The 2013 American College of Cardiology/American Heart Association (ACC/AHA) guideline on cholesterol treatment to prevent atherosclerotic cardiovascular disease (ASCVD)1 has been criticized for lowering the risk thresholds for primary prevention with statin therapy. Although the ≥7.5% (class I) and ≥5% (class IIa) 10-year ASCVD risk thresholds were identified as new indications for treatment by extrapolating results obtained in randomized controlled trials (RCT) of statin therapy, results from population-based European cohorts indicate that >30% of individuals eligible for statin therapy by class I recommendations do not have RCT data supporting statin efficacy2,3. Further, when the US Preventive Services Task Force published their recommendations (based on review of trial evidence), they restricted the indication for statin therapy compared with the ACC/AHA guideline by recommending a higher treatment threshold (10%) combined with ≥1 ASCVD risk factor. However, the evidence base for statins in primary prevention grew substantially in 2016 with publication of the Heart Outcomes Prevention Evaluation-3 (HOPE-3) trial that enrolled intermediate-risk persons for whom a clear indication for statin therapy was still lacking4. The implications of HOPE-3 for the evidence base supporting ACC/AHA risk-based statin allocation remain unknown. Therefore, using MESA (Multiethnic Study of Atherosclerosis, www.mesa-nhlbi.org) - a population-based US cohort - we assessed the extent to which the ACC/AHA recommendations for statin therapy are supported by currently available high-quality RCT evidence: WOSCOPS, AFCAPS/TexCAPS, ASCOT-LLA, CARDS, MEGA, JUPITER and HOPE-3 (ref. 2 and 4 for details). Given the controversy that exists in whom to treat with statins in primary prevention, such information may provide important insights for clinical practice and future updates of guidelines and recommendations.
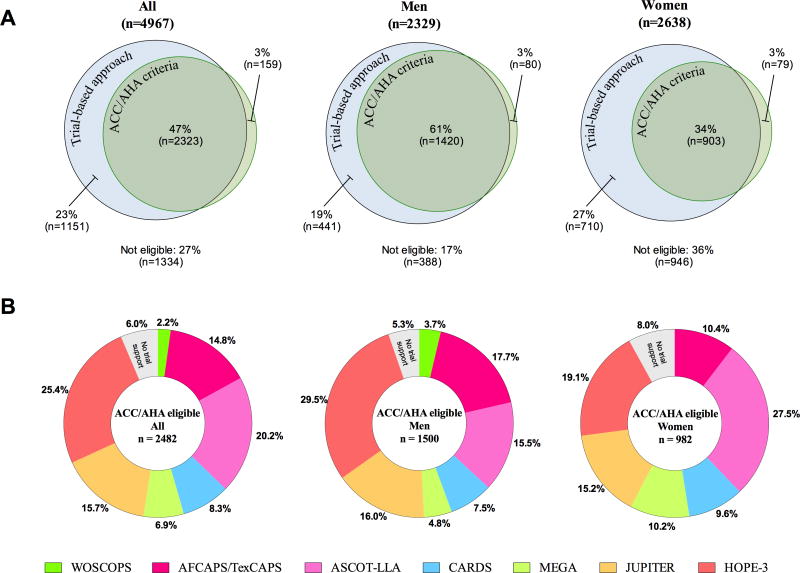
MESA participants (n=4967,age 45-75) free of known ASCVD or lipid-lowering medication at baseline were studied. All provided written informed consent, and the study protocol was approved by the institutional review board of each study site. Overall, 70% were statin eligible based on evidence from the 7 high-quality RCTs of statin therapy, and 63% based upon ACC/AHA guidelines (50% class I and 13% class IIa recommendation). The overlap in statin eligibility based on ACC/AHA class I statin recommendations and RCT evidence documenting efficacy of statin therapy is visualized in Figure 1A. Among individuals with an ACC/AHA class I recommendation (n=2482), 94% had trial evidence supporting statin therapy. The RCTs providing this evidence are shown in Figure 1B. The HOPE-3 trial provided much of the missing trial-based evidence (73% had RCT evidence before HOPE-3). In individuals with a predicted 10-year ASCVD risk from 5% to <7.5% (class IIa), 78% had trial data supporting statin therapy falling to 36% in those with a 10-year risk <5%.
Figure 1. Overlap in eligibility for primary prevention with statin therapy based on trial evidence and ACC/AHA class I recommendations.
A: Area-proportional Venn diagrams demonstrating overlap in statin eligibility based on ACC/AHA class I recommendations (green) and evidence from randomized statin trials (blue). The percentages indicate the fraction of MESA participants aged 45 to 75 years who were eligible for statin therapy, or not eligible (below diagrams). B: Diagrams illustrating trials providing evidence for statin efficacy among ACC/AHA eligible individuals. Trial criteria were applied consecutively in chronological order clockwise starting 12 o'clock, that is, first we selected individuals according to WOSCOPS criteria (1995), then we selected additional individuals according to AFCAPS/TexCAPS criteria (1998), and so on.
The ASCVD (myocardial infarction, resuscitated cardiac arrest, coronary heart disease death plus stroke) event rate varied greatly according to statin eligibility by ACC/AHA class I recommendations and/or trial-based evidence (median follow-up time: 10.0 years). The event rate was highest in the 2482 individuals with an ACC/AHA class I indication (9.2 (95% CI 8.0-10.5) per 1000 person-years), lower in those (n=3474) with a trial-based indication (7.4 (95% CI 6.6-8.5)), and lowest in those (n=1151) with a trial-based indication alone (3.1 (95% CI 2.2-4.4)). Statin uptake during follow-up may have contributed to the generally low event rates but is unlikely to have exaggerated the real differences in event rates.
Like most other guidelines, the ACC/AHA guideline recommends that statin allocation should be based on absolute ASCVD risk assessment. In such a risk-based approach, treatment is targeted to individuals with a 10-year ASCVD risk above a certain guideline-defined threshold to optimize the tradeoff between efficacy and safety of treatment. However, the justification and evidence base for this traditional approach for statin allocation have been questioned as no RCTs of statin therapy have ever enrolled participants based on 10-year ASCVD risk assessment. Indeed, reports from population-based European cohorts suggested that a substantial proportion of individuals eligible for statin therapy by ACC/AHA class I recommendations do not have RCT evidence to support statin efficacy2,3. Thus, as an alternative to the traditional risk-based approach, it has been suggested to allocate statin therapy strictly on the basis of trial-evidence (that is what works and in whom)5. However, with the publication of the HOPE-3 trial4, the evidence base for statin efficacy in primary prevention was expanded substantially as illustrated by our analyses. Among MESA individuals eligible for ACC/AHA class I statin therapy, evidence for statin efficacy increased from 73% to 94% (≈19 out of 20 individuals) with the HOPE-3 trial.
Our results provide important updated insights into the evidence base behind the ACC/AHA recommendations for primary prevention with statin therapy. Although no single RCT of statin therapy has ever enrolled individuals based on 10-year risk assessment, there is now RCT evidence of statin efficacy for nearly all individuals qualifying for statin therapy based on ACC/AHA class I recommendations by combining all currently available RCT evidence. Principally, these results provide strong trial-based evidence in support of the ACC/AHA recommendations as a starting point for a patient-physician discussion on initiation of statin therapy.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding: This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the NIH (National Heart, Lung, and Blood Institute) and by grants UL1-TR-000040 and UL1-TR-001079 from The National Center for Research Resources (NCRR).
Footnotes
Conflict of interest: None.
References
- 1.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Watson K, Wilson PWF, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen W-K, Smith SC, Tomaselli GF. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 2.Mortensen MB, Afzal S, Nordestgaard BG, Falk E. Primary Prevention With Statins: ACC/AHA Risk-Based Approach Versus Trial-Based Approaches to Guide Statin Therapy. Journal of the American College of Cardiology. 2015;66:2699–2709. doi: 10.1016/j.jacc.2015.09.089. [DOI] [PubMed] [Google Scholar]
- 3.Pavlovic J, Greenland P, Deckers JW, Brugts JJ, Kavousi M, Dhana K, Ikram MA, Hofman A, Stricker BH, Franco OH, Leening MJG. Comparison of ACC/AHA and ESC Guideline Recommendations Following Trial Evidence for Statin Use in Primary Prevention of Cardiovascular Disease: Results From the Population-Based Rotterdam Study. JAMA Cardiol. 2016;1:708–713. doi: 10.1001/jamacardio.2016.1577. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L, Pais P, López-Jaramillo P, Leiter LA, Dans A, Avezum A, Piegas LS, Parkhomenko A, Keltai K, Keltai M, Sliwa K, Peters RJG, Held C, Chazova I, Yusoff K, Lewis BS, Jansky P, Khunti K, Toff WD, Reid CM, Varigos J, Sanchez-Vallejo G, McKelvie R, Pogue J, Jung H, Gao P, Diaz R, Lonn E. HOPE-3 Investigators. Cholesterol Lowering in Intermediate-Risk Persons without Cardiovascular Disease. N Engl J Med. 2016;374:2021–2031. doi: 10.1056/NEJMoa1600176. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Wilson PWF. A trial-based approach to statin guidelines. JAMA. 2013;310:1123–1124. doi: 10.1001/jama.2013.276529. [DOI] [PubMed] [Google Scholar]