To the Editor
Mastocytosis is characterized by the proliferation of clonal mast cells and whose clinical features include flushing, pruritus, abdominal pain, diarrhea, hypotension and anaphylaxis. The predominant form of cutaneous mastocytosis (CM) is urticaria pigmentosa (UP, also referred to as maculopapular cutaneous mastocytosis). Other forms of CM include diffuse cutaneous mastocytosis and mastocytomas (1). Systemic mastocytosis (SM) is characterized by multifocal mast cell infiltrates in the bone marrow and other organs, and is subcategorized following WHO criteria (1). One minor criteria for diagnosis of SM is a serum tryptase >20 ng/mL, which generally reflects mast cell expansion and is a useful marker of mast cell activation by established criteria (see (2)). Other mediators where no such criteria have been proposed include heparin, histamine, and prostaglandin D2 and some authors have suggested chromogranin A (CgA) should be among these markers based on limited data;(2, 3).
In particular, serum levels of CgA, have been reported “as fairly specific” to mast cells when evaluating patients for mast cell activation disorder (MCAD) when other causes of elevated chromogranin levels are excluded (3). CgA is a 439-residue granin family protein (48–60 kD) found in the secretory vesicles of neuroendocrine tissues and is a biomarker for assessment of neuroendocrine tumors (NETs) (4). Proton pump inhibitor (PPI) use is associated with an increase in CgA levels, as acid suppression by PPIs promotes hypergastrinemia which leads to increased CgA via gastrin-regulated enterochromaffin-like (ECL) cells and which decreases over time after the drug is discontinued (5, 6). These effects are generally not as pronounced in patients treated with H2 receptor antagonists (5, 7). Patients with mastocytosis are frequently treated with these agents to control symptoms related to mast cell-driven acid hypersecretion.
We thus prospectively determined serum CgA, gastrin and tryptase levels in 20 adults and 17 pediatric patients diagnosed with mastocytosis based on WHO criteria (1) (see Table E1 in the Online Repository). All patients had symptoms consistent with mast cell activation, such has flushing, pruritus, abdominal pain, diarrhea, hypotension and anaphylaxis.
Serum CgA, gastrin, and tryptase levels were measured at the Mayo Clinic Laboratories (Rochester, MN) with a normal reference of <93 ng/ml, <100 pg/ml and <11.5 ng/mL, respectively. Bone marrow, skin and small intestinal biopsies were obtained from adult patients with ISM. Samples were fixed and stained for tryptase and CgA. HMC1.1, HMC1.2 and LAD2 human mast cell lines and the pancreatic beta islet cell carcinoma line, QGP-1, were used to determine relative quantitative expression of CgA using RT-PCR and Western blotting (see methods, Text E1).
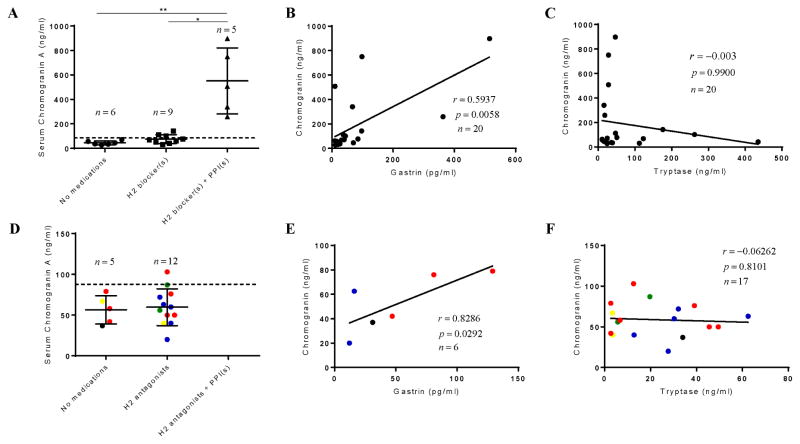
The adult cohort consisted of 10 female and 10 male patients with ISM, with a median age of 52.8 years, and tryptase level of 32.0 ng/mL (Table E1). Serum CgA median, 25th and 75th IQR were 65.0, 38.3 and 135 ng/ml, respectively. Since patients with ISM are often treated with PPIs and H2 antagonists (5), we divided the patients according to medication use. The median, 25th, and 75th IQR CgA serum values for those taking neither H2 antagonists or PPIs were 40.5, 32.5, and 59.8 ng/ml, respectively (n=6); for those on H2 antagonists alone, were 68.0, 46.0, and 107 ng/ml (n=9); and those taking H2 blockers and PPIs were 508, 300, and 824 ng/ml, respectively (n=5). We determined a significant difference in CgA serum values when comparing the no medication group (p<0.001) and the H2 antagonist group (p<0.05) to those taking both PPIs and H2 antagonists but not when comparing the no medication to those taking H2 antagonists alone (Figure 1A). Tryptase levels and D816V allelic frequency were not associated with H2 antagonist or PPI use. (Figure E1). These data are consistent with the conclusions that adult patients with mastocytosis not taking PPIs have serum CgA levels within the normal reference range and that the serum levels of CgA are significantly influenced by the use of PPIs (8). Serum gastrin levels correlated with CgA levels (p=0.020, r = 0.51) (Figure 1B) (5). However, CgA and tryptase levels did not correlate (Figure 1C). CgA levels were similarly measured in patients with pediatric mastocytosis (n=17, age range 3.6 – 15.6 years) (Table E1). All had mediator related symptoms and CgA levels within the normal range (Figure 1D). Also consistent with adult data, we found a positive correlation of CgA with gastrin (p=0.029, r=0.83) (Figure 1E) but not between tryptase and CgA (Figure 1F).
Figure 1. Serum chromogranin levels and correlations in adults and children with mastocytosis.
Adults (upper row) CgA serum levels (A), correlation of CgA with gastrin (B) and Tryptase (C). Pediatric patients (lower row) with parallel analysis shown in (D), (E) and (F). For D–F, ● = UP, ● = mastocytomas, ● =DCM, and ● = ISM, and ● = children with an unknown diagnosis. Data indicates that all subjects with mastocytosis exhibit normal levels of CgA (excluding use of PPIs), which correlates with gastrin but not tryptase. No patients were excluded from analysis. Patients were treated with H2 antagonists alone, and H2 plus PPI, for a median of 6.3 and 5.3 years, respectively.
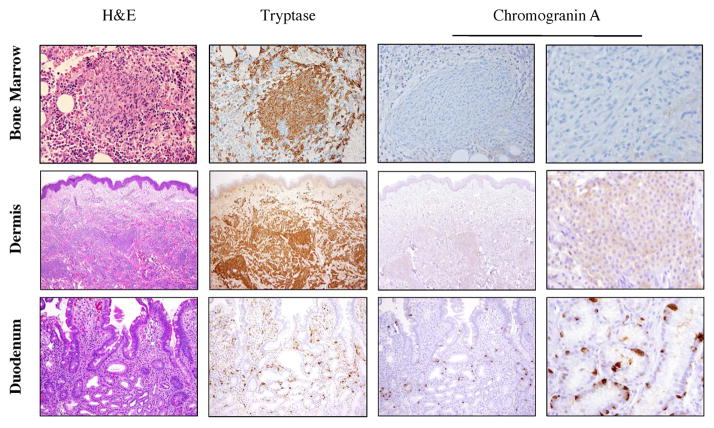
Representative marrow, duodenum and skin biopsy staining for CgA were essentially negative compared to positive controls (Figures 2 and E2). Bone marrow staining with tryptase revealed focal mast cell aggregates with corresponding negative staining for CgA (compare to control, Figure E2). Skin biopsy staining of UP (Figure 2) indicated the presence of infiltrating dermal mast cells with corresponding weak to absent staining for CgA (compared to positive staining of an epidermal Merkel cell; Figure E2). Tryptase staining of the duodenum in a patient with severe GI symptoms who is also treated by PPI highlighted the presence of mast cells in the lamina propria with no significant CgA staining (Figure 2).
Figure 2. Chromogranin immunohistochemistry in adults with ISM.
(Top row) Mast cell aggregate in bone marrow. From left to right: H&E, and tryptase staining identify mast cell aggregate with corresponding absence of CgA staining at 200x, and 600x. (Middle row) Mast cell infiltrate in highly vascularized UP lesion with parallel staining. CgA staining at 100x, and at 600x is scant. (Bottom row): Duodenal section in patient with severe GI symptoms noting absence of CgA in resident mast cells of lamina propria and positive staining of G-cells at 200x, and at 600x.
The relative expression of chromogranin by qPCR and western blotting in mast cell lines compared to positive control (QGP-1 cells) was low, even when cells were activated through the IgE receptor (Figure E3).
We thus determined that CgA is largely not identified in mast cell infiltrates in the bone marrow, skin, and GI tract (Figure 2); and serum levels are within normal reference range in patients with pediatric and adult mastocytosis, except in those patients treated with PPIs (Figures 1A and 1D), whereupon all of these patients exhibited an elevated CgA. Serum tryptase levels and D816V frequencies in peripheral blood, regardless of medication usage, did not associate with CgA levels (Figure E1).
There are reports that suggest serum levels of CgA aids in the diagnosis of patients with MCAD and SM (3, 9). In one study, CgA was found to be elevated in 5 of 8 patients with SM (3) although reference and patients’ CgA values were not reported, whereas in our study a single CLIA approved assay was used in all 37 subjects. Our results are consistent with studies in patients with GERD, which show that acid-suppressive therapy increases serum CgA levels independent of disease activity (5). CgA staining in areas of high mast cell density in bone marrow, skin and GI biopsies was interpreted as absent or unconvincing which is consistent with a previous report (10).
In summary, we found that use of PPIs is the cause of elevated serum CgA in patients with mastocytosis. These results, coupled with the immunohistochemical data, demonstrate that mast cells are not a significant systemic source of serum CgA. Therefore, we recommend that serum CgA not be used as a biomarker of mast cell disease.
Supplementary Material
(A) and (C) Serum tryptase levels vs. medication use in adults and children, respectively; (B) and (D) KIT D816V allelic frequency in peripheral blood vs. medication use in adults and children, respectively. All subjects in (D) with KIT D816V were treated with H2 antagonists. Red dots indicate patients with anaphylaxis. No statistically significant differences were found between adult patients, based on medication use, in tryptase levels or D816V allele burden. Tryptase levels were significantly elevated in pediatric patients treated with H2 antagonists (p=0.0351), as determined by Mann-Whitney test.
Left image shows chromogranin staining for beta islet cells in pancreas at 200x magnification. Right image shows chromogranin staining in Merkel cell of epidermis at 600x magnification (E4).
(A) CgA mRNA expression was significantly greater (>1000-fold) in QGP-1 cells (E5) than in all mast cell lines, with no significant differences in expression among mast cell lines, including LAD2 cells stimulated through the IgE receptor. (B) Significantly lower protein expression at predicted size of CgA (at 70 kD) in mast cell line lysates than in QGP-1 cell lysates (p<0.007), with no significant differences in expression among mast cell lines and minimal protein expression in mast cells (E6). All lysates were normalized to b-actin. Blot is representative of three experiments. No CgA was detected in cell supernatants.
Study population characteristics.
All *creatinine and **eGFR values were within normal limits.
Clinical Implications.
Chromogranin A is not a useful marker to detect mast cell activation in patients with mastocytosis. Elevated serum levels were exclusively associated with PPI use and did not correlate with mast cell burden or activation.
Acknowledgments
Funding statement: This work was supported by the Division of Intramural Research, NIAID, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129(11):1420–7. doi: 10.1182/blood-2016-09-731893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valent P, Akin C, Arock M, Brockow K, Butterfield JH, Carter MC, et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012;157(3):215–25. doi: 10.1159/000328760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vysniauskaite M, Hertfelder HJ, Oldenburg J, Dressen P, Brettner S, Homann J, et al. Determination of plasma heparin level improves identification of systemic mast cell activation disease. PLoS One. 2015;10(4):e0124912. doi: 10.1371/journal.pone.0124912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deftos LJ. Chromogranin A: its role in endocrine function and as an endocrine and neuroendocrine tumor marker. Endocr Rev. 1991;12(2):181–7. doi: 10.1210/edrv-12-2-181. [DOI] [PubMed] [Google Scholar]
- 5.Sanduleanu S, Stridsberg M, Jonkers D, Hameeteman W, Biemond I, Lundqvist G, et al. Serum gastrin and chromogranin A during medium- and long-term acid suppressive therapy: a case-control study. Aliment Pharmacol Ther. 1999;13(2):145–53. doi: 10.1046/j.1365-2036.1999.00466.x. [DOI] [PubMed] [Google Scholar]
- 6.Korse CM, Muller M, Taal BG. Discontinuation of proton pump inhibitors during assessment of chromogranin A levels in patients with neuroendocrine tumours. British journal of cancer. 2011;105(8):1173–5. doi: 10.1038/bjc.2011.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gori G, Spinelli G, Spinelli C, Tuccori M, Blandizzi C, Del Tacca M. Esomeprazole-induced hyperchromograninemia in the absence of concomitant hypergastrinemia. Nature reviews Gastroenterology & hepatology. 2010;7(11):642–6. doi: 10.1038/nrgastro.2010.152. [DOI] [PubMed] [Google Scholar]
- 8.Ferraro S, Borille S, Panteghini M. Reference intervals for the Kryptor second-generation chromogranin A assay. Clinical chemistry and laboratory medicine. 2016;54(11):e335–e7. doi: 10.1515/cclm-2016-0083. [DOI] [PubMed] [Google Scholar]
- 9.Molderings GJ, Brettner S, Homann J, Afrin LB. Mast cell activation disease: a concise practical guide for diagnostic workup and therapeutic options. J Hematol Oncol. 2011;4:10. doi: 10.1186/1756-8722-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horny HP, Kaiserling E. Lymphoid cells and tissue mast cells of bone marrow lesions in systemic mastocytosis: a histological and immunohistological study. British journal of haematology. 1988;69(4):449–55. doi: 10.1111/j.1365-2141.1988.tb02397.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) and (C) Serum tryptase levels vs. medication use in adults and children, respectively; (B) and (D) KIT D816V allelic frequency in peripheral blood vs. medication use in adults and children, respectively. All subjects in (D) with KIT D816V were treated with H2 antagonists. Red dots indicate patients with anaphylaxis. No statistically significant differences were found between adult patients, based on medication use, in tryptase levels or D816V allele burden. Tryptase levels were significantly elevated in pediatric patients treated with H2 antagonists (p=0.0351), as determined by Mann-Whitney test.
Left image shows chromogranin staining for beta islet cells in pancreas at 200x magnification. Right image shows chromogranin staining in Merkel cell of epidermis at 600x magnification (E4).
(A) CgA mRNA expression was significantly greater (>1000-fold) in QGP-1 cells (E5) than in all mast cell lines, with no significant differences in expression among mast cell lines, including LAD2 cells stimulated through the IgE receptor. (B) Significantly lower protein expression at predicted size of CgA (at 70 kD) in mast cell line lysates than in QGP-1 cell lysates (p<0.007), with no significant differences in expression among mast cell lines and minimal protein expression in mast cells (E6). All lysates were normalized to b-actin. Blot is representative of three experiments. No CgA was detected in cell supernatants.
Study population characteristics.
All *creatinine and **eGFR values were within normal limits.