Abstract
Bisphenol A (BPA) is commonly used in the manufacturing of a wide range of consumer products, including polycarbonate plastics, epoxy resin that lines beverage and food cans, and some dental sealants. Consumption of food and beverages containing BPA represents the primary route of human BPA exposure, which is virtually ubiquitous. An increasing number of studies have evaluated the effects of BPA on immune responses in laboratory animals that have reported a variety of effects some of which have been contradictory. To address the divergent findings surrounding BPA exposure, a comprehensive chronic treatment study of BPA was conducted in Sprague-Dawley rats, termed the Consortium Linking Academic and Regulatory Insights on Toxicity of BPA (CLARITY-BPA). As a participant in the CLARITY-BPA project, our studies evaluated the effects of BPA on a broad range of immune function endpoints using spleen cells isolated from BPA or vehicle treated rats. This comprehensive assessment included measurements of lymphoproliferation in response to mitogenic stimuli, immunoglobulin production by B cells, and cellular activation of T cells, NK cells, monocytes, granulocytes, macrophages and dendritic cells. In total, 630 different measurements in BPA treated rats were performed of which 35 measurements were statistically different from vehicle controls. The most substantive alteration associated with BPA treatment was the augmentation of lymphoproliferation in response to pokeweed mitogen stimulations in 1 year old male rats, which was also observed in the reference estrogen ethinyl estradiol treated groups. With the exception of the aforementioned, the statistically significant changes associated with BPA treatment were mostly sporadic and not dose-dependent with only one out of five BPA dose groups showing a statistical difference. In addition, the observed BPA-associated alterations were mostly moderate in magnitude and showed no persistent trend over the one-year time period. Based on these findings, we conclude that the observed BPA-mediated changes observed in this study are unlikely to alter immune competence in adults.
Keywords: Bisphenol A, BPA, CLARITY-BPA, immune system, immunotoxicity
1. Introduction
Immune responses to pathogens are well-orchestrated processes that involve coordination between the innate and adaptive arms of the immune system. Innate immune cells serve as the first line of defense and identify pathogens by recognizing common repetitive molecular structures associated with microorganisms known as pathogen-associated molecular patterns (PAMP), such as lipopolysaccharide, lipoteichoic acid and flagellin. The binding of PAMPs to toll-like receptors or other pattern recognition receptors activates innate immune cells to initiate immune responses (Kumar et al. 2011). Activated macrophages and DCs upregulate MHCII and co-stimulatory molecules (CD80, CD86) to enhance antigen presenting and hence lead to the activation of adaptive immune system (Hume 2008; Leon and Ardavin 2008).
The major cell-types involved in adaptive immune response are B and T lymphocytes, which undergo proliferation and differentiation to exert effector cell functions in response to activation. Activated T cells express elevated levels of cell surface CD25, which is also known as interleukin 2 receptor (IL2R) alpha chain, an essential component of high affinity IL2R (Boyman and Sprent 2012). Signaling through IL2R is critical for T cell proliferation and differentiation into effector cells that participate in direct killing of altered (i.e., infected or neoplastic) target cells and regulation of immune responses (Bachmann and Oxenius 2007). By contrast, effector B cells produce copious amounts of immunoglobulin assist in the elimination of pathogens (Boes 2000). All the aforementioned are critical events in maintaining immune competence and providing host defense against infectious pathogens.
The immune system is a sensitive target for modulation by a variety of drugs, chemicals, and environmental contaminants. Recent studies reported alterations in immune responses in laboratory animals treated with bisphenol A (BPA). BPA is an extensively characterized compound that binds the estrogen receptor. BPA is produced in a massive volume worldwide and is commonly used in the manufacturing of a wide range of consumer products, including polycarbonate plastics, epoxy resin that lines beverage and food cans, and some dental sealants. Consuming foods and beverages containing BPA that leaches from plastic containers or epoxy resin represents a major route of human exposure, which is virtually ubiquitous (Calafat et al. 2008; Sun et al. 2004; Yamada et al. 2002).
There is a growing number of studies that have evaluated the effects of BPA on immune responses with contradictory findings and little consensus (Rogers et al. 2013). For instance, lymphocyte proliferative responses have been reported to be either enhanced (Goto et al. 2004; Yoshino et al. 2003; Youn et al. 2002) or suppressed (Jontell et al. 1995; Sakazaki et al. 2002; Yamazaki et al. 2000) by BPA treatment. Additionally, the reported effects of BPA on CD4+ T cell differentiation are inconsistent with some studies reporting BPA treatment led to Th1 biased responses (Yoshino et al. 2004; Youn et al. 2002) while others reported Th2 polarized responses (Guo et al. 2010; Lee et al. 2003). Moreover, some studies demonstrated that BPA exerted inhibitory effects on macrophage function by suppressing the production of nitric oxide and tumor necrosis factor alpha (Byun et al. 2005; Kim and Jeong 2003). In contract, stimulatory effects of BPA on macrophage function were also reported as evidenced by increased production of nitric oxide and elevated expression of costimulatory molecules and MHCII (Hong et al. 2004; Kuan et al. 2012). In addition, the reported effects of BPA on humoral immune responses have been inconsistent, as some studies found enhancement of antibody responses by BPA treatment (Han et al. 2002; Yoshino et al. 2003; Yurino et al. 2004) while others showed no effects (Takahashi et al. 2002). The reported contradictory effects of BPA on immune responses can putatively be attributed, at least in part, to one or more of the following explanations: 1) differences in biological models spanning a variety of animal species, strains, and cell lines; and 2) differences in concentrations/doses of BPA, duration of exposure, and developmental stages when the exposure occurs. In fact, many of the aforementioned studies were conducted using high BPA concentrations/doses that greatly exceed estimated exposure experienced by people (EFSA Panel on Food Contact Materials Enzymes Flavourings and Processing Aids 2015; US Food and Drug Administration 2017). Due to the above limitations, it has been challenging to establish a comprehensive immunotoxicological profile for BPA.
To comprehensively evaluated the effects of BPA exposure, a chronic toxicity study was conducted using Sprague-Dawley rats in collaboration with regulatory agencies and academic research laboratories, termed the Consortium Linking Academic and Regulatory Insights on Toxicity of BPA (CLARITY-BPA) (Heindel et al. 2015). As a participant in the CLARITY-BPA project, our studies focused on comprehensively assessing the effects of BPA on the immune system. Toward this end, spleens from a total of 484 rats were assayed after being continuously dosed with BPA or vehicle from gestation day 6 for up to one year. The five dose levels of BPA (2.5 to 25000 μg/kg bw/day) employed in this study not only cover the low doses that are relevant to estimated human exposure, but also span the wide range of doses over which BPA-induced effects have been previously reported (Heindel et al. 2015). Splenocytes isolated from BPA or vehicle treated rats were comprehensively assessed for the effects of BPA on immune responses, including lymphoproliferation, immunoglobulin production by B cells, and cellular activation of T cells, NK cells, monocytes, granulocytes, macrophages and dendritic cells.
2. Materials and Methods
2.1 Study Design and Animal Husbandry
Heindel et al. has extensively described the study design, including animal husbandry, diet characterization, dose formulation and euthanization (Heindel et al. 2015). Briefly, Sprague-Dawley rats were treated with vehicle (0.3% aqueous carboxymethylcellulose (CMC)), BPA (2.5, 25, 250, 2,500 or 25,000 μg/kg bw/day), or reference estrogen ethinyl estradiol (EE2) (0.05 or 0.5 μg/kg bw/day). Animals were randomly allocated to treatment groups, dosed by oral gavage continuously and were euthanized on postnatal day (PND) 21, 90, 6 month and 1 year. Comprehensive evaluations of the toxicokinetic of BPA across life stages in Sprague Dawley rats has been reported previously (Churchwell et al. 2014; Doerge et al. 2011). In the present study, spleens on PND 90, 6 month, and 1 year were collected and transferred overnight on ice from the FDA’s National Center for Toxicological Research (NCTR) to Michigan State University in 1640 RPMI medium (Gibco Invitrogen, Carlsbad, CA) supplemented with 5% bovine calf serum (BCS; HyClone, Logan, UT) and penicillin (100 U/ml)/streptomycin (100 g/ml; Gibco Invitrogen) and processed the following day. All tissues were blinded to the respective treatment group.
2.2 Cell culture and activation
Splenocytes were isolated by mechanical disruption and made into single cell suspensions in RPMI 1640 medium supplemented with 10% BCS and penicillin/streptomycin. Red blood cells were lysed using Zap-oglobin prior to counting splenocytes on a Beckman Coulter Counter following manufacturer’s instructions with a size threshold of 4.0 μm. Splenocytes were then cultured at 1 × 106 cells/well in 96 well plates (for proliferation assay) or 2.5 × 106 cell/well in 48 well plates (for ELISA and flow cytometry analyses). Cells were treated with 15 μg/ml LPS (S. typhosa, Sigma Aldrich, St. Louis, MO), 15 μg/ml PWM (Sigma Aldrich), or 1 μg/ml anti-CD3 (clone 145-2C11, Biolegend, San Diego, CA) plus 10 μg/ml anti-CD28 (clone 37.51, Biolegend). For anti-CD3/CD28 treatment, culture plates were coated overnight with 1 μg/ml anti-CD3 at 4 °C, washed twice with 1640 RPMI, and then seeded with cells and 10 μg/ml anti-CD28. Cells were culture at 37 °C with 5% CO2 for up to 72 hours.
Post-activation, cells were harvested by centrifugation at 500 × g for 5 minutes. Supernatants were collected for IgM ELISA, and cells were washed in Hank’s Balanced Salt Solution and stained for flow cytometry.
2.3 ELISA
IgM responses using activated splenocytes were characterized by ELISA. ELISA 96-well plates (Immulon 4 HBX strips, Thermo Scientific, Milford, MA) were coated with 1 μg/ml purified mouse anti-rat IgM primary antibody (clone G53-238, BD Biosciences, San Jose, CA) in 0.1 M NaHCO3 buffer (pH 9.6) at 4°C overnight. Culture supernatants collected from splenic B cell culture were incubated over primary antibody-coated plates for 1 hour at 37°C. Plates were then washed with PBS containing 0.05 % tween-20 and incubated with 3% BSA-PBS at room temperature for 1–2 hours. Plates were washed again as described and samples incubated at 37°C for 1–1.5 hours. Plates were washed three times and incubated with 1 μg/ml of biotin mouse anti-rat IgM secondary antibody (clone G53-238, BD Biosciences) for 1.5 hours. Plates were washed following incubation and developed with 1 mg/ml ABTS buffer (Riche, Branford, CT). Samples were read using BioTek Synergy HT plate reader (BioTek, Winooski, VT) at 405 nm every minutes for 1 hour on kinetic mode.
2.4 Proliferation Assay
Splenocytes were activated with 15 μg/ml LPS, PWM, or 1 μg/ml anti-CD3 and 10 μg/ml anti-CD28 in 96-well plates. Cells were pulsed with [3H]-thymidine (1 μCi/well) 48 hours after activation. Cells were harvested on filter paper 72 hours after activation. The incorporation of [3H]-thymidine into chromosomal DNA during cell proliferation was quantified using Ultima Gold liquid scintillation cocktail (PerkinElmer, Waltham, MA) and Tri-Carb 2100 TR scintillation counter (PerkinElmer).
2.5 Flow Cytometry
Cells were washed using 1X Hank’s Balanced Salt Solution (HBSS, pH 7.4, Invitrogen) and stained using LIVE/DEAD Fixable Near-IR Dead Cell Stain (Gibco Invitrogen) to assess cell viability, according to the manufacturer’s instructions. Cell surface FcRs were blocked with purified mouse anti-rat CD32 (BD Biosciences, San Jose, CA). Cells were incubated in FACS buffer (1× HBSS containing 1% BSA and 0.1% sodium azide) and stained for surface proteins using the following antibodies from BD Biosciences or Biolegend: AF647-CD3 (clone 1F4), PE/Cy7-CD4 (clone W3/25), PerCP-CD8 (clone OX-8), FITC-CD11b/c (clone OX-42), FITC-CD25 (clone OX-39), PE-CD80 (clone 3H5), APC-CD86 (clone 24F), FITC-CD161α (clone 10/78), PE-CD172α (clone OX-41), FITC-IgM (clone G53-238), PerCP-MHCII (clone OX-6). Cells were incubated with the antibodies for 30 min, washed three times with FACS buffer, and fixed by incubating with Cytofix (BD Biosciences) for 10 min. To measure intracellular IgM, cells were washed and incubated with 1x Perm/Wash solution (BD Biosciences) for 30 minutes, then incubated with mouse anti-rat IgM for 30 minutes. Cells were washed and resuspended in FACS buffer. In all cases, flow cytometric analyses were performed on a FACS Canto II cell analyzer (BD Biosciences) and data were analyzed using FlowJo v8.8.6 (Tree Star, Ashland, OR) software.
2.6 Data evaluation and Statistical Analysis
All tissue samples were de-identified with respect to treatment group until results were submitted to a centralized data repository. Statistical analyses were performed using GraphPad Prism version 4.0a (GraphPad Software, La Jolla, CA). Samples were excluded from analysis when the integrity of samples was judged unacceptable in cases where the cell viability upon tissue delivery was less than one standard deviation of the mean. To determine statistically significant changes between the treatment groups and vehicle control within male and female rats, a two-way ANOVA with Dunnett’s posttest was used. The mean ± SE is displayed in all bar graphs. At the 6 month PND time point, treatments were compared to the vehicle-overlap group, due to insufficient number of vehicle control animals.
During the chronic CLARITY-BPA study, the potential for unintentional exposure of vehicle control rats to BPA, termed “overlap” was acknowledged and evaluated (Heindel et al. 2015). It was hypothesized that the unintentional exposure resulted from housing of CLARITY-BPA control rats in the same room as those dosed with 250,000 μg BPA/kg bw/day. These vehicle control and potentially BPA-exposed rats were identified and tracked throughout the study. To eliminate any possibility of confounders and for the purpose of complete transparency, all vehicle control group rats potentially exposed to BPA were excluded from the data analysis, except for the vehicle group at 6 months. The 6-month rats were not excluded because all of vehicle treated female rats at 6 months of age were identified as potential overlap. Therefore, only at the 6-month time point were the vehicle overlap rats included in our analysis but were analyzed as a separate group and identified as “vehicle-overlap” (VH-Ov). At the six-month PND time point, treatments in female rats were compared to the vehicle-overlap group, due to the insufficient number of vehicle control animals.
3. Results
3.1 Characterization of the effects of BPA on lymphoproliferative responses using spleen cells
The spleens of rats continuously administered with vehicle (0.3% aqueous carboxymethylcellulose (CMC)), BPA (2.5, 25, 250, 2500 or 25000 μg/kg bw/day) or EE2 (0.05 or 0.5 μg/kg bw/day) were harvested on postnatal day (PND) 90, PND 180 (6 month) or PND 365 (1 year). Splenocytes were isolated and treated with lipopolysaccharide (LPS), pokeweed mitogen (PWM) or anti-CD3/CD28 to induce lymphoproliferation. Lymphocyte proliferation was quantified using [3H]-thymidine incorporation at 72 hours post treatment. The effects of EE2 and BPA on lymphoproliferation are summarized in Table 1.
Table 1.
Effects of EE2 and BPA on splenocyte proliferation in response to LPS, PWM or anti-CD3/CD28 stimulation.
| PND 90 | PND 6 Month | PND 1 Year | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Activation | Sex | EE2 | BPA | EE2 | BPA | EE2 | BPA |
| LPS | F | ↓0.5 | N | ↑0.05 ↑0.5 |
N | N | ↑2500 |
| M | N | N | N | N | ↑0.5 | ↑2.5 ↑2500 ↑25000 |
|
|
| |||||||
| PWM | F | N | ↓25000 | ↑0.05 | ↑25 ↑2500 |
↓0.05 | N |
| M | ↓0.05 | N | ↓0.5 | ↓2.5 ↓250 ↓2500 |
↑0.05 ↑0.5 |
↑2.5 ↑25 ↑2500 ↑25000 |
|
|
| |||||||
| Anti-CD3/CD28 | F | N | N | N | N | ↓0.05 | ↓2.5 |
| M | N | N | ↓0.5 | N | N | N | |
F: female; M: male. N indicates no significant difference compared to control groups. ↑ indicates increase and ↓ indicates decrease. Numbers designate the dose levels (μg/kg bw/day) at which significant changes were observed.
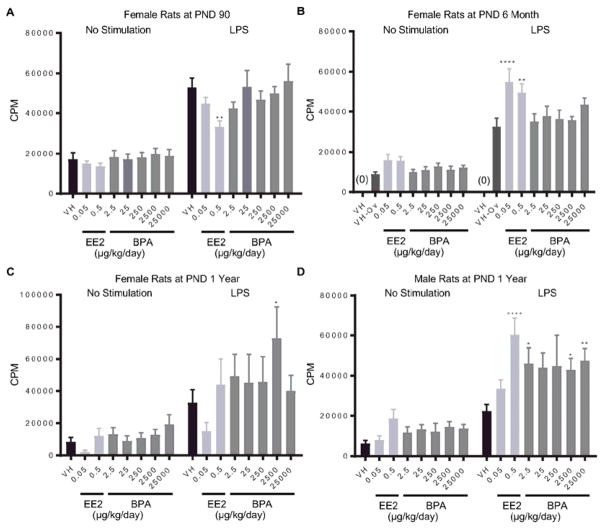
The LPS-induced proliferative response was decreased by EE2 in female rats at PND 90, but increased in 6 month-old female rats and 1 year-old male rats (Figure 1. A, B, D). Consistent with the EE2 effects, BPA increased LPS-induced splenocyte proliferation in one-year old male rats at doses of 2.5, 2500 and 25000 μg/kg bw/day (Figure 1. D). Likewise, the proliferative response was also increased in female rats at 1 year of age in the 2500 μg BPA/kg bw/day dose group (Figure 1. C). No significant differences in LPS-induced splenocyte proliferation were observed in the BPA-treated groups at PND 90 and 6 month as compare to vehicle controls (Table 1 and Supplement Figure 1).
Figure 1. Quantification of LPS-induced spleen cell proliferation by treatment group and sex.
Female (A, B, C) and male (D) rats were administrated vehicle (VH, 0.3% aqueous carboxymethylcellulose), BPA or estrogen ethinyl estradiol (EE2) at the indicated dose level by oral gavage daily and sacrificed at postnatal day (PND) 90 (A), 6 month (B), and 1 year (C, D). Splenocytes were isolated and treated with LPS for 48 h after a 24 h pulse with [3H]-thymidine. Cells were harvested and quantified for [3H]-thymidine incorporation using a Tri-Carb 2100 TR scintillation counter, which is represented as counts per minute (CPM),. Results are presented as mean ± SE. n = 6–10 rats/treatment group/sex. * p < 0.05, ** p < 0.01, **** p < 0.0001 when compared to respective vehicle control (VH-Ov for female rats at PND 6 month) by a two way ANOVA with Dunnett’s posttest. For more details about VH-Ov, please see the Statistical Analysis section in the Materials and Methods.
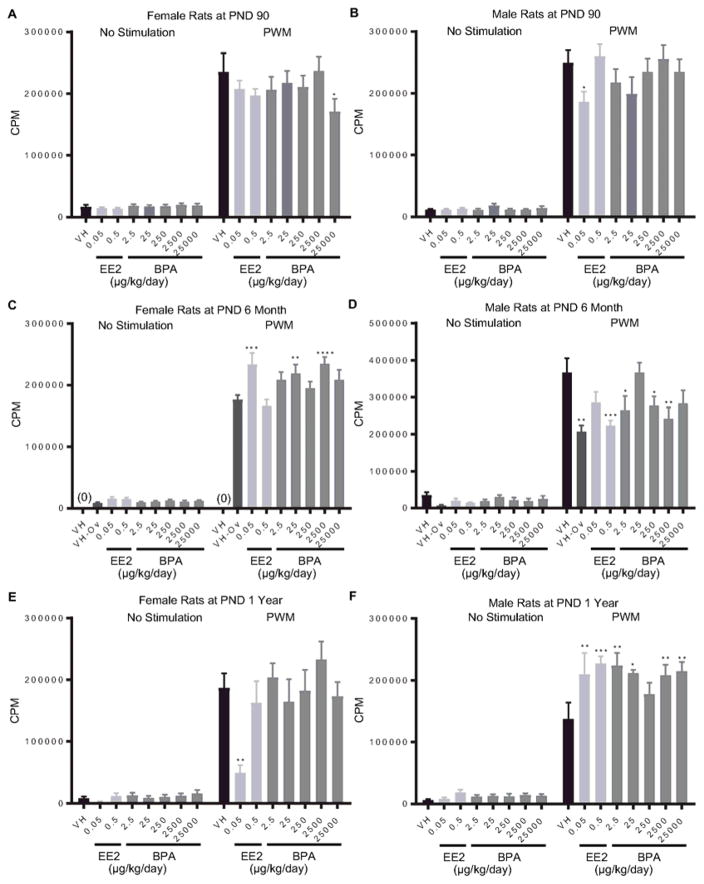
For PWM-induced splenocyte proliferative responses, BPA (25000 μg/kg bw/day) and EE2 (0.05 μg/kg bw/day) decreased lymphoproliferation at PND 90 in female and male rats, respectively (Figure 2. A, B). In addition, proliferative responses were enhanced by EE2 (0.05 μg/kg bw/day) and BPA (25 and 2500 μg/kg bw/day) in 6 month-old female rats, but decreased in male rats by both EE2 (0.5 μg/kg bw/day) and BPA (25, 250 and 2500 μg/kg bw/day) treatment (Figure 2. C, D). In female rats at 1 year of age, 0.05 μg EE2/kg bw/day treatment diminished the PWM-induced splenocyte proliferation, whereas BPA produced no effects on the cellular proliferation at any doses compared to the vehicle control (Figure 2. E). By contrast, both EE2 (0.05 and 0.5 μg/kg bw/day) and BPA (2.5, 25, 2500 and 25000 μg/kg bw/day) significantly augmented the PWM-induced spleen cell proliferation in male rats at 1 year of age (Figure 2. F).
Figure 2. Quantification of pokeweed mitogen (PWM)-induced splenocyte proliferation by treatment group and sex.
Female (A, C, E) and male (B, D, F) rats were administrated vehicle (VH, 0.3% aqueous carboxymethylcellulose), BPA or estrogen ethinyl estradiol (EE2) at the indicated dose level by oral gavage daily and sacrificed at postnatal day (PND) 90 (A, B), 6 month (C, D), and 1 year (E, F). Splenocytes were isolated and treated with PWM for 48 h after a 24 h pulse with [3H]-thymidine. Cells were harvested and quantified for [3H]-thymidine incorporation using a Tri-Carb 2100 TR scintillation counter, which is represented as counts per minute (CPM), Results are presented as mean ± SE. n = 2–10 rats/treatment group/sex, n = 2 rats in PND 1 year 250μg BPA/kg/day treated male group. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 when compared to respective vehicle control (VH-Ov for female rats at PND 6 month) by a two way ANOVA with Dunnett’s posttest. For more details about VH-Ov, please see the Statistical Analysis section in the Materials and Methods.
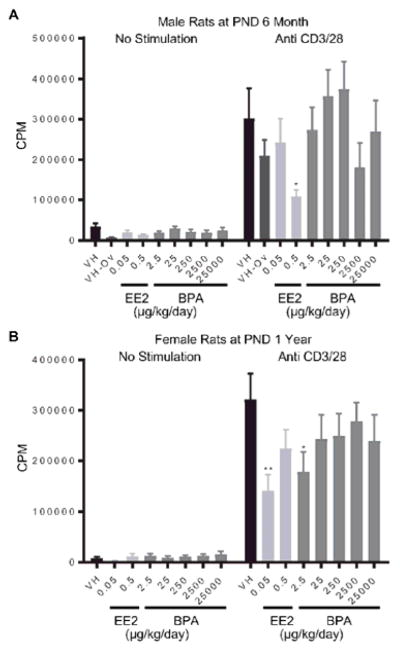
The proliferative response of splenic T cells was stimulated using anti-CD3/CD28 treatment. Administration of 0.5 and 0.05 μg EE2/kg bw/day decreased anti-CD3/CD28 induced T cell proliferation in spleen cells from 6 month-old male rats and 1 year-old female rats, respectively (Figure 3. A, B). Likewise, cellular proliferation was also decreased in female rats at 1 year of age in the 2.5 μg BPA/kg bw/day dose group (Figure 3. B). With the exception of the aforementioned changes, BPA and EE2 produced no effect on the anti-CD3/CD28-induced proliferation responses (Table 1, Supplement Figure 2).
Figure 3. Quantification of anti-CD3/28-induced splenocyte proliferation of rats by treatment group and sex.
Male (A) and female (B) rats were administrated vehicle (VH, 0.3% aqueous carboxymethylcellulose), BPA or estrogen ethinyl estradiol (EE2) at the indicated dose level by oral gavage daily and sacrificed at postnatal day (PND) 6 month (A) and 1 year (B). Splenocytes were isolated and treated with anti-CD3/CD28 for 48 h after a 24 h pulse with [3H]-thymidine. Cells were harvested and quantified for [3H]-thymidine incorporation using a Tri-Carb 2100 TR scintillation counter, which is represented as counts per minute (CPM), Results are presented as mean ± SE. n = 4–10 rats/treatment group/sex. * p < 0.05, ** p < 0.01 when compared to respective vehicle control by a two way ANOVA with Dunnett’s posttest. For more details about VH-Ov, please see the Statistical Analysis section in the Materials and Methods.
3.2 Quantification of the BPA effects on IgM responses of splenic B cell
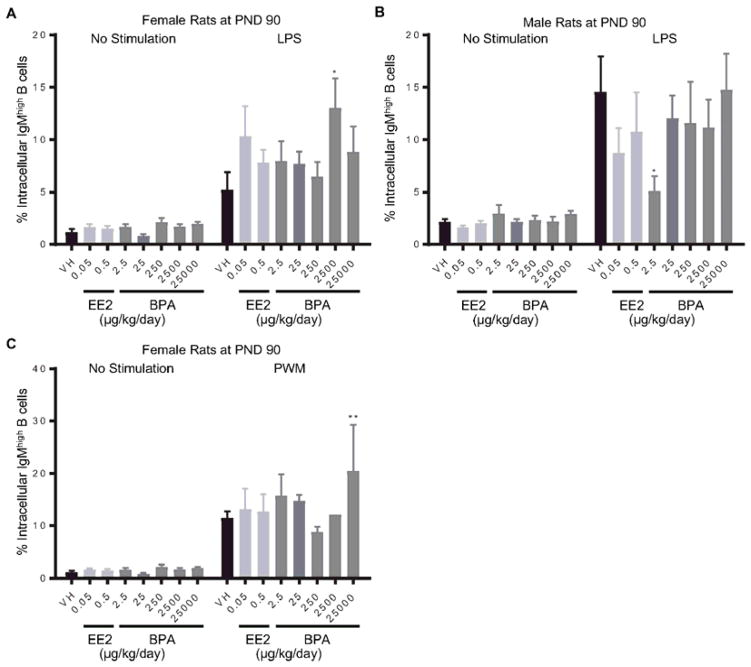
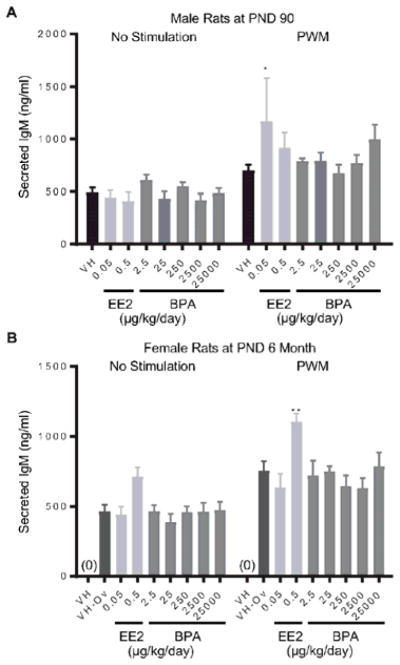
To assess the effects of BPA on IgM responses, splenocytes were stimulated with LPS or PWM to induce IgM responses in B cells. The level of intracellular IgM was quantified at 72 hours post treatment using flow cytometry (Table 2). The LPS-induced increase in the proportion of intracellular IgMhigh cells was further augmented in female rats at PND 90 in the 2500 μg BPA/kg bw/day dose group (Figure 4. A), but reduced in male rats in the 2.5 μg BPA/kg bw/day dose group (Figure 4. B). Moreover, 25000 μg BPA/kg bw/day treatment increased the percentage of intracellular IgMhigh cells as compared to the control after PWM activation in female rats at PND90 (Figure 4. C). In addition to intracellular IgM measurements, IgM secretion by splenic B cell in response to LPS or PWM activation was also quantified using ELISA (Table 2). Compared to the vehicle control groups, the amount of secreted IgM was increased in male rats at PND 90 in the 0.05 μg EE2/kg bw/day dose group and in female rats at 6 month of age in the 0.5 μg EE2/kg bw/day dose group (Figure 5). With the exception of the aforementioned changes, neither BPA nor EE2 produced any effects at any doses compared to the vehicle controls in IgM responses of splenic B cells at any of the time points (Table 2, Supplement Figure 3–8).
Table 2.
Effects of EE2 and BPA on IgM responses at 72 h after LPS or PWM activation.
| PND 90 | PND 6 Month | PND 1 Year | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Endpoints | Activation | Sex | EE2 | BPA | EE2 | BPA | EE2 | BPA |
| Intracellular IgM | LPS | F | N | ↑2500 | N | N | N | N |
| M | N | ↓2.5 | N | N | N | N | ||
|
| ||||||||
| PWM | F | N | ↑25000 | N | N | N | N | |
| M | N | N | N | N | N | N | ||
|
| ||||||||
| Secreted IgM | LPS | F | N | N | N | N | N | N |
| M | N | N | N | N | N | N | ||
|
| ||||||||
| PWM | F | N | N | ↑0.5 | N | N | N | |
| M | ↑0.05 | N | N | N | N | N | ||
F: female; M: male. N indicates no significant difference compared to control groups. ↑ indicates increase and ↓ indicates decrease. Numbers designate the dose levels (μg/kg bw/day) at which significant changes were observed.
Figure 4. Percentage of intracellular IgMhigh splenic B cells by treatment group and sex.
Female (A, C) and male (B) rats were administrated vehicle (VH, 0.3% aqueous carboxymethylcellulose), BPA or estrogen ethinyl estradiol (EE2) at the indicated dose level by oral gavage daily and sacrificed at postnatal day (PND) 90 day. Splenocytes were isolated and treated with LPS (A, B) or PWM (C) for 72 h. The percentage of IgMhigh B cells was quantified by flow cytometry. Results are presented as mean ± SE. n = 1–10 rats/treatment group/sex, n = 1 rat in PWM treated 2500μg BPA/kg/day female group. * p < 0.05, ** p < 0.01 when compared to respective vehicle control by a two way ANOVA with Dunnett’s posttest.
Figure 5. Quantification of pokeweed mitogen (PWM)-induced IgM secretion by splenic B cells by treatment group and sex.
Male (A) and female (B) rats were administrated vehicle (VH, 0.3% aqueous carboxymethylcellulose), BPA or estrogen ethinyl estradiol (EE2) at the indicated dose level by oral gavage daily and sacrificed at postnatal day (PND) 90 day (A) and 6 month (B). Splenocytes were isolated and treated with PWM for 72 h. Post activation, supernatants were collected and the levels of secreted IgM were quantified by ELISA. Results are presented as mean ± SE. n = 2–10 rats/treatment group/sex, n = 2 rats in PWM treated 0.05μg EE/kg/day male group. * p < 0.05, ** p < 0.01 when compared to respective vehicle control (VH-Ov for female rats at PND 6 month) by a two way ANOVA with Dunnett’s posttest. For more details about VH-Ov, please see the Statistical Analysis section in the Materials and Methods.
3.3 Evaluation of the effects of BPA on splenic T cell activation
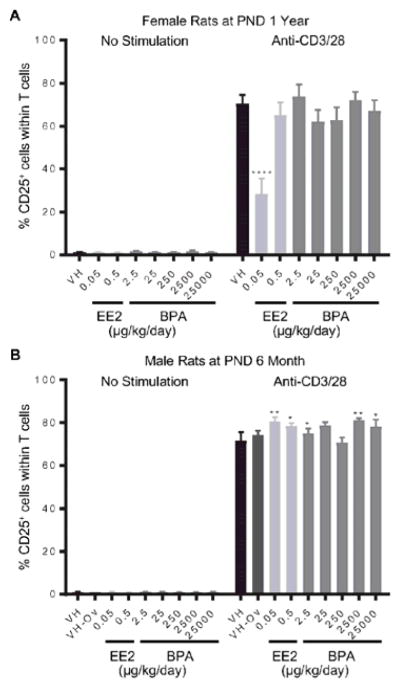
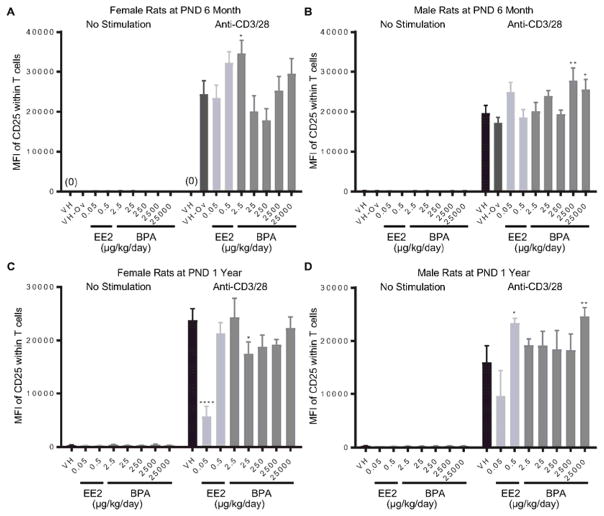
Splenocytes were treated with anti-CD3/CD28 to activate splenic T cells. The expression of CD25 on T cells represents cell activation induced by anti-CD3/CD28 stimulation. The percentage of CD25+ T cells as well as the average expression level of CD25, represented as mean fluorescence intensity (MFI), were quantified using flow cytometry at 48 hours after activation. The effects of BPA and EE2 on splenic T cell activation are summarized in Table 3. Specifically, the percentage of CD25+ T cells was decreased in female rats at 1 year of age in the 0.05 μg EE2/kg bw/day dose group (Figure 6. A). By contrast, both EE2 (0.05 and 0.5 μg/kg bw/day) and BPA (2.5, 2500 and 25000 μg/kg bw/day) increased the percentage of CD25+ T cells in male rats at 6 month of age (Figure 6. B). In addition, BPA treatment increased MFI of CD25 in 6 month-old female rats at the 2.5 μg/kg bw/day dose (Figure 7. A), and in male rats at the 2500 and 25000 μg/kg bw/day doses (Figure 7. B). At the PND 1 year time point, the MFI of CD25 was decreased in female rats in the 0.05 μg EE2/kg bw/day and 25 μg BPA/kg bw/day dose groups (Figure 7. C), but increased in male rats in the 0.5 μg EE2/kg bw/day and 25000 μg BPA/kg bw/day dose groups (Figure 7. D). With the exception of aforementioned changes, neither BPA nor EE2 produced any effects on splenic T cell activation at any of the time points (Table 3, Supplement Figure 9 and 10).
Table 3.
Effects of EE2 and BPA on T cell activation at 48 hr after anti-CD3CD28 stimulation.
| PND 90 | PND 6 Month | PND 1 Year | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Endpoints | Sex | EE2 | BPA | EE2 | BPA | EE2 | BPA |
| % CD25+ cells within T cells | F | N | N | N | N | ↓0.05 | N |
| M | N | N | ↑0.05 ↑0.5 |
↑2.5 ↑2500 ↑25000 |
N | N | |
|
| |||||||
| MFI of CD25 | F | N | N | N | ↑2.5 | ↓0.05 | N |
| M | N | N | N | ↑2500 ↑25000 |
↑0.5 | ↑25000 | |
F: female; M: male. N indicates no significant difference compared to control groups. ↑ indicates increase and ↓ indicates decrease. Numbers designate the dose levels (μg/kg bw/day) at which significant changes were observed.
Figure 6. Percentage of CD25+ T cells post anti-CD3/28 activation by treatment group and sex.
Female (A) and male (B) rats were administrated vehicle (VH, 0.3% aqueous carboxymethylcellulose), BPA or estrogen ethinyl estradiol (EE2) at the indicated dose level by oral gavage daily and sacrificed at postnatal day (PND) 1 year (A) and 6 month (B). Splenocytes were isolated and treated with anti-CD3/28 for 48 h. The percentage of CD25+ cells within splenic T cells was quantified by flow cytometry. Results are presented as mean ± SE. n = 4–10 rats/treatment group/sex. * p < 0.05, ** p < 0.01, **** p < 0.0001 when compared to respective vehicle control by a two way ANOVA with Dunnett’s posttest.
Figure 7. Quantification of the CD25 expression level on T cells post anti-CD3/28 activation by treatment group and sex.
Female (A, C) and male (B, D) rats were administrated vehicle (VH, 0.3% aqueous carboxymethylcellulose), BPA or estrogen ethinyl estradiol (EE2) at the indicated dose level by oral gavage daily and sacrificed at postnatal day (PND) 6 month (A, B) and 1 year (C, D). Splenocytes were isolated and treated with anti-CD3/28 for 48 h. The expression levels of CD25 on splenic T cells, represented as mean fluorescence intensity (MFI), were quantified by flow cytometry. Results are presented as mean ± SE. n = 2–10 rats/treatment group/sex, n = 2 rats in PND 1 year 250μg BPA/kg/day male groups. * p < 0.05, ** p < 0.01, **** p < 0.0001 when compared to respective vehicle control (VH-Ov for female rats at PND 6 month) by a two way ANOVA with Dunnett’s posttest. For more details about VH-Ov, please see the Statistical Analysis section in the Materials and Methods.
3.4 Quantification of the effects of BPA on natural killer (NK) cell activation
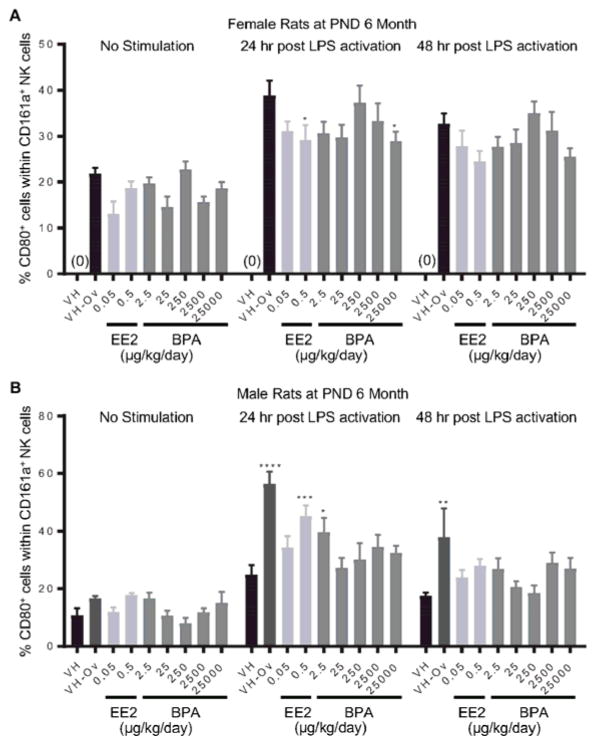
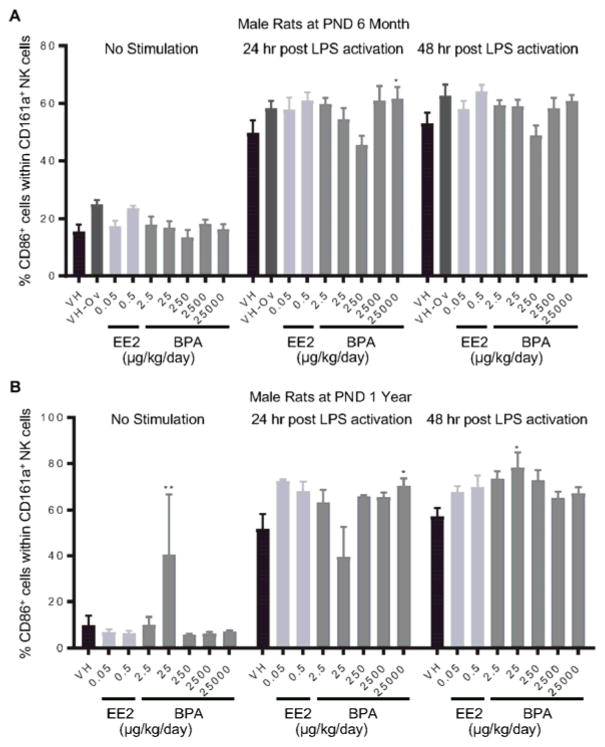
Splenocytes were treated with LPS to activate CD161a+ NK cells. The expression of costimulatory molecules CD80 and CD86 on cell surface represents NK cell activation. The percentage of CD80+ or CD86+ cell populations within CD161a+ cells was quantified using flow cytometry at 24 and 48 hours after LPS treatment. The effects of EE2 and BPA on LPS-induced activation of NK cells are summarized in Table 4. Specifically, the percentage of CD80+ NK cells was decreased in 6-month-old female rats at 24 hours post LPS activation in the 0.5 μg EE2/kg bw/day and 25000 μg BPA/kg bw/day dose groups (Figure 8. A), but increased in male rats in the 0.5 μg EE2/kg bw/day and 25 μg BPA/kg bw/day dose groups (Figure 8. B). In addition, the percentage of CD86+ population in NK cells was increased at 24 hours post LPS activation in the 25000 μg BPA/kg bw/day dose group in both 6 month and 1 year old male rats (Figure 9. A, B). Likewise, an increase in the percentage of CD86+ NK cells was also observed at 48 hours post LPS activation in the 25 μg BPA/kg bw/day dose group in 1 year-old male rats (Figure 9. B). With the exception of aforementioned changes, no significant differences in NK cell activation were observed with BPA or EE2 treatment at any time points when compared to vehicle controls (Table 4, Supplement Figure 11 and 12).
Table 4.
Effects of EE2 and BPA on NK cell activation at 24 or 48 hr after LPS stimulation.
| PND 6 Month | PND 1 Year | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Endpoints | Time points | Sex | EE2 | BPA | EE2 | BPA |
| % CD80+ cells within NK cells | 24hr | F | ↓0.5 | ↓25000 | N | N |
| M | ↑0.5 | ↑2.5 | N | N | ||
|
| ||||||
| 48hr | F | N | N | N | N | |
| M | N | N | N | N | ||
|
| ||||||
| % CD86+ cells within NK cells | 24hr | F | N | N | N | N |
| M | N | ↑25000 | N | ↑25000 | ||
|
| ||||||
| 48hr | F | N | N | N | N | |
| M | N | N | N | ↑25 | ||
F: female; M: male. N indicates no significant difference compared to control groups. ↑ indicates increase and ↓ indicates decrease. Numbers designate the dose levels (μg/kg bw/day) at which significant changes were observed.
Figure 8. Percentage of CD80+ cells within the NK cell population post LPS activation from by treatment group and sex.
Female (A) and male (B) rats were administrated vehicle (VH, 0.3% aqueous carboxymethylcellulose), BPA or estrogen ethinyl estradiol (EE2) at the indicated dose level by oral gavage daily and sacrificed at postnatal day (PND) 6 months. Splenocytes were isolated and treated with LPS for up to 48 h. The percentage of CD80+ cells within CD161a+ NK cells was quantified by flow cytometry. Results are presented as mean ± SE. n = 3–10 rats/treatment group/sex. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 when compared to respective vehicle control (VH-Ov for female rats at PND 6 month) by a two way ANOVA with Dunnett’s posttest. For more details about VH-Ov, please see the Statistical Analysis section in the Materials and Methods.
Figure 9. Percentage of CD86+ cells within the NK cell population post LPS activation by treatment group and sex.
Male rats were administrated vehicle (VH, 0.3% aqueous carboxymethylcellulose), BPA or estrogen ethinyl estradiol (EE2) at the indicated dose level by oral gavage daily and sacrificed at postnatal day (PND) 6 months (A) and 1 year (B). Splenocytes were isolated and treated with LPS for up to 48 h. The percentage of CD86+ cells within CD161a+ NK cells was quantified by flow cytometry. Results are presented as mean ± SE. n = 3–10 rats/treatment group/sex. * p < 0.05, ** p < 0.01 when compared to respective vehicle control by a two way ANOVA with Dunnett’s posttest.
3.5 Characterization of the BPA effects on the cellular activation of spleen-associated myeloid cell lineages
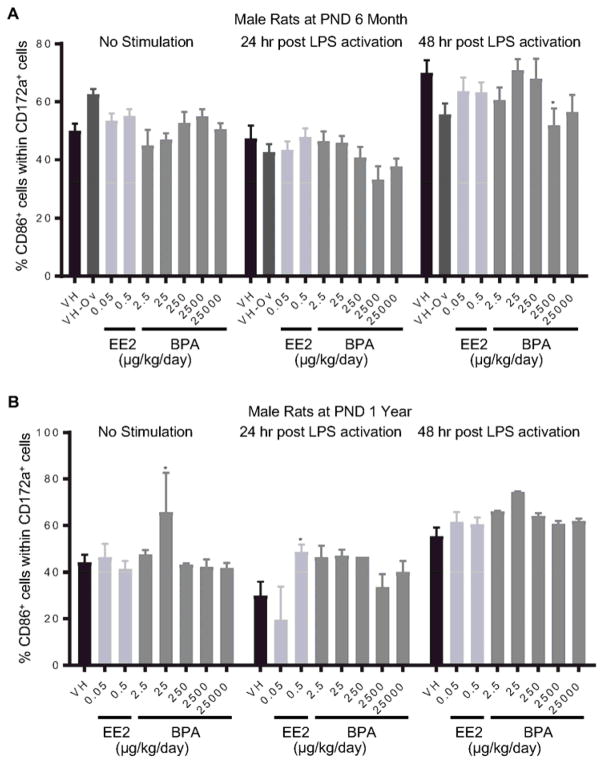
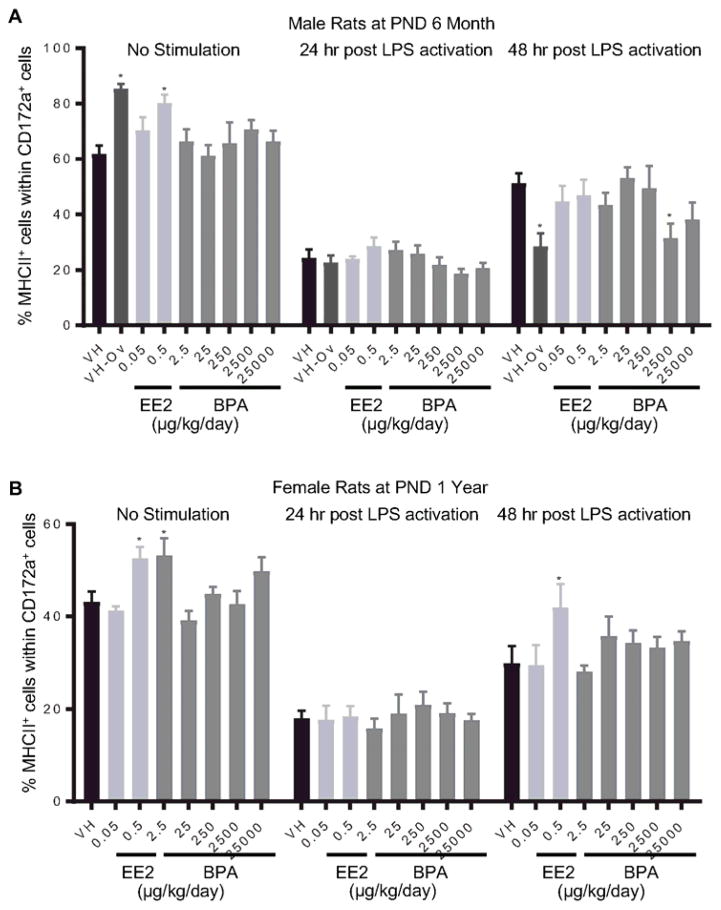
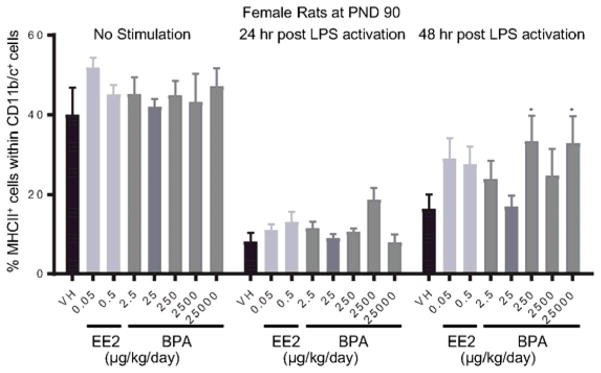
Splenocytes were treated with LPS to activate spleen-associated myeloid cell lineages, including CD172α+ cells (monocytes/macrophages/granulocytes) and CD11b/c+ cells (macrophages/dendritic cells). The expression of MHCII and costimulatory molecule CD86 on cell surface is indicative of cell activation. The percentage of CD86+ or MHCII+ cell populations within CD172α+ or CD11b/c+ cells was quantified using flow cytometry at 24 and 48 hours after LPS treatment. The effects of EE2 and BPA on LPS-induced activation of CD172α+ cells are summarized in Table 5. Specifically, the percentage of CD86+ cells was decreased in 6 month-old male rats in the 2500 μg BPA/kg bw/day dose group at 48 hours post activation (Figure 10. A), and increased in 1 year-old male rats in the 0.5 μg EE2/kg bw/day dose group at 24 hours post activation (Figure. 10B). Moreover, 2500 μg BPA/kg bw/day treatment decreased the percentage of MHCII+ cell population within CD172α+ cells in 6 month-old male rats at 48 hours post LPS-activation (Figure 11. A). An increase in the percentage of MHCII+ cells was observed in 1 year-old female rats in the 0.5 μg EE2/kg bw/day dose group (Figure 11. B). In addition, the effects of EE2 and BPA on LPS-induced activation of CD11b/c+ cells are summarized in Table 6. The only observed changes compared to the vehicle controls were the increases in MHCII+ cell population in female rats at PND 90 in the 250 and 25000 BPA μg/kg bw/day dose groups at 48 hours post LPS activation (Figure 12). With the exception of aforementioned changes, neither BPA nor EE2 produced any effects on CD172α+ or CD11b/c+ cell activation at any of the time points (Table 4, 5, Supplement Figure 13–20).
Table 5.
Effects of EE2 and BPA on the activation of CD172a+ cells (monocytes/macrophages/granulocytes) at 24 or 48 hr after LPS stimulation.
| PND 90 | PND 6 Month | PND 1 Year | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Endpoints | Time points | Sex | EE2 | BPA | EE2 | BPA | EE2 | BPA |
| % CD86+ cells within CD172 + cells | 24hr | F | N | N | N | N | N | N |
| M | N | N | N | N | ↑0.5 | N | ||
|
| ||||||||
| 48hr | F | N | N | N | N | N | N | |
| M | N | N | N | ↓2500 | N | N | ||
|
| ||||||||
| % MHCII+ cells within CD172 + cells | 24hr | F | N | N | N | N | N | N |
| M | N | N | N | N | N | N | ||
|
| ||||||||
| 48hr | F | N | N | N | N | ↑0.5 | N | |
| M | N | N | N | ↓2500 | N | N | ||
F: female; M: male. N indicates no significant difference compared to control groups. ↑ indicates increase and ↓ indicates decrease. Numbers designate the dose levels (μg/kg bw/day) at which significant changes were observed.
Figure 10. Percentage of CD86+ monocyte/macrophage/granulocytes post LPS activation by treatment group and sex.
Male rats were administrated vehicle (VH, 0.3% aqueous carboxymethylcellulose), BPA or estrogen ethinyl estradiol (EE2) at the indicated dose level by oral gavage daily and sacrificed at postnatal day (PND) 6 months (A) and 1 year (B). Splenocytes were isolated and treated with LPS for up to 48 h. The percentage of CD86+ cells within CD172a+ monocyte/macrophage/granulocytes was quantified by flow cytometry. Results are presented as mean ± SE. n = 2–10 rats/treatment group/sex, n = 2 rats in PND 1 year 250μg BPA/kg/day male groups. * p < 0.05 when compared to respective vehicle control by a two way ANOVA with Dunnett’s posttest.
Figure 11. Percentage of MHCII+ monocyte/macrophage/granulocytes post LPS activation by treatment group and sex.
Male (A) and female (B) rats were administrated vehicle (VH, 0.3% aqueous carboxymethylcellulose), BPA or estrogen ethinyl estradiol (EE2) at the indicated dose level by oral gavage daily and sacrificed at postnatal day (PND) 6 months (A) and 1 year (B). Splenocytes were isolated and treated with LPS for up to 48 h. The percentage of MHCII+ cells within CD172a+ monocyte/macrophage/granulocytes was quantified by flow cytometry. Results are presented as mean ± SE. n = 4–10 rats/treatment group/sex. * p < 0.05 when compared to respective vehicle control by a two way ANOVA with Dunnett’s posttest.
Table 6.
Effects of EE2 and BPA on the activation of CD11b/c+ cells (macrophages/dendritic cells) at 24 or 48 hr after LPS stimulation.
| PND 90 | PND 6 Month | PND 1 Year | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Endpoints | Time points | Sex | EE2 | BPA | EE2 | BPA | EE2 | BPA |
| % CD86+ cells within CD11b/c+ cells | 24hr | F | N | N | N | N | N | N |
| M | N | N | N | N | N | N | ||
|
| ||||||||
| 48hr | F | N | N | N | N | N | N | |
| M | N | N | N | N | N | N | ||
|
| ||||||||
| % MHCII+ cells within CD11b/c+ cells | 24hr | F | N | N | N | N | N | N |
| M | N | N | N | N | N | N | ||
|
| ||||||||
| 48hr | F | N | ↑250 ↑25000 |
N | N | N | N | |
| M | N | N | N | N | N | N | ||
F: female; M: male. N indicates no significant difference compared to control groups. ↑ indicates increase and ↓ indicates decrease. Numbers designate the dose levels (μg/kg bw/day) at which significant changes were observed.
Figure 12. Percentage of MHCII+ macrophage/dendritic cells post LPS activation by treatment group and sex.
Female rats were administrated vehicle (VH, 0.3% aqueous carboxymethylcellulose), BPA or estrogen ethinyl estradiol (EE2) at the indicated dose level by oral gavage daily and sacrificed at postnatal day (PND) 90. Splenocytes were isolated and treated with LPS for up to 48 h. The percentage of MHCII+ cells within CD11b/c+ macrophage/dendritic cells was quantified by flow cytometry. Results are presented as mean ± SE. n = 6–10 rats/treatment group/sex. * p < 0.05 when compared to respective vehicle control by a two way ANOVA with Dunnett’s posttest.
4. Discussion
As a participant in the CLARITY-BPA project, the objective of our studies was to evaluate the effects of BPA on the immune system. To our knowledge, this is the first study to evaluate the immunotoxicity of chronic BPA exposure on such a large and comprehensive scale. In this paper we describe the effects of BPA on a broad range of immune function endpoints using spleen cells isolated from Sprague-Dawley rats. A total of 484 rats were assayed after being continuously dosed with BPA or vehicle from gestation day 6 for up to one year. The five dose levels of BPA (2.5 to 25000 μg/kg bw/day) employed in this study not only cover the low doses that are relevant to estimated human exposure, but also span the wide range of doses over which BPA-induced effects have been previously reported (Heindel et al. 2015). Additionally, two dose levels of EE2, which served as a positive control for estrogen, were also included in this study.
Splenocytes isolated from BPA or vehicle treated rats were comprehensively evaluated for lymphoproliferation, immunoglobulin production by B cells, and induction of cellular activation of T cells, NK cells, monocytes, granulocytes, macrophages and dendritic cells. As illustrated in the table 7, male and female rats were continuously administrated one of five doses of BPA for up to 1 year, which gives rise to a total of 30 experimental conditions. For each experimental condition, 21 endpoints were assessed in this immunotoxicological evaluation of BPA, which represents a total of 630 measurements. Within these 630 measurements, only 35 showed a statistical difference due to BPA treatment compared to vehicle controls. The most consistent alterations associated with BPA treatment was the augmentation of cell proliferation in response to LPS or PWM stimulations in 1-year-old male rats, which was also observed in the EE2 treated groups (Table 1). However, neither dose-dependent responses nor persistent trends over time were observed. In fact and by contrast, at the 6-month time point, BPA treatment was associated with a decrease in cell proliferation in response to PWM stimulations in male rats (Table 1). Additionally, enhanced expression of CD25 on T cells in response to anti-CD3/CD28 activation was observed in BPA and EE2 treated 6-month-old male rats (Table 3, Figure 6); however, the alterations were moderate in magnitude and were not dose-dependent. With the exception of the aforementioned, the effects of BPA on all of the immune function endpoints were mostly sporadic with only one out of five BPA dose groups showing statistical differences.
Table 7.
Summary of experimental conditions, end points and the number measurements with observed BPA effects in this study.
| Independent variables | Experimental conditions tested | End points | Measurements (Data points) | Measurements with observed BPA effect | |||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Tissue | Time points | BPA doses | Sex | ||||
| Spleen | 3 | 5 | 2 | 30 | 21 | 630 | 35 |
In the measurement of MHCII expression on CD172α+ cells (monocytes/macrophages/granulocytes) and CD11b/c+ cells (macrophages/dendritic cells), we noticed that the percentage of MHCII+ cells was decreased at 24 and 48 hr after LPS activation as compared to 0 hr naive cells (Figure 11 and 12). This decrease in the MHCII+ cell population after LPS activation could be attributable to several factors. One possibility is that BPA treatment prevents the upregulation of MHCII in CD172α+ cells and CD11b/c+ cells in response to LPS activation. However, since a similar decrease was also observed in vehicle control groups, it is unlikely that the decrease in MHCII+ population results from BPA treatment. The second possibility is that increased cell death after LPS activation leads to the decrease in MHCII+ cell population. This possibility is also unlikely as the cell viability was monitored using Live/Dead staining and no increase in cell death after LPS activation was observed. A third possibility is that CD172α+ cells and CD11b/c+ cells became much more adherent after LPS activation and attached to the culture plate, which leads to poor recovery of activated MHCII+ cells. We believe the third possibility is the most likely explanation.
It is noteworthy to convey that due to the large number of animals employed in this study coupled with the sacrifice of all female rats were terminated at the same period within their estrus cycle, lymphoid tissues were harvested from rats and shipped to our laboratory over the period of 15 months in a staggered manner. Evaluations of immune endpoints were conducted as tissue were received, which occurred on different days even for tissues derived from the same dose groups. To control for potential day-to-day variability as well as variations introduced by shipment, extra control samples were included in each shipment, which were identified as controls and not included in the overall analysis of data. These controls were only used to insure that control responses fell within the historical range. In addition, samples were excluded from analysis when the cell viability upon tissue delivery was less than one standard deviation of the mean. Based on the staggered nature on how tissues were received and assayed, it is quite remarkable how consistent the various immune responses were within treatment groups.
In summary, this study comprehensively evaluated the effects of chronic BPA exposure on immune responses of rat splenocytes. In total, of the 630 measurements in BPA treated rats, 35 measurements were statistically different from vehicle controls, which were mainly associated with the augmentation of cell proliferation in response to LPS or PWM stimulations in 1-year-old male rats. With the exception of the aforementioned, the statistically significant changes associated with BPA treatment were mostly sporadic and not dose-dependent with only one out of five BPA dose groups showing statistical difference. In addition, the observed BPA-associated alterations were mostly moderate in magnitude and showed no persistent trend over the one-year time period. Based on these findings, we conclude that the observed BPA-mediated changes observed are unlikely to compromise immune competence in adults.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the following individual for their important contributions to the experimental design, planning and coordination of the study: Luisa Comacho, Sherry Lewis, Michelle Vanlandingham and K. Barry Delclos, the National Center for Toxicological Research; Retha Newbold, Nigel Walker and John Bucher, the National Toxicology Program; and Thaddeus Schug and Jerrold Heindel the National Institute for Environmental Health Sciences. The authors would like to thank Ms. Kimberly Hambleton for assistance with submission of this manuscript.
Funding
This study was supported by National Institute of Environmental Health Sciences ES020885.
Abbreviations
- PAMP
pathogen-associated molecular patterns
- IL2R
interleukin 2 receptor
- BPA
Bisphenol A
- CLARITY-BPA
Consortium Linking Academic and Regulatory Insights on Toxicity of BPA
- PND
postnatal day
- CMC
aqueous carboxymethylcellulose
- EE2
estrogen ethinyl estradiol
- LPS
lipopolysaccharide
- PWM
pokeweed mitogen
- NK
natural killer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachmann MF, Oxenius A. Interleukin 2: from immunostimulation to immunoregulation and back again. EMBO reports. 2007;8:1142–1148. doi: 10.1038/sj.embor.7401099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes M. Role of natural and immune IgM antibodies in immune responses. Molecular immunology. 2000;37:1141–1149. doi: 10.1016/s0161-5890(01)00025-6. [DOI] [PubMed] [Google Scholar]
- Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nature reviews Immunology. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- Byun JA, Heo Y, Kim YO, Pyo MY. Bisphenol A-induced downregulation of murine macrophage activities in vitro and ex vivo. Environmental toxicology and pharmacology. 2005;19:19–24. doi: 10.1016/j.etap.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environmental health perspectives. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell MI, Camacho L, Vanlandingham MM, Twaddle NC, Sepehr E, Delclos KB, Fisher JW, Doerge DR. Comparison of life-stage-dependent internal dosimetry for bisphenol A, ethinyl estradiol, a reference estrogen, and endogenous estradiol to test an estrogenic mode of action in Sprague Dawley rats. Toxicological sciences: an official journal of the Society of Toxicology. 2014;139:4–20. doi: 10.1093/toxsci/kfu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerge DR, Twaddle NC, Vanlandingham M, Brown RP, Fisher JW. Distribution of bisphenol A into tissues of adult, neonatal, and fetal Sprague-Dawley rats. Toxicology and applied pharmacology. 2011;255:261–270. doi: 10.1016/j.taap.2011.07.009. [DOI] [PubMed] [Google Scholar]
- EFSA Panel on Food Contact Materials Enzymes Flavourings and Processing Aids. Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA Journal. 2015;13:3978. n/a. [Google Scholar]
- Goto M, Ono H, Takano-Ishikawa Y, Shinmoto H, Yoshida M. Mac1 positive cells are required for enhancement of splenocytes proliferation caused by bisphenol a. Biosci Biotechnol Biochem. 2004;68:263–265. doi: 10.1271/bbb.68.263. [DOI] [PubMed] [Google Scholar]
- Guo H, Liu T, Uemura Y, Jiao S, Wang D, Lin Z, Narita Y, Suzuki M, Hirosawa N, Ichihara Y, Ishihara O, Kikuchi H, Sakamoto Y, Senju S, Zhang Q, Ling F. Bisphenol A in combination with TNF-alpha selectively induces Th2 cell-promoting dendritic cells in vitro with an estrogen-like activity. Cellular & molecular immunology. 2010;7:227–234. doi: 10.1038/cmi.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Denison MS, Tachibana H, Yamada K. Effects of estrogenic compounds on immunoglobulin production by mouse splenocytes. Biol Pharm Bull. 2002;25:1263–1267. doi: 10.1248/bpb.25.1263. [DOI] [PubMed] [Google Scholar]
- Heindel JJ, Newbold RR, Bucher JR, Camacho L, Delclos KB, Lewis SM, Vanlandingham M, Churchwell MI, Twaddle NC, McLellen M, Chidambaram M, Bryant M, Woodling K, Gamboa da Costa G, Ferguson SA, Flaws J, Howard PC, Walker NJ, Zoeller RT, Fostel J, Favaro C, Schug TT. NIEHS/FDA CLARITY-BPA research program update. Reprod Toxicol. 2015;58:33–44. doi: 10.1016/j.reprotox.2015.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CC, Shimomura-Shimizu M, Muroi M, Tanamoto K. Effect of endocrine disrupting chemicals on lipopolysaccharide-induced tumor necrosis factor-alpha and nitric oxide production by mouse macrophages. Biol Pharm Bull. 2004;27:1136–1139. doi: 10.1248/bpb.27.1136. [DOI] [PubMed] [Google Scholar]
- Hume DA. Macrophages as APC and the dendritic cell myth. J Immunol. 2008;181:5829–5835. doi: 10.4049/jimmunol.181.9.5829. [DOI] [PubMed] [Google Scholar]
- Jontell M, Hanks CT, Bratel J, Bergenholtz G. Effects of unpolymerized resin components on the function of accessory cells derived from the rat incisor pulp. J Dent Res. 1995;74:1162–1167. doi: 10.1177/00220345950740050401. [DOI] [PubMed] [Google Scholar]
- Kim JY, Jeong HG. Down-regulation of inducible nitric oxide synthase and tumor necrosis factor-alpha expression by bisphenol A via nuclear factor-kappaB inactivation in macrophages. Cancer Lett. 2003;196:69–76. doi: 10.1016/s0304-3835(03)00219-2. [DOI] [PubMed] [Google Scholar]
- Kuan YH, Li YC, Huang FM, Chang YC. The upregulation of tumour necrosis factor-alpha and surface antigens expression on macrophages by bisphenol A-glycidyl-methacrylate. International endodontic journal. 2012;45:619–626. doi: 10.1111/j.1365-2591.2012.02017.x. [DOI] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. International reviews of immunology. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- Lee MH, Chung SW, Kang BY, Park J, Lee CH, Hwang SY, Kim TS. Enhanced interleukin-4 production in CD4+ T cells and elevated immunoglobulin E levels in antigen-primed mice by bisphenol A and nonylphenol, endocrine disruptors: involvement of nuclear factor-AT and Ca2+ Immunology. 2003;109:76–86. doi: 10.1046/j.1365-2567.2003.01631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon B, Ardavin C. Monocyte-derived dendritic cells in innate and adaptive immunity. Immunology and cell biology. 2008;86:320–324. doi: 10.1038/icb.2008.14. [DOI] [PubMed] [Google Scholar]
- Rogers JA, Metz L, Yong VW. Review: Endocrine disrupting chemicals and immune responses: a focus on bisphenol-A and its potential mechanisms. Molecular immunology. 2013;53:421–430. doi: 10.1016/j.molimm.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Sakazaki H, Ueno H, Nakamuro K. Estrogen receptor alpha in mouse splenic lymphocytes: possible involvement in immunity. Toxicol Lett. 2002;133:221–229. doi: 10.1016/s0378-4274(02)00203-5. [DOI] [PubMed] [Google Scholar]
- Sun YV, Boverhof DR, Burgoon LD, Fielden MR, Zacharewski TR. Comparative analysis of dioxin response elements in human, mouse and rat genomic sequences. Nucleic acids research. 2004;32:4512–4523. doi: 10.1093/nar/gkh782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Ohsawa M, Utsumi H. A simple bioassay for evaluating immunotoxic properties of chemicals by use of in vitro antibody production system. J Health Sci. 2002;48:161–167. [Google Scholar]
- US Food and Drug Administration. Bisphenol A (BPA): Use in Food Contact Application. 2017. [Google Scholar]
- Yamada H, Furuta I, Kato EH, Kataoka S, Usuki Y, Kobashi G, Sata F, Kishi R, Fujimoto S. Maternal serum and amniotic fluid bisphenol A concentrations in the early second trimester. Reprod Toxicol. 2002;16:735–739. doi: 10.1016/s0890-6238(02)00051-5. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Okada Y, Hisamatsu Y. Effect of endocrine disrupting chemicals on lymphocyte responses. Organohalogen Compounds. 2000;49:394–396. [Google Scholar]
- Yoshino S, Yamaki K, Li X, Sai T, Yanagisawa R, Takano H, Taneda S, Hayashi H, Mori Y. Prenatal exposure to bisphenol A up-regulates immune responses, including T helper 1 and T helper 2 responses, in mice. Immunology. 2004;112:489–495. doi: 10.1111/j.1365-2567.2004.01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino S, Yamaki K, Yanagisawa R, Takano H, Hayashi H, Mori Y. Effects of bisphenol A on antigen-specific antibody production, proliferative responses of lymphoid cells, and TH1 and TH2 immune responses in mice. Br J Pharmacol. 2003;138:1271–1276. doi: 10.1038/sj.bjp.0705166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn JY, Park HY, Lee JW, Jung IO, Choi KH, Kim K, Cho KH. Evaluation of the immune response following exposure of mice to bisphenol A: induction of Th1 cytokine and prolactin by BPA exposure in the mouse spleen cells. Arch Pharm Res. 2002;25:946–953. doi: 10.1007/BF02977018. [DOI] [PubMed] [Google Scholar]
- Yurino H, Ishikawa S, Sato T, Akadegawa K, Ito T, Ueha S, Inadera H, Matsushima K. Endocrine Disruptors (Environmental Estrogens) Enhance Autoantibody Production by B1 Cells. Toxicological sciences: an official journal of the Society of Toxicology. 2004 doi: 10.1093/toxsci/kfh179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.