Abstract
Epilepsy surgery has seen numerous technological advances in both diagnostic and therapeutic procedures in recent years. This has increased the number of patients who may be candidates for intervention and potential improvement in quality of life. However, the expansion of the field also necessitates a broader understanding of how to incorporate both traditional and emerging technologies into the care provided at comprehensive epilepsy centers. This review summarizes both old and new surgical procedures in epilepsy using an example algorithm. While treatment algorithms are inherently over-simplified, incomplete, and reflect personal bias, they provide a general framework that can be customized to each center and each patient, incorporating differences in provider opinion, patient preference, and the institutional availability of technologies. For instance, the use of minimally-invasive stereotactic electroencephalography has increased dramatically over the past decade, but many cases still benefit from invasive recordings from subdural grids. And although surgical resection remains the gold-standard treatment for focal mesial temporal or neocortical epilepsy, ablative procedures such as laser interstitial thermal therapy or stereotactic radiosurgery may be appropriate and avoid craniotomy in many cases. Furthermore, while palliative surgical procedures were once limited to disconnection surgeries, several neurostimulation treatments are now available to treat eloquent cortical, bitemporal, and even multifocal or generalized epilepsy syndromes. An updated perspective in epilepsy surgery will help guide surgical decision making and lay the groundwork for data collection needed in future studies and trials.
Keywords: decision-making, epilepsy, flowchart, resection, surgery
1. Introduction
Epilepsy surgery has seen numerous changes in recent years, necessitating continued updates to the treatment algorithms for this disorder. This field has achieved technological advances in both diagnostic and therapeutic procedures, and previously unavailable treatment options have been introduced. The core strategy in the evaluation of drug-resistant epilepsy remains relatively consistent: noninvasive presurgical evaluation, with or without invasive intracranial monitoring, followed by a therapeutic intervention [1]. However, many of our diagnostic capabilities have improved, and surgical options now extend beyond subdural electrodes and resection or disconnection. These changes in the new era of epilepsy surgery hinge primarily on the improvement or development of minimally-invasive diagnostic and ablative procedures, as well as the introduction of non-destructive neurostimulation techniques. In addition to subdural grid and strip electrodes, wider use and refinement of stereotactic electroencephalography (SEEG) has permitted invasive electrographic monitoring while avoiding a craniotomy. Beyond lobar or multilobar resection or disconnection, newer ablation procedures include laser interstitial thermal therapy (LITT) guided by magnetic resonance imaging (MRI) and stereotactic radiosurgery (SRS), while neuromodulation techniques now comprise closed-loop responsive neurostimulation (RNS) and open-loop deep brain stimulation (DBS), as well as open- or closed-loop vagus nerve stimulation (VNS). While the expanding armamentarium of surgical interventions in this field is certainly welcomed, it also introduces new challenges in selecting which diagnostic or therapeutic strategy is best for each individual patient.
The goal of this manuscript is to review both novel and traditional interventions in epilepsy surgery, and discuss one possible treatment algorithm for epilepsy surgery in the modern era (Figure 1). With this goal in mind, several disclaimers are in order. The present algorithm reflects the author’s individual opinions and personal biases, and therefore should not be viewed as a definitive clinical guide. Furthermore, no single treatment algorithm is appropriate for every epilepsy center or every patient, as clinical decisions are influenced by institutional availability of technologies and provider opinion and experiences. Also, there are often specific nuances related to individual cases that cannot be captured in a flowchart. While not quite simple, this algorithm is a simplified summary that excludes several clinical scenarios, for the sake of conciseness. Finally, just as quickly as the field of epilepsy surgery has changed in recent decades, we may expect a continued rapid evolution going forward. As such, continued modification and modernization will be required, as has been the case with previous algorithms. The value of this approach, however, is to encourage examination of both old and new surgical options side-by-side, through a critical review of the relevant literature. The timeliness of this topic rests in the fact that despite the introduction of several new anti-epileptic drugs (AEDs) over the past two decades, the proportion of drug resistance amongst epilepsy patients remains at approximately 30–40%, and high rates of morbidity and mortality persist [2]. Furthermore, despite class I evidence and consensus guidelines establishing the efficacy of epilepsy surgery, surgical interventions remain dramatically under-utilized in this disorder, with fewer than 1% of eligible candidates referred for surgical evaluation [2, 3]. Our goal is that an improved understanding of therapeutic options in drug-resistant epilepsy may lead to improved access, utilization, and treatment success.
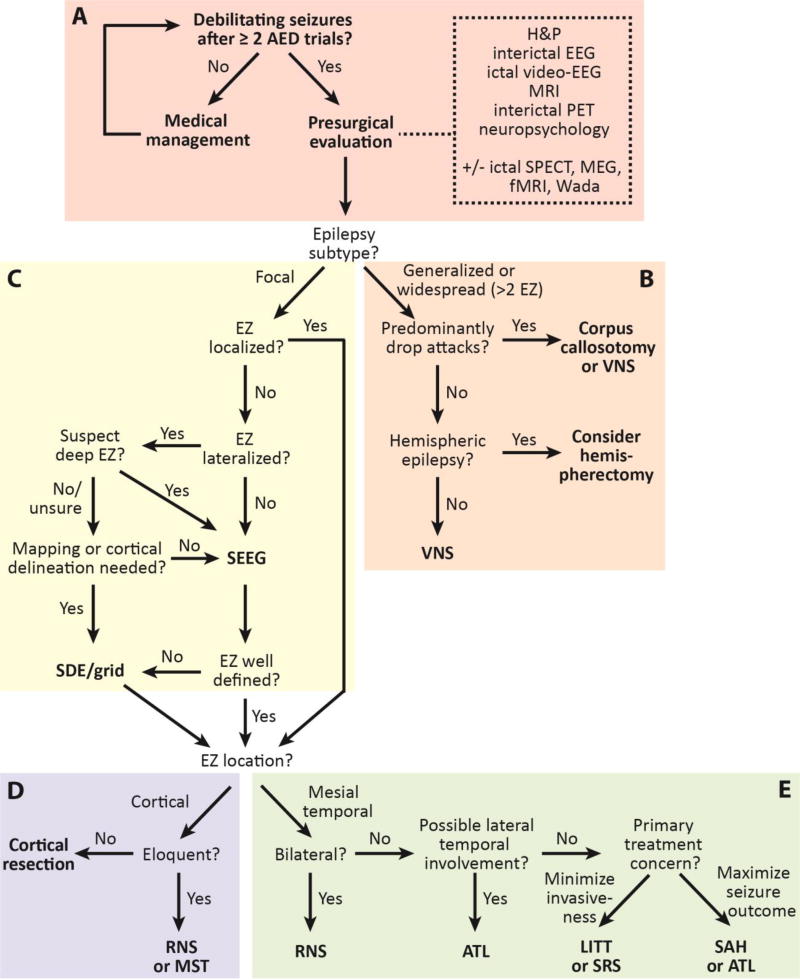
Figure 1. A modern epilepsy surgery treatment algorithm.
The algorithm begins with noninvasive surgical evaluation (A), and the includes treatment of generalized or multifocal epilepsy (B), invasive monitoring decisions (C), and treatment of neocortical (D) or mesial temporal lobe (E) epilepsy. AED: anti-epileptic drug; ATL: anterior temporal lobectomy; EEG: electroencephalography; EZ: epileptogenic zone; fMRI: functional magnetic resonance imaging; LITT: laser interstitial thermal therapy; MEG: magnetoencephalography; MRI: magnetic resonance imaging; MST: multiple subpial transections; PET: position emission tomography; RNS: responsive neurostimulation; SAH: selective amygdalo-hippocampectomy; SDE: subdural electrodes; SEEG: stereotactic electroencephalography; SPECT: single-photon emission computed tomography; SRS: stereotactic radiosurgery
2. Presurgical epilepsy evaluation (Fig. 1A)
Epilepsy patients who continue to have seizures despite treatment trials with two well-tolerated AEDs or drug combinations should be referred to a comprehensive epilepsy center for noninvasive presurgical evaluation. It is well recognized that individuals who fail two AEDs are unlikely to achieve seizure freedom with additional medication trials – a reality that has not changed substantially with the recent introduction of newer AEDs [4, 5]. This recommendation is consistent with consensus guidelines by the American Academy of Neurology and several other North American and international organizations [6–8]. At established epilepsy centers, surgical decision-making involves a multidisciplinary team of neurologists, neurosurgeons, neuropsychologists, neuroradiologists, and other practitioners. When possible, the ultimate goal in epilepsy treatment is to achieve seizure freedom, which is the strongest predictor of quality of life [9].
The goals of the presurgical evaluation are to establish surgical candidacy, epilepsy subtype, and localization of a focal epileptogenic zone (EZ) from which seizures originate, if present. This includes a comprehensive history and physical examination, including detailed evaluation of seizure semiology and frequency, epilepsy duration and symptomatology, and risk factors [10]. Interictal electroencephalography (EEG) and long-term video-EEG are necessary, both to confirm the diagnosis of epilepsy and to correlate with semiology to aid localization.[11] A high-quality 3-Tesla (3T) MRI is equally critical, as the identification of a focal radiographic lesion corresponding to the EZ – such as mesial temporal sclerosis (MTS) – greatly increases the likelihood of surgical candidacy and favorable outcome [12, 13]. Improvements in MRI technology and epilepsy-specific imaging sequences have increased detection rates for radiographic lesion over time [13, 14]. An interictal positron emission tomography (PET) scan is also valuable, as a focal region of hypometabolism may help confirm general EZ location and predict favorable outcome with surgery [15, 16]. For instance, anteromedial temporal lobe hypometabolism in the setting of a normal MRI may portend seizure freedom rates after temporal lobectomy that resemble those with MTS [17]. Furthermore, a comprehensive neuropsychological evaluation is helpful to both localize a symptomatogenic zone, and evaluate neurocognitive performance parameters that may be at risk with certain operative interventions [18].
Several other diagnostic modalities often enter into the presurgical epilepsy work-up, depending on needs of the individual case. Ictal single photon emission computed tomography (SPECT) may be useful to identify an area of hyperperfusion corresponding with focal seizure activity, particularly when subtracting interictal SPECT imaging and overlaying onto an MRI using the SISCOM technique (Subtraction Ictal SPECT Co-registered to MRI) [19]. While subtraction SPECT may have higher positive predictive value than interictal PET, ictal SPECT is more challenging and resource intensive to obtain [20]. In some cases, both PET and SPECT may provide complementary information [20]. Next, interictal magnetoencephalography (MEG) may be a useful tool to help localize epileptogenic spike activity corresponding to the irritative zone, which may also contain the EZ [21]. Interictal spike modelling may not be possible, however, in individuals who have rare discharges. In one study, spikes were modelled in approximately 78% of 132 patients, and were concordant with the presumed EZ in about two-thirds of those individuals [21]. Furthermore, either MEG or functional MRI (fMRI) may be useful for noninvasive localization of eloquent cortex for surgical planning and risk stratification, including motor, sensory, and language neocortices [22]. FMRI is also utilized for lateralization of verbal and visuospatial memory in some centers, although the need for multiple task repetitions make memory localization more challenging than language or sensorimotor localization [22]. For this reason, many centers perform the invasive Wada test – or the intracarotid sodium amobarbital procedure – when memory lateralization is important, such as in mesial temporal lobe epilepsy (TLE) without MTS [23].
The desired outcome of a complete and successful presurgical evaluation is the identification of a focal epilepsy syndrome with a well-localized EZ, as these cases portend the most favorable surgical outcome. However, further study with intracranial EEG may be required for precise EZ localization. Alternatively, data may suggest an epilepsy syndrome with primary generalized seizures or seizures with a diffuse or multifocal seizure onset zone.
3. Surgical treatment of widespread or multifocal EZs and generalized epilepsy (Fig. 1B)
While a multilobar EZ, multifocal EZs, or a generalized epilepsy syndrome presents a therapeutic challenge, surgical options may nonetheless be available. Some individuals, for instance, suffer from frequent, poorly localized or rapidly-generalizing seizures which result in “drop attacks,” including atonic or tonic seizures. Palliative surgical options for these patients include corpus callosotomy or VNS. While complete seizure freedom is relatively uncommon with these therapies, either may reduce the morbidity of atonic or tonic seizures by preventing contralateral seizure spread through commissural fibers (callosotomy), or by decreasing the frequency and severity of drop attacks (VNS).
Corpus callosotomy for frequent drop attacks is most often performed in children, but can also be utilized in adult patients to treat rapidly-generalizing seizures. Section of only the anterior two-thirds of the corpus callosum is frequently performed, while others advocate for a complete callosotomy. One systematic review restricted to children suggested that a complete callosotomy may be associated with a greater reduction in seizures than partial section, although it also carries a somewhat higher risk of transient disconnection syndrome symptoms [24]. In one large patient series, freedom from drop attacks was seen in 67% of individuals after anterior complete callosotomy [25], although long-term studies suggest more modest seizure outcomes, and freedom from all seizures is rare.
Alternatively, VNS represents a neuromodulation approach to treat patients with tonic or atonic seizures. Data from a large, manufacturer-maintained database show a 43% reduction in drop attack frequency 3 months after VNS implantation, with a 75% decrease observed in patients receiving treatment > 2 years [26]. However, one systematic review of 26 case series found that patients were more likely to achieve > 50% reduction in atonic seizure frequency with callosotomy (86%) compared to VNS (58%) [27]. In choosing between these therapies, it is important to consider rates of seizure outcome, adverse events, and patient preference, although class I data are not available for either treatment in this patient population. Finally, a patient with persistent drop attacks after one surgical treatment may remain a candidate for the other approach.
In patients with a catastrophic hemispheric epilepsy syndrome and frequent seizures, corpus callosotomy and VNS are typically insufficient to produce a meaningful reduction in seizures, and an anatomic or functional hemispherectomy procedure may be considered. These patients include those with hemimegalencephaly, Sturge-Weber syndrome, Rasmussen encephalitis, or extensive ischemic, hemorrhagic, traumatic damage to one cerebral hemisphere. While anatomic hemispherectomy involves resection of all lobar gray matter while preserving subcortical structures, functional hemispherectomy (or hemispherotomy) typically includes a large central resection and temporal lobectomy together with disconnection of the frontal and parieto-occipital white matter, together with corpus callosotomy [28]. In appropriately selected patients, seizure outcomes are favorable. In one large series of 170 hemispherectomy procedures in children, approximately two-thirds of patients remained seizure-free 5 years (mean) after surgery [29]. Hemispherectomy is most often performed in infancy or early childhood for a severe, progressive, and drug-resistant epilepsy syndrome associated with contralateral hemiparesis and hemianopia together with marked neurocognitive impairments. However, hemispherectomy in older pediatric or adult patients may be considered when limited neurological function is presumed in the affected hemisphere [30]. Thus, factors to consider in selecting patients for hemispherectomy include epilepsy severity, suspicion of a hemispheric syndrome, patient age and likelihood for plasticity, and baseline neurological deficits.
Patients with multifocal, poorly localized, or generalized epilepsy syndromes that are not appropriate for hemispherectomy or corpus callosotomy may be candidates for neurostimulation with VNS. Notably, most of the patients in the randomized-controlled pivotal trials for VNS suffered from localization-related epilepsy, and United States (US) Food and Drug Administration (FDA) approval includes only focal epilepsy syndromes [31, 32]. However, several observational studies have demonstrated efficacy of VNS in primary generalized epilepsy, and one large meta-analysis suggested a greater reduction in seizure frequency in patients with generalized or multifocal epilepsy (58%) than focal epilepsy (43%, last follow-up) [33]. One major advantage of VNS in generalized/multifocal epilepsy is that neuroanatomical targeting of a specific EZ is not necessary. VNS primarily uses open-loop intermittent stimulation of the left vagus nerve, although closed-loop stimulation utilizing ictal tachycardia as a sign of possible seizure activity is now also being utilized [34]. While the median reduction in seizure frequency with VNS was relatively low in randomized-controlled trials with short 3-month follow-up (25–28%) [31, 32], long-term observational studies have demonstrated progressively improved efficacy over time, with > 50% median seizure reduction after two years of treatment [35]. Nevertheless, complete seizure freedom is uncommon with VNS (approximately 8% after two years) [35], and therefore targeted treatment of the EZ remains favored in patients with a well-localized seizure onset zone who are amenable to an intracranial surgical procedure.
Of note, DBS of the anterior thalamus represents another neurostimulation therapy that does not require EZ localization, and therefore may become an appropriate treatment option for poorly localized epilepsy. However, the pivotal Stimulation of the Anterior Nucleus of Thalamus for Epilepsy (SANTE) trial evaluated only focal epilepsy patients [36], and studies examining DBS in generalized epilepsy patients have not yet been reported. Nevertheless, these clinical investigations will be important in the future.
4. Intracranial monitoring options for EZ localization (Fig. 1C)
After the non-invasive presurgical evaluation, it is often possible to localize the EZ with a high level of confidence and proceed directly to a definitive surgical treatment. This is common in cases with a radiographic lesion on MRI that correlates with other diagnostic data, or when study results and semiology are concordant and strongly suggest mesial TLE. In other cases, intracranial EEG monitoring may be necessary to capture ictal and interictal electrographic data for EZ delineation, with electrode implantation followed by an inpatient stay in the epilepsy monitoring unit of days to weeks. The two most common approaches to intracranial EEG include craniotomy for implantation of subdural electrodes (SDEs), including grid and strip with or without depth electrodes, or SEEG depth electrode placement without craniotomy. Both procedures require hypotheses a priori regarding possible EZ location or network involvement, given that electrode coverage is limited. While multiple burr holes for subdural strip electrode placement is also sometimes performed, this has become less common with the increased availability of SEEG.
SEEG represents a minimally-invasive approach utilizing a stereotactic frame, frameless neuronavigation, stereotactic robot, or 3D-printed disposable frame for depth electrode insertion through small stab incisions and 2–3 mm burr holes. The general technique was developed in France in the 1950s [37] and only became widespread outside of Europe in recent years, where SDE implantation has been the traditional approach. The major strength of SEEG lies in the ability to map three-dimensional epileptogenic networks including spatially distinct and deep regions while avoiding craniotomy [38], although spatially continuous coverage of surface gyri is more challenging than with SDEs [39]. SEEG is associated with decreased peri-operative pain and shorter recovery time than SDEs, and large review studies suggest a lower rate of serious adverse events with the former (1.3%) [40] compared to the latter (3.4%) [41]. An additional convenience of SEEG is that timing of the definitive surgical treatment is not influenced by the need to return to the operating room for grid explantation, as no craniotomy has been performed, and SEEG removal is a minor procedure.
If the EZ is poorly lateralized and sampling of bilateral regions is required, SEEG is typically the preferred approach, to avoid bilateral craniotomies. However, a unilateral craniotomy with contralateral stereotactic depths is possible if extensive neocortical coverage is required on only one side, together with sparser sampling in the second hemisphere. If the regions of greatest interest are in deep locations, such as periventricular or insular regions, SEEG will provide easier access. Sampling of the insula can be performed with SDEs, albeit requiring microsurgical splitting of the Sylvian fissure. Interhemispheric cortex can be sampled with SEEG or with a craniotomy over midline to place SDEs under direct visualization, to avoid venous injury [42]. A craniotomy for grid implantation supplemented by depth electrode placement is also an option for some cases, for example when lateral fronto-temporal cortical coverage is desired together with sampling of mesial temporal structures. However, accuracy may be affected when stereotactically placing depth electrodes once an open craniotomy has allowed brain shift.
If a case requires careful delineation of the EZ borders in a neocortical region, such as cortical regions involving or adjacent to eloquent cortex, a craniotomy for grid placement may be the preferred approach. This more easily allows continuous coverage over a large area of cortical surface, and also permits mapping of eloquent cortex using direct cortical stimulation in the epilepsy monitoring unit, if desired. While both extensive neocortical coverage and mapping of eloquent cortex are possible with SEEG [43], a large number of electrodes must be strategically planned to achieve a similar result. However, it must also be considered that SDEs will not allow sampling of gray matter in sulci, which is achievable with SEEG. Finally, if SEEG results suggest EZ location in an approximate neocortical area, but more detailed cortical delineation is required before definitive treatment, a second monitoring procedure with craniotomy for targeted SDE placement can be considered. Conversely, SEEG of targeted structures after an inconclusive grid procedure is also achievable [44].
5. Surgical treatment of focal neocortical epilepsy (Fig. 1D)
When an EZ is localized to a focal neocortical region, and resection can be safely performed without producing a neurological deficit, resection is the preferred surgical treatment. Unlike surgery for TLE, no randomized, controlled trials have evaluated resection for focal neocortical epilepsy. However, numerous case series have been reported in both adult and pediatric populations, and literature reviews have suggested that post-operative seizure freedom is achieved in approximately 40–60% of patients [45–47]. Furthermore, outcomes are often more favorable in cases with a radiographic neocortical lesion that can be excised completely, such as a brain tumor or vascular malformation, and insufficient extent of resection is the most common reason for surgical failure [12, 48]. Methods for stereotactic ablation of extra-temporal foci have also been described as alternatives to resection. For instance, MRI-guided LITT has been used for ablation of epileptogenic cavernous malformation, hypothalamic hamartomas, and focal cortical dysplasia [49, 50], and stereotactic radiofrequency ablation has been described to treat epilepsy related to periventricular heterotopia [51]. While these offer promising alternatives to craniotomy, further studies examining long-term seizure outcomes in large cohorts are needed.
Complete resection of the EZ may not be possible when it overlaps with eloquent cortex – such as regions serving motor, language, or vision function – but resection to the limits of functional cortex may be considered when guided by extra- or intra-operative mapping with direct cortical stimulation. Nevertheless, for cases in which aggressive resection is not possible without producing a neurological deficit, a tissue-sparing approach such as RNS or multiple subpial transections (MST) should be considered. Either procedure can be performed alone, or together with a resection of adjacent non-eloquent epileptogenic cortex. VNS can also be considered, particularly if a patient prefers to avoid an intracranial procedure.
In the US, RNS received FDA approval in 2013 for the treatment of focal epilepsy in adults, and it has been used off-label in children. As a closed-loop system, stimulation is triggered by early detection of possible epileptogenic activity from constant intracranial EEG recordings, and is customized to each patient. During a 12-week blinded period of a randomized, controlled pivotal trial, greater reduction in seizure frequency was seen with active stimulation (38%) compared to sham treatment (17%) [52]. Over time, epilepsy response rates improve with RNS, as with other neurostimulation-based treatments such as VNS and DBS [53]. In a large observational cohort of 126 patients with focal neocortical epilepsy, median percent seizure reduction was 70% in those with frontal or parietal seizures, and 58% in individuals with lateral temporal lobe epilepsy, with a mean follow-up of 6 years [54]. No stimulation-related neurological deficits were reported and complications, including hemorrhage or infection, were uncommon. Importantly, given that RNS can accommodate two active four-contact depth or strip electrodes, treatment of two suspect EZs is possible [54].
MST was first described by Morrell in 1989 as a focal disconnective procedure for individuals with eloquent neocortical EZ [55]. The procedure requires numerous parallel subpial incisions applied to the neocortex to sever tangential intracortical fibers, with the goal of preventing horizontal seizure spread without interfering with vertical communication of neuronal signals. Early studies reported relatively favorable seizure outcomes in approximately 60% of patients with neocortical epilepsy [56], and a recent meta-analysis of 34 case series observed seizure freedom in approximately 55% of individuals treated with MST together with resection, and 24% of patients receiving MST alone [57]. However, the likelihood of producing a neurological deficit appears higher with MST compared to RNS in treating epileptogenic eloquent cortex [57]. Nevertheless, MST may be a desirable option in these patients who wish to avoid permanently-implanted intracranial hardware, or who are unable to take an active participatory in their care, which is necessary with RNS.
6. Surgical treatment of mesial TLE (Fig. 1D)
Mesial TLE is the most common epilepsy syndrome, and is associated with significant morbidity. The surgical treatment of mesial TLE has been well-studied, and success rates are relatively favorable, particularly in the setting of MTS. However, seizure origination in both hippocampi presents a particular surgical challenge, as resection or ablation of bilateral hippocampi would create devastating neuropsychological consequences. If the vast majority of seizures in a bitemporal epilepsy patient originate on one side, palliative resection or ablation of one temporal lobe may produce favorable results [58]. A brief period of inpatient EEG recordings of one to three weeks, however, may be insufficient to quantify the frequency of bilateral ictal events. RNS represents an option in which leads can be placed longitudinally in the bilateral hippocampi for neurostimulation-based treatment of seizures, and the device can also be used for long-term seizure monitoring, which may in some cases lead to a more definitive surgical procedure.
One large, long-term observational study examined 111 patients with mesial TLE treated with RNS, including 72% with bilateral onset, and 28% with unilateral seizures [59]. For patients receiving a unilateral implant, providers typically had concern for verbal memory decline with resection, or the patient had failed a previous contralateral temporal lobectomy. At mean followup of 6 years, a 70% median decrease in seizure frequency was observed, with no differences seen between unilateral or bilateral patients. In a small number of patients with bitemporal RNS, unilateral resection has been performed after recordings over time suggested predominantly unilateral seizure onset [59, 60]. Thus, RNS may be considered for treatment and/or long-term data collection in patients with bilateral mesial TLE, or in individuals with unilateral TLE who are not candidates for resection or ablation, including those who have had a previous contralateral procedure.
When possible, surgical resection is the gold standard treatment for drug-resistant mesial TLE. The two most common approaches include anterior temporal lobectomy (ATL) – including removal of the anterior hippocampus, amygdala, temporal pole, and anterolateral temporal cortex – or selective amygdalohippocampectomy (SAH). SAH may be performed using several surgical strategies, the most common of which include a transcortical, trans-Sylvian, or subtemporal approach. In addition, ablation of the mesial temporal structures is a relatively new alternative to focal resection.
If lateral temporal epileptogenicity is suspected along with mesial temporal lobe seizure onset, ATL is the preferred surgical approach. Example cases include those with mesial temporal sclerosis together with a basal temporal encephalocele or temporal neocortical lesion (dual pathology), or those with significant lateral epileptiform discharges on intracranial recordings together with hippocampal seizure onset. The efficacy of ATL for TLE has been demonstrated by two randomized, controlled trials comparing surgery to continued medical therapy. In the first trial, seizure freedom was significantly more common among 40 patients randomized to surgery (58%) compared to 40 individuals receiving best medical therapy (8%) at one-year followup.[61] Another trial included only patients with a new (< 2 years) diagnosis of drug-resistant epilepsy, and compared early ATL to continued medication [62]. At 2-year follow-up, seizure freedom was observed in 11 of 15 (73%) individuals in the surgical group, but none (0%) of 23 participants in the medical group, demonstrating superiority of early surgical intervention in TLE.
If lateral temporal involvement is not suspected, selective treatment of mesial temporal structures with resection or ablation may be considered, versus a standard ATL. This surgical decision will inherently be influenced by provider opinion, patient preference, and technology availability. While newer ablative procedures carry the benefit of avoiding craniotomy, reduced hospital stay, and diminished peri-procedural pain, surgical resection remains the option associated with the highest likelihood of seizure freedom, both immediately after surgery and in the long-term. With regard to resection for mesial TLE, there is disagreement regarding the benefit of neocortical tissue removal. However, a large meta-analysis of 1,203 patients in 14 studies suggests somewhat improved seizure outcomes with ATL over SAH, with a 8% increased likelihood of seizure freedom with the former [63]. It is unknown if the increased likelihood of seizure freedom with ATL is related to lateral temporal cortical resection, or a greater extent of mesial structure removal given larger exposure. Furthermore, there is disagreement in the literature about whether SAH carries a lower risk of neuropsychological dysfunction than ATL, particularly with regard to naming and memory, or if outcomes are comparable [64, 65].
If the patient or provider wishes to pursue a less invasive treatment option, MRI-guided LITT has become increasingly popular for the treatment of mesial TLE, and stereotactic radiosurgery (SRS) has also been explored at several centers. LITT is performed with a fiberoptic laser probe, using a longitudinal approach across the long axis of the hippocampus and into the amygdala and uncus. Series ablations can be performed using real-time MRI thermography to track temperatures of ablated tissue and surrounding critical structures. Published clinical series report rates of seizure freedom of 41–54% at approximately one year after surgery, suggesting somewhat less favorable seizure outcomes than resection [50, 66, 67]. Some investigators have observed better naming and object recognition outcomes with LITT compared to resection for mesial TLE [68], although larger clinical series and long-term data for both seizure and neuropsychological outcomes are needed. Furthermore, resection remains an option for patients who have failed LITT.
Finally, SRS is an intervention for mesial TLE that has been explored as an alternative to surgery. SRS is typically performed with gamma knife radiation targeting the mesial temporal structures with a single dose treatment. In a pilot multi-centered prospective trial, seizure freedom was observed in 77% of 13 individuals who received higher dose (24 Gy) therapy and in 59% of 17 patients who received lower dose (20 Gy) treatment, with one-year follow-up [69]. A prospective randomized trial of SRS versus open temporal lobectomy (Radiosurgery or Open Surgery for Epilepsy [ROSE] trial) was recently completed, and publication of the results are pending. Although preliminary results suggest seizure outcome rates may be comparable to surgical resection, the beneficial effects of SRS may be delayed up to 12 months [70]. This creates a period of uncertainty in the months after the procedure, compared to other invasive treatments. While the future role of SRS for mesial TLE remains unclear in light of increasing use of LITT for this disorder, SRS does represent the only intervention that avoids invasive surgery and general anesthesia.
7. Conclusions
In this review, we discussed current surgical procedures for drug-resistant epilepsy, and presented a general framework for integrating traditional and emerging technologies into practice at a comprehensive epilepsy center. However, many clinical scenarios and less common surgical approaches are beyond the scope of this review, and practice patterns differ between centers. In particular, while both children and adults are considered together in this review for the sake of conciseness, the pathology, treatment philosophy, and treatment approach often differs in these patient populations. For example, hemispherectomy may be a reasonable option in a young child with catastrophic epilepsy, but may be associated with unacceptable morbidity in an adult, while RNS is considered more frequently in adult patients than young children. Furthermore, while mesial TLE is the most common epilepsy syndrome in adults, neocortical seizure onset is more commonly observed in pediatric populations.
Finally, treatment option must be considered in light of currently available evidence, and relatively few randomized, controlled trials have been performed in epilepsy surgery, with key trials summarized in Table 1. While resection for TLE and neurostimulation procedures have been evaluated in controlled clinical trials, evidence for many other procedures is limited to observational cases series, including intracranial monitoring, neocortical resection, disconnection procedures, and ablation therapies. While randomized trials may not be practical in all scenarios, rigorous prospective studies are particularly essential now that the number of interventions available has increased. Moving forward, it will be important to develop a framework for outcome data collection in epilepsy surgery, related not only to seizure improvement, but also neuropsychological outcomes, quality of life, and adverse events. Continued education is needed to encourage the referral of drug-resistant epilepsy patients to tertiary epilepsy centers, where the comprehensive tools for evaluation and treatment discussed herein can be offered using a multidisciplinary approach.
Table 1.
Key Randomized, Controlled Trials of Seizure Outcomes after Epilepsy Surgery
| Therapy | Studies |
|---|---|
| Anterior Temporal Lobectomy (ATL) | Engel et al., 2012[62]; Wiebe et al., 2001[61]; |
| Resection in Children | Dwivedi et al., 2017[71] |
| Deep Brain Stimulation (DBS) | Fisher et al., 2010[36] |
| Responsive Neurostimulation (RNS) | Morrel et al., 2011[52] |
| Stereotactic Radiosurgery (SRS) | Barbaro et al. (completed, pending publication) |
| Vagus Nerve Stimulation (VNS) | Amar et al., 1998[72]; Ben-Menachem et al., 1994[31]; DeGiorgio et al., 2005[73]; Handforth et al., 1998[74]; Scherrmann et al., 2011[75] |
Highlights.
-
-
Several new diagnostic and therapeutic surgical procedures for drug-resistant epilepsy have been introduced in recent years.
-
-
In addition to subdural grids and resection, minimally-invasive, non-destructive, and neuromodulation surgical approaches are also now available.
-
-
Choosing between traditional and emerging epilepsy surgery technologies requires comparison of study results and outcome data.
-
-
Epilepsy surgery treatment algorithms may help guide decision-making, but require center-specific customization and personalization.
Acknowledgments
None
Funding: DJE is supported by NIH R00 NS086353.
Abbreviations
- AED
anti-epileptic drug
- ATL
anterior temporal lobectomy
- EEG
electroencephalography
- EZ
epileptogenic zone
- fMRI
functional magnetic resonance imaging
- LITT
laser interstitial thermal therapy
- MEG
magnetoencephalography
- MRI
magnetic resonance imaging
- MST
multiple subpial transections
- PET
position emission tomography
- RNS
responsive neurostimulation
- SAH
selective amygdalo-hippocampectomy
- SDE
subdural electrodes
- SEEG
stereotactic electroencephalography
- SPECT
single-photon emission computed tomography
- SRS
stereotactic radiosurgery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: The author reports no conflicts of interest to disclose.
References
- 1.Jobst BC. Treatment algorithms in refractory partial epilepsy. Epilepsia. 2009;50(Suppl 8):51–6. doi: 10.1111/j.1528-1167.2009.02236.x. [DOI] [PubMed] [Google Scholar]
- 2.Engel J., Jr What can we do for people with drug-resistant epilepsy? The 2016 Wartenberg Lecture. Neurology. 2016;87:2483–2489. doi: 10.1212/WNL.0000000000003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Englot DJ, Ouyang D, Garcia PA, Barbaro NM, Chang EF. Epilepsy surgery trends in the United States, 1990–2008. Neurology. 2012;78:1200–6. doi: 10.1212/WNL.0b013e318250d7ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–9. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 5.Pohlen MS, Jin J, Tobias RS, Maheshwari A. Pharmacoresistance with newer antiepileptic drugs in mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsy Res. 2017;137:56–60. doi: 10.1016/j.eplepsyres.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labiner DM, Bagic AI, Herman ST, Fountain NB, Walczak TS, Gumnit RJ National Association of Epilepsy C. Essential services, personnel, and facilities in specialized epilepsy centers--revised 2010 guidelines. Epilepsia. 2010;51:2322–33. doi: 10.1111/j.1528-1167.2010.02648.x. [DOI] [PubMed] [Google Scholar]
- 7.Engel J, Jr, Wiebe S, French J, Sperling M, Williamson P, Spencer D, Gumnit R, Zahn C, Westbrook E, Enos B. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60:538–47. doi: 10.1212/01.wnl.0000055086.35806.2d. [DOI] [PubMed] [Google Scholar]
- 8.Cross JH, Jayakar P, Nordli D, Delalande O, Duchowny M, Wieser HG, Guerrini R, Mathern GW International League against Epilepsy SfPES, Commissions of N, Paediatrics. Proposed criteria for referral and evaluation of children for epilepsy surgery: recommendations of the Subcommission for Pediatric Epilepsy Surgery. Epilepsia. 2006;47:952–9. doi: 10.1111/j.1528-1167.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- 9.Pauli C, Schwarzbold ML, Diaz AP, de Oliveira Thais MER, Kondageski C, Linhares MN, Guarnieri R, de Lemos Zingano B, Ben J, Nunes JC, Markowitsch HJ, Wolf P, Wiebe S, Lin K, Walz R. Predictors of meaningful improvement in quality of life after temporal lobe epilepsy surgery: A prospective study. Epilepsia. 2017;58:755–763. doi: 10.1111/epi.13721. [DOI] [PubMed] [Google Scholar]
- 10.So EL. Value and limitations of seizure semiology in localizing seizure onset. J Clin Neurophysiol. 2006;23:353–7. doi: 10.1097/01.wnp.0000228498.71365.7b. [DOI] [PubMed] [Google Scholar]
- 11.Guideline twelve: guidelines for long-term monitoring for epilepsy. American Electroencephalographic Society. J Clin Neurophysiol. 1994;11:88–110. [PubMed] [Google Scholar]
- 12.Englot DJ, Chang EF. Rates and predictors of seizure freedom in resective epilepsy surgery: an update. Neurosurg Rev. 2014;37:389–404. doi: 10.1007/s10143-014-0527-9. discussion 404–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deblaere K, Achten E. Structural magnetic resonance imaging in epilepsy. Eur Radiol. 2008;18:119–29. doi: 10.1007/s00330-007-0710-2. [DOI] [PubMed] [Google Scholar]
- 14.Zijlmans M, de Kort GA, Witkamp TD, Huiskamp GM, Seppenwoolde JH, van Huffelen AC, Leijten FS. 3T versus 1.5T phased-array MRI in the presurgical work-up of patients with partial epilepsy of uncertain focus. J Magn Reson Imaging. 2009;30:256–62. doi: 10.1002/jmri.21811. [DOI] [PubMed] [Google Scholar]
- 15.Capraz IY, Kurt G, Akdemir O, Hirfanoglu T, Oner Y, Sengezer T, Kapucu LO, Serdaroglu A, Bilir E. Surgical outcome in patients with MRI-negative, PET-positive temporal lobe epilepsy. Seizure. 2015;29:63–8. doi: 10.1016/j.seizure.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Wong CH, Bleasel A, Wen L, Eberl S, Byth K, Fulham M, Somerville E, Mohamed A. Relationship between preoperative hypometabolism and surgical outcome in neocortical epilepsy surgery. Epilepsia. 2012;53:1333–40. doi: 10.1111/j.1528-1167.2012.03547.x. [DOI] [PubMed] [Google Scholar]
- 17.LoPinto-Khoury C, Sperling MR, Skidmore C, Nei M, Evans J, Sharan A, Mintzer S. Surgical outcome in PET-positive, MRI-negative patients with temporal lobe epilepsy. Epilepsia. 2012;53:342–8. doi: 10.1111/j.1528-1167.2011.03359.x. [DOI] [PubMed] [Google Scholar]
- 18.Loring DW. Neuropsychological evaluation in epilepsy surgery. Epilepsia. 1997;38(Suppl 4):S18–23. doi: 10.1111/j.1528-1157.1997.tb04535.x. [DOI] [PubMed] [Google Scholar]
- 19.Sulc V, Stykel S, Hanson DP, Brinkmann BH, Jones DT, Holmes DR, 3rd, Robb RA, Senjem ML, Mullan BP, Watson RE, Jr, Horinek D, Cascino GD, Wong-Kisiel LC, Britton JW, So EL, Worrell GA. Statistical SPECT processing in MRI-negative epilepsy surgery. Neurology. 2014;82:932–9. doi: 10.1212/WNL.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai A, Bekelis K, Thadani VM, Roberts DW, Jobst BC, Duhaime AC, Gilbert K, Darcey TM, Studholme C, Siegel A. Interictal PET and ictal subtraction SPECT: sensitivity in the detection of seizure foci in patients with medically intractable epilepsy. Epilepsia. 2013;54:341–50. doi: 10.1111/j.1528-1167.2012.03686.x. [DOI] [PubMed] [Google Scholar]
- 21.Englot DJ, Nagarajan SS, Imber BS, Raygor KP, Honma SM, Mizuiri D, Mantle M, Knowlton RC, Kirsch HE, Chang EF. Epileptogenic zone localization using magnetoencephalography predicts seizure freedom in epilepsy surgery. Epilepsia. 2015;56:949–58. doi: 10.1111/epi.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szaflarski JP, Gloss D, Binder JR, Gaillard WD, Golby AJ, Holland SK, Ojemann J, Spencer DC, Swanson SJ, French JA, Theodore WH. Practice guideline summary: Use of fMRI in the presurgical evaluation of patients with epilepsy: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2017;88:395–402. doi: 10.1212/WNL.0000000000003532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abou-Khalil B. An update on determination of language dominance in screening for epilepsy surgery: the Wada test and newer noninvasive alternatives. Epilepsia. 2007;48:442–55. doi: 10.1111/j.1528-1167.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 24.Graham D, Tisdall MM, Gill D. Corpus callosotomy outcomes in pediatric patients: A systematic review. Epilepsia. 2016;57:1053–68. doi: 10.1111/epi.13408. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu H. Our experience with pediatric epilepsy surgery focusing on corpus callosotomy and hemispherotomy. Epilepsia. 2005;46(Suppl 1):30–1. doi: 10.1111/j.0013-9580.2005.461009.x. [DOI] [PubMed] [Google Scholar]
- 26.Englot DJ, Chang EF, Auguste KI. Efficacy of vagus nerve stimulation for epilepsy by patient age, epilepsy duration, and seizure type. Neurosurg Clin N Am. 2011;22:443–8. v. doi: 10.1016/j.nec.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Rolston JD, Englot DJ, Wang DD, Garcia PA, Chang EF. Corpus callosotomy versus vagus nerve stimulation for atonic seizures and drop attacks: A systematic review. Epilepsy Behav. 2015;51:13–7. doi: 10.1016/j.yebeh.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmussen T. Postoperative superficial hemosiderosis of the brain, its diagnosis, treatment and prevention. Trans Am Neurol Assoc. 1973;98:133–7. [PubMed] [Google Scholar]
- 29.Moosa AN, Gupta A, Jehi L, Marashly A, Cosmo G, Lachhwani D, Wyllie E, Kotagal P, Bingaman W. Longitudinal seizure outcome and prognostic predictors after hemispherectomy in 170 children. Neurology. 2013;80:253–60. doi: 10.1212/WNL.0b013e31827dead9. [DOI] [PubMed] [Google Scholar]
- 30.Schusse CM, Smith K, Drees C. Outcomes after hemispherectomy in adult patients with intractable epilepsy: institutional experience and systematic review of the literature. J Neurosurg. 2017:1–9. doi: 10.3171/2016.9.JNS151778. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Menachem E, Manon-Espaillat R, Ristanovic R, Wilder BJ, Stefan H, Mirza W, Tarver WB, Wernicke JF. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35:616–26. doi: 10.1111/j.1528-1157.1994.tb02482.x. [DOI] [PubMed] [Google Scholar]
- 32.Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, Henry TR, Collins SD, Vaughn BV, Gilmartin RC, Labar DR, Morris GL, 3rd, Salinsky MC, Osorio I, Ristanovic RK, Labiner DM, Jones JC, Murphy JV, Ney GC, Wheless JW. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 1998;51:48–55. doi: 10.1212/wnl.51.1.48. [DOI] [PubMed] [Google Scholar]
- 33.Englot DJ, Chang EF, Auguste KI. Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. J Neurosurg. 2011;115:1248–55. doi: 10.3171/2011.7.JNS11977. [DOI] [PubMed] [Google Scholar]
- 34.Fisher RS, Afra P, Macken M, Minecan DN, Bagic A, Benbadis SR, Helmers SL, Sinha SR, Slater J, Treiman D, Begnaud J, Raman P, Najimipour B. Automatic Vagus Nerve Stimulation Triggered by Ictal Tachycardia: Clinical Outcomes and Device Performance--The U.S. E-37 Trial. Neuromodulation. 2016;19:188–95. doi: 10.1111/ner.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Englot DJ, Rolston JD, Wright CW, Hassnain KH, Chang EF. Rates and Predictors of Seizure Freedom With Vagus Nerve Stimulation for Intractable Epilepsy. Neurosurgery. 2016;79:345–53. doi: 10.1227/NEU.0000000000001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, Oommen K, Osorio I, Nazzaro J, Labar D, Kaplitt M, Sperling M, Sandok E, Neal J, Handforth A, Stern J, DeSalles A, Chung S, Shetter A, Bergen D, Bakay R, Henderson J, French J, Baltuch G, Rosenfeld W, Youkilis A, Marks W, Garcia P, Barbaro N, Fountain N, Bazil C, Goodman R, McKhann G, Babu Krishnamurthy K, Papavassiliou S, Epstein C, Pollard J, Tonder L, Grebin J, Coffey R, Graves N Group SS. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 37.Bancaud J, Dell M. Technics and method of stereotaxic functional exploration of the brain structures in man (cortex, subcortex, central gray nuclei) Rev Neurol. 1959;101:213–227. [PubMed] [Google Scholar]
- 38.Gonzalez-Martinez JA. The Stereo-Electroencephalography: The Epileptogenic Zone. J Clin Neurophysiol. 2016;33:522–529. doi: 10.1097/WNP.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 39.Kalamangalam GP, Tandon N. Stereo-EEG Implantation Strategy. J Clin Neurophysiol. 2016;33:483–489. doi: 10.1097/WNP.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 40.Mullin JP, Shriver M, Alomar S, Najm I, Bulacio J, Chauvel P, Gonzalez-Martinez J. Is SEEG safe? A systematic review and meta-analysis of stereo-electroencephalography-related complications. Epilepsia. 2016;57:386–401. doi: 10.1111/epi.13298. [DOI] [PubMed] [Google Scholar]
- 41.Rolston JD, Englot DJ, Cornes S, Chang EF. Major and minor complications in extraoperative electrocorticography: A review of a national database. Epilepsy Res. 2016;122:26–9. doi: 10.1016/j.eplepsyres.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bekelis K, Radwan TA, Desai A, Moses ZB, Thadani VM, Jobst BC, Bujarski KA, Darcey TM, Roberts DW. Subdural interhemispheric grid electrodes for intracranial epilepsy monitoring: feasibility, safety, and utility: clinical article. J Neurosurg. 2012;117:1182–8. doi: 10.3171/2012.8.JNS12258. [DOI] [PubMed] [Google Scholar]
- 43.Trebuchon A, Chauvel P. Electrical Stimulation for Seizure Induction and Functional Mapping in Stereoelectroencephalography. J Clin Neurophysiol. 2016;33:511–521. doi: 10.1097/WNP.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 44.Vadera S, Mullin J, Bulacio J, Najm I, Bingaman W, Gonzalez-Martinez J. Stereoelectroencephalography following subdural grid placement for difficult to localize epilepsy. Neurosurgery. 2013;72:723–9. doi: 10.1227/NEU.0b013e318285b4ae. discussion 729. [DOI] [PubMed] [Google Scholar]
- 45.Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128:1188–98. doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- 46.Englot DJ, Breshears JD, Sun PP, Chang EF, Auguste KI. Seizure outcomes after resective surgery for extra-temporal lobe epilepsy in pediatric patients. J Neurosurg Pediatr. 2013;12:126–33. doi: 10.3171/2013.5.PEDS1336. [DOI] [PubMed] [Google Scholar]
- 47.Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7:525–37. doi: 10.1016/S1474-4422(08)70109-1. [DOI] [PubMed] [Google Scholar]
- 48.Englot DJ, Raygor KP, Molinaro AM, Garcia PA, Knowlton RC, Auguste KI, Chang EF. Factors associated with failed focal neocortical epilepsy surgery. Neurosurgery. 2014;75:648–56. doi: 10.1227/NEU.0000000000000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCracken DJ, Willie JT, Fernald B, Saindane AM, Drane DL, Barrow DL, Gross RE. Magnetic Resonance Thermometry-Guided Stereotactic Laser Ablation of Cavernous Malformations in Drug-Resistant Epilepsy: Imaging and Clinical Results. Oper Neurosurg (Hagerstown) 2016;12:39–48. doi: 10.1227/NEU.0000000000001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis EC, Weil AG, Duchowny M, Bhatia S, Ragheb J, Miller I. MR-guided laser interstitial thermal therapy for pediatric drug-resistant lesional epilepsy. Epilepsia. 2015;56:1590–8. doi: 10.1111/epi.13106. [DOI] [PubMed] [Google Scholar]
- 51.Cossu M, Mirandola L, Tassi L. RF-ablation in periventricular heterotopia-related epilepsy. Epilepsy Res. 2017 doi: 10.1016/j.eplepsyres.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Morrell MJ Group RNSSiES. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 53.Bergey GK, Morrell MJ, Mizrahi EM, Goldman A, King-Stephens D, Nair D, Srinivasan S, Jobst B, Gross RE, Shields DC, Barkley G, Salanova V, Olejniczak P, Cole A, Cash SS, Noe K, Wharen R, Worrell G, Murro AM, Edwards J, Duchowny M, Spencer D, Smith M, Geller E, Gwinn R, Skidmore C, Eisenschenk S, Berg M, Heck C, Van Ness P, Fountain N, Rutecki P, Massey A, O'Donovan C, Labar D, Duckrow RB, Hirsch LJ, Courtney T, Sun FT, Seale CG. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology. 2015;84:810–7. doi: 10.1212/WNL.0000000000001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jobst BC, Kapur R, Barkley GL, Bazil CW, Berg MJ, Bergey GK, Boggs JG, Cash SS, Cole AJ, Duchowny MS, Duckrow RB, Edwards JC, Eisenschenk S, Fessler AJ, Fountain NB, Geller EB, Goldman AM, Goodman RR, Gross RE, Gwinn RP, Heck C, Herekar AA, Hirsch LJ, King-Stephens D, Labar DR, Marsh WR, Meador KJ, Miller I, Mizrahi EM, Murro AM, Nair DR, Noe KH, Olejniczak PW, Park YD, Rutecki P, Salanova V, Sheth RD, Skidmore C, Smith MC, Spencer DC, Srinivasan S, Tatum W, Van Ness P, Vossler DG, Wharen RE, Jr, Worrell GA, Yoshor D, Zimmerman RS, Skarpaas TL, Morrell MJ. Brain-responsive neurostimulation in patients with medically intractable seizures arising from eloquent and other neocortical areas. Epilepsia. 2017;58:1005–1014. doi: 10.1111/epi.13739. [DOI] [PubMed] [Google Scholar]
- 55.Morrell F, Whisler WW, Bleck TP. Multiple subpial transection: a new approach to the surgical treatment of focal epilepsy. J Neurosurg. 1989;70:231–9. doi: 10.3171/jns.1989.70.2.0231. [DOI] [PubMed] [Google Scholar]
- 56.Spencer SS, Schramm J, Wyler A, O'Connor M, Orbach D, Krauss G, Sperling M, Devinsky O, Elger C, Lesser R, Mulligan L, Westerveld M. Multiple subpial transection for intractable partial epilepsy: an international meta-analysis. Epilepsia. 2002;43:141–5. doi: 10.1046/j.1528-1157.2002.28101.x. [DOI] [PubMed] [Google Scholar]
- 57.Rolston JD, Deng H, Wang DD, Englot DJ, Chang EF. Multiple Subpial Transections for Medically Refractory Epilepsy: A Disaggregated Review of Patient-Level Data. Neurosurgery. 2017 doi: 10.1093/neuros/nyx311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aghakhani Y, Liu X, Jette N, Wiebe S. Epilepsy surgery in patients with bilateral temporal lobe seizures: a systematic review. Epilepsia. 2014;55:1892–901. doi: 10.1111/epi.12856. [DOI] [PubMed] [Google Scholar]
- 59.Geller EB, Skarpaas TL, Gross RE, Goodman RR, Barkley GL, Bazil CW, Berg MJ, Bergey GK, Cash SS, Cole AJ, Duckrow RB, Edwards JC, Eisenschenk S, Fessler J, Fountain NB, Goldman AM, Gwinn RP, Heck C, Herekar A, Hirsch LJ, Jobst BC, King-Stephens D, Labar DR, Leiphart JW, Marsh WR, Meador KJ, Mizrahi EM, Murro AM, Nair DR, Noe KH, Park YD, Rutecki PA, Salanova V, Sheth RD, Shields DC, Skidmore C, Smith MC, Spencer DC, Srinivasan S, Tatum W, Van Ness PC, Vossler DG, Wharen RE, Jr, Worrell GA, Yoshor D, Zimmerman RS, Cicora K, Sun FT, Morrell MJ. Brain-responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia. 2017;58:994–1004. doi: 10.1111/epi.13740. [DOI] [PubMed] [Google Scholar]
- 60.DiLorenzo DJ, Mangubat EZ, Rossi MA, Byrne RW. Chronic unlimited recording electrocorticography-guided resective epilepsy surgery: technology-enabled enhanced fidelity in seizure focus localization with improved surgical efficacy. J Neurosurg. 2014;120:1402–14. doi: 10.3171/2014.1.JNS131592. [DOI] [PubMed] [Google Scholar]
- 61.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–8. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 62.Engel J, Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, Sperling MR, Gardiner I, Erba G, Fried I, Jacobs M, Vinters HV, Mintzer S, Kieburtz K. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. 2012;307:922–30. doi: 10.1001/jama.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Josephson CB, Dykeman J, Fiest KM, Liu X, Sadler RM, Jette N, Wiebe S. Systematic review and meta-analysis of standard vs selective temporal lobe epilepsy surgery. Neurology. 2013;80:1669–76. doi: 10.1212/WNL.0b013e3182904f82. [DOI] [PubMed] [Google Scholar]
- 64.Morino M, Uda T, Naito K, Yoshimura M, Ishibashi K, Goto T, Ohata K, Hara M. Comparison of neuropsychological outcomes after selective amygdalohippocampectomy versus anterior temporal lobectomy. Epilepsy Behav. 2006;9:95–100. doi: 10.1016/j.yebeh.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 65.Bujarski KA, Hirashima F, Roberts DW, Jobst BC, Gilbert KL, Roth RM, Flashman LA, McDonald BC, Saykin AJ, Scott RC, Dinnerstein E, Preston J, Williamson PD, Thadani VM. Long-term seizure, cognitive, and psychiatric outcome following trans-middle temporal gyrus amygdalohippocampectomy and standard temporal lobectomy. J Neurosurg. 2013;119:16–23. doi: 10.3171/2013.3.JNS12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Willie JT, Laxpati NG, Drane DL, Gowda A, Appin C, Hao C, Brat DJ, Helmers SL, Saindane A, Nour SG, Gross RE. Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Neurosurgery. 2014;74:569–84. doi: 10.1227/NEU.0000000000000343. discussion 584–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang JY, Wu C, Tracy J, Lorenzo M, Evans J, Nei M, Skidmore C, Mintzer S, Sharan AD, Sperling MR. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia. 2016;57:325–34. doi: 10.1111/epi.13284. [DOI] [PubMed] [Google Scholar]
- 68.Drane DL, Loring DW, Voets NL, Price M, Ojemann JG, Willie JT, Saindane AM, Phatak V, Ivanisevic M, Millis S, Helmers SL, Miller JW, Meador KJ, Gross RE. Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia. 2015;56:101–13. doi: 10.1111/epi.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barbaro NM, Quigg M, Broshek DK, Ward MM, Lamborn KR, Laxer KD, Larson DA, Dillon W, Verhey L, Garcia P, Steiner L, Heck C, Kondziolka D, Beach R, Olivero W, Witt TC, Salanova V, Goodman R. A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Ann Neurol. 2009;65:167–75. doi: 10.1002/ana.21558. [DOI] [PubMed] [Google Scholar]
- 70.Chang EF, Quigg M, Oh MC, Dillon WP, Ward MM, Laxer KD, Broshek DK, Barbaro NM Epilepsy Radiosurgery Study G. Predictors of efficacy after stereotactic radiosurgery for medial temporal lobe epilepsy. Neurology. 2010;74:165–72. doi: 10.1212/WNL.0b013e3181c9185d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dwivedi R, Ramanujam B, Chandra PS, Sapra S, Gulati S, Kalaivani M, Garg A, Bal CS, Tripathi M, Dwivedi SN, Sagar R, Sarkar C, Tripathi M. Surgery for Drug-Resistant Epilepsy in Children. N Engl J Med. 2017;377:1639–1647. doi: 10.1056/NEJMoa1615335. [DOI] [PubMed] [Google Scholar]
- 72.Amar AP, Heck CN, Levy ML, Smith T, DeGiorgio CM, Oviedo S, Apuzzo ML. An institutional experience with cervical vagus nerve trunk stimulation for medically refractory epilepsy: rationale, technique, and outcome. Neurosurgery. 1998;43:1265–76. doi: 10.1097/00006123-199812000-00001. discussion 1276–80. [DOI] [PubMed] [Google Scholar]
- 73.DeGiorgio C, Heck C, Bunch S, Britton J, Green P, Lancman M, Murphy J, Olejniczak P, Shih J, Arrambide S, Soss J. Vagus nerve stimulation for epilepsy: randomized comparison of three stimulation paradigms. Neurology. 2005;65:317–9. doi: 10.1212/01.wnl.0000168899.11598.00. [DOI] [PubMed] [Google Scholar]
- 74.Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, et al. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 1998;51:48–55. doi: 10.1212/wnl.51.1.48. [DOI] [PubMed] [Google Scholar]
- 75.Scherrmann J, Hoppe C, Kral T, Schramm J, Elger CE. Vagus nerve stimulation: clinical experience in a large patient series. J Clin Neurophysiol. 2001;18:408–14. doi: 10.1097/00004691-200109000-00004. [DOI] [PubMed] [Google Scholar]