Abstract
Introduction
This study investigated whether early life trauma mediates genetic effects on the age at onset (AAO) of bipolar disorder.
Method
Data from the BiGS Consortium case samples (N = 1119) were used. Childhood traumatic events were documented using the Childhood Life Events Scale (CLES). Interaction between occurrence of childhood trauma and common genetic variants throughout the genome was tested to identify single nucleotide polymorphic gene variants (SNPs) whose effects on bipolar AAO differ between individuals clearly exposed (CLES ≥ 2) and not exposed (CLES = 0) to childhood trauma.
Results
The modal response to the CLES was 0 (N = 480), but an additional 276 subjects had CLES = 1, and 363 subjects reported 2 or more traumatic lifetime events. The distribution of age at onset showed a broad peak between ages 12 and 18, with the majority of subjects having onset during that period, and a significant decrease in age of onset with the number of traumatic events. No single SNP showed a statistically significant interaction with the presence of traumatic events to impact bipolar age at onset. However, SNPs in or near genes coding for calcium channel activity-related proteins (Gene Ontology: 0005262) were found to be more likely than other SNPs to show evidence of interaction using the INRICH method (p < 0.001).
Limitations
Retrospective ascertainment of trauma and AAO.
Conclusion
Interaction effects of early life trauma with genotype may have a significant effect on the development and manifestation of bipolar disorder. These effects may be mediated in part by genes involved in calcium signaling.
Keywords: Bipolar disorder, Childhood trauma, Age of onset, Genetic, GWAS, Calcium
1. Introduction
The interaction between genetic and environmental factors is recognized to be important in the development and manifestation of complex illnesses such as major psychiatric disorders. Bipolar Disorder (BPD) is a major mental illness, characterized by periods of depression, mania and normal mood, and is known to be strongly heritable. It typically first manifests itself in late adolescent and early adulthood.
Early age of onset (AAO) of BPD has been used to identify a subgroup of patients suffering from the disorder who may have a more severe form of illness, and possibly a stronger genetic liability (Belmonte Mahon et al., 2011; Lin et al., 2006a). There is some evidence for specific genetic variants related to early-onset BPD(Priebe et al., 2012). We hypothesized that further understanding of AAO variability in BPD would be gained by taking specific environmental effects into account along with genetic factors.
While a number of environmental factors could conceivably be important, early life experiences and trauma in particular are known to affect vulnerability to psychiatric illness; this has been noted in particular for mood disorders (Caspi et al., 2003; Gillespie et al., 2005). Though most investigation and reports regarding early-life trauma have involved development of depression, early trauma and stress have also been shown to affect the age of onset, prognosis and course of bipolar disorder (Post and Leverich, 2006). Twin studies have shown a heritability of 0.8–0.9 for BPD, implying that the presence or absence of the disorder is substantially determined by genetic factors (Kieseppä et al., 2004; Nurnberger, 2012). Age of onset appears to be less clearly heritable, though some evidence for familiality has emerged (Lin et al., 2006b; Schulze et al., 2006). A number of studies have shown that early-life trauma, such as sexual or physical abuse, is associated with an earlier age of onset of bipolar illness as well as a more complicated and treatment-resistant course (Brown et al., 2005; Garno et al., 2005; Gilman et al., 2015; Leverich et al., 2002; Leverich and Post, 2006; Romero et al., 2009). Importantly, presence or absence of childhood trauma and family history of BPD have been reported to demonstrate interaction in their influence on the age of onset and course of bipolar disorder; the effect of trauma upon age of onset was observed to be substantially larger when family history is present (Post and Leverich, 2006).
Genome wide association studies (GWAS) investigating the association between common single nucleotide polymorphisms (SNPs) and BPD have currently identified 14 genomewide significant susceptibility loci (Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011). Common SNPs in aggregate appear to explain 25–40% of liability to BP (Lee et al., 2011), and current studies suggest that rare variants and gene-gene interactions explain substantial fractions of the remaining genetic contribution to BPD risk (Belmonte Mahon et al., 2011; Gershon et al., 2010; Judy et al., 2013). We hypothesized that the inclusion of environmental factors, such as early life trauma, in GWAS analyses would allow us to account for some additional unexplained variability in age of onset not modeled in other study designs.
2. Materials and methods
The GWAS SNPs and phenotypic data analyzed (N = 1119 subjects; Table 1) were available from the NIMH Bipolar Disorder Genetic Association Information Network (GAIN-BP) and the Translational Genomics Institute (TGEN) bipolar case samples. The ascertainment and assessment procedures of the NIMH-BP sample are described elsewhere (Dick et al., 2003; Kassem et al., 2006). All subjects were assessed with the Diagnostic Interview for Genetic Studies (DIGS) and this was combined with family informant data and medical records to assign diagnoses based on DSM-III-R or DSM-IV criteria. Unrelated cases were genotyped in two separate efforts described in detail elsewhere, the Genetic Association Information Network Bipolar Sample (GAIN-BP, Smith et al., 2009) and the Bipolar Genome Study (Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011). Genotyping in both efforts was performed using the Affymetrix 6.0 array, providing genotype data on approximately 700,000 SNPs.
Table 1.
Subjects demographics and trauma history.
| CLES = 0 | CLES =1 | CLES ≥ 2 | Total | |
|---|---|---|---|---|
| Males | ||||
| AAO ≤ 18 | 98 | 38 | 61 | 197 |
| AAO > 18 | 109 | 46 | 55 | 210 |
| Total | 207 | 84 | 116 | 407 |
| Females | ||||
| AAO ≤ 18 | 147 | 99 | 161 | 407 |
| AAO > 18 | 126 | 93 | 86 | 305 |
| Total | 273 | 192 | 247 | 712 |
| Combined | ||||
| AAO ≤ 18 | 245 | 137 | 222 | 604 |
| AAO > 18 | 235 | 139 | 141 | 515 |
| Total | 480 | 276 | 363 | 1119 |
Note: GWAS SNP genotypes in all subjects with AAO ≥ 12 were tested for interaction effect upon AAO with CLES as a continuous measure (n = 1119) and classified as CLES = 0 versus CLES ≥ 2 (combined n= 843). GWAS: Genome-wide association study; AAO: Age at onset; CLES: Childhood Life Events Scale.
2.1. Age of onset
Detailed information regarding age of onset of bipolar disorder (AAO) was available for 1119 of the genotyped cases, and AAO was determined best estimate using DIGS interview and medical records. The criterion was the age of occurrence of the first mood episode that met diagnostic criteria for either depression or mania.
2.2. Early-life trauma
Early-life trauma was documented using the Childhood Life Events Scale (CLES) (Lawson and Gershon, unpublished, Supplementary Table 3) an 11-point scale that asks the subject about various traumatic events that may have happened between ages 3 and 12. Scoring is the number of accumulated traumas during childhood (range 0–8 events, Table 1). The distributions of AAO of BPD and the number of traumatic events per individual in the GAIN and TGEN samples are shown in Fig. 1. As we were investigating the effects of trauma on age of onset, only subjects with bipolar onset at age 12 or older were included.
Fig. 1.
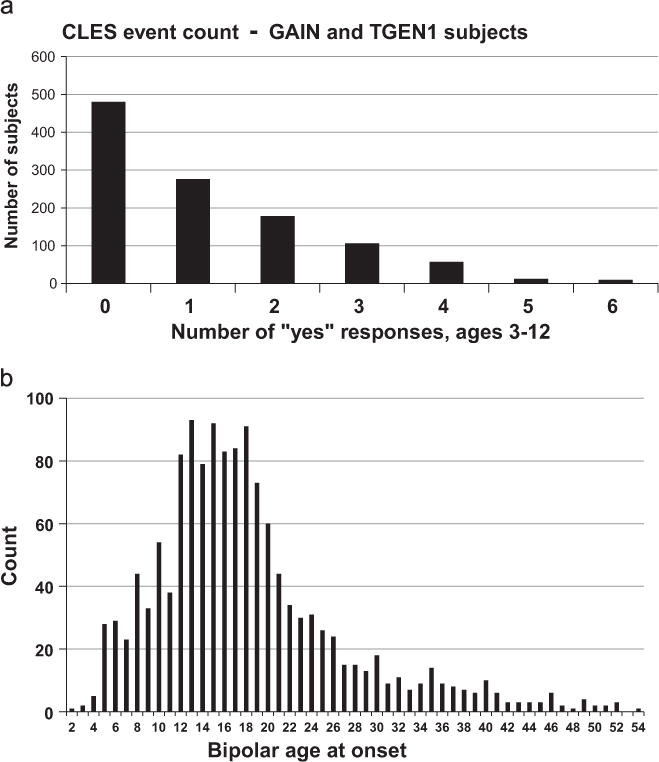
Distributions of (a) childhood traumatic event count, 0 to 8 events per subject as measured by Childhood Life Events Scale (CLES); and (b) bipolar disorder age of onset, in the combined GAIN (Genetic Association Information Network for Bipolar Disorder) and TGEN (Translational Genomics Institute) case cohorts; total N = 1119.
2.3. Statistical analysis
Early life trauma was considered as a binary measure-no trauma (CLES score = 0) vs. two or more reported traumatic events (CLES score ≥ 2), omitting subjects with a single traumatic event to more clearly separate the two subgroups differing in degree of trauma exposure. A linear model was fitted to test the interaction of SNP genotype and early-life trauma upon age of onset, with main effects for the SNP and trauma variables included simultaneously. This corresponds to a comparison of the strength and direction of the SNP-AAO relationship between the trauma subgroups. To aid in interpretation of the genotype by trauma interaction results for a particular SNP, the main effect of SNP genotype on age of onset, covarying for trauma but omitting the interaction term, was also tested. The most significant SNP-trauma interactions in the binary analysis were also tested with the CLES score modeled as a continuous variable. This analysis was best powered to detect SNPs with an additive genetic effect upon bipolar AAO in subjects in the trauma group, but no effect (or an opposite effect) in the other group.
2.4. Pathway analysis
Enrichment of our top interaction GWAS findings within particular molecular pathways, as defined by Gene Ontology (GO) terms, was examined using the INRICH software (http://atgu.mgh.harvard.edu/inrich). This was performed for GO terms identified previously for other GWAS studies of psychiatric disorders (Cross-Disorder Group of the Psychiatric Genomics, 2013). Linkage disequilibrium (LD)-based clumping in PLINK was conducted to identify independent or nearly independent association regions (LD r2 < 0.25) containing the top GWAS findings (p < 10−4). A permutation approach implemented in INRICH was then used to approximate the nominal probability of the gene(s) in these regions intersecting with the gene set associated with each GO term by chance.
3. Results
Onset of bipolar disorder for the majority of the 1119 genotyped subjects with AAO and CLES data available occurred between ages 12 and 18 (Fig. 1A). All subjects were from the United States. The distribution of the CLES score in this sample is shown in Fig. 1B. The percentages for AAO less than 12 yrs, 12–18 yrs and greater than 18 yrs were 19%, 43% and 38% respectively. A significant decrease in age of onset with an increasing number of traumatic events ascertained via the CLES was observed (p = 0.01, R2 = 1.4%; Fig. 2). A total of 843 subjects were included in the binary SNP-trauma genetic interaction analysis. This represented the 1119 subjects detailed above, but omitting those with intermediate trauma history (CLES = 1, N = 276) (Fig. 1). Demographic and subject characteristics in regard to AAO and CLES scores for the included sample are depicted in Table 1.
Fig. 2.
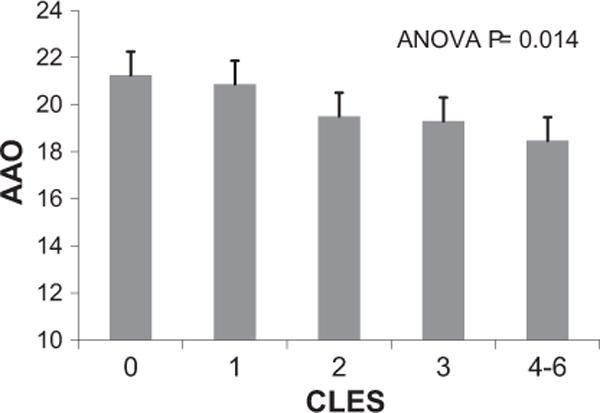
Mean values of age at onset (AAO, years) by number of childhood traumatic events from the Childhood Life Events Scale (CLES). N = 1119 subjects in the study, all AAO ≥ 12. Error bars indicate standard error of the mean for each CLES level. Results of one-way ANOVA analysis are shown.
3.1. GWAS analysis for genotype and trauma interaction for age of onset
The genes nearest the top 50 SNPs in the interaction analysis (p < 10−4 or below for the interaction test) were examined. Of the SNP-trauma interactions identified, the top SNP was that near to the TRPC5 gene (p = 10−6) on the X chromosome. Among these top 50 SNP-trauma interactions, we observed several other genes known to be related to calcium ion channels and signal transduction mechanisms. The data for the top 100 SNPs are shown in Supplementary Table 1, including p-values for interaction and for main effect. None of the individual p-values was significant at a genome-wide level. SNP-trauma interaction p-values for this group of SNPs with CLES modeled as a continuous measure were similar to those observed using the binary classification, also shown in Supplementary Table 1.
3.2. Pathway analysis results
The SNPs with p < 10−4 in the GWAS for SNP* trauma interaction effects upon bipolar AAO were used in a comparison with the Gene Ontology category (GO:0005262; calcium channel activity) identified by similar methods in other psychiatric GWAS studies as described above. Statistical support for calcium pathway enrichment among these interaction findings was observed with the same GO term (GO:0005262; calcium channel activity) reported as enriched in the results of these other studies (Lancet 2013; 381: 1371–1379; Nature Genetics 2011; 43:977–983). This calcium channel activity gene group had the smallest nominal significance level in our study (0.0016) among the 6000 GO terms forming the null hypothesis to which it was compared. In addition, the calcium ion transport term (GO:0006816; p = 0.029) was one of only 3 other terms with nominal p-values below 0.05 in our results. Genes annotated with molecular function “calcium channel activity” (GO:0005262) and those genes for which the AAO/life events interaction results were suggestive are depicted in Supplementary Table 2.
4. Discussion
This study investigated the interaction of early childhood trauma and specific genetic variants in influencing age of onset in bipolar disorder. We detected a significant relationship between age of onset and childhood trauma, with greater numbers of traumatic events being associated with bipolar onset at earlier ages. Our genetic screen thus sought to identify particular variants, which magnify or reduce this effect of trauma upon age of bipolar onset for individuals who carry them. Even in this relatively modest group of bipolar cases, several suggestive interactions between genotype and childhood trauma on age of onset were found. Several of the genes near the genetic variants identified here are known to be involved in calcium and other signal transduction pathways. It is notable that a recent meta-analysis of GWAS from 5 psychiatric disorders (Cross-Disorder Group of the Psychiatric Genomics, 2013)(BPD, Schizophrenia, Major Depression, Autism, and Attention Deficit Hyperactivity Disorder-33,332 cases and 27,888 controls) showed that a particular pathway composed of calcium channel genes was enriched for significant SNP associations in a meta-analysis of all five disorders (CrossDisorder Group of the Psychiatric Genomics, 2013). The top SNPs from our GWAS, with greatest evidence for interaction with trauma affecting BP age of onset for bipolar disorder, were tested in the same manner with this group of genes (GO:0005262; calcium channel activity) and found to have significant enrichment with this pathway as well.
The most highly replicated association in samples of cases with bipolar disorder is that with CACNA1C (Ferreira et al., 2008; Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011). Variants in the calcium channel protein TRPM2 have been associated with BP (McQuillin et al., 2005) as well. Variants in P2RX7, which codes for a calcium-stimulated ATPase, are also associated with BP (Lucae et al., 2006). Bigos et al. (2010) and Perrier et al. (2011) reported differences in subcortical morphology in BP cases carrying the CACNA1C risk variant compared to controls, and there is also evidence for reduced corticolimbic connectivity in carriers (Wang et al., 2011). Finally, L-type calcium channels have been clearly implicated in meta-analysis of the BP GWAS data (Cross-Disorder Group of the Psychiatric Genomics, 2013) and their importance in the present analysis suggests that they affect the age of onset of bipolar disorder as well as the presence or absence of that disorder in a given individual. The genes implicated in our study, and in particular their potential interaction with childhood trauma and other potential environmental exposures, may be involved in vulnerability to BPD in the presence of significant trauma and stress during development. Early-life trauma is very likely to affect the age of onset of bipolar illness—either as a pure environmental factor or, more likely, as a factor which interacts with genetic factors to either advance or delay the age of onset of the illness. Recent studies have raised the awareness of epigenetic factors in the development of psychiatric illnesses (Robison and Nestler, 2011). Environmental factors such as early-life trauma may influence gene expression and thereby change the trajectory of the illness.
5. Limitations
The behavioral data collected for this particular analysis was retrospective in nature, in regard to recall of childhood traumatic events or the age of onset of the disorder. Prospective studies will be illuminating in offspring at high risk for bipolar disorder because of family history. Such studies are in progress (Nurnberger et al., 2011).
In conclusion, the results of this study provide preliminary data identifying environmental factors that may interact with genetic vulnerability to bipolar disorder, thereby influencing the course and prognosis of the illness. Calcium channel gene variants appear to affect onset age as well as presence of bipolar illness per se; these variants interact with traumatic events to lower age of onset.
Supplementary Material
Acknowledgments
Bipolar Genome Study (BiGS) Co-authors: John R. Kelsoe, Tiffany A. Greenwood, Caroline M. Nievergelt, Rebecca McKinney, Paul D. Shilling - University of California, San Diego, CA, USA; Nicholas J. Schork, Erin N. Smith, Cinnamon S. Bloss - Scripps Translational Science Institute, La Jolla, CA, USA; John I. Nurnberger, Jr., Howard J. Edenberg, Tatiana Foroud, Daniel L. Koller - Indiana University, Indianapolis, IN, USA; Elliot S. Gershon, Chunyu Liu, Judith A. Badner - University of Chicago, Chicago, IL, USA; William A. Scheftner - Rush University Medical Center, Chicago, IL, USA; William B. Lawson, Evaristus A. Nwulia, Maria Hipolito - Howard University, Washington, D.C., USA; William Coryell - University of Iowa, Iowa City, IA, USA; John Rice - Washington University, St. Louis, MO, USA; William Byerley - University of California, San Francisco, CA, USA; Francis J. McMahon, Thomas G. Schulze - National Institute of Mental Health Intramural Research Program, Bethesda, MD, USA; Wade H. Berrettini - University of Pennsylvania, Philadelphia, PA, USA; James B. Potash, Peter P. Zandi, Pamela B. Mahon - Johns Hopkins School of Medicine, Baltimore, MD, USA; Melvin G. McInnis, Sebastian Zöllner, Peng Zhang - University of Michigan, Ann Arbor, MI, USA; David W. Craig, Szabolcs Szelinger - The Translational Genomics Research Institute, Phoenix, AZ, USA; Thomas B. Barrett - Portland Veterans Affairs Medical Center, Portland, OR, USA; Thomas G. Schulze - Georg-August-University Göttingen, Göttingen, Germany.
Role of funding source
There is no role of a funding source.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/jjad.2015.02.029.
Footnotes
Conflict of interest
The authors report no conflict of interest.
References
- Belmonte Mahon P, Pirooznia M, Goes FS, Seifuddin F, Steele J, Lee PH, Huang J, Hamshere ML, The Bipolar Genome Study Consortium, The Wellcome Trust Case Control Consortium Bipolar Disorder Group. DePaulo ML, JR, Kelsoe JR, Rietschel M, Nöthen M, CiChon S, Gurling H, Purcell S, Smoller JW, Craddock N, Schulze TG, McMahon FJ, Potash JB, Zandi PP. Genome-wide association analysis of age at onset and psychotic symptoms in bipolar disorder. Am J Med Genet Part B: Neuropsychiatr Genet. 2011;156:370–378. doi: 10.1002/ajmg.b.31172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigos KL, Mattay VS, Callicott JH, et al. Genetic variation in cacnalc affects brain circuitries related to mental illness. Arch Gen Psychiatry. 2010;67:939–945. doi: 10.1001/archgenpsychiatry.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GR, McBride L, Bauer MS, Williford WO. Impact of childhood abuse on the course of bipolar disorder: a replication study in US veterans. J Affect Disord. 2005;89:57–67. doi: 10.1016/j.jad.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-htt gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics C. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Foroud T, Flury L, Bowman ES, Miller MJ, Rau NL, Moe PR, Samavedy N, El-Mallakh R, Manji H, Glitz DA, Meyer ET, Smiley C, Hahn R, Widmark C, McKinney R, Sutton L, Ballas C, Grice D, Berrettini W, Byerley W, Coryell W, DePaulo R, MacKinnon DF, Gershon ES, Kelsoe JR, McMahon FJ, McInnis M, Murphy DL, Reich T, Scheftner W, Nurnberger JI., Jr Genomewide linkage analyses of bipolar disorder: a new sample of 250 pedigrees from the national institute of mental health genetics initiative. Am J Hum Genet. 2003;73:107–114. doi: 10.1086/376562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MA, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, Fan J, Kirov G, Perlis RH, Green EK, Smoller JW, Grozeva D, Stone J, Nikolov I, Chambert K, Hamshere ML, Nimgaonkar VL, Moskvina V, Thase ME, Caesar S, Sachs GS, Franklin J, Gordon-Smith K, Ardlie KG, Gabriel SB, Fraser C, Blumenstiel B, Defelice M, Breen G, Gill M, Morris DW, Elkin A, Muir WJ, McGhee KA, Williamson R, MacIntyre DJ, MacLean AW, StC D, Robinson M, Van Beck M, Pereira AC, Kandaswamy R, McQuillin A, Collier DA, Bass NJ, Young AH, Lawrence J, Ferrier IN, Anjorin A, Farmer A, Curtis D, Scolnick EM, McGuffin P, Daly MJ, Corvin AP, Holmans PA, Blackwood DH, Gurling HM, Owen MJ, Purcell SM, Sklar P, Craddock N. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garno JL, Goldberg JF, Ramirez PM, Ritzler BA. Impact of childhood abuse on the clinical course of bipolar disorder. Br J Psychiatry. 2005;186:121–125. doi: 10.1192/bjp.186.2.121. [DOI] [PubMed] [Google Scholar]
- Gershon ES, Alliey-Rodriguez N, Liu C. After GWAS: searching for genetic risk for schizophrenia and bipolar disorder. Am J Psychiatry. 2010;168:253–256. doi: 10.1176/appi.ajp.2010.10091340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie NA, Whitfield JB, Williams B, Heath AC, Martin NG. The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychol Med. 2005;35:101–111. doi: 10.1017/s0033291704002727. [DOI] [PubMed] [Google Scholar]
- Gilman SE, Ni MY, Dunn EC, Breslau J, McLaughlin KA, Smoller JW, Perlis RH. Contributions of the social environment to first-onset and recurrent mania. Mol Psychiatry. 2015;20(3):329–336. doi: 10.1038/mp.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judy JT, Seifuddin F, Pirooznia M, Mahon PB, Jancic D, Goes FS, Schulze T, Cichon S, Noethen M, Rietschel M, Depaulo JR, Jr, Potash JB, Zandi PP. Converging evidence for epistasis between ANK3 and potassium channel gene KCNQ2 in bipolar disorder. Front Genet. 2013;4(87) doi: 10.3389/fgene.2013.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem PDL, Lopez MDV, Hedeker PDD, Steele BEJ, Zandi PDP, Consortium N.G.I.B.D. McMahon MDF. Familiality of polarity at illness onset in bipolar affective disorder. Am J Psychiatry. 2006;163:1754–1759. doi: 10.1176/ajp.2006.163.10.1754. [DOI] [PubMed] [Google Scholar]
- Kieseppä T, Partonen T, Haukka J, Kaprio J, Lönnqvist J. High concordance of bipolar I disorder in a nationwide sample of twins. Am J Psychiatry. 2004;161:1814–1821. doi: 10.1176/ajp.161.10.1814. [DOI] [PubMed] [Google Scholar]
- Lee Sang H, Wray Naomi R, Goddard Michael E, Visscher Peter M. Estimating missing heritability for disease from genome-wide association studies. Am J Hum Genet. 2011;88:294–305. doi: 10.1016/j.ajhg.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverich GS, McElroy SL, Suppes T, Keck PE, Jr, Denicoff KD, Nolen WA, Altshuler LL, Rush AJ, Kupka R, Frye MA, Autio KA, Post RM. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51:288–297. doi: 10.1016/s0006-3223(01)01239-2. [DOI] [PubMed] [Google Scholar]
- Leverich GS, Post RM. Course of bipolar illness after history of childhood trauma. Lancet. 2006;367:1040–1042. doi: 10.1016/S0140-6736(06)68450-X. [DOI] [PubMed] [Google Scholar]
- Lin PI, McInnis MG, Potash JB, Willour V, MacKinnon DF, DePaulo JR, Zandi PP. Clinical correlates and familial aggregation of age at onset in bipolar disorder. Am J Psychiatry. 2006a;163:240–246. doi: 10.1176/appi.ajp.163.2.240. [DOI] [PubMed] [Google Scholar]
- Lin PI, McInnis MG, Potash JB, Willour V, MacKinnon DF, DePaulo JR, Zandi PP. Clinical correlates and familial aggregation of age at onset in bipolar disorder. Am J Psychiatry. 2006b;163:240–246. doi: 10.1176/appi.ajp.163.2.240. [DOI] [PubMed] [Google Scholar]
- Lucae S, Salyakina D, Barden N, Harvey M, Gagné B, Labbé M, Binder EB, Uhr M, Paez-Pereda M, Sillaber I, Ising M, Brückl T, Lieb R, Holsboer F, Müller-Myhsok B. P2RX7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Hum Mol Genet. 2006;15:2438–2445. doi: 10.1093/hmg/ddl166. [DOI] [PubMed] [Google Scholar]
- McQuillin A, Bass NJ, Kalsi G, Lawrence J, Puri V, Choudhury K, Detera-Wadleigh SD, Curtis D, Gurling HMD. Fine mapping of a susceptibility locus for bipolar and genetically related unipolar affective disorders, to a region containing the C21ORF29 and TRPM2 genes on chromosome 21q22.3. Mol Psychiatry. 2005;11:134–142. doi: 10.1038/sj.mp.4001759. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI., Jr . General genetics of bipolar disorder. In: Strakowski S, editor. The Bipolar Brain: Integrating Neuroimaging and Genetics. Oxford University Press; 2012. [Google Scholar]
- Perrier E, Pompei F, Ruberto G, Vassos E, Collier D, Frangou S. Initial evidence for the role of CACNA1C on subcortical brain morphology in patients with bipolar disorder. Eur Psychiatry. 2011;26:135–137. doi: 10.1016/j.eurpsy.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Post RM, Leverich GS. The role of psychosocial stress in the onset and progression of bipolar disorder and its comorbidities: the need for earlier and alternative modes of therapeutic intervention. Dev Psychopathol. 2006;18:1181–1211. doi: 10.1017/S0954579406060573. [DOI] [PubMed] [Google Scholar]
- Priebe L, Degenhardt FA, Herms S, Haenisch B, Mattheisen M, Nieratschker V, Weingarten M, Witt S, Breuer R, Paul T, Alblas M, Moebus S, Lathrop M, Leboyer M, Schreiber S, Grigoroiu-Serbanescu M, Maier W, Propping P, Rietschel M, Nothen MM, Cichon S, Muhleisen TW. Genome-wide survey implicates the influence of copy number variants (CNVs) in the development of early-onset bipolar disorder. Mol Psychiatry. 2012;17:421–432. doi: 10.1038/mp.2011.8. [DOI] [PubMed] [Google Scholar]
- Psychiatric GWAS Consortium Bipolar Disorder Working Group. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011;43:977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero S, Birmaher B, Axelson D, Goldstein T, Goldstein BI, Gill MK, Iosif AM, Strober MA, Hunt J, Esposito-Smythers C, Ryan ND, Leonard H, Keller M. Prevalence and correlates of physical and sexual abuse in children and adolescents with bipolar disorder. J Affect Disord. 2009;112:144–150. doi: 10.1016/j.jad.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze TG, Hedeker D, Zandi P, Rietschel M, McMahon FJ. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry. 2006;63:1368–1376. doi: 10.1001/archpsyc.63.12.1368. [DOI] [PubMed] [Google Scholar]
- Wang F, McIntosh AM, He Y, Gelernter J, Blumberg HP. The association of genetic variation in CACNA1C with structure and function of a frontotemporal system. Bipolar Disord. 2011;13:696–700. doi: 10.1111/j.1399-5618.2011.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


