation and tethering of actin to adherens junctions. This should alter the cellular response to LPA such that effects of Gαi/o- and Gα11/q-dependent signaling cascades are prevalent. Indeed, we found that downregulation of E-Cadherin results in reduced effects of LPA on directionality and consistent movement of cells. One could speculate that LPA might have a different effect in E-Cadherin negative cells as E-Cadherin might be replaced by other cell adhesion proteins or integrins, resulting in yet another migratory response.
We suggest that balancing cell motility via a signaling network involving multiple pathways as opposed to regulating it via a single pathway can result in several qualitatively distinct migratory responses. Such qualitatively distinct responses have been described for LPA-induced MDA-MB-231 motility. MDA-MB-231 cell invasion requires Rho-, Rac- and MAPK-signaling, while MDA-MB-231 cell migration only requires Rac- and MAPK-signaling [33]. In MCF10CA1a cells the interplay between Rho / ROCK- and Gαi/o- and Gα11/q-dependent signaling pathways balances LPA’s effect on cell motility.
Cells are tethered to the sheet by E-Cadherin, which is described as a suppressor of invasion [47], if Rho / ROCK signaling is predominant. This leads to the formation of well-established, cohesive sheets. In contrast, prevalent Gαi/o- and Gα11/q signaling results in heterogeneous sheets. Here, cells can more easily migrate away from the original cell cluster, leading to dispersal of tumor cells. Thus, a shift of LPA-signaling from the Rho / ROCK- to the Gαi/o- and Gα11/q-signaling pathway will increase independent, less directional movement of cells and consequently cell dispersal. Because cells under these circumstances can switch positions in the sheet more easily, lost cells might be more easily replaced, and expansion of clones might happen more readily. Such changes in the dynamics of tumor growth and development, in addition to cell dispersal, has been postulated in a model of tumor cell dispersal [1].
The integration of signaling activity in a network that relies on multiple receptors and downstream signaling cascades is not only influenced by the concentration of a single ligand such as LPA, but also by the activation status of other components. For example, the cell line used here, MCF10CA1a cells, expresses Ha-RAS [21] and has an activating PI3K mutation [48], which results in high basal Akt phosphorylation [49]. Thus, the Gαi/o- and Gα11/q- dependent signaling pathways are active. The inhibition of JNK resulted in a more epithelial phenotype, indicating that this signaling pathway facilitates independent cell movement. The LPA response in cells with reduced JNK signaling was accentuated, illustrating a shift from the Gαi/o- and Gα11/q-dependent signaling pathway towards the Rho / ROCK pathway. In another example, we describe that the LPA-mediated migratory response of MCF10CA1a cells is mostly mediated by LPA1 and LPA3, while LPA2 does not appear to play a relevant role. On the other hand, in pancreatic cancer cells LPA1 mediates stimulation and LPA2 mediates inhibition of cell migration [9]. The apparently contradictory contribution of the different LPA receptor on cell migration might arise from several reasons. First, we used breast cancer cells to show that LPA1 and LPA3 mediate LPA effects on cell migration, while in pancreatic cancer cells LPA1 and LPA2 have opposing effects on cell migration. Second, different migratory parameters were assessed. Here we analyzed cell migration after uniform stimulation with LPA, while chemoattraction towards LPA in the presence of EGF was analyzed in the pancreatic cancer cells. Thus, the LPA network is a complex signaling network. Multiple ligands can bind to multiple receptors with different affinity. Receptors in turn can activate multiple signaling pathways. Cellular computation of multiple signals in such a complex network may allow intricate cellular responses to an ever-changing environment as has been shown in the context of BMP signaling and EMT [50–53]. The biological response to ligands may be further modulated by transactivation of additional pathways. For example, the EGF receptor can be transactivated by LPA receptors [54,55].
The LPA signaling cascade is dysregulated in tumors and has been implicated in tumor progression [2,16,32,56], making it a potential therapeutic target. However, we have shown here that, depending on the cellular signaling context, interference with LPA-signaling might lead to either increased or decreased tumor cell dispersal as well as tumor progression. In addition to the cellular responses studied here, LPA can also have mitogenic effects, can alter cell survival, and modulate cell differentiation [16]. Consequently, targeting LPA signaling for therapeutic purposes, e.g. in tumor treatment, requires a precise understanding of the LPA signaling network. Determining the state of the LPA signaling network in individual tumor tissues may allow us to predict more precisely whether enhancing or blocking LPA signaling will reduce tumor cell dispersal. This could be done by profiling phosphorylation patterns of Rho / ROCK- and Gαi/o- and Gα11/q- dependent pathways in response to LPA, E-Cadherin expression patterns, and, if feasible, LPA-induced motility of cells in patient derived material. In addition, it may be necessary to further assess expression and activity of other signaling molecules and pathways that are known to interact with the LPA signaling network, such as EGF- or TGF-β signaling [9,57–59], and to consider that the tissue of origin can influence the cellular response to LPA. Further in vitro and in vivo experiments are needed to tease out the effect of LPA on cell motility and dispersal in different cellular contexts, and to determine how LPA-induced changes in cell motility affect tumor growth, invasion and metastasis.
5. Conclusions
The ubiquitous lipid mediator LPA alters motility of MCF10CA1a breast cancer cell sheets via two major pathways: LPA1 / Rho / ROCK signaling increases E-Cadherin containing cell-cell adhesions and cortical actomyosin arrangement to promote the observed net effect of LPA on cell migration - slow, directional, coherent and consistent movement. In contrast, Gαi/o- and Gα11/q- dependent signaling cascades lessen directionality and increase independent movement, fostering cell dispersal. It is the balance between these two major pathways that determines the migratory response of MCF10CA1a cells to LPA. Thus, LPA might support or oppose tumor cell motility and dispersal depending on the cellular signaling. A thorough understanding of the regulation of LPA-induced cell motility and cell dispersal is therefore necessary if the LPA signaling network is to be exploited for treatment of tumor disease and undesired responses are to be avoided.
Supplementary Material
Highlights.
LPA induces slow, directional, coherent and consistent movement of MCF10CA1a cell sheets
The observed effect of LPA depends on the balance of signaling activity between two pathways
Rho / ROCK signaling is the predominant pathway to mediate observed LPA effects on MCF10CA1a cells
The Gαi/o- and Gαq/11- dependent signaling pathway opposes the Rho / ROCK signaling pathway
Acknowledgments
We would like to thank Paul Randazzo for insightful discussions of data and extensive help with writing the manuscript, Bhagawat Subramanian for help with the generation of RhoAKO cell lines and comments on the manuscript, and Olga Aprelikova for reading and commenting on the manuscript.
Funding: This work was funded by the Intramural Research Program, National Cancer Institute, National Institutes of Health. R.M.L. was supported in part by NCI/NIH Award Number T32CA154274. W.L. was supported by AFOSR grant FA9550-16-1-0052
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration if Interest
The authors report no conflicts of interest in this work.
Author Contributions:
The study was designed by C.H.S. and C.A.P. Experiments were performed by C.H.S.. MATLAB codes for analysis of time-lapse imaging data and clustering were provided and maintained by R.M.L. and W.L; PIV analysis of time-lapse imaging data was performed by CHL; cluster analysis was performed by R.M.L. Analysis of all other data was performed by C.H.S. The manuscript was written by C.H.S., and read and edited by all authors.
Bibliography
- 1.Waclaw B, Bozic I, Pittman ME, Hruban RH, Vogelstein B, Nowak MA. A spatial model predicts that dispersal and cell turnover limit intratumour heterogeneity. Nature. 2015;525:261–264. doi: 10.1038/nature14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houben AJS, Moolenaar WH. Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis Rev. 2011;30:557–565. doi: 10.1007/s10555-011-9319-7. [DOI] [PubMed] [Google Scholar]
- 3.Leblanc R, Peyruchaud O. New insights into the autotaxin/LPA axis in cancer development and metastasis. Exp Cell Res. 2015;333:183–189. doi: 10.1016/j.yexcr.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Yung YC, Stoddard NC, Chun J. LPA receptor signaling: pharmacology, physiology, and pathophysiology. J Lipid Res. 2014;55:1192–1214. doi: 10.1194/jlr.R046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willier S, Butt E, Grunewald TGP. Lysophosphatidic acid (LPA) signalling in cell migration and cancer invasion: a focussed review and analysis of LPA receptor gene expression on the basis of more than 1700 cancer microarrays. Biol Cell. 2013;105:317–333. doi: 10.1111/boc.201300011. [DOI] [PubMed] [Google Scholar]
- 6.Boucharaba A, Serre C-M, Grès S, Saulnier-Blache JS, Bordet J-C, Guglielmi J, et al. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest. 2004;114:1714–1725. doi: 10.1172/JCI22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boucharaba A, Serre C-M, Guglielmi J, Bordet J-C, Clézardin P, Peyruchaud O. The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases. Proc Natl Acad Sci U S A. 2006;103:9643–9648. doi: 10.1073/pnas.0600979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen M, Towers LN, O’Connor KL. LPA2 (EDG4) mediates Rho-dependent chemotaxis with lower efficacy than LPA1 (EDG2) in breast carcinoma cells. Am J Physiol, Cell Physiol. 2007;292:C1927–33. doi: 10.1152/ajpcell.00400.2006. [DOI] [PubMed] [Google Scholar]
- 9.Komachi M, Tomura H, Malchinkhuu E, Tobo M, Mogi C, Yamada T, et al. LPA1 receptors mediate stimulation, whereas LPA2 receptors mediate inhibition, of migration of pancreatic cancer cells in response to lysophosphatidic acid and malignant ascites. Carcinogenesis. 2009;30:457–465. doi: 10.1093/carcin/bgp011. [DOI] [PubMed] [Google Scholar]
- 10.Tanikawa T, Kurohane K, Imai Y. Regulatory effect of lysophosphatidic acid on lymphocyte migration. Biol Pharm Bull. 2010;33:204–208. doi: 10.1248/bpb.33.204. [DOI] [PubMed] [Google Scholar]
- 11.Muinonen-Martin AJ, Susanto O, Zhang Q, Smethurst E, Faller WJ, Veltman DM, et al. Melanoma cells break down LPA to establish local gradients that drive chemotactic dispersal. PLoS Biol. 2014;12:e1001966. doi: 10.1371/journal.pbio.1001966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahay D, Leblanc R, Grunewald TGP, Ambatipudi S, Ribeiro J, Clézardin P, et al. The LPA1/ZEB1/miR-21-activation pathway regulates metastasis in basal breast cancer. Oncotarget. 2015;6:20604–20620. doi: 10.18632/oncotarget.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jongsma M, Matas-Rico E, Rzadkowski A, Jalink K, Moolenaar WH. LPA is a chemorepellent for B16 melanoma cells: action through the cAMP-elevating LPA5 receptor. PLoS ONE. 2011;6:e29260. doi: 10.1371/journal.pone.0029260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shida D, Kitayama J, Yamaguchi H, Okaji Y, Tsuno NH, Watanabe T, et al. Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer Res. 2003;63:1706–1711. [PubMed] [Google Scholar]
- 15.David M, Ribeiro J, Descotes F, Serre C-M, Barbier M, Murone M, et al. Targeting lysophosphatidic acid receptor type 1 with Debio 0719 inhibits spontaneous metastasis dissemination of breast cancer cells independently of cell proliferation and angiogenesis. Int J Oncol. 2012;40:1133–1141. doi: 10.3892/ijo.2011.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi JW, Herr DR, Noguchi K, Yung YC, Lee C-W, Mutoh T, et al. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–186. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 17.Stuelten CH, Busch JI, Tang B, Flanders KC, Oshima A, Sutton E, et al. Transient tumor-fibroblast interactions increase tumor cell malignancy by a TGF-Beta mediated mechanism in a mouse xenograft model of breast cancer. PLoS ONE. 2010;5:e9832. doi: 10.1371/journal.pone.0009832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiger MC, Vedham V, Stuelten CH, Shou K, Herrera M, Sato M, et al. Real-time motion analysis reveals cell directionality as an indicator of breast cancer progression. PLoS ONE. 2013;8:e58859. doi: 10.1371/journal.pone.0058859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee RM, Stuelten CH, Parent CA, Losert W. Collective cell migration over long time scales reveals distinct phenotypes. Convergent Science Physical Oncology. 2016;2:025001. doi: 10.1088/2057-1739/2/2/025001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang B, Vu M, Booker T, Santner SJ, Miller FR, Anver MR, et al. TGF-beta switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J Clin Invest. 2003;112:1116–1124. doi: 10.1172/JCI18899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santner SJ, Dawson PJ, Tait L, Soule HD, Eliason J, Mohamed AN, et al. Malignant MCF10CA1 cell lines derived from premalignant human breast epithelial MCF10AT cells. Breast Cancer Res Treat. 2001;65:101–110. doi: 10.1023/A:1006461422273. [DOI] [PubMed] [Google Scholar]
- 22.Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11:783–784. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee RM, Kelley DH, Nordstrom KN, Ouellette NT, Losert W. Quantifying stretching and rearrangement in epithelial sheet migration. New J Phys. 2013;15 doi: 10.1088/1367-2630/15/2/025036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bazellières E, Conte V, Elosegui-Artola A, Serra-Picamal X, Bintanel-Morcillo M, Roca-Cusachs P, et al. Control of cell-cell forces and collective cell dynamics by the intercellular adhesome. Nat Cell Biol. 2015;17:409–420. doi: 10.1038/ncb3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian L, Xu Y, Hasegawa Y, Aoki J, Mills GB, Prestwich GD. Enantioselective responses to a phosphorothioate analogue of lysophosphatidic acid with LPA3 receptor-selective agonist activity. J Med Chem. 2003;46:5575–5578. doi: 10.1021/jm034207p. [DOI] [PubMed] [Google Scholar]
- 27.Hasegawa Y, Erickson JR, Goddard GJ, Yu S, Liu S, Cheng KW, et al. Identification of a phosphothionate analogue of lysophosphatidic acid (LPA) as a selective agonist of the LPA3 receptor. J Biol Chem. 2003;278:11962–11969. doi: 10.1074/jbc.M209168200. [DOI] [PubMed] [Google Scholar]
- 28.Zegers MM, Friedl P. Rho GTPases in collective cell migration. Small Gtpases. 2014;5:e28997. doi: 10.4161/sgtp.28997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovács M, Tóth J, Hetényi C, Málnási-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 30.Lehmann DM, Seneviratne AMPB, Smrcka AV. Small molecule disruption of G protein beta gamma subunit signaling inhibits neutrophil chemotaxis and inflammation. Mol Pharmacol. 2008;73:410–418. doi: 10.1124/mol.107.041780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shida D, Watanabe T, Aoki J, Hama K, Kitayama J, Sonoda H, et al. Aberrant expression of lysophosphatidic acid (LPA) receptors in human colorectal cancer. Lab Invest. 2004;84:1352–1362. doi: 10.1038/labinvest.3700146. [DOI] [PubMed] [Google Scholar]
- 32.Sun K, Cai H, Duan X, Yang Y, Li M, Qu J, et al. Aberrant expression and potential therapeutic target of lysophosphatidic acid receptor 3 in triple-negative breast cancers. Clin Exp Med. 2015;15:371–380. doi: 10.1007/s10238-014-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrison SMW, Knifley T, Chen M, O’Connor KL. LPA, HGF, and EGF utilize distinct combinations of signaling pathways to promote migration and invasion of MDAMB-231 breast carcinoma cells. BMC Cancer. 2013;13:501. doi: 10.1186/1471-2407-13-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bian D, Su S, Mahanivong C, Cheng RK, Han Q, Pan ZK, et al. Lysophosphatidic Acid Stimulates Ovarian Cancer Cell Migration via a Ras-MEK Kinase 1 Pathway. Cancer Res. 2004;64:4209–4217. doi: 10.1158/0008-5472.CAN-04-0060. [DOI] [PubMed] [Google Scholar]
- 35.Badri L, Lama VN. Lysophosphatidic acid induces migration of human lung-resident mesenchymal stem cells through the β-catenin pathway. Stem Cells. 2012;30:2010–2019. doi: 10.1002/stem.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao J, He D, Berdyshev E, Zhong M, Salgia R, Morris AJ, et al. Autotaxin induces lung epithelial cell migration through lysoPLD activity-dependent and -independent pathways. Biochem J. 2011;439:45–55. doi: 10.1042/BJ20110274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JH, Adelstein RS. LPA(1) -induced migration requires nonmuscle myosin II light chain phosphorylation in breast cancer cells. J Cell Physiol. 2011;226:2881–2893. doi: 10.1002/jcp.22631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du J, Sun C, Hu Z, Yang Y, Zhu Y, Zheng D, et al. Lysophosphatidic acid induces MDA-MB-231 breast cancer cells migration through activation of PI3K/PAK1/ERK signaling. PLoS ONE. 2010;5:e15940. doi: 10.1371/journal.pone.0015940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu B, Shi S, Ma Y-G, Fan F, Yao Z-Z. Lysophosphatidic acid enhances human hepatocellular carcinoma cell migration, invasion and adhesion through P38 MAPK pathway. Hepatogastroenterology. 2012;59:785–789. doi: 10.5754/hge11427. [DOI] [PubMed] [Google Scholar]
- 40.Hao F, Tan M, Xu X, Han J, Miller DD, Tigyi G, et al. Lysophosphatidic acid induces prostate cancer PC3 cell migration via activation of LPA(1), p42 and p38alpha. Biochim Biophys Acta. 2007;1771:883–892. doi: 10.1016/j.bbalip.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stähle M, Veit C, Bachfischer U, Schierling K, Skripczynski B, Hall A, et al. Mechanisms in LPA-induced tumor cell migration: critical role of phosphorylated ERK. J Cell Sci. 2003;116:3835–3846. doi: 10.1242/jcs.00679. [DOI] [PubMed] [Google Scholar]
- 42.Smith AL, Dohn MR, Brown MV, Reynolds AB. Association of Rho-associated protein kinase 1 with E-cadherin complexes is mediated by p120-catenin. Mol Biol Cell. 2012;23:99–110. doi: 10.1091/mbc.E11-06-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newell-Litwa KA, Badoual M, Asmussen H, Patel H, Whitmore L, Horwitz AR. ROCK1 and 2 differentially regulate actomyosin organization to drive cell and synaptic polarity. J Cell Biol. 2015;210:225–242. doi: 10.1083/jcb.201504046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ratheesh A, Yap AS. A bigger picture: classical cadherins and the dynamic actin cytoskeleton. Nat Rev Mol Cell Biol. 2012;13:673–679. doi: 10.1038/nrm3431. [DOI] [PubMed] [Google Scholar]
- 45.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010;67:545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruner HC, Derksen PWB. Loss of E-Cadherin-Dependent Cell-Cell Adhesion and the Development and Progression of Cancer. Cold Spring Harb Perspect Biol. 2017 doi: 10.1101/cshperspect.a029330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Birchmeier W. E-cadherin as a tumor (invasion) suppressor gene. Bioessays. 1995;17:97–99. doi: 10.1002/bies.950170203. [DOI] [PubMed] [Google Scholar]
- 48.Kadota M, Yang HH, Gomez B, Sato M, Clifford RJ, Meerzaman D, et al. Delineating genetic alterations for tumor progression in the MCF10A series of breast cancer cell lines. PLoS ONE. 2010;5:e9201. doi: 10.1371/journal.pone.0009201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lim S-J, Choi HG, Jeon CK, Kim SH. Increased chemoresistance to paclitaxel in the MCF10AT series of human breast epithelial cancer cells. Oncol Rep. 2015;33:2023–2030. doi: 10.3892/or.2015.3775. [DOI] [PubMed] [Google Scholar]
- 50.Antebi YE, Linton JM, Klumpe H, Bintu B, Gong M, Su C, et al. Combinatorial signal perception in the BMP pathway. Cell. 2017;170:1184–1196. e24. doi: 10.1016/j.cell.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jolly MK, Tripathi SC, Jia D, Mooney SM, Celiktas M, Hanash SM, et al. Stability of the hybrid epithelial/mesenchymal phenotype. Oncotarget. 2016;7:27067–27084. doi: 10.18632/oncotarget.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu M, Jolly MK, Levine H, Onuchic JN, Ben-Jacob E. MicroRNA-based regulation of epithelial-hybrid-mesenchymal fate determination. Proc Natl Acad Sci U S A. 2013;110:18144–18149. doi: 10.1073/pnas.1318192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Antebi YE, Nandagopal N, Elowitz MB. An operational view of intercellular signaling pathways. Current Opinion in Systems Biology. 2017;1:16–24. doi: 10.1016/j.coisb.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao Y, He D, Saatian B, Watkins T, Spannhake EW, Pyne NJ, et al. Regulation of lysophosphatidic acid-induced epidermal growth factor receptor transactivation and interleukin-8 secretion in human bronchial epithelial cells by protein kinase Cdelta, Lyn kinase, and matrix metalloproteinases. J Biol Chem. 2006;281:19501–19511. doi: 10.1074/jbc.M511224200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liebmann C. EGF receptor activation by GPCRs: an universal pathway reveals different versions. Mol Cell Endocrinol. 2011;331:222–231. doi: 10.1016/j.mce.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 56.Marshall J-CA, Collins JW, Nakayama J, Horak CE, Liewehr DJ, Steinberg SM, et al. Effect of inhibition of the lysophosphatidic acid receptor 1 on metastasis and metastatic dormancy in breast cancer. J Natl Cancer Inst. 2012;104:1306–1319. doi: 10.1093/jnci/djs319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shida D, Fang X, Kordula T, Takabe K, Lépine S, Alvarez SE, et al. Cross-talk between LPA1 and epidermal growth factor receptors mediates up-regulation of sphingosine kinase 1 to promote gastric cancer cell motility and invasion. Cancer Res. 2008;68:6569–6577. doi: 10.1158/0008-5472.CAN-08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu MY, Porte J, Knox AJ, Weinreb PH, Maher TM, Violette SM, et al. Lysophosphatidic acid induces alphavbeta6 integrin-mediated TGF-beta activation via the LPA2 receptor and the small G protein G alpha(q) Am J Pathol. 2009;174:1264–1279. doi: 10.2353/ajpath.2009.080160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sauer B, Vogler R, Zimmermann K, Fujii M, Anzano MB, Schäfer-Korting M, et al. Lysophosphatidic acid interacts with transforming growth factor-beta signaling to mediate keratinocyte growth arrest and chemotaxis. J Invest Dermatol. 2004;123:840–849. doi: 10.1111/j.0022-202X.2004.23458.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.