Abstract
Electrical synapses with diverse configurations and functions occur at a variety of interneuronal appositions, thereby significantly expanding the physiological complexity of neuronal circuitry over that provided solely by chemical synapses. Gap junctions between apposed dendritic and somatic plasma membranes form “purely electrical” synapses that allow for electrical communication between coupled neurons. In addition, gap junctions at axon terminals synapsing on dendrites and somata allow for “mixed” (dual chemical + electrical) synaptic transmission. “Dual transmission” was first documented in the autonomic nervous system of birds, followed by its detection in the central nervous systems of fish, amphibia, and reptiles. Subsequently, mixed synapses have been detected in several locations in the mammalian CNS, where their properties and functional roles remain undetermined. Here, we review available evidence for the presence, complex structural composition, and emerging functional properties of mixed synapses in the mammalian CNS.
Keywords: Neuronal gap junctions, Connexins, Nerve terminals, Dye coupling, Electrical coupling
1. Introduction
Over the past five decades, hundreds of reports using a variety of methodological approaches have provided evidence that gap junctions between neurons form electrical synapses that occur widely and abundantly in most major regions of the mammalian central nervous system (CNS). “Purely electrical” synapses, which we define as gap junctions without associated structural components for chemical neurotransmission, occur at dendro-dendritic, dendro-somatic or somato-somatic contacts, where they are proposed to contribute to neural networks as coincidence detectors, to provide for high-speed intercellular transmission of information, and to reduce signaling noise. A well-established property of these synapses is their capacity to synchronize the activity of the electrically-coupled ensemble through synchronization of the collective subthreshold membrane depolarizations toward or away from the threshold for firing action potentials [1]. This property is now recognized as a hallmark of how purely electrical synapses contribute to synchronized and oscillatory network activities that are physiologically important features of information processing in many areas of the mammalian CNS. Especially prominent and widely distributed are the relatively larger dendro-dendritic gap junctions between inhibitory GABAergic interneurons [2–6], with electrical coupling proposed in part to orchestrate the synchronous and/or rhythmic activity of local principal cells. We also note the possible occurrence of axo-axonic gap junctions along axon shafts as another form of purely electrical synapses. So far, axo-axonic electrical synapses have been convincingly demonstrated only in lower vertebrates and invertebrates (but see [7]). However, gap junctions are also formed by axon terminals at axo-dendritic and axo-somatic contacts that link different types of neurons in both lower vertebrates and mammals. These were first identified in lower vertebrates and subsequently designated as classical “mixed” synapses in studies of mammalian CNS [8]. Recent studies involving cellular localization of neuronally-expressed connexin proteins, specifically connexin36 (Cx36), in ultrastructurally-identified gap junctions at nerve terminals have greatly extended those early results and have provided evidence for the presence of mixed synapses in several additional mammalian brain regions. In addition, a few gap junctions have been found to link the lateral aspect of an axon terminal to a nearby dendrite, away from the active zone/PSD complex, potentially representing purely electrical synapses involving a “three-cell” arrangement that was then designated a “functionally-mixed synapse” to indicate separate chemical and electrical interaction of the three structurally-linked cells. However, the use of the modifier “functional” did not imply that electrophysiological analysis had shown functionality of either the chemical or electrical component. Rather, “functional” was used solely to distinguish one of several possible three-cell configurations, differing from both the normal two-cell configurations of purely electrical synapses and classical mixed synapse. In turn, this phrase was incorrectly presumed by many to require the separate use of the unnecessary modifier “morphological” (or “morphologically”) to indicate that ultrastructural analysis had been conducted without companion functional analysis, which as yet remains to be done for any mixed synapse in mammals. Finally, a unique form of dendro-dendritic “reciprocal” mixed synapse [9–11], so far found only in the olfactory bulb, utilizes glutamate bi-directional neurotransmitter release between apposed dendrites, as demonstrated pharmacologically and electrophysiologically [9], as well as by NMDA receptor labeling of their postsynaptic densities [10,11], with electrical transmission via Cx36-containing gap junctions [9,11] proposed to augment rapid oscillatory synchronization during initial odorant discrimination [9]. However, in this review, we address primarily the evidence for the presence of classical mixed synapses in mammalian CNS, and summarize the several approaches that have been used to garner that evidence. Further, we discuss the anatomical sites and properties of mixed synapses that have been described in non-mammalian systems, the anatomically comparable as well as novel sites where these have been found in mammalian CNS, and consider possible functional roles of those synapses in mammals.
2. Configurations of electrical and mixed synapses
Gap junctions in both non-mammalian (see Section 3 below) and mammalian CNS are deployed at a variety of neuronal plasma membrane appositions. Most of the larger vertebrate neuronal gap junctions found to date by thin-section transmission electron microscopy (TS-TEM) involve, on both sides, a neuronal soma or dendrite [2–6,12–18]. These mostly large and relatively abundant dendro-dendritic (and somato-somatic) gap junctions have been described at what are referred to as “purely electrical synapses” [2,12–15,19]). Also not uncommon are gap junctions formed at axon terminal synapses onto soma or dendrites, forming classical “mixed synapses” [8,20–26]. To date, the gap junctions at axon terminals have been found to occur solely at excitatory terminals, with none observed at confirmed inhibitory synapses. As an aid to anatomical identification of diverse types of chemical synapses, parallel studies revealed that excitatory (primarily glutamatergic and cholinergic) nerve terminals contained round (spherical) synaptic vesicles, distinctive active zones, and asymmetric (i.e., ultrastructural Gray type 1), thickened, electron-dense postsynaptic membranes, whereas inhibitory chemical synapses (primarily GABAergic and glycinergic) had either large or small flattened “pleomorphic” synaptic vesicles and symmetric (i.e., ultrastructural Gray type 2), weakly-stained presynaptic and postsynaptic densities (active zones and PSDs), allowing the potential for further discrimination of axon terminal types having gap junctions. It is also noteworthy that multiple structural components of chemical synapses (synaptic vesicles, active zones, postsynaptic densities, and cytoplasmic machinery for endocytosis and recycling of synaptic vesicles), each independently and separately specified in the genome, have all been retained from fish, through amphibian, reptilian, and mammalian lineages, further supporting their maintained functionality and physiological importance of the chemical component of mixed synapses.
3. Mixed synapses in the non-mammalian nervous system
Mixed, combined electrical and chemical, synaptic transmission was first discovered in birds [27] and lower vertebrates, including various species of fish[1,28–33]. In fact, among the first examples of electrical neurotransmission found were those where gap junctions link axon terminals with somata and dendrites. The circuits found to harbor mixed synapses included the electromotor system in electric fish [34], and vestibular afferent terminals in the vestibular nuclei in lamprey [35–37], goldfish [38], toadfish [39], frog [40–41], lizard [42], pigeon [43] and chick [27,44,45], and the Club ending synapses on brainstem Mauthner cells in goldfish brain [29,30,46,47]. Several anatomical systems that utilize these synapses consist of neurons with relatively large somata, large diameter and long-projecting axons, and large axon terminals. These factors contributed to the amenability of these systems for electrophysiological detection and detailed analyses of mixed synaptic transmission.
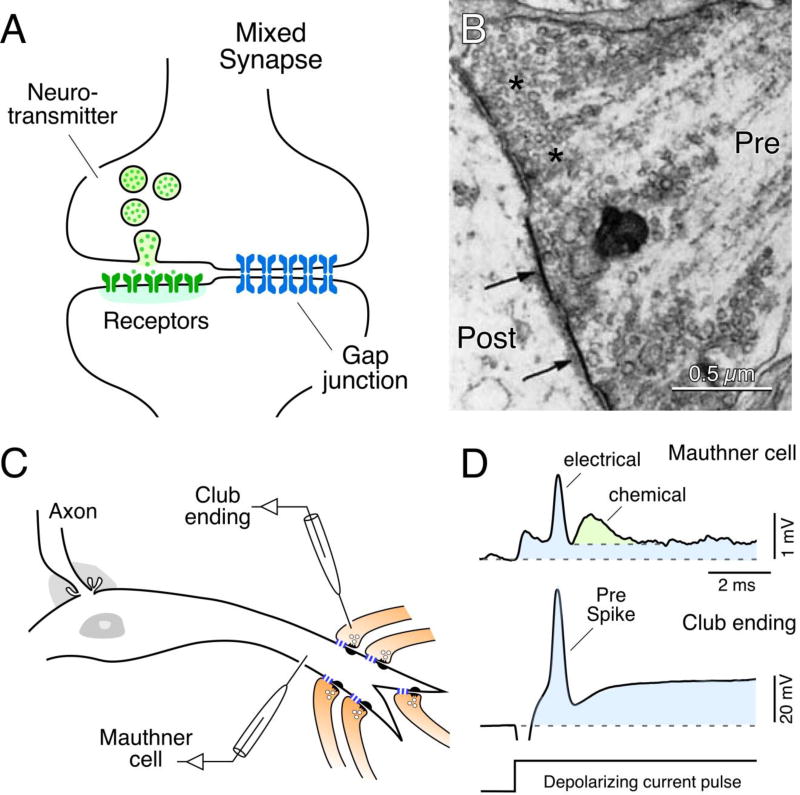
The most intensively studied of these were the easily-identifiable Club ending terminals that form scores of individual gap junction plaques with their postsynaptic Mauthner cell dendrite [29,30,46–48] (Fig. 1A,B). At the club ending / Mauthner cell synapse and other similar systems examined, mediation of transmission by the electrical component was readily distinguishable from the chemical component by virtue of the temporal separation of these components (Fig. 1C,D), in part arising from the lower speed of chemical transmission at the lower body temperature of fish, and from the bi-directionality of the electrical component identified as antidromically-transmitted responses that can be recorded in the presynaptic terminal [49,50]. The chemical component was identified as glutamatergic by immunolabeling [51–55] and by pharmacological means [54,56]. More recently, the composition of the electrical component, the gap junctions, was also identified using freeze-fracture replica immunogold labeling (FRIL) [55,57], as detailed in Section 4.3. In contrast to mammals endowed with twenty connexin family members, identification of connexins that form gap junctions in teleost fish (representing the vast majority of fish species) is made more complicated by an evolutionary genome duplication, apparently doubling in fish the number of connexin genes extant in mammals. As discussed in greater detail elsewhere [58], the fish ortholog Cx35 of mammalian Cx36, as identified even earlier in skate [59], was localized at the club ending / Mauthner cell synapses [55]. Subsequently, an additional Cx36-related connexin, namely Cx34.7, was found at these synapses, and surprisingly, gap junctional hemiplaques containing Cx35 were located exclusively at the presynaptic Club endings, and those containing Cx34.7 were exclusive to the postsynaptic Mauthner cell [57]. This asymmetric/heterotypic distribution of connexin proteins within gap junctions is likely to mediate the rectification of electrical transmission observed at these synapses (see Ref. 57 for details), which has been proposed to act as a mechanism of lateral excitation by favoring the antidromic spread of dendritic depolarizations to other nearby presynaptic Club endings [57,60]. This asymmetric pre- and postsynaptic distribution of Cx35 vs. Cx34.7 was also found to apply to other synaptic contacts in goldfish brain [53], suggesting that this heterotypic configuration is typical of at least some mixed synapses in fish. In summary, the ability to correlate physiological properties with ultrastructural and biochemical features in Club endings provided unambiguous evidence for the existence of mixed synaptic transmission, and at the same time established a set of prospective criteria for characterization of these contacts in mammalian CNS.
Fig. 1.
Mixed synapses in goldfish brain and their electrophysiological properties. (A) Diagram of a mixed synapse, at which a nerve terminal transmits both chemically via neurotransmitter and electrically via a gap junction. (B) Early thin-section TEM image of a portion of a Club ending synapse on a goldfish Mauthner cell, showing specializations for chemical transmission and nearby gap junctions (arrows) at contacts between presynaptic auditory afferents and postsynaptic Mauthner cell. Modified from [30], with permission. (C) Simultaneous in vivo pre- and postsynaptic recordings from a Club ending (recorded intracranially) and the lateral dendrite of the Mauthner cell. (D) Club endings exhibit mixed synaptic transmission. Presynaptic current injection (depolarizing current pulse) evokes a depolarization of the presynaptic terminal that is suprathreshold for spike generation (Pre spike). Both the coupling of the presynaptic spike and membrane depolarization were recorded in the postsynaptic lateral dendrite (blue overlay). A slower glutamate-mediated response (green) follows the electrical potential (blue area under the peak), or spikelet, evoked by the presynaptic spike. Traces represent the average of at least 10 individual responses. Panels C and D from [55].
Co-transmission by multiple chemical neurotransmitters has been established to occur at individual synaptic terminals; therefore it is perhaps not surprising that co-transmission of chemical and electrical signals could also take place at this additional type of nerve terminal contacts. There are probably several advantages for combining co-transmission of chemical and electrical signals. The speed and reliability of electrical synapses is likely to compensate for the longer delays of chemical transmission in cold blooded species [61]. Also, by virtue of the bi-directionality of electrical synapses, retrograde spread of postsynaptic depolarizations via mixed synapses can mediate mechanisms of lateral excitation [57,62]. Electrophysiological investigations of mixed synapses in fish revealed the close functional relationship between chemical and electrical transmission. Activation of glutamate receptors by afferent activity was shown to trigger changes in the strength of electrical coupling at mixed synapses [63,64] and, conversely, electrical synapses are likely to contribute to the release of glutamate from the presynaptic terminal [62], as regulated by instantaneous changes in potential of the axon terminal [65]. We further discuss some of these mechanisms below in relation to possible functional contributions of mixed synapses in mammals.
The abundance and wide distribution of mixed synapses in lower vertebrates indicates that this synaptic configuration is functionally relevant and evolutionary advantageous. Since teleost fish alone account for half of the ~40,000 vertebrate species [66], one could conclude that most vertebrates rely on the presence of mixed synapses in their CNS neural circuitry. Moreover, mixed synaptic transmission was also identified in invertebrates [67–70], indicating that it constitutes a feature of all nervous systems.
4. Identification of electrical vs. mixed synapses
In considering the occurrence and identification of mixed vs. purely electrical synapses, and notwithstanding the cellular heterogeneity and morphological complexity of CNS tissue, these two distinct coupling configurations can be recognized by various direct and indirect approaches. Among the most definitive means for identification of gap junction subcellular location is ultrastructurally, either by thin-section (TS) or freeze-fracture (FF) transmission electron microscopy (TS-TEM or FF-TEM). Because of resolution limited to 5–10 nm by imaging backscatter or secondary electrons, serial block-face scanning electron microscopy methods [71] have only recently been applied to detecting and mapping neuronal gap junctions and mixed synapses in mammalian CNS tissues [72]. However, rapid improvements in immunogold labeling within millimeter-dimension blocks may soon overcome these limitation and allow large-scale 3-dimensional mapping of ultrastructurally-identified, immunogold-labeled mixed synapses.
4.1. Thin-section transmission electron microscopy (TS-TEM)
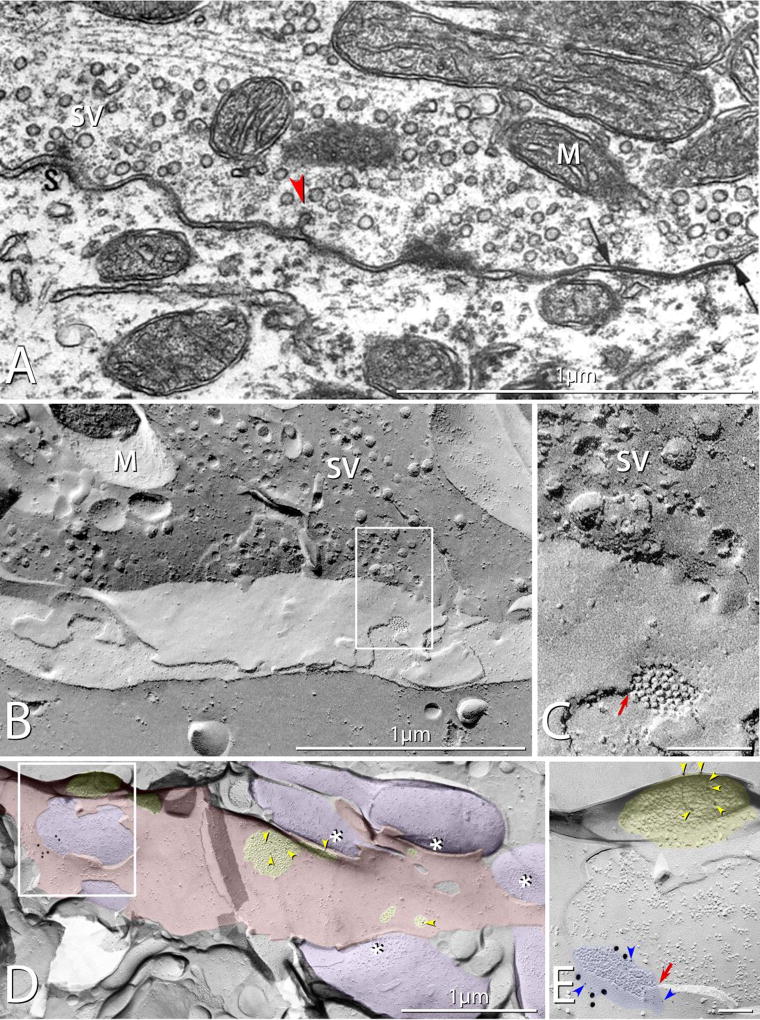
Gap junctions of sufficient diameter, and with appropriate tilting, visualized by TS-TEM can reveal their prototypical heptalaminar arrangement of plasma membrane leaflets and intervening membrane separations at apposing dendrites and at axon terminals. Nerve terminals forming gap junctions were shown to conform to ultrastructural criteria for identification as mixed synapses, including their content of uniform-diameter spherical synaptic vesicles, active-zone transmitter release sites, and associated sites of endocytosis of coated pits (Fig. 2A, arrowhead). However, a single thin section from within a large axon terminals may not always include an active zone/PSD complex in the same plane of section as the gap junction, potentially creating the false impression that one or the other may not be present in the same terminal. This deficiency that can be overcome by serial-section reconstruction. While axon terminals may be readily recognized by TS-TEM, a limitation of this approach is that very small gap junctions, represented essentially by points of contact between plasma membranes, are easily overlooked, and even when recognized, defy positive identification as gap junctions because they would be indistinguishable from small ripples commonly seen in many plasma membranes.
Fig. 2.
Mixed synapses in rat. (A) Thin-section TEM image of a mixed synapse in rat lateral vestibular nucleus, showing axon terminal filled with round synaptic vesicles and displaying a gap junction (arrows). Also evident is a synaptic active zone (S) with attached synaptic vesicles (SV) A nearby coated pit (red arrowhead) provides evidence for prior exocytosis and ongoing endocytosis / membrane recycling. M, mitochondrion. (Modified from [8], with permission). (B) Correlative freeze-fracture image of cross-fractured axon terminal in lumbosacral adult rat spinal cord. The axon terminal is filled with synaptic vesicles (SV), and exhibits a gap junction (box) shared with a motoneuron. (C) Higher magnification of boxed area in B, showing the gap junction, with diagnostic narrowing of the extracellular space (red arrow) at step from axonal E-face to motor neuron P-face, and regular arrays of P-face particles and E-face pits. (D,E) Low- and high-magnification images of multiple synaptic terminals onto unidentified spiny dendrite in stratum oriens in CA3 region of adult rat hippocampus. Active zones on some axon terminals are indicated by asterisks; PSDs (yellow overlays) contain immunogold-labeled NMDA receptors. (E) A higher magnification image of boxed area in D, showing a gap junction linking one of the axon terminals (purple overlays) to the dendrite (red overlay). The gap junction is heavily labeled for Cx36 (21 6-nm gold beads [blue arrowheads] and six 18-nm gold beads), and the nearby glutamate receptor-containing PSD, recognized as clustered 9–10 nm E-face particles (yellow overlay), is immunogold labeled for NMDA receptors (12-nm gold beads; yellow arrowheads). Note that the axon terminal shares the gap junction and the PSD with the overlying E-face of the small-diameter dendrite. (Modified from [105])
4.2. Freeze-fracture transmission electron microscopy (FF-TEM)
In contrast to TS-TEM, axon terminals are identified in freeze-fracture replicas (Fig. 2B,C) by visualization of one or more diagnostic features. In fortuitous fractures through the axon terminal cytoplasm, numerous uniform-diameter synaptic vesicles are usually observed (Fig. 2B). Additional diagnostic features of axon terminals are illustrated in Section 5.3. By FF, even the smallest gap junctions are positively identified by their characteristic regular hexagonal arrays of 9- to 10-nm-diameter intramembrane particles (IMPs) in the P-face (i.e., replicated protoplasmic membrane leaflet), with each gap junction P-face IMP called a “connexon”, corresponding to the visualized half of a previously-intact intercellular channel that existed before fracturing). Likewise, similar hexagonal arrays of pits are detected in each gap junction E-face (i.e., replicated extraplasmic membrane leaflet). Where the fracture plane within a gap junction steps from P-face of the lower cell to the E-face of the upper cell, the extracellular space is demonstrably narrowed from 10–20 nm to ca. 3 nm (Fig. 2C, red arrow). While freeze fracture allows visualization of the tiniest of gap junctions containing just a few connexons, the fracture plane may not always enter the axon terminal cytoplasm to reveal synaptic vesicles, nor does it always expose sufficient area of either presynaptic or postsynaptic markers to reveal either active zones or PSDs. Thus, both TS-TEM and FF-TEM have their separate limitations for detecting or recognizing mixed synapses.
4.3 Freeze fracture replica immunogold labeling (FRIL)
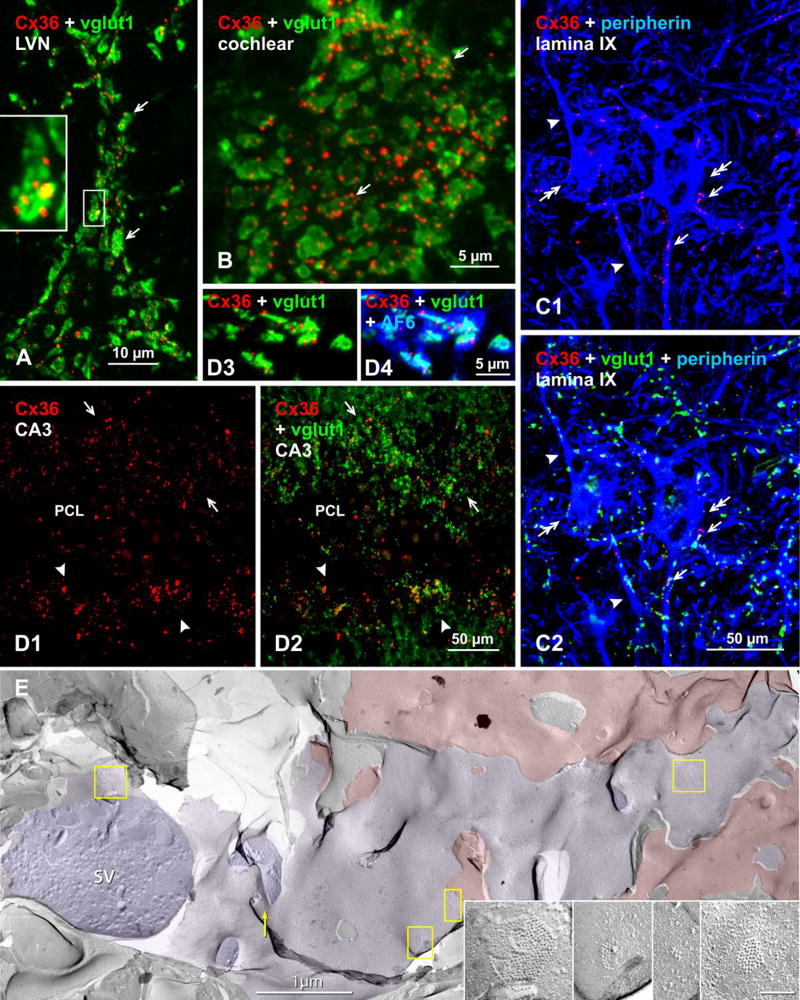
A major advance to the freeze-fracture approach was the use of FF-TEM in combination with immunohistochemical labelling of membrane proteins to yield the procedure known as FRIL. This method allows simultaneous high-resolution visualization of subcellular membrane structures such as gap junctions, in combination with easy detection of immunogold labeling of their protein constituents over broad expanses of tissue (Fig. 2D,E). Application of FRIL (as modified from SDS-FRL [73] by combining SDS-FRIL with confocal grid-mapping [74]) to studies of neuronal gap junctions has firmly established the connexin constituents of literally thousands of neuronal gap junctions and has revealed their diverse subcellular locations and structural configurations [73,75]. Specifically, among the twenty members of the connexin family of proteins in mammals, Cx36 and connexin45 (Cx45) were found to be expressed or even co-expressed in individual neuronal gap junctions [76–78], and FRIL studies have contributed to identification of Cx36 as a major constituent of most neuronal gap junctions that have been ultrastructurally documented in many areas of mammalian brain and spinal cord. Combined FRIL and high-resolution immunofluorescence studies have confirmed the presence of mixed synapses in mammalian brain, including demonstration of close proximity of immunogold-labeled gap junctions to labeled and unlabeled, ultrastructurally-distinctive glutamate-receptor-containing PSDs (Fig. 2D,E), as separately shown by demonstration of Cx36 immunofluorescent puncta in close proximity to immunostaining for vesicular glutamate transporter (vglut1) (Fig. 3) [11,52,79].
Fig. 3.
Immunofluorescence evidence for mixed synapses in various adult mouse and rat CNS regions, based on co-localization of Cx36 with the vglut1 marker of axon terminals. Color code for immunolabeled protein in overlay images is indicated in the upper left of each panel. (A) Lateral vestibular nucleus, where overlay of labeling for Cx36 and vglut1 shows a single neuronal cell body (not counterstained) contacted by large vglut1-positive axon terminals co-localized with labeling for Cx36 (arrows). The boxed area showing a single terminal is magnified in the inset. (B) Cochlear nucleus, where a similar overlay image shows vglut1-positive terminals decorated with Cx36-puncta (arrows) on a root neuron. (C) Spinal cord thoracic ventral horn lamina IX motor nucleus, where labeling for the motoneuron marker peripherin shows motoneurons (C1, double arrows), with Cx36-puncta decorating the somata (C1, arrows) and dendrites (C1, arrowheads) of those motoneurons, and in the same field labeled in addition for vglut1 showing Cx36-puncta co-localized with vgut1-positive axon terminals (C2, arrows). (D) Rat ventral hippocampal CA3 stratum lucidum, where Cx36-puncta straddling the pyramidal cell layer (PCL) dorsally (D1, arrows) and ventrally (D1, arrowheads) are largely localized to vglut1-positive mossy fiber terminals, as shown by labeling for Cx36/vglut1 in overlay (D2). Association of Cx36-puncta with vglut1, shown at higher magnification (D3), is also associated with labeling of the gap junction and adherens junction associated protein AF6 (aka, afadin) (D4). (E) Early freeze fracture image of mixed synapse between large axon terminal P-face (purple overlay) and overlying interneuron E-face (red overlay) in lamina VI of the lumbosacral adult rat spinal cord. (A small portion of this mixed synapse was published as a stereoscopic pair in Fig. 10 in [74]). Four gap junctions shared between the axon terminal and the interneuron are indicated (yellow boxes), and enlarged in insets.
4.4. Immunofluorescence localization of connexins
Immunofluorescence localization of the connexin constituents of neuronal gap junctions have added to the repertoire of approaches for identification of mixed synapses and for distinguishing these from purely electrical synapses. Unlike immunolabeling of many other proteins expressed in neurons, where immunolabel can be distributed throughout cell bodies, dendrites, and other cellular locations, detection of Cx36 is highly restricted and is visualized exclusively as immunofluorescent puncta restricted to the surfaces of identified neurons. Reasons for the failure to detect intracellular Cx36 are unknown, but this absence of diffuse cytoplasmic staining fortuitously allows immunofluorescence labeling of Cx36 to sites of confirmed neuronal gap junctions. Correspondence of immunofluorescent puncta to sites where Cx36 is present in gap junctions has been deduced from the known locations of ultrastructurally-detected neuronal gap junction in brain, and by correlation of size and distribution of immunolabeling of Cx36 by immunofluorescence and FRIL in selected CNS regions [75]. Demonstrating the correspondence of Cx36-puncta to sites of gap junctions is also advantageous for quantitative comparison of the density of these junctions over larger regions of the CNS, which cannot be done by TS-TEM or by FRIL, and has been of value in providing evidence for mixed synapses in several CNS regions (discussed below).
Ultimately, electrophysiological approaches will be necessary to establish that mixed synapses in mammals, as in lower vertebrates, have the characteristic features that define these structures, namely, direct evidence for dual chemical and electrical transmission. Altogether, all these criteria have been met at some mixed synapses in only a few non-mammalian vertebrates (Fig. 1C,D). In mammalian systems, however, those locations where evidence for mixed synapses has been reported have not yet been subjected to comprehensive investigations of the occurrence of even electrical transmission, let alone dual transmission. Several other points concerning electrophysiological studies of mixed synapses and warranting consideration are discussed below.
5. Evidence for mixed synapses in mammalian CNS
The broad distribution of mixed synapses in lower vertebrates contrasts with the situation in the mammalian brain, where mixed synapses have so far been found only in a relatively few locations vs. the dozens of regions in which purely electrical synapses have been described. It may be that functional requirements for mixed synapses were largely abandoned in mammalian CNS; however, their presence in notable abundance where they already have been shown to occur in mammals suggests their functional relevance. Moreover, an exhaustive search, yet to be done, for mixed synapses in mammalian brain may reveal other locations where these synapses occur, in addition to the growing evidence for their presence in several mammalian CNS regions described below.
Demonstrations of electrical synapses in lower vertebrates, including those encountered early on as mixed synapses, spurred efforts to search for mixed synapses in mammalian CNS. In early studies, anatomical locations most promising to examine in mammalian brain were presumed to correspond to CNS systems where mixed synapses were originally found in lower vertebrates, notwithstanding that many additional regions harboring mixed synapses have more recently been found throughout goldfish midbrain and hindbrain [53]. The most obvious of these in mammals were brainstem nuclei receiving eighth cranial nerve primary afferent auditory and vestibular input from the cochlea, via the cochlear and vestibular nerves, respectively. Early studies of mammalian mixed synapses involved the exclusive use of TS-TEM and freeze-fracture approaches. More recently, Cx36 puncta associated with axon terminals have been demonstrated in a number of brain and spinal cord regions (Fig. 3), as discussed below.
5.1. Vestibular system
In both adult rat and mouse, gap junctions are formed between large axon terminals and either the somata or dendrites of large neurons in the lateral vestibular nucleus (LVN) [8,20,21,24,25] that themselves appear not to be directly coupled by purely electrical synapses (i.e., absence of dendro-dendritic or somato-somatic gap junctions). Using immunofluorescence dual labeling for Cx36 and the axon terminal marker vesicular glutamate transporter1 (vglut1), we recently reported [79] the localization of Cx36-puncta to vglut1-positive axon terminals in the LVN of adult rat and mouse (Fig. 3A), which represents a new way to demonstrate the presence of Cx36-containing gap junctions at identified glutamatergic mixed synapses. This Cx36/vglut1 association was present not only in the LVN, but was also widely distributed in the entire vestibular nuclear complex, including the spinal, medial and superior vestibular nuclei. Further, the large terminals, together with the high incidence of multiple Cx36-puncta associated with individual terminals, were both eliminated after deafferentation of the vestibular nuclear complex by labyrinthectomy. This indicated that many/most of these abundant mixed synapses in this complex are of primary afferent origin [79], similar to the primary afferents forming club ending mixed synapses on Mauthner cells in goldfish.
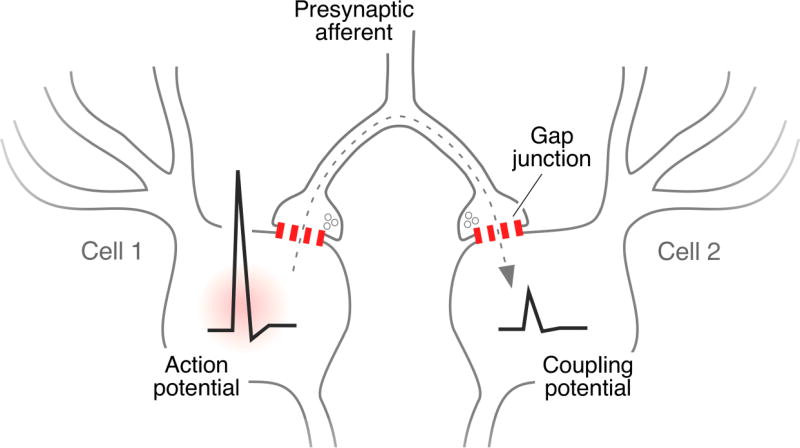
In contrast, there are only a few reports supporting the possibility that vestibular primary afferents mediate electrical coupling in mammals. Indirect evidence for such coupling in rat was derived by recording from individual LVN neurons following antidromic stimulation in vivo of their projections to the spinal cord at stimulus strengths that were subthreshold for activating all of these projections [8]. Neurons not activated antidromically nevertheless showed small depolarizations, suggesting their electrical coupling to those neurons that were antidromically activated. The involvement of mixed synapses in this coupling was invoked because, while these synapses were readily found in the LVN, no ultrastructural evidence was found for gap junctions directly linking the LVN neurons themselves [8], which is also consistent with findings that large LVN neurons did not display somato-somatic, dendro-dendritic, or dendro-somatic immunofluorescent Cx36-puncta that would have indicated their direct coupling by purely electrical synapses [79]. Instead, the coupling was suggested to be mediated by presynaptic fibers forming mixed synapses on separate neurons, as illustrated in Figure 4. More direct evidence for mixed transmission by vestibular afferents was provided by in vivo stimulation of these afferents from the ear [80]. Labyrinth stimulation evoked a synaptic potential in which two components with different latency, early and late, could be distinguished. When tested, the amplitude of the early component was not sensitive to changes in membrane potential, whereas that of the late component was consistent with variations in the driving force of an excitatory neurotransmitter. While the evidence was consistent with mixed synaptic transmission, this possibility was not confirmed pharmacologically.
Fig. 4.
Possible mechanism for electrical coupling of neurons via their innervation by mixed synapses. A single afferent fiber forms collaterals with terminals, producing mixed synapses on different neurons that themselves are not coupled by gap junctions. Depolarizations or action potentials generated in one neurons are transmitted retrogradely into an axon terminal via the gap junction component of one mixed synapse, travel antidromically along the collateral axon to the branch point, and orthodromically to the other mixed synapse (dotted arrow), where changes in membrane potential are transmitted to the second neuron causing a coupling potential. This pathway for electrical coupling was originally proposed to occur in fish [39,100] and later in rat [8]. Hypothetically, ensembles of neurons may be coupled in this fashion depending on the degree of collateralization of a single fiber to form mixed synapses on multiple neurons. Redrawn from [8].
5.2. Auditory system
Mixed synapses in the ventral cochlear nucleus of adult rat were first identified by TS-TEM, and were especially abundant at terminal contacts on neuronal somata [24]. This was recently confirmed by us [81], as in the case of the LVN, by combined immunofluorescence labeling for Cx36 and vglut1 (Fig. 3B), where Cx36 was localized to vglut1-positive terminals forming mixed synapses on multiple neuron types, including on bushy cells, octopus cells and auditory root neurons in adult mouse and rat. Further, it was established that these synapses are formed by terminals of primary afferent origin, with those on bushy cells likely corresponding to primary afferent endbulbs of Held. Evidence for mixed synapses also was found in other regions of the auditory system based on Cx36/vglut1 co-localization [81]. One of these was the medial nucleus of the trapezoid body (MNTB), where labeling of Cx36 was distributed on neuronal somata, strongly suggestive of abundant mixed synapses formed on MNTB neurons by calyx of Held terminations originating from glutamatergic globular bushy cells in the anteroventral cochlear nucleus. Another was the lateral superior olivary complex, where labeling of Cx36 associated with vglut1-positive terminals was found around neuronal somata, suggesting mixed synapses that link these neurons with glutamatergic axon terminals of as-yet-unknown origin. Electrophysiological evidence for an electrical component to transmission by mixed synapses in the above auditory centers is currently lacking.
5.3. Spinal cord and trigeminal motor nucleus
In the trigeminal motor nucleus, intermediate laminae along the length of the spinal cord, and in the spinal cord ventral horn at all rostro-caudal levels of adult mouse and rat, large numbers of Cx36-puncta were found to be co-localized with vglut1-containing nerve terminals on neuronal somata and dendrites (Fig. 3C), indicative of glutamatergic mixed synapses [82,83]. In lamina IX of the spinal cord ventral horn, mixed synapses (defined by co-localization of Cx36 and vglut1) were prominent on somata and dendrites of motoneurons, and were considered to be formed by terminals of monosynaptic connections between Ia muscle spindle afferents and motoneurons, based on elimination of these terminals and their associated Cx36 after dorsal rhizotomy [82]. These results derived from immunofluorescence observations of Cx36 in various spinal regions were remarkably consistent both qualitatively and quantitatively with an earlier freeze-fracture study that demonstrated an abundance of ultrastructurally-identified gap junctions at mixed synapses formed by nerve terminals in the spinal cord, including those on motoneuron somata and proximal dendrites [84]. Those axon terminals having gap junctions were further characterized by surface fractures within apposing presynaptic and postsynaptic membranes (Fig. 3E). In the axon terminal membrane, distinctive active zones of two types were observed: those with complex invaginations called “synaptic sombreros” showed evidence for ongoing synaptic vesicle exocytosis in the crown of the sombrero, with gap junctions located primarily in the brim of the sombreros [84]. In the second type, synaptic vesicle exocytosis was abundant in flat-topped presynaptic invaginations designated “synaptic mesas” [84]. Where the fracture plane stepped from axon P-face to motor neuron E-face, the postsynaptic membranes near synaptic sombreros (but not near synaptic mesas) had dense clusters of E-face IMPs corresponding to classical postsynaptic densities (PSDs) of glutamatergic synapses (not shown here, but see labeled glutamate receptor PSDs in Fig. 2D,E). In contrast, no mixed synapses have yet been seen having PSDs as clusters of P-face IMPs, as may occur in cholinergic synapses and in inhibitory (GABAergic and glycinergic) chemical synapses.
Despite these findings, and despite that motoneurons and their synaptology have been extensively studied for decades by TS-TEM, it is baffling that gap junctions associated with axon terminals on these neurons have not otherwise been reported. TS-TEM studies of neuronal gap junctions in mammalian spinal cord identified gap junctions / electrical synapses (dendro-dendritic, somato-somatic, and dendro-somatic) in two small nuclei in the lower lumbosacral enlargement, where gap junctions were thought to be involved in the synchronous muscle contractions of sexually dimorphic pelvic musculature [85,86], although some of the putative neuronal gap junctions in the included freeze-fracture images [86] were in fact images of oligodendrocyte and astrocyte gap junctions, as subsequently identified based on published criteria [87].) Possible explanations for the failure to detect what we have found to be relatively abundant mixed synapses throughout the spinal cord are that most of the gap junctions at mixed synapses are too small to be unambiguously distinguished from other close membrane appositions; that equivalent searches requiring especially high TEM magnification had not been conducted in wider regions of the spinal cord [84]; or that the abundant large axon terminals containing gap junctions were not examined using TS-TEM goniometric tilting. Moreover, even though two morphological types of mixed synapses (i.e., those with synaptic mesas and those with synaptic sombreros) have been discerned in spinal cord [84], only the “sombrero” type, with E-face postsynaptic IMPs characteristic of glutamatergic transmission, have been positively identified by FRIL [11,52,88]. Synapses having “synaptic mesas” likely constitute a second type of excitatory mixed synapses based on their content of round synaptic vesicles [84]. Likewise, in the trigeminal motor nucleus, the mixed synapses were found on large motoneurons, although the source of the axon terminals forming mixed synapses has not yet been determined; but they also are likely to be primary afferents.
Currently, there are few published reports regarding examination of electrical transmission by primary afferent fibers in the spinal cord of adult mouse or rat, including those ending on ventral horn motoneurons. Motoneurons are known to be electrically coupled at early postnatal ages [89], but this coupling decreases and was suggested (but not explicitly shown) to disappear later during development [90]. If coupling between these neurons does disapear, this would eliminate the possibility that mixed synapses on adult motoneurons contribute to electrical coupling via presynaptic fibers innervating multiple neurons, at least under the conditions used for electrophysiological recording from motoneurons during development. Although we have not yet examined cat spinal cord for Cx36 distribution and localization, there have been some controversial findings that raised the possibility of primary afferent electrical transmission in the spinal cord of this species. While direct evidence for mixed trasnmission is still lacking, circumstantial evidence for electrical coupling between motoneurons and Ia afferents was suggested by findings that when a coincident antidromic action potential is evoked in the postsynaptic motoneuron, these afferents can be either excited or their thresholds to electrical activation reduced at a very short latency [91–93]. Moreover, electrical coupling was proposed as a possible explanation for the short latency excitation between some motoneurons in the cat [94].
5.4. Hippocampus: CA3 and dentate gyrus
Gap junctions as purely electrical synapses between various types of interneurons in the hippocampus have been well documented, and Cx36 expression in this structure has been extensively studied in rodents. In our studies of Cx36 distribution in rat and mouse CNS, it therefore came as a surprise to find a strikingly high density of immunofluorescent puncta for Cx36 localized in the stratum lucidum of the rat hippocampus [95], where gap junctions had not been described previously. This labeling was similar to the Cx36-puncta visualized on neuronal somata elsewhere in brain. These puncta had a highly-restricted regional distribution, being present in sub-regions of the CA3 stratum lucidum in rat ventral but not dorsal hippocampus. Curiously, comparable ventral regions in mouse hippocampus did not display Cx36 labeling. The stratum lucidum represents the projection area of axons arising from granule cells in the dentate gyrus, where these axons end as mossy fiber terminals, in part on CA3 pyramidal cells. The Cx36-puncta in the stratum lucidum were highly localized to these mossy fiber terminals labeled for their glutamatergic marker vglut1 (Fig. 3D) [95]). TS-TEM studies have not as yet definitively identified gap junctions between mossy fiber terminals and postsynaptic pyramidal cells, although these terminals were described to form close membrane appositions with their synaptic targets, where these appositions were considered putative gap junctions [88]. However, one gap junction was found at a dendro-dendritic mixed synapse linking two GABAergic interneurons in the hippocampus [4], potentially representing the first example of an inhibitory mixed synapse. Studies by FRIL have reported a variety of gap junction configurations in the hippocampus of rat, including glutamatergic mixed synapses on spiny dendrites in the stratum oriens (Fig. 2D,E), but none of these could be conclusively associated with mossy fiber terminal-pyramidal cell contacts [88]. Thus, ultrastructural searches for mixed synapses formed by mossy fibers in optimally fixed tissue allowing definitive gap junction identification at defined subcellular location remain to be conducted.
In agreement with the presence of Cx36 puncta, one report has provided electrophysiological evidence for co-existence of electrical and chemical transmission in mossy fibers [96]. The authors showed in slices of rat hippocampus that mossy fiber activation can evoke in pyramidal cells a fast spikelet (coupling of a presynaptic action potential), followed by a chemical synaptic potential. However, the preceding spikelet was observed in only about 5% of the recordings. Moreover, when present, the spikelet behaved in an all-or-none fashion, contrasting with that of the chemical synaptic potential, whose amplitude was proportional to the increase in stimulation strength, which progressively recruited mossy fibers. This property suggests that only one of the stimulated mossy fibers (about 50 in number) had boutons electrically coupled to the postsynaptic pyramidal cell, which could be taken to indicate that electrical transmission at mossy fiber terminals is rare, or conversely, that the mixed synapses detected by immunofluorescence are abundant but that most neurons had been de-afferented in the tissue slices. Such low incidence of electrical coupling is puzzling when considering the high density of Cx36 found to be associated with mossy terminals specifically in the rat ventral hippocampus, but could be related to the precise areas of the hippocampal CA3 area examined electrophysiologically (i.e., dorsal vs. ventral), or to the in-vitro recording conditions used, which could potentially alter the functional state of the gap junction channels.
5.5. Retina
Gap junctions coexist with chemical synapses in various retinal cell types, including at ribbon synapses that were immunogold labeled for NMDA glutamate receptors [75], making these a form of glutamatergic mixed synapses. Although neuronal gap junctions composed of Cx36, and to a lesser extent, Cx45, are abundant in adult rat, mouse, and cat retina [75,78], usually at ultrastructurally-defined mixed synapses, even a limited description of the unique cell types and diversity of synaptic configurations is outside the scope of this brief review of mixed synapses in the brain and spinal cord of mammals.
5.6. Olfactory bulb
Classical mixed synapses with gap junctions composed of Cx36 and Cx45 have been described in rat olfactory bulb [11]. In addition, dendro-dendritic “reciprocal” mixed synapses in olfactory bulb of adult rats [11] have gap junctions containing Cx36, and are closely co-associated with neurotransmitter receptor clusters, as identified by FRIL using antibodies against NMDA glutamate receptors [11]. However, those bidirectionally-transmitting dendro-dendritic synapses are outside the scope of this review. Thus, we note here only that at least some of the canonical mixed synapses in olfactory bulb are glutamatergic, as identified by ultrastructural and FRIL markers.
5.7. Red nucleus
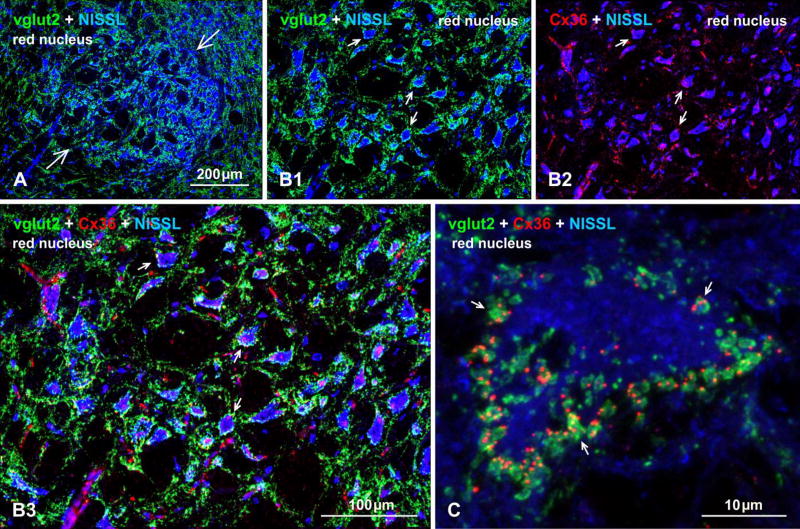
In addition to the above examples, it is likely that additional sites harboring mixed synapses will be found in the mammalian CNS. Indeed, inspection of just a few CNS regions has revealed Cx36 association not with vglut1-containing, but with abundant vglut2-containing nerve terminals on large neuronal somata and dendrites in the red nucleus of mouse (Fig. 5), further broadening the scope of possible axon terminal types forming mixed synapses to include those using an alternative vesicular glutamate transporter (i.e., vglut2) at these putative glutamatergic mixed synapses. The anatomical source of the vglut2-containing terminals that show labeling for Cx36 remains to be determined.
Fig. 5.
Immunofluorescence evidence for mixed synapses formed by axon terminals and neurons in the red nucleus of adult mouse brain. (A) Image of the red nucleus (arrows) with its distinctive large neurons counterstained with blue fluorescence Nissl, and showing a high density of immunolabeling for vglut2 within the nucleus. (B) Higher magnification of the red nucleus, showing vglut2-positive nerve terminals heavily concentrated around blue Nissl-stained neuronal somata and initial dendrites (B1, arrows) and, in the same field, immunolabeling of Cx36 associated with the same Nissl-stained neurons (B2, arrows) and often displaying overlap with labelling of vglut2, as shown in three-color overlay (B3, arrows). (C) Confocal image of a single large red nucleus neuron (blue fluorescence Nissl counterstained), showing the periphery of the neuronal somata heavily invested with prominent vglut2-positive terminals that frequently display co-localization with fine punctate labelling for Cx36 (arrows). Immunofluorescence labelling of Cx36 was absent in the red nucleus of Cx36 null mice (not shown).
6. Functional considerations
While physiological evidence for electrical transmission at mammalian mixed synapses is still preliminary, we and others can only speculate about the functional roles of these mixed synapses based on knowledge of their contributions to synaptic circuitry in fish [50,57,62]. Combining electrical with chemical transmission is advantageous in cold-blooded vertebrates, where electrical synapses provide higher speed (shorter synaptic delay) and reliability (they are not probabilistic), and provide higher fidelity to networks that require fast signaling, including those involved in circuits governing behaviors such as escape from predators [97]. Network output depends however on whether inputs reach threshold for generating action potentials. These particular advantages are less relevant in mammals wherein transmitter release occurs with much shorter synaptic delay, and where reliable transmission can be achieved by adding multiple transmitter release sites. However, other properties observed at fish mixed synapses can still be advantageous in mammals, and some are based on the bi-directional nature of electrical transmission. The synaptic potentials and action potentials produced in the cell that is postsynaptic to a mixed synapse can actually travel back to the presynaptic and neighboring electrically-coupled terminals, with their depolarizations likely increasing transmitter release [65,98], and perhaps even promoting synchronization in the closely-adjacent mixed terminals [50,57,62]. Antidromic spread of synaptic potentials to nearby inactive mixed terminals can also increase their excitability and lead to their firing, acting as a mechanism of lateral excitation (also observed in invertebrates [99]). Retrograde communication via electrical synapses can also lead to synchronization of several neurons that are innervated by collaterals of the same presynaptic fiber via mixed synapses (Fig. 4). Given the prevalence and importance of synchronous neuronal activity in electrically-coupled systems discussed above, it is possible that mixed synapses promote synchrony via this mechanism. For example, previously described electrical coupling between lateral vestibular neurons in rat appears to be mediated by presynaptic fibers [8], where collaterals of a single fiber innervate and form mixed synapses on two or more different neurons, allowing electrical activity in one neuron to be transmitted to another via the gap junctions at each end of the collateral, as has also been described in fish [39,100]. It might also be considered that in a system with highly collateralized primary afferents, such as the vestibular nuclear complex, motoneurons in the spinal cord, and in pyramidal cells in the hippocampus, coupling of postsynaptic neurons via presynaptic fibers could promote synchronous responses in ensembles of target neurons receiving a functionally common afferent input.
7. Conclusions
Following the initial descriptions of mixed synapses in the vestibular and cochlear nucleus of rodents beginning in the late 1960’s and during the ensuing decade (early review in [8]), there was subsequently little attention paid to these synapses, possibly due in part to what appeared to be their restricted distribution to only a few brain regions, and in part to prior and current technical limitations in assessing their physiological relevance. More recently, application of additional immunocytochemical approaches to the detection of gap junctions at axon terminals has provided evidence for their presence in several additional brain regions, which may serve to revive interest in these enigmatic structures. However, there are currently no examples in which mixed synaptic transmission has been firmly established in mammals by combining ultrastructural and electrophysiological evidence, as has been done in fish. While co-localization of immunofluorescence for neuronal connexins and presynaptic markers is beginning to suggest a wider distribution of mixed synapses, this possibility needs to be confirmed ultrastructurally and electrophysiologically. Along these lines, it is interesting that in several of the mammalian systems in which evidence for mixed synapses has so far been found, such as the Ia primary afferents on motoneurons, vestibular and auditory primary afferents, cochlear bushy cell projections to MNTB, and hippocampal mossy fiber terminals, these are well characterized for the high fidelity of their synaptic transmission, as exemplified by designation of mossy fiber terminals as “detonator” synapses [101]. With as yet little evidence for electrical transmission at mixed synapses in these system, we can only wonder if their electrical component contributes to this fidelity of transmission. Despite the fact that two of the above-mentioned anatomical sites, namely the calyx of Held terminals on MNTB neurons and hippocampal mossy fiber terminals, have been extensively explored electrophysiologically and pharmacologically, with little resulting evidence of electrical transmission, further investigations of the functional relevance of Cx36 localization at axon terminals in mammalian neural systems are nevertheless warranted. Obtaining evidence for functionality of mixed synapses might be a challenge, but both mossy fiber terminals and the calyx of Held terminals are amenable to electrophysiological recording from axon terminals themselves [102–104], which could allow direct assessment of electrical coupling between pre- and postsynaptic elements by dual recording approaches. Difficulties in the detection of electrical coupling might result as a consequence of the preparation of brain or spinal cord slices for in-vitro recordings, which could potentially alter the functional state of the gap junction channels in some structures and not others (e.g., release of a modulatory substances during slice preparation, or deafferentation). Alternatively, the gap junction component at mixed synapses could represent non-conductive functions of gap junction channels, which have been shown to act as adhesion molecules in both vertebrate and invertebrate nervous systems. Future, investigations are likely to shed light on these and other possibilities.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research and the Natural Sciences and Engineering Research Council to J. I. Nagy; National Institutes of Health (NIH) grants DC03186, DC011099, NS055726, NS085772 and NS0552827 to A. E. Pereda; and NS31027, NS44010, and NS44295 to J. E. Rash. We thank B. McLean, K. Vanderpool and T. Yasumura for unexcelled technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Bennett MVL, Aljure E, Nakajima Y, Pappas GD. Electrotonic junctions between teleost spinal neurons: Electrophysiology and ultrastructure. Science. 1963;141:262–264. doi: 10.1126/science.141.3577.262. [DOI] [PubMed] [Google Scholar]
- 2.Fukuda T, Kosaka T, Singer W, Galuske RAW. Gap junctions among dendrites of cortical GABAergic neurons establish a dense and widespread intercolumnar network. J. Neurosci. 2006;26:3434–3443. doi: 10.1523/JNEUROSCI.4076-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukuda T, Kosaka T. The dual network of GABAergic interneurons linked by both chemical and electrical synapses: a possible infrastructure of the cerebral cortex. Neurosci. Res. 2000;38:123–130. doi: 10.1016/s0168-0102(00)00163-2. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda T, Kosaka T. Gap junctions linking the dendritic network of GABAergic interneurons in the hippocampus. J. Neurosci. 2000;20:1519–1528. doi: 10.1523/JNEUROSCI.20-04-01519.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katsumaru H, Kosaka T, Heizmann CW, Hama K. Gap junctions on GABAergic neurons containing the calcium-binding protein parvalbumin in the rat hippocampus (CA1 region) Exp. Brain Res. 1988;72:363–370. doi: 10.1007/BF00250257. [DOI] [PubMed] [Google Scholar]
- 6.Kita H, Kosaka T, Heizmann CW. Parvalbumin-immunoreactive neurons in the rat neostriatum: a light and electron microscopic study. Brain Research. 1990;536:1–15. doi: 10.1016/0006-8993(90)90002-s. [DOI] [PubMed] [Google Scholar]
- 7.Hamzei-Sichani F, Kamasawa N, Jannsen WGM, Yasumura T, Davidson KGV, Hof PR, Wearne SL, Stewart MG, Young SR, Whittington MA, Rash JE, Traub RD. Gap junctions on hippocampal mossy fiber axons demonstrated using thin-section electron microscopy and freeze-fracture replica immunogold labeling. Proc. Natl. Acad. Sci. (USA) 2007;130:12548–12553. doi: 10.1073/pnas.0705281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korn H, Sotelo C, Crepel F. Electrotonic coupling between neurons in the rat lateral vestibular nucleus. Exp. Brain Res. 1973;16:255–275. [PubMed] [Google Scholar]
- 9.Christie JM, Bark C, Hormuzdi SG, Helbig I, Monyer H, Westbrook GL. Connexin36 mediates spike synchrony in olfactory bulb glomeruli. Neuron. 2005;46:761–772. doi: 10.1016/j.neuron.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 10.Kamasawa N, Yasumura T, Davidson KGV, Furman CS, Nagy JI, Rash JE. Differential distribution of connexin45 (Cx45), Cx36 in neuronal gap junctions of adult rat retina olfactory bulb, cerebral cortex, Abstracts. ASCB Annual Meeting. 2004;44:110. [Google Scholar]
- 11.Rash JE, Davidson KGV, Kamasawa N, Yasumura T, Kamasawa M, Zhang C, Michaels R, Restrepo D, Ottersen OP, Olson C, Nagy JI. Ultrastructural localization of connexins (Cx36 Cx43, Cx45), glutamate receptors aquaporin-4 in rodent olfactory mucosa, olfactory nerve and olfactory bulb. J. Neurocytol. 2005;34:307–341. doi: 10.1007/s11068-005-8360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosaka T. Gap junctions between non-pyramidal cell dendrites in the rat hippocampus (CA1 and CA3 regions) Brain Res. 1983;271:157–161. doi: 10.1016/0006-8993(83)91377-x. [DOI] [PubMed] [Google Scholar]
- 13.Kosaka T, Hama K. Gap junctions between non-pyramidal cell dendrites in the rat hippocampus (CA1 and CA3 regions): a combined golgi-electron microscopy study. J. Comp. Neurol. 1985;231:150–161. doi: 10.1002/cne.902310203. [DOI] [PubMed] [Google Scholar]
- 14.Kosaka T, Kosaka K. Intraglomerular dendritic link connected by gap junctions and chemical synapses in the mouse main olfactory bulb: Electron microscopic serial section analyses. Neuroscience. 2005;131:611–625. doi: 10.1016/j.neuroscience.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 15.Kosaka T, Kosaka K. Neuronal gap junctions between intraglomerular mitral/tufted cell dendrites in the mouse main olfactory bulb. Neurosci. Res. 2004;49:373–378. doi: 10.1016/j.neures.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Sloper JJ. Gap junctions between dendrites in the primate cortex. Brain Research. 1972;44:641–646. doi: 10.1016/0006-8993(72)90327-7. [DOI] [PubMed] [Google Scholar]
- 17.Sloper JJ, Powell TPS. Gap junctions between dendrites and somata of neurons in the primate sensori-motor cortex. Proc. R. Soc. London,Ser. B. 1978;203:39–47. doi: 10.1098/rspb.1978.0089. [DOI] [PubMed] [Google Scholar]
- 18.Kosaka T. Neuronal gap junctions in the polymorph layer of the rat dentate gyrus. Brain Res. 1983;277:347–351. doi: 10.1016/0006-8993(83)90943-5. [DOI] [PubMed] [Google Scholar]
- 19.Kosaka T, Kosaka K. Neuronal gap junctions in the rat main olfactory bulb, with special reference to intraglomerular gap junctions. Neurosci. Res. 2003;45:189. doi: 10.1016/s0168-0102(02)00222-5. [DOI] [PubMed] [Google Scholar]
- 20.Sotelo C, Korn H. Morphological correlates of electrical and other interactions through low-resistance pathways between neurons of the vertebrate central nervous system. Internat. Rev. Cytol. 1978;55:67–107. doi: 10.1016/s0074-7696(08)61887-2. [DOI] [PubMed] [Google Scholar]
- 21.Sotelo C, Palay SL. The fine structure of the lateral vestibular nucleus in the rat. II. Synaptic organization. Brain Res. 1970;18:93–115. doi: 10.1016/0006-8993(70)90459-2. [DOI] [PubMed] [Google Scholar]
- 22.Llinás R, Baker R, Sotelo C. Electronic coupling between neurons in cat inferior olive. J. Neurophysiol. 1974;37:560–571. doi: 10.1152/jn.1974.37.3.560. [DOI] [PubMed] [Google Scholar]
- 23.Sotelo C, Gentschev T, Zamora AJ. Gap junctions in ventral cochlear nucleus of the rat. A possible new example of electrotonic junctions in the mammalian CNS. Neurosci. 1976;1 doi: 10.1016/0306-4522(76)90041-5. 5-IN2. [DOI] [PubMed] [Google Scholar]
- 24.Sotelo C, Triller A. Morphological correlates of electrical, chemical and dual modes of transmission. Chemical neurotransmission. 1981;75:13–28. [Google Scholar]
- 25.Sotelo C. Morphological correlates of electrotonic coupling between neurons in mammalian nervous system. Raven Press; New York: 1975. pp. 355–365. [Google Scholar]
- 26.Sotelo C, Llinás R, Baker R. Structural study of inferior olivary nucleus of the cat: Morphological correlates of electrotonic coupling. J. Neurophysiol. 1974;37:541–559. doi: 10.1152/jn.1974.37.3.541. [DOI] [PubMed] [Google Scholar]
- 27.Martin AR, Pilar G. Transmission through the ciliary ganglion of the chick. J. Physiol. (London) 1963;168:464–475. doi: 10.1113/jphysiol.1963.sp007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett MVL, Crain SM, Grundfest H. Electrophysiology of Supramedullary Neurons in Spheroides maculatus III. Organization of the supramedulary neurons. J. Gen. Physiol. 1959;43:221–250. doi: 10.1085/jgp.43.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson JD. The occurrence of a subunit pattern in the unit membranes of club endings in Mauthner cell synapses in goldfish brains. J. Cell Biol. 1963;19:201–221. doi: 10.1083/jcb.19.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson JD, Bodenheimer TS, Stage DE. The ultrastructure of Mauthner cell synapses and nodes in goldfish brains. J. Cell Biol. 1963;19:159–199. doi: 10.1083/jcb.19.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett MV, Pappas GD, Gimenez M, Nakajima Y. Physiology and ultrastructure of electrotonic junctions. IV. Medullary electromotor nuclei in gymnotid fish. J. Neurophysiol. 1967;30:236–300. doi: 10.1152/jn.1967.30.2.236. [DOI] [PubMed] [Google Scholar]
- 32.Bennett MVL, Pappas GD, Aljure E, Nakajima Y. Physiology and ultrastructure of electrotonic junctions. II. Spinal and medullary electromotor nuclei in mormyrid fish. J. Neurophysiol. 1967;30:180–208. doi: 10.1152/jn.1967.30.2.180. [DOI] [PubMed] [Google Scholar]
- 33.Kriebel ME, Bennett MVL, Waxman SG, Pappas GD. Oculomotor neurons in fish: electrotonic coupling and multiple sites of impulse initiation. Science. 1969;166:520–524. doi: 10.1126/science.166.3904.520. [DOI] [PubMed] [Google Scholar]
- 34.Bennett MVL. Gap junctions as electrical synapses. J. Neurocytol. 1997;26:349–366. doi: 10.1023/a:1018560803261. [DOI] [PubMed] [Google Scholar]
- 35.Stefanelli A, Caravita S. Ultrastructural features of the synaptic complex of the vestibular nuclei of Lampetra planeri (Bloch) Zeit. Zellforsc. Mikrosk. Anat. 1970;108:282–296. doi: 10.1007/BF00335299. [DOI] [PubMed] [Google Scholar]
- 36.Rovainen CM. Synaptic interactions of reticulospinal neurons and nerve cells in the spinal cord of the sea lamprey. J. Comp. Neurol. 1974;154:207–224. doi: 10.1002/cne.901540207. [DOI] [PubMed] [Google Scholar]
- 37.Rovainen CM. Synaptic interactions of identified nerve cells in the spinal cord of the sea lamprey. J. Comp. Neurol. 1974;154:189–206. doi: 10.1002/cne.901540206. [DOI] [PubMed] [Google Scholar]
- 38.Hinojosa R. Synaptic ultrastructure in the tangential nucleus of the goldfish (Carassius auratus) Am. J. Anat. 1973;137:159–185. doi: 10.1002/aja.1001370204. [DOI] [PubMed] [Google Scholar]
- 39.Korn H, Sotelo C, Bennett MVL. The lateral vestibular nucleus of the toadfish Opsanus tau: Ultrastructural and electrophysiological observations with special reference to electrotonic transmission. Neuroscience. 1977;2:851–884. [Google Scholar]
- 40.Precht W, Richter A, Ozawa S, Shimazu H. Intracellular study of frog’s vestibular neurons in relation to the labyrinth and spinal cord. Exptl. Brain Res. 1974;19:377–393. doi: 10.1007/BF00234462. [DOI] [PubMed] [Google Scholar]
- 41.Babalian AL, Shapovalov AI. Mode of synaptic transmission between vestibular afferents and neurons of the vestibular nucleus in the frog. Brain Res. 1984;309:163–167. doi: 10.1016/0006-8993(84)91023-0. [DOI] [PubMed] [Google Scholar]
- 42.Richter A, Precht W, Ozawa S. Responses of neurons of lizard’s, Lacerta viridis, vestibular nuclei to electrical stimulation of the ipsi-and contralateral VIIIth nerves. Pflügers Arch. 1975;355:85–94. doi: 10.1007/BF00584802. [DOI] [PubMed] [Google Scholar]
- 43.Wilson VJ, Wylie RM. A short-latency labyrinthine input to the vestibular nuclei in the pigeon. Science. 1970;168:124–127. doi: 10.1126/science.168.3927.124. [DOI] [PubMed] [Google Scholar]
- 44.Hinojosa R, Robertson JD. Ultrastructure of the spoon type synaptic endings in the nucleus vestibularis tangentialis of the chick. J. Cell Biol. 1967;34:421. doi: 10.1083/jcb.34.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peusner KD. The development of synapses and “spoon” synaptic terminal space in the tangential vestibular nucleus: a quantitative electron microscope study. J. Comp. Neurol. 1984;230:372–385. doi: 10.1002/cne.902300306. [DOI] [PubMed] [Google Scholar]
- 46.Nakajima Y, Tuttle R, Masuko S, Betz H, Pfeiffer F. Synapses on the Mauthner cell of the goldfish: Thin section freeze-fracture, and immunocytochemical studies. J. Electron Microsc. Tech. 1987;6:143–153. [Google Scholar]
- 47.Tuttle R, Masuko S, Nakajima Y. Freeze-fracture study of the large myelinated club ending synapse on the goldfish Mauthner cell: Specialized reference to the quantitative analysis of gap junctions. J. Comp. Neurol. 1986;246:202–211. doi: 10.1002/cne.902460206. [DOI] [PubMed] [Google Scholar]
- 48.Nakajima Y. Fine structure of the synaptic endings on the Mauthner cell of the goldfish. J. Comp. Neurol. 1974;156:375–402. [PubMed] [Google Scholar]
- 49.Furshpan EJ. “Electrical transmission” at an excitatory synapse in a vertebrate brain. Science. 1964;144:878–880. doi: 10.1126/science.144.3620.878. [DOI] [PubMed] [Google Scholar]
- 50.Pereda AE, Bell TD, Faber DS. Retrograde synaptic communication via gap junctions coupling auditory afferents to the Mauthner cell. J. Neurosci. 1995;15:5943–5955. doi: 10.1523/JNEUROSCI.15-09-05943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rash JE, Yasumura T, Furman CS, Ottersen OP, Pereda AE, Dudek FE, Whalen LR, Staines WA, Nagy JI. Excitatory mixed synapses in goldfish and rat: Freeze-fracture replica immunogold labeling of glutamate receptor NR1 and connexin. 2000;36:1390. [Google Scholar]
- 52.Rash JE, Pereda A, Kamasawa N, Furman CS, Yasumura T, Davidson KGV, Dudek FE, Olson C, Nagy JI. High-resolution proteomic mapping in the vertebrate central nervous system: Close proximity of connexin35 to NMDA glutamate receptor clusters and co-localization of connexin36 with immunoreactivity for zonula occludens protein-1 (ZO-1) J. Neurocytol. 2004;33:131–152. doi: 10.1023/B:NEUR.0000029653.34094.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rash JE, Kamasawa N, Vanderpool KG, Yasumura T, O’Brien J, Nannapaneni S, Pereda A, Nagy JI. Heterotypic gap junctions at glutamatergic mixed synapses are abundant in goldfish brain. Neuroscience. 2014;285:166–193. doi: 10.1016/j.neuroscience.2014.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sur C, Wenthold R, Triller A. Differential cellular distribution of excitatory amino acid receptor subunits on the M-cell of teleost. 1994:489. [Google Scholar]
- 55.Pereda A, O’Brien J, Nagy JI, Bukauskas F, Davidson KGV, Kamasawa N, Yasumura T, Rash JE. Connexin35 mediates electrical transmission at mixed synapses on Mauthner cells. J. Neurosci. 2003;23:7489–7503. doi: 10.1523/JNEUROSCI.23-20-07489.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolszon LR, Pereda AE, Faber DS. A fast synaptic potential mediated by NMDA and non-NMDA receptors. J. Neurophysiol. 1997;78:2693–2705. doi: 10.1152/jn.1997.78.5.2693. [DOI] [PubMed] [Google Scholar]
- 57.Rash JE, Curti S, Vanderpool KG, Kamasawa N, Nannapaneni S, Palacios-Prado N, Flores CE, Yasumura T, O’Brien J, Lynn BD, Bukauskas FF, Nagy JI, Pereda AE. Molecular and functional asymmetry at a vertebrate electrical synapse. Neuron. 2013;79:957–969. doi: 10.1016/j.neuron.2013.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller AC, Pereda AE. The electrical synapse – molecular complexities at the gap and beyond. Dev. Neurobiol. 2017 doi: 10.1002/dneu.22484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Brien J, Al-Ubaidi MR, Ripps H. Connexin35: a gap junctional protein expressed preferentially in the skate retina. Mol. Biol. Cell. 1996;7:233–243. doi: 10.1091/mbc.7.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pereda AE, Curti S, Hoge G, Cachope R, Flores CE, Rash JE. Gap junction-mediated electrical transmission: Regulatory mechanisms and plasticity. Biochim. Biophys. Acta (BBA) - Biomembranes. 2013;1828:134–146. doi: 10.1016/j.bbamem.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sabatini BL, Regehr WG. Timing of neurotransmission at fast synapses in the mammalian brain. Nature. 1996;384:170–172. doi: 10.1038/384170a0. [DOI] [PubMed] [Google Scholar]
- 62.Curti S, Pereda AE. Voltage-dependent enhancement of electrical coupling by a subthreshold sodium current. J. Neurosci. 2004;24:3999–4010. doi: 10.1523/JNEUROSCI.0077-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang XD, Korn H, Faber DS. Long-term potentiation of electrotonic coupling at mixed synapses. Nature. 1990;348:542–545. doi: 10.1038/348542a0. [DOI] [PubMed] [Google Scholar]
- 64.Pereda A, Faber DS. Activity dependent short-term plasticity of intercellular coupling. J. Neurosci. 1996;16:983–992. doi: 10.1523/JNEUROSCI.16-03-00983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zbili M, Rama S, Debanne D. Dynamic control of neurotransmitter release by presynaptic potential. Front. Cell. Neurosci. 2016;10:278. doi: 10.3389/fncel.2016.00278. electronic citation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Volff J-N. Genome evolution and biodiversity in teleost fish. Heredity. 2005;94:280–294. doi: 10.1038/sj.hdy.6800635. [DOI] [PubMed] [Google Scholar]
- 67.Baylor DA, Nicholls JG. Chemical and electrical synaptic connexions between cutaneous mechanoreceptor neurones in the central nervous system of the leech. J. Physiol. 1969;203:591–609. doi: 10.1113/jphysiol.1969.sp008881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Graubard K, Hartline DK. Full-wave rectification from a mixed eectrical-chemical synapse. Science. 1987;237:535–537. doi: 10.1126/science.2885921. [DOI] [PubMed] [Google Scholar]
- 69.Johnson BR, Peck JH, Harris-Warrick RM. Differential modulation of chemical and electrical components of mixed synapses in the lobster stomatogastric ganglion. J. Comp. Physiol. A. 1994;175:233–249. doi: 10.1007/BF00215119. [DOI] [PubMed] [Google Scholar]
- 70.Todd KL, Kristan WB, Jr, French KA. Gap junction expression is required for normal chemical synapse formation. J. Neurosci. 2010;30:15277–15285. doi: 10.1523/JNEUROSCI.2331-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Denk W, Horstmann H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2004;2:e329. doi: 10.1371/journal.pbio.0020329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pallotto M, Watkins PV, Fubara B, Singer JH, Briggman KL. Extracellular space preservation aids the connectomic analysis of neural circuits. Elife. 2015;4:e08206. doi: 10.7554/eLife.08206. 20 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fujimoto K. Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins. Application to the immunogold labeling of intercellular junctional complexes. J. Cell Sci. 1995;108:3443–3449. doi: 10.1242/jcs.108.11.3443. [DOI] [PubMed] [Google Scholar]
- 74.Rash JE, Dillman RK, Morita M, Whalen LR, Guthrie PB, Fay-Guthrie D, Wheeler DW. Grid-mapped freeze fracture: Correlative confocal laser scanning microscopy and freeze-fracture electron microscopy of preselected cells in tissue slices. In: Severs NJ, Shotton DM, editors. Rapid Freezing, Freeze Fracture, and Deep Etching. Wiley-Liss, Inc.; New York, NY: 1995. pp. 127–150. [Google Scholar]
- 75.Kamasawa N, Furman CS, Davidson KGV, Sampson JA, Magnie AR, Gebhardt B, Kamasawa M, Morita M, Yasumura T, Pieper M, Zumbrunnen JR, Pickard GE, Nagy JI, Rash JE. Abundance and ultrastructural diversity of neuronal gap junctions in the OFF and ON sublaminae of the inner plexiform layer of rat and mouse retina. Neuroscience. 2006;142:1093–1117. doi: 10.1016/j.neuroscience.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Condorelli DF, Parenti R, Spinella F, Salinaro AT, Belluardo N, Cardile V, Cicirata F. Cloning of a new gap junction gene (Cx36) highly expressed in mammalian brain neurons. Eur. J. Neurosci. 1998;10:1202–1208. doi: 10.1046/j.1460-9568.1998.00163.x. [DOI] [PubMed] [Google Scholar]
- 77.Condorelli DF, Belluardo N, Trovato-Salinaro A, Mudo G. Expression of Cx36 in mammalian neurons. Brain Res. Brain Res. Rev. 2000;32:72–85. doi: 10.1016/s0165-0173(99)00068-5. [DOI] [PubMed] [Google Scholar]
- 78.Li X, Kamasawa N, Ciolofan C, Olson CO, Lu S, Davidson KGV, Yasumura T, Shigemoto R, Rash JE, Nagy JI. Connexin45-containing neuronal gap junctions in rodent retina also contain connexin36 in both apposing hemiplaques forming bi-homotypic gap junctions, with scaffolding contributed by zonula occludens-1. J. Neurosci. 2008;28:9769–9789. doi: 10.1523/JNEUROSCI.2137-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nagy JI, Bautista W, Blakley B, Rash JE. Morphologically mixed chemical-electrical synapses formed by primary afferents in rodent vestibular nuclei as revealed by immunofluorescence detection of connexin36 and vesicular glutamate transporter-1. Neuroscience. 2013;252:468–488. doi: 10.1016/j.neuroscience.2013.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wylie RM. Evidence of electrotonic transmission in the vestibular nuclei of the rat. Brain Res. 1973;50:179–183. doi: 10.1016/0006-8993(73)90605-7. [DOI] [PubMed] [Google Scholar]
- 81.Rubio ME, Nagy JI. Connexin36 expression in major centers of the auditory system in the CNS of mouse and rat: Evidence for neurons forming purely electrical synapses and morphologically mixed synapses. Neuroscience. 2015;303:604–629. doi: 10.1016/j.neuroscience.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bautista W, McCrea DA, Nagy JI. Connexin36 identified at morphologically mixed chemical/electrical synapses on trigeminal motoneurons and at primary afferent terminals on spinal cord neurons in adult mouse and rat. Neuroscience. 2014;263:159–180. doi: 10.1016/j.neuroscience.2013.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bautista W, Rash JE, Vanderpool KG, Yasumura T, Nagy JI. Re-evaluation of connexins associated with motoneurons in rodent spinal cord, sexually dimorphic motor nuclei and trigeminal motor nucleus. Eur. J. Neurosci. 2013;39:757–770. doi: 10.1111/ejn.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rash JE, Dillman RK, Bilhartz BL, Duffy HS, Whalen LR, Yasumura T. Mixed synapses discovered and mapped throughout mammalian spinal cord. Proc. Natl. Acad. Sci. (USA) 1996;93:4235–4239. doi: 10.1073/pnas.93.9.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Matsumoto A, Arnold AP, Micevych PE. Gap junctions between lateral spinal motoneurons in the rat. Brain Res. 1989;495:362–366. doi: 10.1016/0006-8993(89)90229-1. [DOI] [PubMed] [Google Scholar]
- 86.Matsumoto A, Arnold AP, Zampighi G, Micevych PE. Androgenic regulation of gap junctions between motoneurons in the rat spinal cord. J. Neurosci. 1988;8:4177–4138. doi: 10.1523/JNEUROSCI.08-11-04177.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rash JE, Duffy HS, Dudek FE, Bilhartz BL, Whalen LR, Yasumura T. Grid-mapped freeze-fracture analysis of gap junctions in gray white matter of adult rat central nervous system, with evidence for a “panglial syncytium” that is not coupled to neurons. J. Comp. Neurol. 1997;388:265–292. doi: 10.1002/(sici)1096-9861(19971117)388:2<265::aid-cne6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 88.Hamzei-Sichani F, Davidson KGV, Yasumura T, Janssen WGM, Wearne SL, Hof PR, Traub RD, Gutierrez R, Ottersen OP, Rash JE. Mixed electrical-chemical synapses in adult rat hippocampus are primarily glutamatergic and coupled by connexin-36. Front. Neuroanat. 2012;6:1–26. doi: 10.3389/fnana.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fulton BP, Miledi R, Takahashi T. Electrical synapses between motoneurons in the spinal cord of the newborn rat. Proc. R. Soc. London, Ser. B. 1980;206:115–120. doi: 10.1098/rspb.1980.0045. [DOI] [PubMed] [Google Scholar]
- 90.Walton KD, Navarette R. Postnatal changes in motoneurone electronic coupling studied in the in vitro rat lumbar spinal cord. J. Physiol. 1991;433:283–305. doi: 10.1113/jphysiol.1991.sp018426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Decima EE, Goldberg LJ. Centrifugal dorsal root discharges induced by motoneurone activation. J. Physiol. 1970;207:103. doi: 10.1113/jphysiol.1970.sp009051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Decima EE, Goldberg LJ. Motoneuron-presynaptic interaction in the spinal cord of the cat: rebuttal to a denial. Brain Res. 1976;110:387–391. doi: 10.1016/0006-8993(76)90413-3. [DOI] [PubMed] [Google Scholar]
- 93.Curtis DR, Lodge D, Headley PM. Electrical interaction between motoneurons and afferent terminals in cat spinal cord. J. Neurophysiol. 1979;42:635–641. doi: 10.1152/jn.1979.42.3.635. [DOI] [PubMed] [Google Scholar]
- 94.Gogan P, Gueritaud JP, Horcholle-Bossavit G, Tyc-Dumont S. Direct excitatory interactions between spinal motoneurones of the cat. J. Physiol. 1977;272:755–767. doi: 10.1113/jphysiol.1977.sp012071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nagy JI. Evidence for connexin36 localization at hippocampal mossy fiber terminals suggesting mixed chemical/electrical transmission by granule cells. Brain Res. 2012;1487:107–122. doi: 10.1016/j.brainres.2012.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vivar C, Traub RD, Gutierrez R. Mixed electrical-chemical transmission between hippocampal mossy fibers and pyramidal cells. Eur. J. Neurosci. 2012;35:76–82. doi: 10.1111/j.1460-9568.2011.07930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Korn H, Faber DS. The Mauthner cell half a century later: A neurobiological model for decision-making? Neuron. 2005;47:13–28. doi: 10.1016/j.neuron.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 98.Pereda A, Rash JE, Nagy JI, Bennett MVL. Dynamics of electrical transmission at club endings on the Mauthner cells. Brain Res. Brain Res. Rev. 2004;47:227–244. doi: 10.1016/j.brainresrev.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 99.Herberholz J, Antonsen BL, Edwards DH. A lateral excitatory network in the escape circuit of crayfish. J. Neurosci. 2002;22:9078–9085. doi: 10.1523/JNEUROSCI.22-20-09078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pappas GD, Bennett MVL. Specialized junctions involved in electrical transmission between neurons. Ann. N. Y. Acad. Sci. 1966;166:495–508. doi: 10.1111/j.1749-6632.1966.tb50177.x. [DOI] [PubMed] [Google Scholar]
- 101.Henze DA, Wittner L, Buzsáki G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat. Neurosci. 2002;5:790–795. doi: 10.1038/nn887. [DOI] [PubMed] [Google Scholar]
- 102.Alle H, Roth A, Geiger JRP. Energy-efficient action potentials in hippocampal mossy fibers. Science. 2009;325:1405–1408. doi: 10.1126/science.1174331. [DOI] [PubMed] [Google Scholar]
- 103.Forsythe ID. Direct patch recording from identified presynaptic terminals mediating glutamatergic EPSCs in the rat CNS, in vitro. J. Physiology. 1994;479:381–387. doi: 10.1113/jphysiol.1994.sp020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Borst JG, Helmchen F, Sakmann B. Pre-and postsynaptic whole-cell recordings in the medial nucleus of the trapezoid body of the rat. J. Physiol. 1995;489:825. doi: 10.1113/jphysiol.1995.sp021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hamzei-Sichani F, Davidson KGV, Yasumura T, Janssen WGM, Wearne SL, Hof PR, Traub RD, Gutierrez R, Ottersen OP, Rash JE. Mixed electrical-chemical synapses in adult rat hippocampus are primarily glutamatergic and coupled by connexin-36. Front. Neuroanat. 2012;6:1–26. doi: 10.3389/fnana.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]