Abstract
Pancreatic ductal adenocarcinoma (PDAC) is still one of the deadliest types of cancer. The worldwide estimates of its incidence and mortality in the general population are 8 cases per 100,000 person-years and 7 deaths per 100,000 person-years, and they are significantly higher in the United States than the rest of the world. The incidence of this disease in the United States (US) is over 50,000 new cases in 2017. Indeed, total deaths due to PDAC are projected to increase dramatically to become the second leading cause of cancer-related deaths before 2030. Considering the failure to date to efficiently treat existing PDAC, increased effort should be undertaken to prevent this disease. A better understanding of the risk factors leading to PDAC development is of utmost importance to identify and formulate preventive strategies. Large epidemiological and cohort studies have identified risk factors for the development of PDAC, including obesity and type-2 diabetes mellitus (T2DM). This review highlights the current knowledge of obesity and T2DM as risk factors for PDAC development and progression, their interplay and underlying mechanisms, the relation to diet, as well as outlines research gaps and opportunities to address this deadly disease.
Keywords: Pancreatic cancer, obesity, type 2 diabetes mellitus, prevention, review
Introduction
It is estimated that about one third of cases of cancer, the second leading cause of death in the United States (US), are caused by dietary factors.1,2 One of the deadliest types of cancer has been and still is pancreatic ductal adenocarcinoma (PDAC), the most common histologic type of pancreatic cancer. The worldwide estimates of its incidence and mortality in the general population are eight cases per 100,000 person-years and seven deaths per 100,000 person-years, and they are significantly higher in the US than the rest of the world.3 The projected incidence of this disease in the US is over 50,000 new cases in 2017 and it is currently the third leading cause of cancer mortality in both men and women.2 Despite advances in understanding the biology of PDAC, molecularly targeted therapy (such as epidermal growth factor receptor inhibitors) has not translated into substantially improved prognosis. Indeed, total deaths due to PDAC are projected to increase considerably to become the second leading cause of cancer-related deaths before 2030.4 Considering the failure to date to efficiently treat existing PDAC, increased effort should be undertaken to prevent this disease. Consequently, the focus of research has shifted gradually towards its prevention and interception, which encompasses halting transformed cells from becoming malignant cancers.5–9 In this context, a better understanding of the risk factors leading to PDAC development is of great importance to identify and formulate preventive and interceptive strategies and to ultimately educate the public. Large epidemiological and cohort studies have identified risk factors for the development of PDAC,10–13 including obesity and type-2 diabetes mellitus (T2DM). This review highlights the current knowledge of obesity and T2DM as risk factors for PDAC development and progression, their interplay and underlying mechanisms, the relation to dietary influences, as well as outlines research gaps and opportunities to address this deadly disease.
Epidemiology of obesity, diabetes mellitus, and pancreatic cancer
Obesity and Diabetes as Risk Factors for PDAC
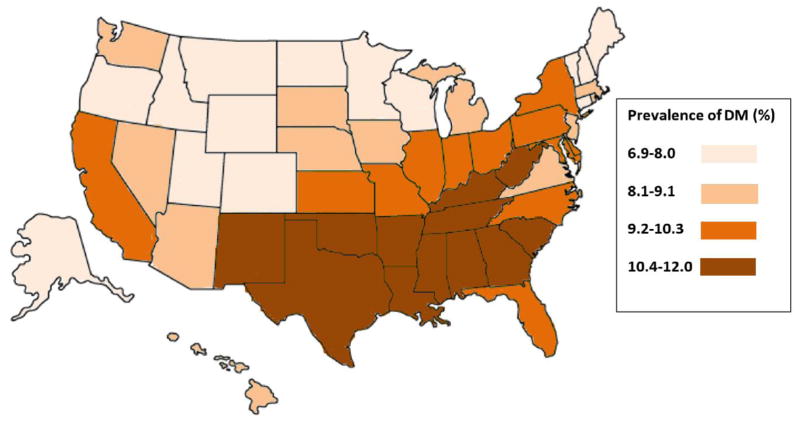
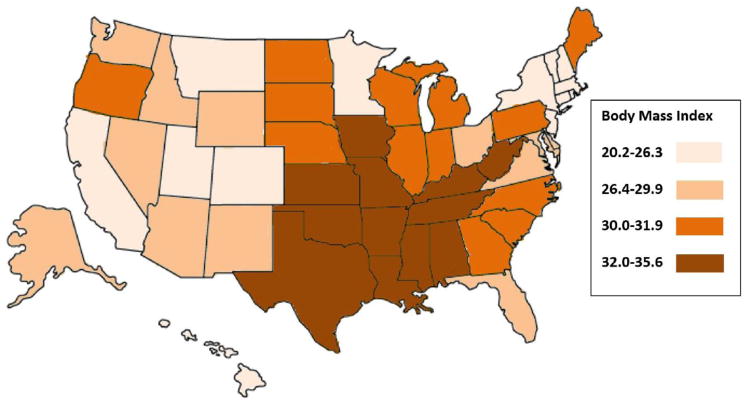
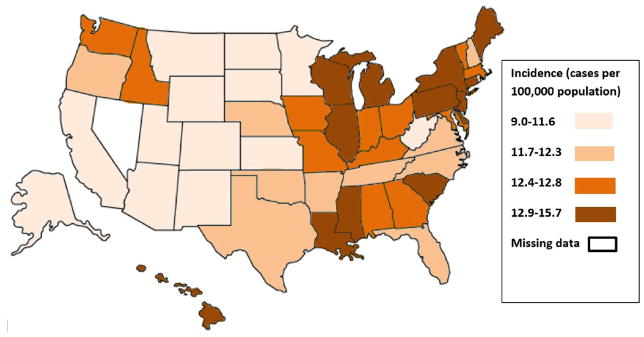
T2DM and obesity are among the small number of known modifiable risk factors for PDAC. There is a complex relationship between T2DM and obesity as they often coexist, but independently increase the risk for developing PDAC. An association of PDAC with T2DM and obesity is strongly suggested when the geographic prevalence of all three diseases is examined (Figure 1). The epidemiologic support for and proposed mechanisms of increased risk for PDAC in both longstanding T2DM and new-onset DM have been previously reviewed,14–17 so the connection with obesity is further emphasized here.
Figure 1. Prevalence of diabetes mellitus (DM), Prevalence of Obesity, and Incidence of Pancreatic Cancer.
Figure 1A (Top): Prevalence of DM (in quartiles) 2014 (from http://www.cdc.gov/diabetes/data). Figure 1B (Middle): Prevalence of Obesity expressed as Body Mass Index (in quartiles) 2015 (from http://www.cdc.gov/obesity/data). Figure 1C (Bottom): Incidence of Pancreatic Cancer, age adjusted, all races (in quartiles) 2009–2013 (from http://statecancerprofiles.cancer.gov/data-topics/incidence.html).
Epidemiological evidence from various study types has consistently shown that obesity is a dose-dependent risk factor for the development of PDAC.18–24 In a population study of over 900,000 adults, a 52% increased death rate from all cancers was observed in men and a 62% increased death rate in women with a body mass index (BMI) greater than 40 kg/m2 compared to normal weight controls.21 The relative risk (RR) of PDAC for subjects with BMI > 40 kg/m2 was 2.61 (95% CI, 1.3–5.4; P=.001) for men and 2.76 (95% CI, 1.74–4.36; P=.001) for women. Additionally, an increased BMI was associated with an increased risk of death from several other cancers (such as esophagus, liver, and colon)25–27 in which DM is less prevalent supporting an independent role of obesity in cancer development. It is important to acknowledge that the effect size of obesity as a PDAC risk factor is likely diluted when only BMI is considered because the distribution of fat appears to also influence cancer risk. For example, an increased waist to hip ratio is associated with a greater than 70% increased risk of PDAC.23
Evidence from clinical studies shows that weight loss, induced by dietary restriction, exercise, or bariatric surgery, reduces the risk of cancer.28–33 Adams et al reported that the incidence of obesity-related cancers decreased by 50% in 6,596 bariatric surgery patients compared to 9,442 obese controls followed for an average of 12.5 years.31 Similarly, in the Swedish Obesity Subjects study involving 2,010 bariatric surgery patients and 2,037 un-operated controls, Sjöström et al reported that the overall mortality was reduced by 24% in the surgery cohort, but the number of deaths from individual cancers was too small to assess organ-specific effects.29,33
Interaction of Obesity with Diabetes and PDAC
Although many epidemiologic studies have been confounded by the frequent coexistence of T2DM in the obese groups, larger studies indicate that obesity confers a significant cancer risk independent of the presence of T2DM. For example, Jiao et al studied a pooled cohort of over 900,000 subjects in which there were 2,454 who developed PDAC.34 The incidence of PDAC increased by 19% in the group with a BMI 30–35 kg/m2, and was not affected by the presence of T2DM.
Studies probing the contribution of metabolic alterations associated with obesity have corroborated the risk and suggest that increased insulin levels due to the insulin resistance of obesity are an important factor. Stolzenberg-Solomon et al studied levels of glucose and insulin and measures of insulin resistance in 29,133 Finnish male smokers followed for almost two decades.35 Fasting glucose, insulin levels, and insulin resistance (estimated with HOMA-IR) were positively associated with PDAC. Importantly, the RR of PDAC was 2.71 (95% CI, 1.19–6.18; P=.006) in subjects with the highest quartile of insulin resistance. As obesity is associated with insulin resistance in virtually all subjects, hyperinsulinemia is believed to have a causative role in PDAC tumorigenesis.36 In an autopsy study, Butler et al evaluated the influence of DM and obesity on pancreatic duct cell proliferation determined by the expression of Ki67, a nuclear protein strictly associated with cellular proliferation.37 Lean non-diabetic, obese non-diabetic, lean diabetic, and obese diabetic subjects demonstrated progressive increases in Ki67 expression, suggesting that obesity increased pancreatic duct cell proliferation, and the magnitude of this effect was further increased by the presence of T2DM. When hyperinsulinemia can no longer adequately compensate for the insulin resistance, persistent hyperglycemia results, and further increases the risk. In this way, both hyperinsulinemia and hyperglycemia are related factors which promote dysplasia and neoplasia in the pancreas.
Overview of mechanisms underlying the increased risk of PDAC by T2DM and obesity
A variety of overlapping and distinct mechanisms exist, by which T2DM and obesity can promote PDAC development. Patients with T2DM and the overwhelming majority of obese subjects are characterized by insulin resistance with ensuing hyperinsulinemia and high levels of insulin-like growth factor-1 (IGF-1),38–45 which can act as potent growth-promoting factors. The effects of insulin and/or IGF-1 are mediated by binding to insulin receptors, IGF-1 receptors, and hybrid insulin/IGF-1 receptors with subsequent activation of the PI3K signaling cascade.46 Of note, insulin/IGF-1 receptors have been described to be expressed on human pancreatic cancer cells.47 The importance of elevated insulin/IGF-1 levels in PDAC development is highlighted by the potential anti-tumor effects of metformin, an anti-diabetic drug that lowers circulating insulin/IGF-1 levels. Recent preclinical and clinical studies indicate that metformin use lowers the risk of PDAC, inhibits cancer cell growth, and improves survival of patients.7,48–55 The observation that several clinical trials did not find a beneficial effect of metformin in patients with advanced PDAC56–58 suggests a potential role of metformin more in the primary/secondary prevention and early interception settings.59
Obesity and T2DM are increasingly recognized as chronic, systemic, low-grade inflammatory conditions with elevations in reactive oxygen species, pro-inflammatory cytokines, adipokines, and eicosanoids.40,41,45 This systemic and local inflammatory milieu may be conducive to tumor initiation and/or promotion.60,61 The inflammatory microenvironment also is thought to be the major mechanism, by which chronic pancreatitis leads to PDAC development.62–65 Targeting pancreatic inflammation by inhibiting cyclooxygenase activity, using aspirin, or targeted blockade of inflammatory cytokines has been shown to attenuate cancer development and/or growth.66–71 Mouse models have demonstrated that obesity is associated with increased pancreatic inflammation, immune cell infiltration, and accelerated neoplasia.72–74 Targeting obesity by caloric restriction decreased pancreatic inflammation and reduced PDAC incidence and progression.75,76 Similarly, T2DM and accompanying hyperglycemia have been shown to lead to chronic inflammation and increased cancer risk,77 while inhibition of inflammatory signaling pathways reduced PDAC growth in a diabetic animal model.78 On a molecular level, a novel cross-talk between the inflammatory prostaglandin signaling pathway and mTORC-1, which is implicated in insulin resistance during obesity and T2DM, highlights the intricate cross-talk between obesity, T2DM, and inflammation.79 There is recent evidence indicating the nuclear receptor PPAR-γ to be at the crossroads of obesity, T2DM, and PDAC by regulating metabolism, inflammation, insulin and adipokine production.80 Dietary fish oil and omega-3 polyunsaturated fatty acids (PUFAs) have been shown in some laboratory and clinical studies to be correlated with a reduced PDAC incidence and decreased cancer growth,81–86 while some reports showed no beneficial effects.87 The Pancreatic Cancer 2012 Report of the Continuous Update Project (CUP) described a marginal correlation between total fat intake and PDAC risk, but no conclusions could be made for PUFAs.88 Consumption of fish oil has been correlated with the prevention of obesity and the improvement of insulin resistance, non-alcoholic fatty liver disease, and cardiovascular disease.89–94 Fish oil rich in omega-3 PUFAs has thereby been shown to promote an anti-inflammatory microenvironment by altering adipokine/cytokine production and attenuating pro-inflammatory immune cells.95–98 Importantly, omega-3 PUFAs are known ligands of PPAR-γ, again placing this nuclear receptor in a central role orchestrating metabolism, inflammation, and cancer risk.
Chronic pancreatic inflammation is characterized by a desmoplastic (dense fibroblastic) reaction. Conflicting reports have described both a pro- and anti-tumorigenic role of desmoplasia (dense connective tissue).99,100 Recent studies have highlighted the importance of pancreatic desmoplasia in the context of obesity-associated PDAC.101 In addition, a connection between hyperinsulinemia, pancreatic stellate cell activation, and islet fibrosis in a diet-induced obesity model of PDAC has been described.102 Other mechanisms have been described, by which obesity can promote the development of cancers, including changes in autophagic processes, gastrointestinal peptide secretion, and gut microbiota.39,43,45,103 A recent study reported that a membrane protein from a specific gut bacterium improved metabolism in obese and diabetic mice.104 However, a current meta-analysis questioned whether there are specific microbiome-based markers that can be associated with human obesity.105 This analysis reported that the ability to reliably classify individuals as obese solely on the basis of the composition of their microbiome was limited due to a lack of power to detect modest effect sizes.105 It is therefore currently unclear whether obesity leads to significant changes in the gut microbiome with subsequent deleterious health effects. Overall, there are many possible mechanisms, by which obesity and T2DM can promote PDAC development and enhance risk factor-induced tumor formation, including changes in signaling and metabolic pathways and fibro-inflammatory processes.
The central role of adipose tissue in mediating the increased risk of PDAC by T2DM and obesity
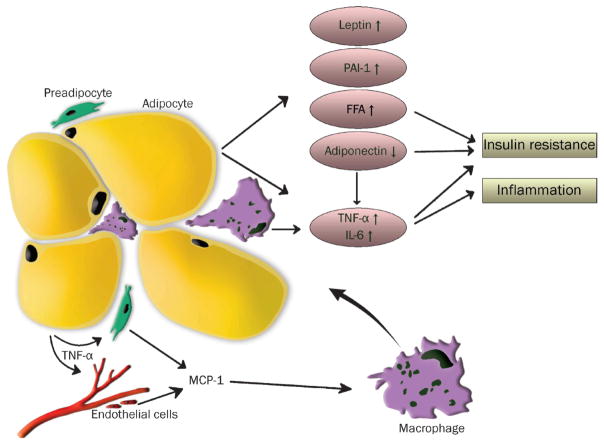
During obesity, the white adipose tissue may become dysfunctional and fail to meet the storage capacity needed for the excess caloric intake. This may lead to deleterious sequelae, including inflammation, fibrosis, hypoxia, and dysregulated adipokine secretion (Figure 2).106 This adaptive failure of adipose tissue may also result in ectopic fat deposition in other metabolically active tissues, e.g. liver, skeletal muscle, endocrine and exocrine pancreas, leading to progressive insulin resistance and T2DM.106,107 Obesity-associated adipose tissue inflammation is characterized by an elevation of pro-inflammatory cytokines and adipokines, e.g. TNF-α, IL-1β, IL-6, MCP-1, leptin, and resistin, and a decrease in anti-inflammatory molecules, e.g. IL-10, adiponectin.108 The inflammatory milieu in white adipose tissue during obesity is also characterized by profound immune cell changes. Adipose tissue macrophages (ATM) play a critical role in obesity-associated adipose tissue inflammation.109–115 In the lean state, ATMs display an anti-inflammatory M2-polarized phenotype, which is maintained by Th2-type cytokines produced by other tissue-resident immune and stromal cells. During obesity, the adipose tissue is characterized by a reduction of anti-inflammatory immune cells and cytokines, a predominance of pro-inflammatory M1-polarized macrophages, and an increase in Th1-type cytokines. Inhibiting the pro-inflammatory macrophage phenotype has been demonstrated to improve obesity-associated metabolic dysfunctions.116,117
Figure 2. Adipose tissue dysfunction during obesity.
Schematic overview of obesity-associated changes in white adipose tissue leading to hyperplasia/hypertrophy of adipocytes, recruitment and proliferation of immune cells, increased secretion of pro-inflammatory cytokines (e.g. TNF-α, IL-6) and adipokines (leptin), reduction of adiponectin, increase of free fatty acids (FFA), ultimately leading to insulin resistance and systemic and local inflammation (from van Kruijsdijk et al. Cancer Epidemiol Biomarkers Prev 2009; 18(10): 2569–78 with permission).
Adipose tissue inflammation is considered to significantly contribute to the development of obesity-associated cancers, including PDAC.41,43,101,118,119 A recent report has demonstrated that in the conditional KrasG12D mouse model of PDAC diet-induced obesity induced robust inflammation in the visceral white adipose tissue with an increase in crown-like structures, histological features of adipose tissue inflammation (necrotic adipocytes surrounded by macrophages) and elevated levels of pro-inflammatory cytokines and adipokines.120 This was associated with an acceleration of PDAC development.120 Interestingly, the observed obesity-associated inflammatory changes in this model were more robustly seen in mesenteric (peri-pancreatic) visceral adipose tissue than in other depots, emphasizing the importance of distinct anatomical locations of white adipose tissue.120 This notion is supported by human studies showing a stronger correlation between visceral adiposity (in contrast to generalized whole body fat), metabolic dysfunction, and PDAC.121–126
Recent studies have highlighted the importance of adipokines in PDAC risk and progression. Elevated levels of leptin, which are commonly seen in obese patients (with or without T2DM), were found to be associated with an increased risk of PDAC.127,128 Increased plasma concentrations of leptin were also detected in a diet-induced obesity model of PDAC,72 whereas caloric restriction decreased circulating leptin levels, which was accompanied by a delay of PDAC development.129 Experimental studies have linked leptin signaling to PDAC progression.130–132 Fewer studies have focused on the role of adiponectin in PDAC but lower circulating adiponectin levels are correlated to an increased PDAC risk.133–135
Intra-pancreatic fat accumulation, termed non-alcoholic fatty pancreas disease (NAFPD), ranging from simple fat deposition to pancreatic inflammation and fibrosis, has been associated with other diseases of obesity.107 A recent meta-analysis of clinical studies has shown that NAFPD may promote pancreatic endocrine dysfunction associated with insulin resistance and T2DM, and have links to the development of PDAC.136–141 Taken together, the available literature strongly indicates an important role of dysfunctional white adipose tissue, including inflammation and ectopic fat deposition due to obesity on metabolic disorders and PDAC development.
Dietary factors and Pancreatic Ductal Adenocarcinoma
Dietary factors that may affect the risk for PDAC include carbohydrate intake, fat intake, meat, fish, fruits and vegetables.12 It has been difficult to definitively establish risk factors because selection bias (e.g. studies limited to females only, or individuals of certain ethnicity only) complicates such investigations.24 The other common problem with studies on dietary factors is recall bias, which is a particular concern in retrospective studies. Further, individual studies of various designs and questionable methodological quality are frequently evaluated by meta-analysis,142 which may yield biased estimates. While more than 100 articles are published annually on dietary factors associated with risk of developing PDAC, we elected to present findings from the most robust individual epidemiological studies, i.e. prospective cohort studies conducted in a general population. PubMed was searched for articles published between January 1, 1990, and December 31, 2016. Only prospective, population-based, cohort studies that investigated dietary factors in relation to the development of PDAC are summarized below. Studies had to include adult individuals of both sexes living in a given geographic area. Studies were excluded if they were retrospective cohort, (nested) case-control, cross-sectional, or interventional studies or were not representative of the general population (e.g., insurance claims, tertiary setting only, cohorts limited to a particular ethnicity or occupation).
Carbohydrate intake
A prospective cohort study by Mueller et al.143 followed up a total of 60,524 individuals in Singapore for 14 years (on average), of which 140 developed incident PDAC. In multivariate analysis, the authors found that individuals consuming ≥2 soft drinks/week had a statistically significant increased risk of PDAC (hazard ratio or HR, 1.87; 95% CI, 1.10–3.15) compared with individuals who did not consume soft drinks. No statistically significant association was found between juice consumption and PDAC risk. A prospective cohort study by Larsson et al.144 followed a total of 77,797 individuals in Sweden for 7.2 years (on average), of which 131 developed incident PDAC. In multivariate analysis, the authors found significant associations between consumption of added sugar and soft drinks and risk of pancreatic cancer. The HRs for the highest compared with the lowest consumption categories were 1.95 (95% CI, 1.10–3.46; P = 0.03) for added sugar and 2.30 (95% CI, 1.35–3.92; P = 0.006) for soft drinks. However, there were no associations between consumption of fruit soups, or stewed fruit jam/marmalade, or sweets and PDAC risk. In a prospective cohort study by Bao et al.145 a total of 487,922 individuals in the US were followed up for 7.2 years (on average), of which 1258 developed incident PDAC. In multivariate analysis, the authors found no statistically significant association between high intake of total added sugar or sugar-sweetened foods/beverages and PDAC risk. The median intakes for the lowest and highest quintiles of total added sugar intake were 12.6 (3 tsp/d) and 96.2 (22.9 tsp/d) g/d, respectively. A prospective cohort study by Jiao et al.146 followed up a total of 482,362 individuals in the US for 7.2 years (on average), of which 1151 developed incident PDAC. In multivariate analysis, the authors found no statistically significant association between total or available carbohydrates or glycemic load/glycemic index and risk of pancreatic cancer. However, individuals with high free fructose intake were at a significantly higher risk of developing PDAC (highest compared with lowest quintile, RR, 1.29; 95% CI, 1.04–1.59; P = 0.004). Similarly, individuals with high free glucose intake were at a significantly higher risk of developing PDAC (RR, 1.35; 95% CI, 1.10–1.67; P = 0.005). These data are in agreement with another large meta-analysis describing a positive correlation between high fructose intake (but not total carbohydrates, glycemic index) and pancreatic cancer.142 In another prospective cohort study by Patel et al.147 a total of 124,907 individuals were followed up in the US for a maximum of nine years, of which 401 developed incident PDAC. In multivariate analysis, the authors found no statistically significant association between glycemic load/glycemic index or carbohydrate intake and PDAC risk. Finally, an important meta-analysis reported that high fructose intake is associated with an increased incidence of PDAC.142
Fat intake
A prospective cohort study by Thiebaut et al.148 followed up a total of 525,473 individuals in the US for 6.3 years (on average), of which 1337 developed incident PDAC. In multivariate analysis, the authors found that the intakes of total fat (highest vs lowest quintile, HR, 1.23; 95% CI, 1.03–1.46; P = 0.03), saturated fat (HR, 1.36; 95% CI, 1.14–1.62; P = 0.001), and monounsaturated fat (HR, 1.22; 95% CI, 1.02–1.46; P = 0.05) were associated with statistically significant higher PDAC risks. The associations were strongest for saturated fat from animal food sources (HR, 1.43; 95% CI, 1.20–1.70; P < .001); specifically, intakes from red meat and dairy products.
Fish intake
A prospective cohort study by He et al.85 followed up a total of 66,616 individuals in the US for 6.8 years (on average), of which 151 developed incident PDAC. In multivariate analysis, the authors found that long-chain (n-3) polyunsaturated fatty acids (LC-PUFAs) were associated with a statistically significant lower PDAC risk (HR, 0.62; 95% CI, 0.40–0.98; P = 0.04). Similarly, non-fried fish intake was associated with a statistically significant lower PDAC risk (HR, 0.55; 95% CI, 0.34–0.88; P = 0.04). Also, docosahexaenoic acid showed a greater inverse association with PDAC than eicosapentaenoic acid. However, no statistically significant associations were observed with fried fish and shellfish consumption.
Meat intake
A prospective cohort study by Stolzenberg-Solomon et al.149 followed up a total of 537,302 individuals in the US for a maximum of five years, of which 831 developed incident PDAC. In multivariate analysis, the authors found total, red, and high-temperature cooked meat intake was significantly associated with PDAC among men (fifth versus first quintile: HR, 1.41, 95% CI, 1.08–1.83, P = 0.001; HR, 1.42, 95% CI, 1.05–1.91, P = 0.01; and HR, 1.52, 95% CI, 1.12–2.06, P = 0.005, respectively). However, no statistically significant associations were observed among women.
Fruits and vegetables
A prospective cohort study by Larsson et al.150 followed up a total of 81,922 individuals in Sweden for 6.8 years (on average), of which 135 developed incident PDAC. In multivariate analysis, the authors found that cabbage consumption was associated with a statistically significant lower PDAC risk (≥1 serving/week versus never consumption: HR, 0.62; 95% CI, 0.39–0.99). However, the HR for the highest compared with the lowest category of intake was not statistically significant for total vegetables (HR, 1.08; 95% CI, 0.63–1.85). Similarly, the HR for the highest compared with the lowest category of intake was not statistically significant for total fruits (HR 1.10; 95% CI, 0.64–1.88). While some studies found no association between fruit and vegetable intake and PDAC, it is possible that measurement errors may obscure an association as one large cohort found an inverse association between vitamin C and carotenoids (as markers of fruit and vegetable intake) and PDAC risk.151
It is now well-established that metabolic pathways are altered in cancer.152 However, it is less readily evident how chronic alterations in our system biology enhance cancer risk. Excessive caloric intake due to excessive dietary fat and red meat, sugar-containing soft drinks, and fructose can lead to an increasing prevalence of obesity of diabetes, and secondarily to a higher incidence of PDAC. However, there are likely also direct effects. Thus, high intake of sugars leads to elevated chronic elevation in insulin levels, excessive fat intake may cause abnormal lipid metabolism and promote reactive oxygen generation, whereas the chronic absence of green vegetables and fruits can lead to alterations in regulatory immune pathways and the microbiome. All these alterations can enhance cancer risk in the context of polymorphisms and genetic susceptibilities. An update of the current evidence on food, nutrition and physical activity relating to the prevention of pancreatic cancer has been published as part of the Continuous Update Project in 2012.88 An improved understanding of these alterations that precede neoplastic transformation in the pancreas would allow for the development of more precise preventive strategies.
Animal models of obesity and diabetes to study PDAC
Animal models that mimic aspects of diabetes, in particular, T2DM and obesity are important tools for the understanding of how these metabolic diseases can increase the risk of developing other diseases such as PDAC. The complexity of the processes involved with T2DM and obesity and the time it takes for individuals to develop PDAC makes it challenging to conduct longitudinal studies in humans focused on specific molecular mechanisms related to these diseases. Therefore, using animal models that recapitulate in part obesity and T2DM as surrogates are an option for researchers interested in understanding the molecular basis of these diseases. Unfortunately, animal models are imperfect and most do not recapitulate all features of human obesity and T2DM. The selection of the models used for pre-clinical studies depends on the research hypothesis and a detailed understanding of how the model was generated including its phenotype. Of the many models currently available, the diet-induced obesity (DIO) model is one of the most commonly used as well as genetically engineered mouse models (GEMM) for which pathways linked to the control of body weight and appetite signals are altered. Currently available mouse models to study obesity are comprehensively reviewed elsewhere.153,154 Here we describe the models most commonly used to study these diseases as they relate to PDAC.
In the DIO model mice are fed a diet high in fats and calories that closely mimics what is commonly referred to as a Western-style diet, which contains 40–60% of energy derived from fats and is consumed ad libitum.155 These diets have been used extensively in obesity research with great success76,156 and recapitulate obesity-induced by diet in humans. The most commonly used mouse strain is C57BL/6, which is among the most sensitive to DIO and results in increased glucose levels. In this strain, males develop more severe obesity than females. Using this model injection of PDAC cells led to increased tumor burden in diet-induced obese mice.132,157 A number of studies have tested these diets using GEMM that recapitulate the human stages of PDAC development, and shown an acceleration of the initiation and progression of PDAC and reduced survival.72,74,76,120,158,159 Moreover, these studies have been able to identify the molecular mechanisms for which obesity increases the risk of developing PDAC, including an increase in inflammatory pathways.
Among the most commonly used GEMM of obesity and diabetes are those affecting the leptin pathway leading to hyperphagia, decreased energy expenditure, hyperglycemia, and insulin resistance. Mice with a single base spontaneous mutation in the obese (ob) gene (Lepob/Lepob) are not able to secrete leptin from adipose tissues.160,161 These mice become obese and exogenous administration of leptin prevents obesity development and metabolic syndrome. Recently this model was used to study the effects of obesity-induced inflammation in PDAC where PDAC cell lines were injected orthotopically and an accelerated tumor growth was observed.101 Another useful model is one, in which mice lack the leptin receptor (LepRdb/LepRdb)162–164 due to a point mutation in a stop codon that shortens the intracellular domain and abolishes signaling. PDAC cells implanted subcutaneously in this model developed larger tumors compared to lean mice.165 However, the obesity and insulin-resistant phenotype observed in these mice is dependent upon the genetic background.153 These mice are often used in T2DM studies, but do not recapitulate all aspects of human disease, such as pancreatic amyloid deposition.153
Despite marked similarities between mouse models and human disease, differences exist that need to be considered before designing animal experiments. Even though the mouse models of obesity and T2DM (in the context of PDAC) discussed here do not recapitulate all features seen in humans, they are still an invaluable resource to investigate important aspects of these diseases that can then be translated into humans. With obesity and obesity-related metabolic diseases representing a growing socioeconomic burden globally and PDAC being a relatively rare cancer, mouse models are needed to explore the molecular mechanisms that link these diseases.
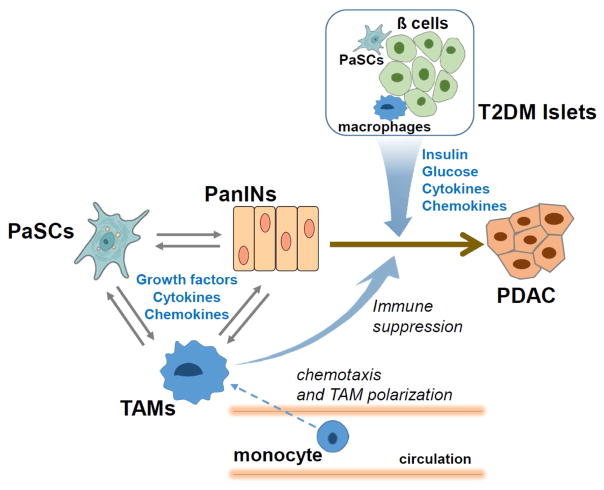
Effect of T2DM and obesity on the pancreatic microenvironment - crosstalk between stellate cells and islets
PDAC presents a unique microenvironment for a cancer with a substantial desmoplastic response. The desmoplastic response is due to proliferation and production of extracellular matrix proteins by tumor-associated fibroblasts, which are activated pancreatic stellate cells (PaSCs). This desmoplasia or microenvironment contains immune cells, nerve cells, blood vessels and extracellular matrix (ECM) proteins and factors that regulate ECM production.166 The identification of activated PaSCs as key participants in the desmoplastic microenvironment of PDAC was first made in 2004 by Apte et al.167 They showed that the activated PaSCs, which contribute to the fibrosis of chronic pancreatitis,168 were also present in PDAC. In their normal, inactive and non-pathologic state, PaSCs produce small amounts of ECM and are involved in tissue repair.169 In both in human tissue and in mouse experimental models of PDAC activated PaSCs are present both in early intraepithelial neoplastic lesions (PanIN) as well as in advanced PDAC.72,166,170 Related to obesity and diabetes, in a mouse model of high fat, high calorie diet there were increased activated PaSCs and fibrosis associated with the advancement of the tumor lesions.72
Regarding the effect of T2DM on PaSC function a recent publication examined the effects on insulin and glucose on PaSCs in mice and humans.102 In this study, activated PaSC were observed both in the islets and the peri-islet exocrine tissue in both T2DM patients and in mice fed a high-fat, high-calorie diet. These findings were absent in control-fed animals. These findings support those of previous studies showing mild fibrosis in patients with T2DM which has been referred to as diabetes-associated exocrine pancreatopathy.171 This study also found that PaSCs respond to high concentrations of both insulin and glucose with increased cell proliferation and ECM production. Further, the effects of insulin were mediated by insulin receptors and insulin-like growth factor receptors that were present on PaSCs and by Akt/mTOR downstream signaling. The study concluded that obesity and T2DM through effects on PaSCs can contribute to pancreatic fibrogenesis, desmoplasia and promotion of PDAC. Several other studies have demonstrated that islet macrophages, particularly M1-like macrophages, contribute to islet inflammation and beta-cell dysfunction in T2DM.172 Therefore, it is plausible that in T2DM multiple local cytokines secreted by islet macrophages and PaSCs modulate macrophage polarization, islet inflammation, and peri-islet fibrosis, all factors increasing T2DM pathology and the risk of PDAC.
There are studies designed to delineate mechanistic relationships between PaSCs, cancer cells and immune cells in PDAC.166,173 In brief, these studies show that PDAC cells stimulate proliferation and migration of PaSC in culture, as well as their production of ECM components.166,174 Secretions from pancreatic duct cells isolated from GEMM stimulate activation, proliferation and fibrogenic responses in isolated mouse PaSC170 supporting the role of the cancer cell in regulating the PaSC responses in development.
A question that remains under debate is whether the activated PaSCs promote or retard cancer growth. Early studies showed that PaSCs stimulate cell proliferation; inhibit apoptosis; and promote migration as well as epithelial mesenchymal transition (EMT) in cancer cells.166,175,176 Also, studies have shown that ECM proteins made by PaSCs are necessary to prevent apoptosis of cancer cells.177–181 A recent study182 using a chronic pancreatitis model where the tissue has similar characteristics to the desmoplasia of PDAC activated PaSCs secrete cytokines IL-4 and IL-13 promoting the transition of monocytes and macrophages to pro-tumor associated macrophages (TAMs). These macrophages are present in PanIN lesions and PDAC.183 Furthermore, TAMs in PDAC are associated with poor outcomes as they inhibit normal immune surveillance.184,185 Because TAMs secrete TGF-β, which promotes the activated state of PaSCs, a feed forward promotion of PaSC activation and TAM maintenance has been proposed for advancement of PDAC.173
There are recent reports186,187 showing that genetic methods eliminating PaSCs or pharmacological targeting of the Sonic hedgehog pathway, which is known to promote PaSC function in a PDAC mouse model, increased invasive and undifferentiated tumors and decreased survival. These results have resulted in a consensus that PaSCs can have either PDAC promoting or inhibiting effects. Current work is directed toward understanding the molecular details of these differences which could lead to important therapeutic interventions.
Gaps in knowledge and research opportunities
Although the risk factors promoting PDAC development have been known for several decades their underlying molecular mechanisms and interactions have just recently begun to be explored. High quality epidemiological studies associate obesity with an elevated risk of PDAC. Nevertheless, it is currently unclear whether “metabolically healthy obesity” also carries an increased risk of PDAC. In addition, the beneficial effects of weight reduction and bariatric surgery on improving insulin resistance are known, but their role in decreasing PDAC incidence is still essentially unknown. Although the general pathways linking obesity and T2DM to PDAC have been described, including chronic tissue and systemic inflammation, cytokines/adipokines, hyperinsulinemia and elevated IGF-1, the exact molecular and signaling pathways and their intricate interaction are still underexplored. Adipose tissue plays a central role in obesity and T2DM. However, the precise contribution and molecular signals of different adipose tissue depots and possible sex differences on PDAC development are not known. Several genetically engineered animal models are now available to study early PDAC development and risk factors and to investigate preventive strategies. Diet-induced obesity models are valuable tools to explore the role of obesity and metabolic disturbances in PDAC. However, very few studies exist that comprehensively investigate and compare the effects of individual nutritional components on PDAC development. It is currently unknown whether obesity experimentally induced by a high fat or high carbohydrate diet differs in increasing PDAC incidence or whether the simply increased caloric intake is the essential component. Altogether, given the high mortality of PDAC and expected increase in obesity and diabetes over the next few decades, efforts should be undertaken to mechanistically understand the link between obesity, diabetes, and PDAC. Preclinical animal models are available that will facilitate the study of these important interactions to advance our knowledge, so that the obesity- and diabetes-driven burden of PDAC can be curbed.
The Consortium for the Study of Chronic Pancreatitis, Diabetes and Pancreatic Cancer (CPDPC) is a multi-center program jointly sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Cancer Institute, which is pursuing a variety of studies to further identify mechanisms and biomarkers of PDAC in order to increase early detection of the disease and to inform intervention strategies.188 CPDPC investigators are applying lessons learned from studies such as those described here to gain insights into the mechanisms by which diabetes and inflammation contribute to the incidence of PDAC.
Figure 3. Promotion of PDAC by T2DM: Potential Role of Islet Factors.
Illustration of the interplay and crosstalk between pancreatic pre-cancer (PanIN) and cancer (PDAC) cells, pancreatic stellate cells (PaSCs), immune cells (e.g. tumor-associated macrophages: TAMs), and islets.
Research Snapshot.
Research Question
What is the current research status on the link between obesity, type 2 diabetes mellitus, dietary issues, and pancreatic cancer?
Key Findings
This narrative review describes a clear epidemiological association between obesity, type 2 diabetes mellitus and pancreatic cancer risk. Major pathophysiological mechanisms underlying this link, including inflammation and adipose tissue dysfunction, are discussed. Available animal models to study the impact of these risk factors on pancreatic cancer development are summarized, and research gaps and opportunities to advance the field presented.
Acknowledgments
Grant support: Research reported in this publication was supported by National Cancer Institute and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award numbers U01DK108300, U01DK108314, U01DK108323, U01DK108326, U01DK108327, P01CA163200, P01DK098108, R01CA075059. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author contributions: GE, ZCM, MK, MSP, MOG, WEF, AH, AL, SJP, PAH, DKA wrote the first draft of manuscript chapters. All authors reviewed and commented on subsequent drafts of the manuscript, and approved the final manuscript.
Conflict of Interest disclosures: All authors declared no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Guido Eibl, Professor, Department of Surgery, David Geffen School of Medicine at UCLA, 10833 LeConte Avenue, Los Angeles, CA 90095; phone: 310-794-9577.
Zobeida Cruz-Monserrate, Assistant Professor, Department of Internal Medicine, Division of Gastroenterology, Hepatology, and Nutrition, and Arthur G. James Comprehensive Cancer Center, The Ohio State University Wexner Medical Center, 400 W 12th Ave 2041 Wiseman Hall, Columbus, OH, 43210; phone 614-685-8266.
Murray Korc, Departments of Medicine, Biochemistry and Molecular Biology, Division of Endocrinology, Indiana University School of Medicine, the Melvin and Bren Simon Cancer Center and the Pancreatic Cancer Signature Center, Indianapolis, IN 46202; phone: 317-278-6410.
Maxim S. Petrov, Senior Lecturer, Department of Surgery, University of Auckland; Room 12.085A, Auckland City Hospital, Private Bag 92019, Victoria Street West, Auckland 1142, New Zealand; phone: +64 9-923-2776.
Mark O. Goodarzi, Professor, Division of Endocrinology, Diabetes & Metabolism, Cedars-Sinai Medical Center, 8700 Beverly Blvd, Room B-131; Los Angeles, CA 90048; phone: 310-423-4774.
William E. Fisher, Professor, Department of Surgery, Baylor College of Medicine, 6620 Main Street, Suite 1350, Houston, TX 77030; phone: 713-798-8371.
Aida Habtezion, Assistant Professor, Division of Gastroenterology and Hepatology, Stanford University School of Medicine, 900 Blake Wilbur Dr., Palo Alto, CA 94304; phone: 650-736-5555.
Aurelia Lugea, Adjunct Associate Professor, Departments of Medicine and Biomedical Sciences, Cedars-Sinai Medical Center; Department of Medicine, David Geffen School of Medicine at UCLA; 8700 Beverly Blvd, Los Angeles, CA 90048; phone 310-248-7691.
Stephen J. Pandol, Professor, Departments of Medicine and Biomedical Sciences, Cedars-Sinai Medical Center; Department of Medicine, David Geffen School of Medicine at UCLA; 8700 Beverly Blvd., Los Angeles, CA 90048; phone 310-248-7691.
Phil A. Hart, Assistant Professor of Medicine, Division of Gastroenterology, Hepatology, and Nutrition, The Ohio State University Wexner Medical Center, 410 West Tenth Ave, Columbus, OH 43210; phone: 614-293-6255.
Dana K. Andersen, Division of Digestive Diseases and Nutrition, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, 6707 Democracy Blvd, 6th Floor, Bethesda, MD 20892; phone: 410-868-0638.
References
- 1.Hine RJ, Srivastava S, Milner JA, Ross SA. Nutritional links to plausible mechanisms underlying pancreatic cancer: a conference report. Pancreas. 2003;27(4):356–366. doi: 10.1097/00006676-200311000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Xiao AY, Tan MLY, Wu LM, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. The Lancet Gastroenterology & Hepatology. 1(1):45–55. doi: 10.1016/S2468-1253(16)30004-8. [DOI] [PubMed] [Google Scholar]
- 4.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 5.Meyskens FL, Jr, Mukhtar H, Rock CL, et al. Cancer Prevention: Obstacles, Challenges and the Road Ahead. J Natl Cancer Inst. 2016;108(2) doi: 10.1093/jnci/djv309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kensler TW, Spira A, Garber JE, et al. Transforming Cancer Prevention through Precision Medicine and Immune-oncology. Cancer Prev Res (Phila) 2016;9(1):2–10. doi: 10.1158/1940-6207.CAPR-15-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albini A, DeCensi A, Cavalli F, Costa A. Cancer Prevention and Interception: A New Era for Chemopreventive Approaches. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-0695. [DOI] [PubMed] [Google Scholar]
- 8.Umar A, Dunn BK, Greenwald P. Future directions in cancer prevention. Nat Rev Cancer. 2012;12(12):835–848. doi: 10.1038/nrc3397. [DOI] [PubMed] [Google Scholar]
- 9.Miller MS, Allen P, Brentnall TA, et al. Pancreatic Cancer Chemoprevention Translational Workshop: Meeting Report. Pancreas. 2016;45(8):1080–1091. doi: 10.1097/MPA.0000000000000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jansen RJ, Tan XL, Petersen GM. Gene-by-Environment Interactions in Pancreatic Cancer: Implications for Prevention. Yale J Biol Med. 2015;88(2):115–126. [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen DK, Andren-Sandberg A, Duell EJ, et al. Pancreatitis-diabetes-pancreatic cancer: summary of an NIDDK-NCI workshop. Pancreas. 2013;42(8):1227–1237. doi: 10.1097/MPA.0b013e3182a9ad9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maisonneuve P, Lowenfels AB. Risk factors for pancreatic cancer: a summary review of meta-analytical studies. Int J Epidemiol. 2015;44(1):186–198. doi: 10.1093/ije/dyu240. [DOI] [PubMed] [Google Scholar]
- 13.Edderkaoui M, Eibl G. Risk factors for pancreatic cancer: underlying mechanisms and potential targets. Front Physiol. 2014;5:490. doi: 10.3389/fphys.2014.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sah RP, Nagpal SJ, Mukhopadhyay D, Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol. 2013;10(7):423–433. doi: 10.1038/nrgastro.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart PA, Bellin MD, Andersen DK, et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. The Lancet Gastroenterology & Hepatology. 1(3):226–237. doi: 10.1016/S2468-1253(16)30106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui Y, Andersen DK. Diabetes and pancreatic cancer. Endocr Relat Cancer. 2012;19(5):F9–F26. doi: 10.1530/ERC-12-0105. [DOI] [PubMed] [Google Scholar]
- 17.Fisher WE. Diabetes: risk factor for the development of pancreatic cancer or manifestation of the disease? World J Surg. 2001;25(4):503–508. doi: 10.1007/s002680020344. [DOI] [PubMed] [Google Scholar]
- 18.Genkinger JM, Kitahara CM, Bernstein L, et al. Central adiposity, obesity during early adulthood, and pancreatic cancer mortality in a pooled analysis of cohort studies. Ann Oncol. 2015;26(11):2257–2266. doi: 10.1093/annonc/mdv355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L, Dyer A. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA. 2000;283(19):2552–2558. doi: 10.1001/jama.283.19.2552. [DOI] [PubMed] [Google Scholar]
- 20.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA. 2001;286(8):921–929. doi: 10.1001/jama.286.8.921. [DOI] [PubMed] [Google Scholar]
- 21.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 22.Johansen D, Stocks T, Jonsson H, et al. Metabolic factors and the risk of pancreatic cancer: a prospective analysis of almost 580,000 men and women in the Metabolic Syndrome and Cancer Project. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2307–2317. doi: 10.1158/1055-9965.EPI-10-0234. [DOI] [PubMed] [Google Scholar]
- 23.Arslan AA, Helzlsouer KJ, Kooperberg C, et al. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan) Arch Intern Med. 2010;170(9):791–802. doi: 10.1001/archinternmed.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alsamarrai A, Das SL, Windsor JA, Petrov MS. Factors that affect risk for pancreatic disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Clin Gastroenterol Hepatol. 2014;12(10):1635–1644. e1635. doi: 10.1016/j.cgh.2014.01.038. quiz e1103. [DOI] [PubMed] [Google Scholar]
- 25.Dixon JL, Copeland LA, Zeber JE, et al. Association between diabetes and esophageal cancer, independent of obesity, in the United States Veterans Affairs population. Dis Esophagus. 2016;29(7):747–751. doi: 10.1111/dote.12402. [DOI] [PubMed] [Google Scholar]
- 26.Aggarwal G, Kamada P, Chari ST. Prevalence of diabetes mellitus in pancreatic cancer compared to common cancers. Pancreas. 2013;42(2):198–201. doi: 10.1097/MPA.0b013e3182592c96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang WS, Va P, Bray F, et al. The role of pre-existing diabetes mellitus on hepatocellular carcinoma occurrence and prognosis: a meta-analysis of prospective cohort studies. PLoS One. 2011;6(12):e27326. doi: 10.1371/journal.pone.0027326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teucher B, Rohrmann S, Kaaks R. Obesity: focus on all-cause mortality and cancer. Maturitas. 2010;65(2):112–116. doi: 10.1016/j.maturitas.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 29.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 30.Birks S, Peeters A, Backholer K, O’Brien P, Brown W. A systematic review of the impact of weight loss on cancer incidence and mortality. Obes Rev. 2012;13(10):868–891. doi: 10.1111/j.1467-789X.2012.01010.x. [DOI] [PubMed] [Google Scholar]
- 31.Adams TD, Stroup AM, Gress RE, et al. Cancer incidence and mortality after gastric bypass surgery. Obesity (Silver Spring) 2009;17(4):796–802. doi: 10.1038/oby.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christou NV, Lieberman M, Sampalis F, Sampalis JS. Bariatric surgery reduces cancer risk in morbidly obese patients. Surg Obes Relat Dis. 2008;4(6):691–695. doi: 10.1016/j.soard.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 33.Sjostrom L, Gummesson A, Sjostrom CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10(7):653–662. doi: 10.1016/S1470-2045(09)70159-7. [DOI] [PubMed] [Google Scholar]
- 34.Jiao L, Berrington de Gonzalez A, Hartge P, et al. Body mass index, effect modifiers, and risk of pancreatic cancer: a pooled study of seven prospective cohorts. Cancer Causes Control. 2010;21(8):1305–1314. doi: 10.1007/s10552-010-9558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stolzenberg-Solomon RZ, Graubard BI, Chari S, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA. 2005;294(22):2872–2878. doi: 10.1001/jama.294.22.2872. [DOI] [PubMed] [Google Scholar]
- 36.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 37.Butler AE, Galasso R, Matveyenko A, Rizza RA, Dry S, Butler PC. Pancreatic duct replication is increased with obesity and type 2 diabetes in humans. Diabetologia. 2010;53(1):21–26. doi: 10.1007/s00125-009-1556-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bracci PM. Obesity and pancreatic cancer: overview of epidemiologic evidence and biologic mechanisms. Mol Carcinog. 2012;51(1):53–63. doi: 10.1002/mc.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allott EH, Hursting SD. Obesity and cancer: mechanistic insights from transdisciplinary studies. Endocr Relat Cancer. 2015;22(6):R365–386. doi: 10.1530/ERC-15-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes. 2013;2013:291546. doi: 10.1155/2013/291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, Inflammation, and Cancer. Annu Rev Pathol. 2016;11:421–449. doi: 10.1146/annurev-pathol-012615-044359. [DOI] [PubMed] [Google Scholar]
- 42.Goodwin PJ, Stambolic V. Impact of the obesity epidemic on cancer. Annu Rev Med. 2015;66:281–296. doi: 10.1146/annurev-med-051613-012328. [DOI] [PubMed] [Google Scholar]
- 43.Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10(8):455–465. doi: 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Preziosi G, Oben JA, Fusai G. Obesity and pancreatic cancer. Surg Oncol. 2014;23(2):61–71. doi: 10.1016/j.suronc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 45.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15(8):484–498. doi: 10.1038/nrc3967. [DOI] [PubMed] [Google Scholar]
- 46.Rozengurt E. Mechanistic target of rapamycin (mTOR): a point of convergence in the action of insulin/IGF-1 and G protein-coupled receptor agonists in pancreatic cancer cells. Front Physiol. 2014;5:357. doi: 10.3389/fphys.2014.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ireland L, Santos A, Ahmed MS, et al. Chemoresistance in Pancreatic Cancer Is Driven by Stroma-Derived Insulin-Like Growth Factors. Cancer Res. 2016;76(23):6851–6863. doi: 10.1158/0008-5472.CAN-16-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jang WI, Kim MS, Kang SH, et al. Association between metformin use and mortality in patients with type 2 diabetes mellitus and localized resectable pancreatic cancer: a nationwide population-based study in korea. Oncotarget. 2017 doi: 10.18632/oncotarget.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duan W, Chen K, Jiang Z, et al. Desmoplasia suppression by metformin-mediated AMPK activation inhibits pancreatic cancer progression. Cancer Lett. 2017;385:225–233. doi: 10.1016/j.canlet.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 50.Elgogary A, Xu Q, Poore B, et al. Combination therapy with BPTES nanoparticles and metformin targets the metabolic heterogeneity of pancreatic cancer. Proc Natl Acad Sci U S A. 2016;113(36):E5328–5336. doi: 10.1073/pnas.1611406113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amin S, Mhango G, Lin J, et al. Metformin Improves Survival in Patients with Pancreatic Ductal Adenocarcinoma and Pre-Existing Diabetes: A Propensity Score Analysis. Am J Gastroenterol. 2016;111(9):1350–1357. doi: 10.1038/ajg.2016.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ming M, Sinnett-Smith J, Wang J, et al. Dose-Dependent AMPK-Dependent and Independent Mechanisms of Berberine and Metformin Inhibition of mTORC1, ERK, DNA Synthesis and Proliferation in Pancreatic Cancer Cells. PLoS One. 2014;9(12):e114573. doi: 10.1371/journal.pone.0114573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gong J, Robbins LA, Lugea A, Waldron RT, Jeon CY, Pandol SJ. Diabetes, pancreatic cancer, and metformin therapy. Front Physiol. 2014;5:426. doi: 10.3389/fphys.2014.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kisfalvi K, Moro A, Sinnett-Smith J, Eibl G, Rozengurt E. Metformin inhibits the growth of human pancreatic cancer xenografts. Pancreas. 2013;42(5):781–785. doi: 10.1097/MPA.0b013e31827aec40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cifarelli V, Lashinger LM, Devlin KL, et al. Metformin and Rapamycin Reduce Pancreatic Cancer Growth in Obese Prediabetic Mice by Distinct MicroRNA-Regulated Mechanisms. Diabetes. 2015;64(5):1632–1642. doi: 10.2337/db14-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kordes S, Pollak MN, Zwinderman AH, et al. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16(7):839–847. doi: 10.1016/S1470-2045(15)00027-3. [DOI] [PubMed] [Google Scholar]
- 57.Reni M, Dugnani E, Cereda S, et al. (Ir)relevance of Metformin Treatment in Patients with Metastatic Pancreatic Cancer: An Open-Label, Randomized Phase II Trial. Clin Cancer Res. 2016;22(5):1076–1085. doi: 10.1158/1078-0432.CCR-15-1722. [DOI] [PubMed] [Google Scholar]
- 58.Chaiteerakij R, Petersen GM, Bamlet WR, et al. Metformin Use and Survival of Patients With Pancreatic Cancer: A Cautionary Lesson. J Clin Oncol. 2016;34(16):1898–1904. doi: 10.1200/JCO.2015.63.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang YX, Rustgi AK. Impact of Metformin on Advanced Pancreatic Cancer Survival: Too Little, Too Late? Clin Cancer Res. 2016;22(5):1031–1033. doi: 10.1158/1078-0432.CCR-15-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bronte V, Tortora G. Adipocytes and Neutrophils Give a Helping Hand to Pancreatic Cancers. Cancer Discov. 2016;6(8):821–823. doi: 10.1158/2159-8290.CD-16-0682. [DOI] [PubMed] [Google Scholar]
- 61.Harvey AE, Lashinger LM, Hursting SD. The growing challenge of obesity and cancer: an inflammatory issue. Ann N Y Acad Sci. 2011;1229:45–52. doi: 10.1111/j.1749-6632.2011.06096.x. [DOI] [PubMed] [Google Scholar]
- 62.Greer JB, Whitcomb DC. Inflammation and pancreatic cancer: an evidence-based review. Curr Opin Pharmacol. 2009;9(4):411–418. doi: 10.1016/j.coph.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 63.Algul H, Treiber M, Lesina M, Schmid RM. Mechanisms of disease: chronic inflammation and cancer in the pancreas--a potential role for pancreatic stellate cells? Nat Clin Pract Gastroenterol Hepatol. 2007;4(8):454–462. doi: 10.1038/ncpgasthep0881. [DOI] [PubMed] [Google Scholar]
- 64.Swidnicka-Siergiejko AK, Gomez-Chou SB, Cruz-Monserrate Z, et al. Chronic inflammation initiates multiple forms of K-Ras-independent mouse pancreatic cancer in the absence of TP53. Oncogene. 2016 doi: 10.1038/onc.2016.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guerra C, Schuhmacher AJ, Canamero M, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11(3):291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 66.Eibl G, Reber HA, Wente MN, Hines OJ. The selective cyclooxygenase-2 inhibitor nimesulide induces apoptosis in pancreatic cancer cells independent of COX-2. Pancreas. 2003;26(1):33–41. doi: 10.1097/00006676-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 67.Mace TA, Shakya R, Pitarresi JR, et al. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut. 2016 doi: 10.1136/gutjnl-2016-311585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mohammed A, Janakiram NB, Madka V, et al. Targeting pancreatitis blocks tumor-initiating stem cells and pancreatic cancer progression. Oncotarget. 2015;6(17):15524–15539. doi: 10.18632/oncotarget.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan XL, Reid Lombardo KM, Bamlet WR, et al. Aspirin, nonsteroidal anti-inflammatory drugs, acetaminophen, and pancreatic cancer risk: a clinic-based case-control study. Cancer Prev Res (Phila) 2011;4(11):1835–1841. doi: 10.1158/1940-6207.CAPR-11-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Risch HA, Lu L, Streicher SA, et al. Aspirin Use and Reduced Risk of Pancreatic Cancer. Cancer Epidemiol Biomarkers Prev. 2017;26(1):68–74. doi: 10.1158/1055-9965.EPI-16-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang YP, Wan YD, Sun YL, Li J, Zhu RT. Aspirin might reduce the incidence of pancreatic cancer: A meta-analysis of observational studies. Sci Rep. 2015;5:15460. doi: 10.1038/srep15460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dawson DW, Hertzer K, Moro A, et al. High-fat, high-calorie diet promotes early pancreatic neoplasia in the conditional KrasG12D mouse model. Cancer Prev Res (Phila) 2013;6(10):1064–1073. doi: 10.1158/1940-6207.CAPR-13-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Incio J, Tam J, Rahbari NN, et al. PlGF/VEGFR-1 Signaling Promotes Macrophage Polarization and Accelerated Tumor Progression in Obesity. Clin Cancer Res. 2016;22(12):2993–3004. doi: 10.1158/1078-0432.CCR-15-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Philip B, Roland CL, Daniluk J, et al. A high-fat diet activates oncogenic Kras and COX2 to induce development of pancreatic ductal adenocarcinoma in mice. Gastroenterology. 2013;145(6):1449–1458. doi: 10.1053/j.gastro.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harvey AE, Lashinger LM, Hays D, et al. Calorie restriction decreases murine and human pancreatic tumor cell growth, nuclear factor-kappaB activation, and inflammation-related gene expression in an insulin-like growth factor-1-dependent manner. PLoS One. 2014;9(5):e94151. doi: 10.1371/journal.pone.0094151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lashinger LM, Harrison LM, Rasmussen AJ, et al. Dietary energy balance modulation of Kras- and Ink4a/Arf+/--driven pancreatic cancer: the role of insulin-like growth factor-I. Cancer Prev Res (Phila) 2013;6(10):1046–1055. doi: 10.1158/1940-6207.CAPR-13-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang SC, Yang WV. Hyperglycemia, tumorigenesis, and chronic inflammation. Crit Rev Oncol Hematol. 2016;108:146–153. doi: 10.1016/j.critrevonc.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 78.Wang L, Bai YY, Yang Y, et al. Diabetes mellitus stimulates pancreatic cancer growth and epithelial-mesenchymal transition-mediated metastasis via a p38 MAPK pathway. Oncotarget. 2016;7(25):38539–38550. doi: 10.18632/oncotarget.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang HH, Young SH, Sinnett-Smith J, et al. Prostaglandin E2 activates the mTORC1 pathway through an EP4/cAMP/PKA- and EP1/Ca2+-mediated mechanism in the human pancreatic carcinoma cell line PANC-1. Am J Physiol Cell Physiol. 2015;309(10):C639–649. doi: 10.1152/ajpcell.00417.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Polvani S, Tarocchi M, Tempesti S, Bencini L, Galli A. Peroxisome proliferator activated receptors at the crossroad of obesity, diabetes, and pancreatic cancer. World J Gastroenterol. 2016;22(8):2441–2459. doi: 10.3748/wjg.v22.i8.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hidaka A, Shimazu T, Sawada N, et al. Fish, n-3 PUFA consumption, and pancreatic cancer risk in Japanese: a large, population-based, prospective cohort study. Am J Clin Nutr. 2015;102(6):1490–1497. doi: 10.3945/ajcn.115.113597. [DOI] [PubMed] [Google Scholar]
- 82.Yu M, Liu H, Duan Y, Zhang D, Li S, Wang F. Four types of fatty acids exert differential impact on pancreatic cancer growth. Cancer Lett. 2015;360(2):187–194. doi: 10.1016/j.canlet.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 83.Ma YJ, Yu J, Xiao J, Cao BW. The consumption of omega-3 polyunsaturated fatty acids improves clinical outcomes and prognosis in pancreatic cancer patients: a systematic evaluation. Nutr Cancer. 2015;67(1):112–118. doi: 10.1080/01635581.2015.976315. [DOI] [PubMed] [Google Scholar]
- 84.Mohammed A, Janakiram NB, Brewer M, et al. Endogenous n-3 polyunsaturated fatty acids delay progression of pancreatic ductal adenocarcinoma in Fat-1-p48(Cre/+)-LSL-Kras(G12D/+) mice. Neoplasia. 2012;14(12):1249–1259. doi: 10.1593/neo.121508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.He K, Xun P, Brasky TM, Gammon MD, Stevens J, White E. Types of fish consumed and fish preparation methods in relation to pancreatic cancer incidence: the VITAL Cohort Study. Am J Epidemiol. 2013;177(2):152–160. doi: 10.1093/aje/kws232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Funahashi H, Satake M, Hasan S, et al. Opposing effects of n-6 and n-3 polyunsaturated fatty acids on pancreatic cancer growth. Pancreas. 2008;36(4):353–362. doi: 10.1097/MPA.0b013e31815ccc44. [DOI] [PubMed] [Google Scholar]
- 87.MacLean CH, Newberry SJ, Mojica WA, et al. Effects of omega-3 fatty acids on cancer risk: a systematic review. JAMA. 2006;295(4):403–415. doi: 10.1001/jama.295.4.403. [DOI] [PubMed] [Google Scholar]
- 88.World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Report. Food, Nutrition, Physical Activity, and the Prevention of Pancreatic Cancer. 2012 Available at http://www.dietandcancerreport.org.
- 89.Simopoulos AP. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients. 2016;8(3) doi: 10.3390/nu8030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ellulu MS, Khaza’ai H, Abed Y, Rahmat A, Ismail P, Ranneh Y. Role of fish oil in human health and possible mechanism to reduce the inflammation. Inflammopharmacology. 2015;23(2–3):79–89. doi: 10.1007/s10787-015-0228-1. [DOI] [PubMed] [Google Scholar]
- 91.Nobili V, Alisi A, Musso G, Scorletti E, Calder PC, Byrne CD. Omega-3 fatty acids: Mechanisms of benefit and therapeutic effects in pediatric and adult NAFLD. Crit Rev Clin Lab Sci. 2016;53(2):106–120. doi: 10.3109/10408363.2015.1092106. [DOI] [PubMed] [Google Scholar]
- 92.Martinez-Fernandez L, Laiglesia LM, Huerta AE, Martinez JA, Moreno-Aliaga MJ. Omega-3 fatty acids and adipose tissue function in obesity and metabolic syndrome. Prostaglandins Other Lipid Mediat. 2015;121(Pt A):24–41. doi: 10.1016/j.prostaglandins.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 93.Monteiro J, Leslie M, Moghadasian MH, Arendt BM, Allard JP, Ma DW. The role of n - 6 and n - 3 polyunsaturated fatty acids in the manifestation of the metabolic syndrome in cardiovascular disease and non-alcoholic fatty liver disease. Food Funct. 2014;5(3):426–435. doi: 10.1039/c3fo60551e. [DOI] [PubMed] [Google Scholar]
- 94.Ulven T, Christiansen E. Dietary Fatty Acids and Their Potential for Controlling Metabolic Diseases Through Activation of FFA4/GPR120. Annu Rev Nutr. 2015;35:239–263. doi: 10.1146/annurev-nutr-071714-034410. [DOI] [PubMed] [Google Scholar]
- 95.Flock MR, Rogers CJ, Prabhu KS, Kris-Etherton PM. Immunometabolic role of long-chain omega-3 fatty acids in obesity-induced inflammation. Diabetes Metab Res Rev. 2013;29(6):431–445. doi: 10.1002/dmrr.2414. [DOI] [PubMed] [Google Scholar]
- 96.Titos E, Claria J. Omega-3-derived mediators counteract obesity-induced adipose tissue inflammation. Prostaglandins Other Lipid Mediat. 2013;107:77–84. doi: 10.1016/j.prostaglandins.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 97.Oliver E, McGillicuddy F, Phillips C, Toomey S, Roche HM. The role of inflammation and macrophage accumulation in the development of obesity-induced type 2 diabetes mellitus and the possible therapeutic effects of long-chain n-3 PUFA. Proc Nutr Soc. 2010;69(2):232–243. doi: 10.1017/S0029665110000042. [DOI] [PubMed] [Google Scholar]
- 98.Masoodi M, Kuda O, Rossmeisl M, Flachs P, Kopecky J. Lipid signaling in adipose tissue: Connecting inflammation & metabolism. Biochim Biophys Acta. 2015;1851(4):503–518. doi: 10.1016/j.bbalip.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 99.Waghray M, Yalamanchili M, di Magliano MP, Simeone DM. Deciphering the role of stroma in pancreatic cancer. Curr Opin Gastroenterol. 2013;29(5):537–543. doi: 10.1097/MOG.0b013e328363affe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Neesse A, Algul H, Tuveson DA, Gress TM. Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut. 2015;64(9):1476–1484. doi: 10.1136/gutjnl-2015-309304. [DOI] [PubMed] [Google Scholar]
- 101.Incio J, Liu H, Suboj P, et al. Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discov. 2016;6(8):852–869. doi: 10.1158/2159-8290.CD-15-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang J, Waldron RT, Su HY, et al. Insulin promotes proliferation and fibrosing responses in activated pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2016;311(4):G675–G687. doi: 10.1152/ajpgi.00251.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Font-Burgada J, Sun B, Karin M. Obesity and Cancer: The Oil that Feeds the Flame. Cell Metab. 2016;23(1):48–62. doi: 10.1016/j.cmet.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 104.Plovier H, Everard A, Druart C, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23(1):107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 105.Sze MA, Schloss PD. Looking for a Signal in the Noise: Revisiting Obesity and the Microbiome. MBio. 2016;7(4) doi: 10.1128/mBio.01018-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kusminski CM, Bickel PE, Scherer PE. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat Rev Drug Discov. 2016;15(9):639–660. doi: 10.1038/nrd.2016.75. [DOI] [PubMed] [Google Scholar]
- 107.Singh RG, Yoon HD, Wu LM, Lu J, Plank LD, Petrov MS. Ectopic fat accumulation in the pancreas and its clinical relevance: A systematic review, meta-analysis, and meta-regression. Metabolism - Clinical and Experimental. 69:1–13. doi: 10.1016/j.metabol.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 108.Singh RG, Pendharkar SA, Gillies NA, Miranda-Soberanis V, Plank LD, Petrov MS. Associations between circulating levels of adipocytokines and abdominal adiposity in patients after acute pancreatitis. Clin Exp Med. 2017 doi: 10.1007/s10238-017-0453-6. [DOI] [PubMed] [Google Scholar]
- 109.Castoldi A, Naffah de Souza C, Camara NO, Moraes-Vieira PM. The Macrophage Switch in Obesity Development. Front Immunol. 2015;6:637. doi: 10.3389/fimmu.2015.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dam V, Sikder T, Santosa S. From neutrophils to macrophages: differences in regional adipose tissue depots. Obes Rev. 2016;17(1):1–17. doi: 10.1111/obr.12335. [DOI] [PubMed] [Google Scholar]
- 111.Wensveen FM, Valentic S, Sestan M, Turk Wensveen T, Polic B. The “Big Bang” in obese fat: Events initiating obesity-induced adipose tissue inflammation. Eur J Immunol. 2015;45(9):2446–2456. doi: 10.1002/eji.201545502. [DOI] [PubMed] [Google Scholar]
- 112.Bai Y, Sun Q. Macrophage recruitment in obese adipose tissue. Obes Rev. 2015;16(2):127–136. doi: 10.1111/obr.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hill AA, Reid Bolus W, Hasty AH. A decade of progress in adipose tissue macrophage biology. Immunol Rev. 2014;262(1):134–152. doi: 10.1111/imr.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vieira-Potter VJ. Inflammation and macrophage modulation in adipose tissues. Cell Microbiol. 2014;16(10):1484–1492. doi: 10.1111/cmi.12336. [DOI] [PubMed] [Google Scholar]
- 115.Boutens L, Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia. 2016;59(5):879–894. doi: 10.1007/s00125-016-3904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim J, Chung K, Choi C, et al. Silencing CCR2 in Macrophages Alleviates Adipose Tissue Inflammation and the Associated Metabolic Syndrome in Dietary Obese Mice. Mol Ther Nucleic Acids. 2016;5:e280. doi: 10.1038/mtna.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Romeo GR, Lee J, Shoelson SE. Metabolic syndrome, insulin resistance, and roles of inflammation--mechanisms and therapeutic targets. Arterioscler Thromb Vasc Biol. 2012;32(8):1771–1776. doi: 10.1161/ATVBAHA.111.241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gucalp A, Iyengar NM, Hudis CA, Dannenberg AJ. Targeting obesity-related adipose tissue dysfunction to prevent cancer development and progression. Semin Oncol. 2016;43(1):154–160. doi: 10.1053/j.seminoncol.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Conroy MJ, Dunne MR, Donohoe CL, Reynolds JV. Obesity-associated cancer: an immunological perspective. Proc Nutr Soc. 2016;75(2):125–138. doi: 10.1017/S0029665115004176. [DOI] [PubMed] [Google Scholar]
- 120.Hertzer KM, Xu M, Moro A, et al. Robust Early Inflammation of the Peripancreatic Visceral Adipose Tissue During Diet-Induced Obesity in the KrasG12D Model of Pancreatic Cancer. Pancreas. 2016;45(3):458–465. doi: 10.1097/MPA.0000000000000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vongsuvanh R, George J, Qiao L, van der Poorten D. Visceral adiposity in gastrointestinal and hepatic carcinogenesis. Cancer Lett. 2013;330(1):1–10. doi: 10.1016/j.canlet.2012.11.038. [DOI] [PubMed] [Google Scholar]
- 122.Kwee TC, Kwee RM. Abdominal adiposity and risk of pancreatic cancer. Pancreas. 2007;35(3):285–286. doi: 10.1097/MPA.0b013e318068fca6. [DOI] [PubMed] [Google Scholar]
- 123.Aune D, Greenwood DC, Chan DS, et al. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23(4):843–852. doi: 10.1093/annonc/mdr398. [DOI] [PubMed] [Google Scholar]
- 124.Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34(1):1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Muller MJ, Lagerpusch M, Enderle J, Schautz B, Heller M, Bosy-Westphal A. Beyond the body mass index: tracking body composition in the pathogenesis of obesity and the metabolic syndrome. Obes Rev. 2012;13(Suppl 2):6–13. doi: 10.1111/j.1467-789X.2012.01033.x. [DOI] [PubMed] [Google Scholar]
- 126.Walker GE, Marzullo P, Ricotti R, Bona G, Prodam F. The pathophysiology of abdominal adipose tissue depots in health and disease. Horm Mol Biol Clin Investig. 2014;19(1):57–74. doi: 10.1515/hmbci-2014-0023. [DOI] [PubMed] [Google Scholar]
- 127.Babic A, Bao Y, Qian ZR, et al. Pancreatic Cancer Risk Associated with Prediagnostic Plasma Levels of Leptin and Leptin Receptor Genetic Polymorphisms. Cancer Res. 2016;76(24):7160–7167. doi: 10.1158/0008-5472.CAN-16-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stolzenberg-Solomon RZ, Newton CC, Silverman DT, et al. Circulating Leptin and Risk of Pancreatic Cancer: A Pooled Analysis From 3 Cohorts. Am J Epidemiol. 2015;182(3):187–197. doi: 10.1093/aje/kwv041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lanza-Jacoby S, Yan G, Radice G, LePhong C, Baliff J, Hess R. Calorie restriction delays the progression of lesions to pancreatic cancer in the LSL-KrasG12D; Pdx-1/Cre mouse model of pancreatic cancer. Exp Biol Med (Maywood) 2013;238(7):787–797. doi: 10.1177/1535370213493727. [DOI] [PubMed] [Google Scholar]
- 130.Harbuzariu A, Rampoldi A, Daley-Brown DS, et al. Leptin-Notch signaling axis is involved in pancreatic cancer progression. Oncotarget. 2016 doi: 10.18632/oncotarget.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fan Y, Gan Y, Shen Y, et al. Leptin signaling enhances cell invasion and promotes the metastasis of human pancreatic cancer via increasing MMP-13 production. Oncotarget. 2015;6(18):16120–16134. doi: 10.18632/oncotarget.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mendonsa AM, Chalfant MC, Gorden LD, VanSaun MN. Modulation of the leptin receptor mediates tumor growth and migration of pancreatic cancer cells. PLoS One. 2015;10(4):e0126686. doi: 10.1371/journal.pone.0126686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stolzenberg-Solomon RZ, Weinstein S, Pollak M, et al. Prediagnostic adiponectin concentrations and pancreatic cancer risk in male smokers. Am J Epidemiol. 2008;168(9):1047–1055. doi: 10.1093/aje/kwn221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nogueira LM, Newton CC, Pollak MN, et al. Serum C-peptide, total and high molecular weight adiponectin, and pancreatic cancer: Do associations differ by smoking? Cancer Epidemiol Biomarkers Prev. 2017 doi: 10.1158/1055-9965.EPI-16-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bao Y, Giovannucci EL, Kraft P, et al. A prospective study of plasma adiponectin and pancreatic cancer risk in five US cohorts. J Natl Cancer Inst. 2013;105(2):95–103. doi: 10.1093/jnci/djs474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alempijevic T, Dragasevic S, Zec S, Popovic D, Milosavljevic T. Non-alcoholic fatty pancreas disease. Postgrad Med J. 2017 doi: 10.1136/postgradmedj-2016-134546. [DOI] [PubMed] [Google Scholar]
- 137.Carter R, Mouralidarane A, Soeda J, et al. Non-alcoholic fatty pancreas disease pathogenesis: a role for developmental programming and altered circadian rhythms. PLoS One. 2014;9(3):e89505. doi: 10.1371/journal.pone.0089505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu WC, Wang CY. Association between non-alcoholic fatty pancreatic disease (NAFPD) and the metabolic syndrome: case-control retrospective study. Cardiovasc Diabetol. 2013;12:77. doi: 10.1186/1475-2840-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gotoh K, Inoue M, Shiraishi K, et al. Spleen-derived interleukin-10 downregulates the severity of high-fat diet-induced non-alcoholic fatty pancreas disease. PLoS One. 2012;7(12):e53154. doi: 10.1371/journal.pone.0053154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fraulob JC, Ogg-Diamantino R, Fernandes-Santos C, Aguila MB, Mandarim-de-Lacerda CA. A Mouse Model of Metabolic Syndrome: Insulin Resistance, Fatty Liver and Non-Alcoholic Fatty Pancreas Disease (NAFPD) in C57BL/6 Mice Fed a High Fat Diet. J Clin Biochem Nutr. 2010;46(3):212–223. doi: 10.3164/jcbn.09-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Grippo PJ, Fitchev PS, Bentrem DJ, et al. Concurrent PEDF deficiency and Kras mutation induce invasive pancreatic cancer and adipose-rich stroma in mice. Gut. 2012;61(10):1454–1464. doi: 10.1136/gutjnl-2011-300821. [DOI] [PubMed] [Google Scholar]
- 142.Aune D, Chan DS, Vieira AR, et al. Dietary fructose, carbohydrates, glycemic indices and pancreatic cancer risk: a systematic review and meta-analysis of cohort studies. Ann Oncol. 2012;23(10):2536–2546. doi: 10.1093/annonc/mds076. [DOI] [PubMed] [Google Scholar]
- 143.Mueller NT, Odegaard A, Anderson K, et al. Soft drink and juice consumption and risk of pancreatic cancer: the Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev. 2010;19(2):447–455. doi: 10.1158/1055-9965.EPI-09-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Larsson SC, Bergkvist L, Wolk A. Consumption of sugar and sugar-sweetened foods and the risk of pancreatic cancer in a prospective study. Am J Clin Nutr. 2006;84(5):1171–1176. doi: 10.1093/ajcn/84.5.1171. [DOI] [PubMed] [Google Scholar]
- 145.Bao Y, Stolzenberg-Solomon R, Jiao L, et al. Added sugar and sugar-sweetened foods and beverages and the risk of pancreatic cancer in the National Institutes of Health-AARP Diet and Health Study. Am J Clin Nutr. 2008;88(2):431–440. doi: 10.1093/ajcn/88.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jiao L, Flood A, Subar AF, Hollenbeck AR, Schatzkin A, Stolzenberg-Solomon R. Glycemic index, carbohydrates, glycemic load, and the risk of pancreatic cancer in a prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1144–1151. doi: 10.1158/1055-9965.EPI-08-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Patel AV, McCullough ML, Pavluck AL, Jacobs EJ, Thun MJ, Calle EE. Glycemic load, glycemic index, and carbohydrate intake in relation to pancreatic cancer risk in a large US cohort. Cancer Causes Control. 2007;18(3):287–294. doi: 10.1007/s10552-006-0081-z. [DOI] [PubMed] [Google Scholar]
- 148.Thiebaut AC, Jiao L, Silverman DT, et al. Dietary fatty acids and pancreatic cancer in the NIH-AARP diet and health study. J Natl Cancer Inst. 2009;101(14):1001–1011. doi: 10.1093/jnci/djp168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stolzenberg-Solomon RZ, Cross AJ, Silverman DT, et al. Meat and meat-mutagen intake and pancreatic cancer risk in the NIH-AARP cohort. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2664–2675. doi: 10.1158/1055-9965.EPI-07-0378. [DOI] [PubMed] [Google Scholar]
- 150.Larsson SC, Hakansson N, Naslund I, Bergkvist L, Wolk A. Fruit and vegetable consumption in relation to pancreatic cancer risk: a prospective study. Cancer Epidemiol Biomarkers Prev. 2006;15(2):301–305. doi: 10.1158/1055-9965.EPI-05-0696. [DOI] [PubMed] [Google Scholar]
- 151.Jeurnink SM, Ros MM, Leenders M, et al. Plasma carotenoids, vitamin C, retinol and tocopherols levels and pancreatic cancer risk within the European Prospective Investigation into Cancer and Nutrition: a nested case-control study: plasma micronutrients and pancreatic cancer risk. Int J Cancer. 2015;136(6):E665–676. doi: 10.1002/ijc.29175. [DOI] [PubMed] [Google Scholar]
- 152.Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016;23(1):27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lutz TA, Woods SC. Overview of animal models of obesity. Curr Protoc Pharmacol. 2012;Chapter 5(Unit5):61. doi: 10.1002/0471141755.ph0561s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tschop M, Heiman ML. Rodent obesity models: an overview. Exp Clin Endocrinol Diabetes. 2001;109(6):307–319. doi: 10.1055/s-2001-17297. [DOI] [PubMed] [Google Scholar]
- 155.Wang CY, Liao JK. A mouse model of diet-induced obesity and insulin resistance. Methods Mol Biol. 2012;821:421–433. doi: 10.1007/978-1-61779-430-8_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hursting SD. Obesity, energy balance, and cancer: a mechanistic perspective. Cancer Treat Res. 2014;159:21–33. doi: 10.1007/978-3-642-38007-5_2. [DOI] [PubMed] [Google Scholar]
- 157.White PB, True EM, Ziegler KM, et al. Insulin, leptin, and tumoral adipocytes promote murine pancreatic cancer growth. J Gastrointest Surg. 2010;14(12):1888–1893. doi: 10.1007/s11605-010-1349-x. discussion 1893–1884. [DOI] [PubMed] [Google Scholar]
- 158.Khasawneh J, Schulz MD, Walch A, et al. Inflammation and mitochondrial fatty acid beta-oxidation link obesity to early tumor promotion. Proc Natl Acad Sci U S A. 2009;106(9):3354–3359. doi: 10.1073/pnas.0802864106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gomez-Chou S, Swidnicka-Siergiejko A, Badi N, et al. Lipocalin-2 Promotes Pancreatic Ductal Adenocarcinoma by Regulating Inflammation in the Tumor Microenvironment. Cancer Res. 2017 doi: 10.1158/0008-5472.CAN-16-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 161.Mayer J, Bates MW, Dickie MM. Hereditary diabetes in genetically obese mice. Science. 1951;113(2948):746–747. doi: 10.1126/science.113.2948.746. [DOI] [PubMed] [Google Scholar]
- 162.Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14(3):141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- 163.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153(3740):1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 164.Koenig RJ, Cerami A. Synthesis of hemoglobin AIc in normal and diabetic mice: potential model of basement membrane thickening. Proc Natl Acad Sci U S A. 1975;72(9):3687–3691. doi: 10.1073/pnas.72.9.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zyromski NJ, Mathur A, Pitt HA, et al. Obesity potentiates the growth and dissemination of pancreatic cancer. Surgery. 2009;146(2):258–263. doi: 10.1016/j.surg.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 166.Apte MV, Wilson JS, Lugea A, Pandol SJ. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144(6):1210–1219. doi: 10.1053/j.gastro.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Apte MV, Park S, Phillips PA, et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29(3):179–187. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 168.Apte MV, Haber PS, Darby SJ, et al. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut. 1999;44(4):534–541. doi: 10.1136/gut.44.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117(1):50–59. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pandol S, Gukovskaya A, Edderkaoui M, Dawson D, Eibl G, Lugea A. Epidemiology, risk factors, and the promotion of pancreatic cancer: role of the stellate cell. J Gastroenterol Hepatol. 2012;27(Suppl 2):127–134. doi: 10.1111/j.1440-1746.2011.07013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mohapatra S, Majumder S, Smyrk TC, et al. Diabetes Mellitus Is Associated With an Exocrine Pancreatopathy: Conclusions From a Review of Literature. Pancreas. 2016;45(8):1104–1110. doi: 10.1097/MPA.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Eguchi K, Nagai R. Islet inflammation in type 2 diabetes and physiology. J Clin Invest. 2017;127(1):14–23. doi: 10.1172/JCI88877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Habtezion A, Edderkaoui M, Pandol SJ. Macrophages and pancreatic ductal adenocarcinoma. Cancer Lett. 2016;381(1):211–216. doi: 10.1016/j.canlet.2015.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Apte MV, Wilson JS. Dangerous liaisons: pancreatic stellate cells and pancreatic cancer cells. J Gastroenterol Hepatol. 2012;27(Suppl 2):69–74. doi: 10.1111/j.1440-1746.2011.07000.x. [DOI] [PubMed] [Google Scholar]
- 175.Vonlaufen A, Joshi S, Qu C, et al. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res. 2008;68(7):2085–2093. doi: 10.1158/0008-5472.CAN-07-2477. [DOI] [PubMed] [Google Scholar]
- 176.Kikuta K, Masamune A, Watanabe T, et al. Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochem Biophys Res Commun. 2010;403(3–4):380–384. doi: 10.1016/j.bbrc.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 177.Vaquero EC, Edderkaoui M, Nam KJ, Gukovsky I, Pandol SJ, Gukovskaya AS. Extracellular matrix proteins protect pancreatic cancer cells from death via mitochondrial and nonmitochondrial pathways. Gastroenterology. 2003;125(4):1188–1202. doi: 10.1016/s0016-5085(03)01203-4. [DOI] [PubMed] [Google Scholar]
- 178.Edderkaoui M, Hong P, Vaquero EC, et al. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am J Physiol Gastrointest Liver Physiol. 2005;289(6):G1137–1147. doi: 10.1152/ajpgi.00197.2005. [DOI] [PubMed] [Google Scholar]
- 179.Edderkaoui M, Hong P, Lee JK, Pandol SJ, Gukovskaya AS. Insulin-like growth factor-I receptor mediates the prosurvival effect of fibronectin. J Biol Chem. 2007;282(37):26646–26655. doi: 10.1074/jbc.M702836200. [DOI] [PubMed] [Google Scholar]
- 180.Lee JK, Edderkaoui M, Truong P, et al. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology. 2007;133(5):1637–1648. doi: 10.1053/j.gastro.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 181.Edderkaoui M, Nitsche C, Zheng L, Pandol SJ, Gukovsky I, Gukovskaya AS. NADPH oxidase activation in pancreatic cancer cells is mediated through Akt-dependent up-regulation of p22phox. J Biol Chem. 2011;286(10):7779–7787. doi: 10.1074/jbc.M110.200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Xue J, Sharma V, Hsieh MH, et al. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun. 2015;6:7158. doi: 10.1038/ncomms8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67(19):9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 184.Ino Y, Yamazaki-Itoh R, Shimada K, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108(4):914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sugimoto M, Mitsunaga S, Yoshikawa K, et al. Prognostic impact of M2 macrophages at neural invasion in patients with invasive ductal carcinoma of the pancreas. Eur J Cancer. 2014;50(11):1900–1908. doi: 10.1016/j.ejca.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 186.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25(6):719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25(6):735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.http://cpdpc.mdanderson.org