Abstract
Objective
We aimed to estimate the proportion of underdiagnosis and undertreatment of cardiovascular risk factors (CVRF) in an international multicentre cohort of patients with psoriasis (PsC) and psoriatic arthritis (PsA).
Methods
A cross-sectional analysis of patients with psoriatic disease from the International Psoriasis and Arthritis Research Team (IPART) cohort was conducted. The presence of modifiable CVRF (diabetes, hypertension, dyslipidemia, smoking, elevated BMI and central obesity) and the use of appropriate therapies for hypertension and dyslipidemia were determined. The 10-year CV risk was calculated according to the Framingham Risk Score (FRS). Physician adherence with guidelines for the treatment of dyslipidemia and hypertension was assessed. Regression analysis was used to assess predictors of undertreatmnet of hypertension and dyslipidemia.
Results
A total of 2254 patients (58.9% PsA, 41.1% PsC) from 8 centres in Canada, US and Israel were included. Their mean age was 52±13.8 years and 53% were males. 87.6% of the patients had at least one modifiable CVRF. 45.1% of the patients had hypertension, 49.4% dyslipidemia, 13.3% diabetes, 75.3% were overweight or obese, 54.3% central obesity and 17.3% were current smokers. We found 59.2% of patients with hypertension and 65.6% of patients with dyslipidemia were undertreated. Under-treatment was associated with younger age (≤50 years), having PsC and male gender.
Conclusions
In real-world settings, a large proportion of patients with psoriasis and PsA were underdiagnosed and undertreated for hypertension and dyslipidemia. Strategies to improve the management of CVRF in psoriatic patients are warranted.
Patients with psoriasis and psoriatic arthritis (PsA) have an increased cardiovascular (CV) risk. Based on recent meta-analyses, patients with psoriasis and PsA have 29% and 55% higher risk of developing incident myocardial infarction, respectively (1, 2). While the increased CV risk in psoriatic patients is attributed in part to the chronic systemic inflammation related to skin and joint disease (3, 4), the high prevalence of traditional CV risk factors (CVRF) in psoriatic patients significantly contributes to their high CV risk (5-7). Indeed, the presence of traditional CVRF, including hypertension, smoking, dyslipidemia and diabetes mellitus (DM), is associated with higher burden of atherosclerosis and increased risk of developing cardiovascular diseases (CVD) in patients with psoriatic disease (4, 7-10). Thus, despite limited data, it is conceivable that controlling CVRF could reduce the risk of CVD in PsA, as has been shown in the general population.
Extensive literature supports the strong link between obesity and its related metabolic abnormalities and psoriatic disease. Patients with psoriatic disease, particularly those with severe psoriasis and PsA, have a higher prevalence of DM, hypertension and dyslipidemia compared with the general population (5, 11-13). This association is stronger than with other chronic inflammatory arthritic conditions, such as rheumatoid arthritis (RA) and ankylosing spondylitis (14-16).
The recent EULAR recommendations for CVD risk management suggest that all patients with PsA should be assessed for CVRF every 5 years (17). The Canadian Cardiology Society considers PsA as an independent risk factor for CVD (18). Stratification of patients according to their predicted CV risk, as calculated by validated clinical algorithms, such as the Framingham risk score, is the first step in the formulation of treatment decisions. However, unlike the recent suggestion to use a multiplication factor of 1.5 for adjusting the estimated CV risk in patients with RA (17), there is no parallel modification for CV risk estimation in patients with psoriatic disease. Clinicians are encouraged to use the general population guidelines for CV risk estimation and for the management of CVRF in patients with psoriasis and PsA.
Recent publications found major gaps in the management of CVRF in RA patients (19-21), however, little evidence exists about the extent of underdiagnosis and undertreatment of CVRF in psoriasis and PsA patients. Analysis of psoriasis patients enrolled in clinical trials found that 19%, 22% and 39% of patients with DM, hypertension and dyslipidemia, respectively, were untreated for these conditions (22). This study also found major gaps in the treatment of hypertension and dyslipidemia, however, the generalizability of the results may be limited since the analysis was based on a highly selective population of patients recruited for clinical trials. Little information exists about the adherence to treatment recommendations in PsA and psoriasis patients in the clinic setting. Additionally, to the best of our knowledge, no study thus far has compared the treatment practices for CV risk management in psoriatic patients across different heath care systems in different countries. Investigating the physician adherence to screening and treatment guidelines for CVRF among psoriatic patients could identify gaps in care and provide support to develop strategies that address these potential gaps.
The aim of this study was to estimate the proportion of underdiagnosis and undertreatment of CVRF in an international multicentre cohort of patients with psoriasis and PsA and to investigate factors associated with undertreatment of hypertension and dyslipidemia in these patients.
Methods
Population and Setting
A cross-sectional analysis of patients with psoriatic disease from the International Psoriasis and Arthritis Research Team (IPART) cohort was conducted. IPART is an international network of dermatologists and rheumatologists from 10 sites mostly in North America, that was established to investigate disease-related outcomes in patients with psoriatic disease. Adult patients with PsA and those with psoriasis alone (PsC) are enrolled and assessed according to a standardized protocol. For the present study, information from 8 sites (5 Canadian, 2 US and 1 Israeli) that enrolled more than 50 patients with PsC and/or PsA was extracted from the last available study visit. The majority of the patients were enrolled in specialty clinics in academic centres, however, community-based dermatology and rheumatology clinics and family medicine clinics were additional sources of recruitment. The health systems and access to health services varies across the three countries. The US has a multipayer healthcare system (public and private systems) and access to health care services and coverage of medication varies widely between individuals. In contrast, the Canadian and Israleli healthcare systems are largely publicly funded and health services are widely available to all citizens.
Data collection
Patient evaluation included a history of lifestyle habits, medications and co-morbidities, physical examination with blood pressure measurement (measured once), anthropometric measures and laboratory assessment including non-fasting glucose and lipid profile. Detailed joint examination was conducted including the assessment of tender and swollen joint counts in 68/66 joints, respectively, the number of dactylitic digits and tender entheseal count according to the SPARCC enthesitis index. Low disease activity was defined as having tender, swollen joint counts and enthesitis counts of 1 or less in patients with PsA. Psoriasis Area and Severity Index (PASI) was used to estimate psoriasis activity. Severe psoriasis was defined as PASI ≥10.
Evaluation of traditional CV risk factors
The presence of traditional modifiable CVRF (DM, hypertension, dyslipidemia, smoking, elevated BMI and central obesity) and the use of appropriate therapies for hypertension and dyslipidemia were determined based on patients' report, physical examination and laboratory tests.
Patient height and weight were used to calculate Body Mass Index (BMI) and determine the proportion of patients who were overweight (BMI≥25) or obese (BMI≥30). Central obesity was defined by a waist circumference >88 cm for women or >102 cm for men among Caucasians and >80 cm for women or >94 cm for men in other ethnic groups (23).
Hypertension was defined as either a former medical history of hypertension, elevated blood pressure measurement (systolic blood pressure (SBP) ≥140 mm Hg or diastolic (DBP)≥ 90 mm Hg) or the use of blood pressure lowering drugs. Patients with elevated blood pressure in the absence of a former medical history of hypertension or use of appropriate mediations were considered to have undiagnosed hypertension.
DM was defined as either elevated non-fasting glucose levels (glucose ≥11.1 mmol/L or 200 mg/dL), previous medical history of DM or use of glucose lowering medications. Patients with elevated levels of blood glucose in the absence of a previous medical history of DM or use of appropriate mediations were considered to have undiagnosed DM.
Dyslipidemia was defined as either a medical history of dyslipidemia, use of lipid lowering therapy or abnormal lipid profile (LDL-c > 3.5 mmol/L (135 mg/dL) or non-HDL-c >4.3 mmol/L (166 mg/dL)) regardless of other CVRF (18). Patients with an abnormal lipid profile in the absence of previous medical history of dyslipidemia or use of appropriate mediations were considered to have undiagnosed dyslipidemia.
The 10-year CV risk was calculated according to the Framingham Risk Score (FRS) derived from an update of the Framingham Heart Study based on the following variables: age, sex, smoking status, total cholesterol, HDL-c and SBP (24). When lipid profile was missing (44% of the study population), a BMI-based Framingham global 10-year CV risk score was calculated using the following variables: age, sex, smoking status, SBP and BMI (25). Patients were stratified into three risk categories: low (<10%), intermediate (10-20%), high (>20%). Patients with pre-existing clinical CV disease or DM were included in the high-risk group.
Adherence with treatment recommendations for CV risk factors
Adherence with guidelines for the treatment of dyslipidemia and hypertension was assessed. The results were reported for the entire study population and by disease status (PsA or PsC). Treatment goals for hypertension were defined using the 2014 guidelines for the management of hypertension in adults (JNC 8) (26). The treatment goals were SBP less than 140 mm Hg (or less then 150 mm Hg if age >65) and DBP less than 90 mm Hg for the overall population and SBP less than 130 mm Hg and DBP less than 80 mm Hg for patients at high CV risk. The treatment goals for dyslipidemia were defined according to the 2013 ACC/AHA guideline for the treatment of blood cholesterol. Lipid lowering therapy was indicated in any of the following: 1) Clinical CVD; 2) DM and LDL-c≥70 mg/dL (1.8 mmol/L); 3) 10-year CV risk according to FRS ≥7.5% in people 40 to 75 years of age; 4) LDL-c ≥190 mg/dL (4.9 mmol/L)(27). Since HbA1c or fasting glucose levels were not available for the majority of the study patients, adherence to treatment guidelines of diabetes was not assessed.
Statistical analysis
Statistical analyses were performed using SAS (version 9.4). Descriptive statistics were computed with continuous variables summarized by their means and standard deviations and categorical variables summarized by proportions. The following categorical variables were assessed as predictors of undertreatment of hypertension and dyslipidemia: age group (≤50 vs. >50 years), sex, disease status (PsC vs. PsA), severe psoriasis, low PsA disease activity, NSAIDs use and corticosteroid use. Logistic regression models were used to assess the association between the above detailed variables and undertreatment of hypertension and dyslipidemia.
The initial regression model included only a single co-variate. All variables that achieved significance at the 5% level were then included in a more comprehensive multivariable model to identify independent predictors of undertreatment. The effect of each factor was measured with an odds ratio (OR) that was estimated along with the respective 95% Confidence Intervals (CI).
Results
A total of 2254 patients (58.9% PsA, 41.1% PsC) from 8 centres in Canada, US and Israel were included in the analysis. The mean age was 52±13.8 years and 53% were males. The mean duration of psoriasis and PsA were 21.5±15 years and 13.7±12.1 years, respectively. The characteristics of the study population are shown in Table 1.
Table 1. Characteristics of the study population.
| Total (N=2254) | Male (N=1200) | Female (N=1054) | |
|---|---|---|---|
|
| |||
| Site: | |||
| Toronto, ON, Canada | 1362 (60.5%) | 791 (65.9%) | 571 (54.2%) |
| Vancouver, BC, Canada | 73 (3.2%) | 38 (3.2%) | 35 (3.3%) |
| Ann Arbor, MI, USA | 77 (3.4%) | 39 (3.3%) | 38 (3.6%) |
| St. Johns, NF, Canada | 127 (5.6%) | 57 (4.8%) | 70 (6.6%) |
| Rochester, NY, USA | 295 (13.1%) | 125 (10.4%) | 170 (16.1%) |
| London, ON, Canada | 58 (2.6%) | 36 (3%) | 22 (2.1%) |
| Winnipeg, MN, Canada | 181 (8%) | 85 (7.1%) | 96 (9.1%) |
| Haifa, Israel | 81 (3.6%) | 29 (2.4%) | 52 (4.9%) |
|
| |||
| Disease status: | |||
| PsA | 1327 (58.9%) | 442 (42%) | 612 (58%) |
| PsC | 927 (41.1%) | 485 (40.1%) | 715 (59.9%) |
|
| |||
| Age (years) | 52±13.8 | 52.2±13.7 | 51.6±13.9 |
|
| |||
| Ethnicity: | |||
| Caucasian | 1800 (82.4%) | 958 (82%) | 842 (82.7%) |
| South Asian | 85 (3.9%) | 65 (5.6%) | 20 (2%) |
| Chinese | 59 (2.7%) | 38 (3.3%) | 21 (2.1%) |
| Other | 240 (10.9%) | 139 (11.6%) | 171 (16.2%) |
|
| |||
| Duration of Psoriasis (years) | 21.5±15 | 21.2±14.2 | 21.8±15.8 |
|
| |||
| Duration of PsA (years) | 13.7±12.1 | 14±11.8 | 13.2±12.4 |
|
| |||
| Tender joint count* | 6.9±13.2 | 5.5±11.7 | 8.5±14.5 |
|
| |||
| Swollen joint count* | 3.9±9.8 | 3.5±9.1 | 4.4±10.5 |
|
| |||
| Damaged joint count* | 4.82±9.96 | 5.4±10.7 | 4.1±8.9 |
|
| |||
| PASI | 4.13±5.75 | 4.7±6.4 | 3.4±4.8 |
|
| |||
| Current use of NSAIDs (%) | 922 (40.9%) | 470 (39.2%) | 452 (42.9%) |
|
| |||
| Current use of DMARDs (%) | 882 (39.1%) | 442 (36.8%) | 440 (41.8%) |
|
| |||
| Current use of oral corticosteroids (%) | 91 (4%) | 46 (3.8%) | 45 (4.3%) |
|
| |||
| Current use of biologics (%) | 709 (31.5%) | 385 (32.1%) | 324 (30.7%) |
In PsA patients
CV Risk factors in the study population
83.2% of the patients had at least one modifiable CVRF. Based on the FRS classification, 30%, 18% and 52% of patients were in a high, moderate or low risk category, respectively. 6.5% of the patients had ischemic heart disease, 45.1% hypertension, 49.4% dyslipidemia, 13.3% DM, 54.3% central obesity, 75.3% were overweight or obese and 17.3% were current smokers.
The distribution of the different CVRF varied widely across the different sites (Figure 1).
Figure 1. The prevalence of CV Risk Factors by site (%).

The majority of the patients had at least 1 or more CVRF (87.6%). The proportions of patients with more than one CVRF were: ≥2: 70.6%; ≥3: 52.9%; ≥4: 35.4%; ≥5: 16.7%.29.3% of patients were using antihypertensive medications, 20.5% were using lipid lowering drugs and 10.4% were using anti-diabetic drugs. The frequency of underdiagnosis of diabetes was low. Only 9 (4.9%) of 177 patients diabetes were previously undiagnosed.
Underdiagnosis and undertreatment of Hypertension
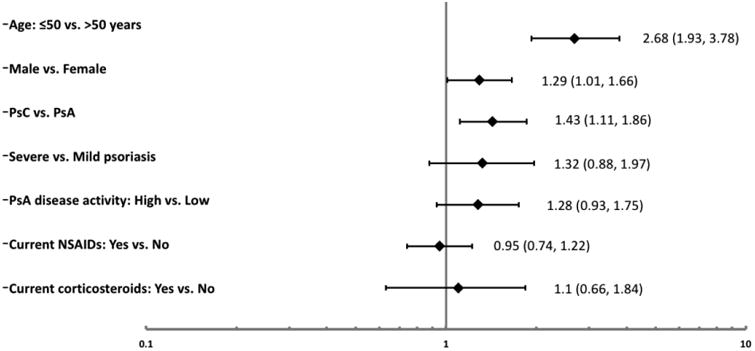
Underdiagnosis and undertreatment of hypertension were observed in a significant proportion of the patients across all sites. A total of 1017 (45.1%; PsA: 48.5%; PsC 40.2%) of the study patients had hypertension; however, 233 (23%; PsA: 19.9%; PsC: 39.1%) of these patients did not have a previous diagnosis of hypertension and were not taking any blood pressure lowering medications. Adherence to treatment recommendations was found to be low with 602 (59.2%; PsA: 55.9%, PsC: 64.8%) patients found to be untreated or undertreated. 60.9% of patients with clinical CV disease or DM were undertreated for hypertension. Among the patients with hypertension, the prevalence of undertreatment was lowest in Israel (42.2%) and highest in Winnipeg, Canada (75.2%). We assessed the association between a variety of demographic and disease-related factors and undertreatment of hypertension (Figure 2). The following factors were associated with undertreatment of hypertension among patients with hypertension in univariate regression analysis: younger age (≤50 years of age: OR 2.68 (95% CI 1.94, 3.71)), male sex (OR 1.29 (95% CI 1.01, 1.66)) and disease status (PsC vs. PsA OR 1.43 (95% CI 1.11, 1.86)). In multivariable regression analysis, younger age (≤50 years of age: OR 2.59 (95% CI 1.87, 3.58)) and disease status (PsC vs. PsA OR 1.36 (95% CI 1.05, 1.78)) remained independent predictors of undertreatment of hypertension.
Figure 2. Factors associated with Undertreatement of Hypertension Odds Ratio (95% Confidence Intervals).
Underdiagnosis and undertreatment of dyslipidemia
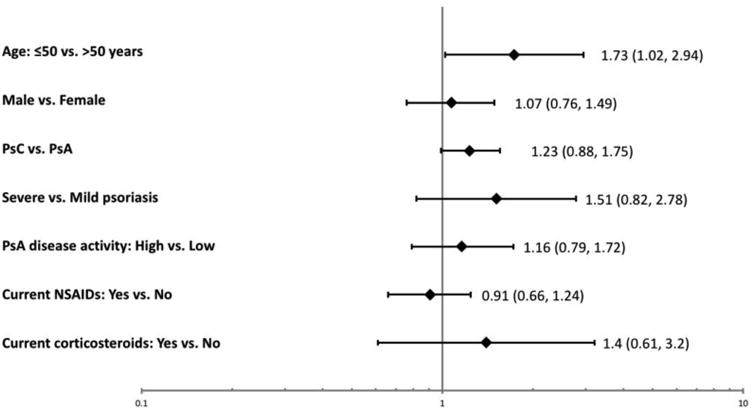
The analysis was limited to 1260 patients with information about their lipid profile. A total of 623 (49.4%) of the study patients had dyslipidemia. However, 35.8% of them did not have a previous diagnosis of dyslipidemia (PsA: 33.6%; PsC: 40.3%). Based on the ACC/AHA guidelines, 696 (55.2%) patients had an indication for using lipid lowering medications. However, adherence to treatment recommendations was low, as only 34.4% of these patients reported using lipid-lowering drugs (PsA: 36.2%; PsC: 31.2%). Adherence prevalence was higher in patients with clinical CVD (79%) and DM (61.5%). The prevalence of undertreatment of dyslipidemia was lowest in Israel (38.6%) and highest in Vancouver, Canada (80%). Among the various factors assessed (Figure 3), only younger age was associated with undertreatment of dyslipidemia among patients with an indication for lipid-lowering therapy (≤50 years of age OR 1.72 (95% CI 1.02, 2.94)).
Figure 3. Factors associated with Undertreatement of Deyslipidemia Odds Ratio (95% Confidence Intervals).
Sensitivity analysis
Since the Toronto cohort dominated the study population, comprising over 50% of the study participants, we performed a sensitivity analysis to assess the proportion of underdiagnosis and undertreatment of CVRF by excluding the patients from Toronto. The results were remarkably similar: underdiagnosis and undertreatment of hypertension were found in 26.2% and 62.2% of the patients, respectively. Underdiagnosis and undertreatment of dyslipidemia were found in 25.2% and 41% of the patients, respectively.
Discussion
In this large international study, we found a high prevalence of underdiagnosis and undertreatment of hypertension and dyslipidemia among patients with PsC and PsA. Despite the known association between CV morbidity and psoriatic disease, a significant proportion of the patients with hypertension and dyslipidemia were undiagnosed and among those who had an indication for treatment, sub-optimal adherence to treatment recommendations was found. Wide variations in adherence to treatment recommendations for hypertension and dyslipidemia were observed across the different sites. These results are in line with recent studies that found inadequate management of CVRF in patients with psoriasis and other rheumatic diseases that may contribute to the increased CV risk observed in these patients (20, 22).
In accordance with previous studies, a high burden of CVRF was found in the study population. 87.6% of the patients had at least one modifiable CVRF and a third of the patients were classified as having high 10-year risk for CV events. Although the increased CV risk in psoriatic disease is partially independent of traditional CVRF, their presence is associated with a higher burden of atherosclerosis and a greater risk of developing clinical CVD (7, 28, 29). Thus, intense control of traditional CVRF is likely the best approach to reduce CV morbidity in psoriatic patients despite the lack of specific primary prevention studies in this population. Observational studies and subgroup analysis of clinical trials suggest that lipid lowering therapy could potentially reduce CV risk in patients with inflammatory arthritis, supporting efforts to improve control of abnormal lipid levels as an effective measure to reduce CV morbidity (30, 31).
Gaps in care of CV risk have been extensively investigated in RA patients; however, only few studies have assessed this in psoriatic patients. Slightly lower rates of undertreatment of hypertension (21.8% untreated, 40.4% not meeting treatment targets) and dyslipidemia (38.6% untreated) were reported in patients with severe psoriasis enrolled in three clinical trials (22). Jafri et al. reported slightly higher rates of pharmacologic treatment of hypertension and dyslipidemia in patients with PsA compared with the general population in a population-based study using administrative data (11). However, due to the design of this study, the rates of underdiagnosis of these risk factors could not be assessed. As we found in our study, underdiagnosis could be high in psoriatic patients (35.7% of patients with dyslipidemia and 23% of patients with hypertension). Therefore, the rates of undertreatment of hypertension and dyslipidemia in PsA patients were likely underestimated in the study by Jafri et al. Our study did not include a control group, however, it could be argued that even if the level of adherence is similar to the general population, the higher CV risk in psoriatic patients requires special attention to primary prevention of CV events, as per current recommendations for other high risk populations, such as patients with DM.
Potential explanations for the gaps in screening and management of CV risk in psoriatic patients may include unawareness of the increased CV risk in psoriatic disease, time constraints especially when attention is directed to the management of active psoriatic disease, limited knowledge about CV prevention strategies and lack of specific guidelines for CV prevention in psoriatic patients. Furthermore, there is disagreement in the rheumatology and dermatology communities about the role of these specialists or family doctors in the management of CVRF due to some of the reasons mentioned above (32). The factors responsible for this gap in care have been identified in other rheumatic diseases. Studies in RA suggested that awareness of the increased CV risk has not translated into adherence to treatment recommendations (20). In our study, we did not find any disease-related factors to explain the gaps in care of hypertension and dyslipidemia. However, patients with PsC were more likely to be undertreated for hypertension and dyslipidemia, although the latter did not reach statistical significance. This finding may suggest different adherence to CV risk management across the two specialties, rheumatology and dermatology. Additional factors that were associated with undertreatment included younger age and male gender. It is possible that younger patients who are followed by dermatologists or rheumatologists do not visit primary care physicians as often as older patients do.
The study provides unique comparative data about CV prevention practices across different countries. Variations were observed in the prevalence of hypertension (40.2% to 65.5%), DM (6.9% to 29.6%), dyslipidemia (46.8% to 72%) and smoking (8.6% to 30.9%). The prevalence of overweight and central obesity was markedly elevated across all sites, measuring over 80% in some sites. Differences were also observed in screening and managing hypertension and dyslipidemia across the different sites. These variations could reflect differences in ethnicities and genetic susceptibility to CVRF or differences related to health care systems and access to care. However, it should be noted that the majority of patients were recruited from specialty clinics in academic medical centers in which a high degree of awareness of CV risk could be expected, thus the gaps in care of patients in community rheumatology and dermatology clinics may be even higher.
The study has several limitations including the lack of non-psoriatic controls, the significant proportion of missing lipid profile data, mostly from the two US centers, that precluded us from providing a complete assessment of the management of dyslipidemia. As indicated above, the study population came mostly from specialty clinics in academic centers which may limit the generalizability of the results. However, it could be expected that gaps in care are at least similar if not larger in the general population of psoriasis and PsA patients. Due to the lack of information about fasting glucose or HbA1C, we used a less accurate measure to determine whether a patent has undiagnosed DM, which was based on non-fasting glucose levels. Only 9 patients (4.9%) were found to have previously undiagnosed DM. More sensitive measures of DM may have revealed a higher prevalence of undiagnosed DM. Additionally, no information was available on the type and dose of lipid lowering and anti-hypertensive medications used by the study participants, thus, we could not assess the impact of various treatment approaches on CVRF. Lastly, by its design this study did not assess the management of obesity, smoking and sedentary lifestyle that contribute to CV risk. Given the high proportion of overweight and obesity (>75%) in psoriatic patients, weight reduction could be another effective measure to manage CVRF. The study has several strengths. To our knowledge this is the first study that has assessed gaps in care of CVRF in both psoriasis and PsA patients. The patients are well phenotyped, allowing us to explore the association between a variety of disease-related and demographic factors and adherence to treatment recommendations. Lastly, the study provides data about the prevalence of CVRF and the quality of care of these factors across different populations of psoriatic patients and health care systems.
In summary, in this large international study we found significant gaps in screening and treating CVRF in patients with PsC and PsA. Additionally, we report a high burden of CVRF in our study population. This high-risk group could benefit from improvement in the screening and management of CVRF to address the gaps in care identified in our study. Although questions exist regarding the optimal treatment targets for CVRF in psoriatic patients, adherence by physicians to, at a minimum, the general treatment recommendations for primary CV prevention is warranted. The high burden of CVRF and the gaps in care observed across all sites highlight the need for new strategies for quality improvement in prevention of CV morbidity in psoriatic patients. Future strategies should consider local resources, type of health care systems and all physicians involved, including dermatologists, rheumatologists, primary care physicians and cardiologists.
References
- 1.Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and major adverse cardiovascular events: a systematic review and meta-analysis of observational studies. J Am Heart Assoc. 2013;2:e000062. doi: 10.1161/JAHA.113.000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polachek A, Touma Z, Anderson M, Eder L. Risk of Cardiovascular Morbidity in Patients With Psoriatic Arthritis: A Meta-Analysis of Observational Studies. Arthritis Care Res (Hoboken) 2017;69:67–74. doi: 10.1002/acr.22926. [DOI] [PubMed] [Google Scholar]
- 3.Maradit-Kremers H, Icen M, Ernste FC, Dierkhising RA, McEvoy MT. Disease severity and therapy as predictors of cardiovascular risk in psoriasis: a population-based cohort study. J Eur Acad Dermatol Venereol. 2012;26:336–43. doi: 10.1111/j.1468-3083.2011.04071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–41. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 5.Miller IMea. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors -supplement. doi: 10.1016/j.jaad.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 6.Jamnitski A, Symmons D, Peters MJ, Sattar N, McInnes I, Nurmohamed MT. Cardiovascular comorbidities in patients with psoriatic arthritis: a systematic review. Ann Rheum Dis. 2013;72:211–6. doi: 10.1136/annrheumdis-2011-201194. [DOI] [PubMed] [Google Scholar]
- 7.Eder L, Wu Y, Chandran V, Cook R, Gladman DD. Incidence and predictors for cardiovascular events in patients with psoriatic arthritis. Ann Rheum Dis. 2016;75:1680–6. doi: 10.1136/annrheumdis-2015-207980. [DOI] [PubMed] [Google Scholar]
- 8.Lin YC, Dalal D, Churton S, Brennan DM, Korman NJ, Kim ES, et al. Relationship between metabolic syndrome and carotid intima-media thickness: cross-sectional comparison between psoriasis and psoriatic arthritis. Arthritis Care Res (Hoboken) 2014;66:97–103. doi: 10.1002/acr.22144. [DOI] [PubMed] [Google Scholar]
- 9.Eder L, Jayakar J, Shanmugarajah S, Thavaneswaran A, Pereira D, Chandran V, et al. The burden of carotid artery plaques is higher in patients with psoriatic arthritis compared with those with psoriasis alone. Ann Rheum Dis. 2013;72:715–20. doi: 10.1136/annrheumdis-2012-201497. [DOI] [PubMed] [Google Scholar]
- 10.Yiu KH, Yeung CK, Zhao CT, Chan JC, Siu CW, Tam S, et al. Prevalence and extent of subclinical atherosclerosis in patients with psoriasis. J Intern Med. 2013;273:273–82. doi: 10.1111/joim.12002. [DOI] [PubMed] [Google Scholar]
- 11.Jafri K, Bartels CM, Shin D, Gelfand JM, Ogdie A. Incidence and Management of Cardiovascular Risk Factors in Psoriatic Arthritis and Rheumatoid Arthritis: A Population-Based Study. Arthritis Care Res (Hoboken) 2017;69:51–7. doi: 10.1002/acr.23094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA dermatology. 2013;149:84–91. doi: 10.1001/2013.jamadermatol.406. [DOI] [PubMed] [Google Scholar]
- 13.Phan C, Sigal ML, Lhafa M, Barthelemy H, Maccari F, Esteve E, et al. Metabolic comorbidities and hypertension in psoriasis patients in France. Comparisons with French national databases. Ann Dermatol Venereol. 2016;143:264–74. doi: 10.1016/j.annder.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 14.Radner H, Lesperance T, Accortt NA, Solomon DH. Incidence and Prevalence of Cardiovascular Risk Factors Among Patients With Rheumatoid Arthritis, Psoriasis, or Psoriatic Arthritis. Arthritis Care Res (Hoboken) 2016 doi: 10.1002/acr.23171. [DOI] [PubMed] [Google Scholar]
- 15.Castaneda S, Martin-Martinez MA, Gonzalez-Juanatey C, Llorca J, Garcia-Yebenes MJ, Perez-Vicente S, et al. Cardiovascular morbidity and associated risk factors in Spanish patients with chronic inflammatory rheumatic diseases attending rheumatology clinics: Baseline data of the CARMA Project. Semin Arthritis Rheum. 2015;44:618–26. doi: 10.1016/j.semarthrit.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Bhole VM, Choi HK, Burns LC, Vera Kellet C, Lacaille DV, Gladman DD, et al. Differences in body mass index among individuals with PsA, psoriasis, RA and the general population. Rheumatology (Oxford) 2012;51:552–6. doi: 10.1093/rheumatology/ker349. [DOI] [PubMed] [Google Scholar]
- 17.Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJ, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76:17–28. doi: 10.1136/annrheumdis-2016-209775. [DOI] [PubMed] [Google Scholar]
- 18.Anderson TJ, Gregoire J, Hegele RA, Couture P, Mancini GB, McPherson R, et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2013;29:151–67. doi: 10.1016/j.cjca.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 19.van Breukelen-van der Stoep DF, van Zeben D, Klop B, van de Geijn GJ, Janssen HJ, van der Meulen N, et al. Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology (Oxford) 2016;55:1210–6. doi: 10.1093/rheumatology/kew039. [DOI] [PubMed] [Google Scholar]
- 20.Barber CE, Esdaile JM, Martin LO, Faris P, Barnabe C, Guo S, et al. Gaps in Addressing Cardiovascular Risk in Rheumatoid Arthritis: Assessing Performance Using Cardiovascular Quality Indicators. J Rheumatol. 2016;43:1965–73. doi: 10.3899/jrheum.160241. [DOI] [PubMed] [Google Scholar]
- 21.Emanuel G, Charlton J, Ashworth M, Gulliford MC, Dregan A. Cardiovascular risk assessment and treatment in chronic inflammatory disorders in primary care. Heart. 2016;102:1957–62. doi: 10.1136/heartjnl-2016-310111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimball AB, Szapary P, Mrowietz U, Reich K, Langley RG, You Y, et al. Underdiagnosis and undertreatment of cardiovascular risk factors in patients with moderate to severe psoriasis. J Am Acad Dermatol. 2012;67:76–85. doi: 10.1016/j.jaad.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 23.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 24.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 25.D'Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 26.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 27.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000–6. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eder L, Thavaneswaran A, Chandran V, Cook R, Gladman DD. Increased burden of inflammation over time is associated with the extent of atherosclerotic plaques in patients with psoriatic arthritis. Ann Rheum Dis. 2015;74:1830–5. doi: 10.1136/annrheumdis-2014-205267. [DOI] [PubMed] [Google Scholar]
- 30.Rollefstad S, Ikdahl E, Hisdal J, Olsen IC, Holme I, Hammer HB, et al. Rosuvastatin-Induced Carotid Plaque Regression in Patients With Inflammatory Joint Diseases: The Rosuvastatin in Rheumatoid Arthritis, Ankylosing Spondylitis and Other Inflammatory Joint Diseases Study. Arthritis & rheumatology. 2015;67:1718–28. doi: 10.1002/art.39114. [DOI] [PubMed] [Google Scholar]
- 31.Semb AG, Kvien TK, DeMicco DA, Fayyad R, Wun CC, LaRosa JC, et al. Effect of intensive lipid-lowering therapy on cardiovascular outcome in patients with and those without inflammatory joint disease. Arthritis Rheum. 2012;64:2836–46. doi: 10.1002/art.34524. [DOI] [PubMed] [Google Scholar]
- 32.Ogdie A, Eder L. Improving cardiovascular health and metabolic comorbidities in patients with psoriatic arthritis. Int J Clin Rheumtol. 2015;10:451–9. doi: 10.2217/ijr.15.45. [DOI] [PMC free article] [PubMed] [Google Scholar]


