Abstract
Objective
Psychogenic non-epileptic seizures (PNES) is a common diagnosis after evaluation of medication resistant or atypical seizures with video-electroencephalographic monitoring (VEM), but usually follows a long delay after the development of seizures, during which patients are treated for epilepsy. Therefore, more readily available diagnostic tools are needed for earlier identification of patients at risk for PNES. A tool based on patient-reported psychosocial history would be especially beneficial because it could be implemented in the outpatient clinic.
Methods
Based on data from 1,375 patients with VEM-confirmed diagnoses, we used logistic regression to compare the frequency of specific patient-reported historical events, demographic information, age of onset and delay from first seizure until VEM in five mutually exclusive groups of patients: epileptic seizures (ES), PNES, physiologic non-epileptic seizure-like events (PSLE), mixed PNES plus ES, and inconclusive monitoring. To determine the diagnostic utility of this information to differentiate PNES only from ES only, we used multivariate piecewise-linear logistic regression trained using retrospective data from chart review and validated based on data from 246 prospective standardized interviews.
Results
The prospective area under the curve of our weighted multivariate piecewise-linear by-sex score was 73%, with the threshold that maximized overall retrospective accuracy resulting in a prospective sensitivity of 74% (95% CI 70-79%) and prospective specificity of 71% (95% CI 64-82%). The linear model and piecewise linear without an interaction term for sex had very similar performance statistics. In the multivariate piecewise-linear sex-split predictive model, the significant factors positively associated with ES were history of febrile seizures, current employment or active student status, history of traumatic brain injury, and longer delay from first seizure until VEM. The significant factors associated with PNES were female sex, older age of onset, mild traumatic brain injury, significant stressful events with sexual abuse, in particular, increasing the likelihood of PNES. Delays longer than 20 years, age of onset after 31 years for men, and age of onset after 40 years for women had no additional effect on the likelihood of PNES.
Discussion
Our promising results suggest that an objective score has the potential to serve as an early outpatient screening tool to identify patients with greater likelihood of PNES when considered in combination with other factors. In addition, our analysis suggests that sexual abuse, more than other psychological stressors including physical abuse, is more associated with PNES. There was a trend of increasing frequency of PNES for women during childbearing years and plateauing outside those years that was not observed in men.
Keywords: dissociative seizures, diagnostic score, logistic regression, multiple imputation
1. Introduction
To an untrained observer, psychogenic non-epileptic seizures (PNES) appear similar behaviorally to epileptic seizures (ES) but their cause and treatment are entirely dissimilar.[1] Patients with PNES, without comorbid ES, often are diagnosed mistakenly as having ES, but they do not benefit from treatment with anti-seizure medications.[2, 3] Therefore accurate and early differentiation between PNES and ES facilitates the initiation of targeted treatment.[4–8] The average delay to diagnosis varies widely across centers from 2 years in the PNES-treatment trials to over 8 years at our center [3], during which time patients have diminished quality of life and high health care utilization [9, 10].
There are multiple challenges to the early identification of PNES including unreliable patient or witness reported details regarding seizure behavior [11, 12] and the limited sensitivity and specificity of interictal scalp electroencephalography [13]. In comparison to seizure behavior, patients’ reports of other medical and social history typically are more accurate and detailed when the patient is interviewed sensitively.
The literature describing historical risk factors for PNES and ES is rich, and has been comprehensively reviewed recently [1, 14]. In brief, the most common features of patients with PNES are female sex, presenting to specialty epilepsy care in the fourth decade of life, history of sexual abuse and a mild traumatic brain injury (TBI), and frequent, disabling seizures for many years. However, none of these features exclude the diagnosis of ES. In contrast, risk factors for ES include a history of severe TBI [15–20], meningitis or encephalitis [21], neurotoxin exposure [22, 23], complex febrile seizures in childhood [24], premature birth [25, 26], and a family history of epilepsy [27]. None of these risk factors exclude the diagnosis of PNES.
While historically associated factors have been demonstrated in small and moderate size populations, this investigation aims to evaluate prospectively an objective screening score based on the combination of these factors in a large, unselected population using patient-reported data that is available in an outpatient neurology or primary care clinic. By combining associated factors in a large population, the conditionally-independent diagnostic utility of each factor can be evaluated more accurately. Additionally, this investigation assesses the prevalence of these factors in the understudied populations of patients with mixed ES plus PNES and physiologic non-epileptic seizure-like episodes (PSLE) [28].
2. Methods
The patient population constitutes all patients admitted to the UCLA adult video-electroencephalography monitoring (VEM) unit from January 2006 to November 2016. Clinical diagnosis was based on expert clinical opinion determined from the available clinical history, physical exam, VEM, MRI, FDG-PET, and sometimes MEG and SPECT. MEG and SPECT were obtained when the treating physicians decided it would be clinically useful. We placed patients in five mutually exclusive categories: psychogenic non-epileptic seizures (PNES), physiologic non-epileptic seizure-like episodes (PSLE), epileptic seizures (ES), mixed non-epileptic plus epileptic seizures, and inconclusive monitoring. Although the populations are heterogeneous, with many important subtypes, the description of subtypes within PNES and ES is outside the scope of this article. We define PSLE as non-epileptic seizures caused by non-psychological factors including syncope, complex migraines, dementia, and tremors [29]. Throughout this manuscript, mixed seizures indicate patients with both PNES and ES. Differentiating between mixed seizures and PNES is important because patients with mixed seizures would benefit from anti-seizure medication treatment, and because there is insufficient evidence to suggest that the mechanisms and risk factors for PNES are the same in mixed seizures and isolated PNES [28].
Inconclusive monitoring occurred when patients did not have sufficiently informative episodes during monitoring to yield a definitive diagnosis for all types of seizures that a patient reported, if these patients reported more than one characteristic seizure type. While patients with inconclusive monitoring represent a mixture of the other groups, we separate this group to provide information about its relative composition. Inclusion of these patients also allows inclusion of all patients in our analysis, thereby reducing the potential for selection bias, and improves the control for confounding variables while otherwise not affecting the results or conclusions regarding the other diagnostic categories.
Although all patients were adults during VEM, they were not necessarily adults during the clinical interview. There is evidence that pediatric PNES and late-onset PNES may differ from adult-onset PNES, but the existing literature uses varying criteria to define pediatric and late-onset. Therefore, we opted to include all subjects in our analysis, with the recognition that if the factors associated with pediatric and late-onset PNES differed from adult-onset PNES, this would result in reduced predictive performance that could be explored in more depth in later studies.
2.1 Clinical Databases Descriptions
Our population includes two sets of patients based on whether their data were acquired retrospectively (January 2006-April 2015) or prospectively (May 2015-November 2016). Records from patients admitted prior to May 2015 were acquired though retrospective chart review. In the interest of developing an early screening tool, if multiple notes were available, we used a single neurology note from the earliest clinical encounter that provided a description of the patients’ seizures and pertinent history. This included both outpatient and inpatient encounters. Detailed social history including psychological stressors and history of abuse was obtained only if deemed appropriate by the neurologist that authored the note. Patients admitted after this date underwent standardized interview with a trained non-neurologist researcher (E.A.J., S.D., W.T.K., or M.AB.) within 48 hours of VEM admission. To simulate the data that would be available during an outpatient visit, no information from the health record was used to supplement the patient-provided history except height, weight, sex and age data. Information from the neurologist’s admission note was not included or referenced prior to interview. If retrospective patients were re-admitted during the prospective period (i.e. due to inconclusive initial monitoring), they were excluded from the prospective analysis. Information from the standardized interview was not used, and their diagnosis was updated in the retrospective dataset. This reduced the frequency of inconclusive monitoring in the retrospective group and ensured that the historical information was blinded to VEM results. Age was recorded as the age at the time of the clinical note or standardized interview that was used for data collection.
Our analysis focuses on the frequency of patient-reported historical factors excluding co-morbidities (for an analysis of co-morbidities, see [30]). These potential risk factors were selected based on published research papers on PNES. The complete list of studied factors is displaying in Table 1. Based on recent guidelines, the definitions for concussion and mild TBI were identical and therefore we use the two terms interchangeably [31]. Please refer to Supplemental Text for a detailed discussion of each factor and references.
Table 1.
| Factor Description |
|---|
| Age of seizure onset |
| Delay to assessment |
| Febrile seizures |
| Family history seizures |
| Neuroinfection |
| Neurotoxin |
| Premature birth |
| Traumatic Brain Injury (TBI) |
| Concussion/mild TBI |
| TBI with prolonged deficits |
| Precipitating event |
| Psychological stressor |
| Sexual abuse |
| Physical abuse |
| Obesity (BMI≥30) |
| Employed or Student |
| Handedness |
Patient reported historical factors considered to potentially contribute to our model. Indentation reflect additional details within a larger category. Abbreviations: body mass index (BMI), traumatic brain injury (TBI).
2.2 Statistical Methods
We analyzed the relationship of historical factors using both population-level descriptive statistics and individual-level predictive statistics. Predictive statistics ask if the seizure etiology of an individual patient can be predicted by the presence of a specific historical factor; whereas descriptive statistics asks the reverse: is the probability of a historical factor associated with a particular seizure etiology on a population level. We controlled for patient's sex and age in all regressions. For the individual-level predictive statistics, we used multivariate piecewise-linear logistic regression to be able to interpret if the contribution of each specific historical factor was conditionally independent of other studied factors.
For predictive statistics, we trained our multivariate piecewise-linear logistic regression model on the patients with either PNES alone or ES alone in the retrospective dataset so that we could assess our performance on independently collected prospective data. Instead of reporting positive and negative predictive values, we report the predictive value of PNES and ES that are defined similarly because our population lacks healthy negative controls. Statistically, the binary comparison of PNES versus ES is both well-posed and well-studied but there are not yet well-established, highly effective statistical methods to accomplish multinomial prediction between all five diagnostic classes. We report the predicted composition of PSLE, mixed PNES plus ES and patients with inconclusive monitoring based on the predictive algorithm for PNES versus ES.
In addition to linear analysis of age of onset and delay to VEM, we also utilized piecewise-linear logistic regression to improve our model of the nonlinear effects of men and women separately that were apparent on univariate display of the data (Figure 1). Based on these visualized trends, the piecewise-linear regressions were structured as follows: values below a cut-off contributed linearly to the odds of PNES versus ES, whereas all values above that cut-off were considered equivalent (Supplemental Figure 1). This cut-off and the odds of PNES versus ES above the cut-off were chosen to minimize the deviance of the overall model in each imputation separately. When results are summarized, we quote the average cut-off across imputations. Due to the differing trends in age of onset in men and women, we also fit a piecewise-linear by sex regression where this age of onset cutoff was determined separately in men and women.
Figure 1.
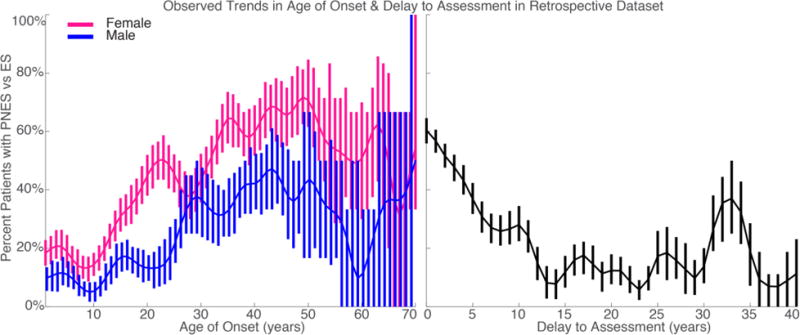
The frequency of PNES as compared to ES with respect to patient-reported age of onset and delay from seizure onset to presentation. Error bars reflect exact binomial standard error. Data Gaussian smoothed with full-width half-max of 2.4 years for delay and 5 years for age of onset. A) Age of onset split by patient sex. B) Delay.
For patients with mixed ES plus PNES and inconclusive monitoring, we report the rate that our score predicted that the patient had ES only for both retrospective and prospective patients because these patients did not contribute to the overall model that was trained on retrospective patients with PNES only and ES only.
For population level descriptive statistics, we combined the retrospective and prospective datasets because this practice results in the best linear and unbiased estimate of the effect of each studied factor [32]. We recognize that the method of data collection and presence of missing data differed between retrospective and prospective groups, and have included details of the difference in prevalence between the groups in Supplemental Table 1.
For prospective patients, the standardized interview ensured that all factors were discussed with all patients. For retrospective patients, the presence of our studied historical risk factors was based on review of clinical notes that did not discuss all studied factors uniformly. With a few exceptions, if a historical factor was not mentioned, it was assumed to be absent, because medical record authors may not mention all pertinent negatives if they do not contribute to the overall evaluation. The exceptions to this include age of onset, delay to assessment and handedness, because these factors are present for each patient but were not discussed in all notes. For population-level analysis, we excluded patients with missing entries that pertained to the factor modeled in that regression. For predictive analysis, we conservatively assumed that the probability that age of onsets and handedness were missing was unrelated to diagnosis, age of onset or handedness, and therefore used multiple imputation to fill in these missing values stochastically based on collinearity with other studied factors in the retrospective dataset [33, 34]. No information from the prospective dataset was used for multiple imputation. If missingness were correlated with diagnosis, then this approach would underestimate the effect of the imputed factor. If the probabilities of missingness were correlated to the missing value, then imputation introduced a bias that underestimates the importance of the imputed factor. Based on the available data, it is impossible to evaluate if this bias exists. We validated our score using prospective standardized interviews without missing data. In the rare case that a factor was missed during a prospective interview, the prediction of each independent imputation model was used to fill in these missing values. For more details regarding the implementation of multiple imputation, please refer to Supplemental Text.
For all tests of statistical significance, we utilized permutation tests to estimate the empirical probability distribution of the null hypothesis. P-values based on this analysis are indicated as . In permutation testing the diagnostic class labels were shuffled, without replacement, to remove any relationship between diagnostic classes and input data. The entire analysis was repeated on 50,000 independently-permuted null datasets to estimate the empirical null probability distributions of all summary statistics. This was done to avoid distributional assumptions that may be made less valid by multiple imputation. Supplemental Figure 2 summarizes the data processing and modeling scheme with all data.
All patients consented for the use of their records in research, and the UCLA Institutional Review Board approved this study. This work is consistent with Declaration of Helsinki. De-identified raw data, code and online predictive score for this study is available at http://www.brainmapping.org/MarkCohen/research.html.
3. Results
The following factors were reported more frequently in the patient-reported prospective database compared to the neurologist-documented retrospective database (Supplemental Table 1, p<0.05): TBI in ES, inconclusive and PNES; significant psychological stressors in all groups except PSLE; physical and sexual abuse in inconclusive; precipitating event in all groups except PSLE; obesity in ES and PNES; and neurotoxin exposure in ES and inconclusive. (Of course, each of these factors could have been reported during the interview, but they were not included in the note because the author judged that they were not relevant.) There were no other significance differences within group between the retrospective databases, including no significant differences in the rate of concussion or long-term sequelae of TBI despite the increased TBI rate (Fisher exact test, p>0.1). Inconclusive monitoring occurred more often in the prospective dataset (26% vs 10%, Fisher exact test, p<0.01).
The proportions of missing data for the following factors were: age of onset (5%, 72/1372), delay to VEM (5%, 72/1372), and handedness (15%, 199/1372). Standardized interviews were performed with 83% (246/296) of patients admitted during the prospective period. Patients were not interviewed due to declining the interview, inability of the patient or family to communicate with the interviewer (i.e. language barrier and declined translation services), or no trained interviewers were available during admission.
3.1 Individual-Level Prediction
The performance of our piecewise by-sex score compared to that of permuted datasets and the naïve assumption that all patient have ES is displayed in Figure 2. Information regarding all models is summarized in Supplemental Table 2. The prospective binary performances of each of our regression models were nearly identical. The piecewise linear model without separating men and women improved the fit of the model significantly (deviance difference 28, df=2, p<0.05). This was reflected in a non-significantly increased accuracy (71% linear, 72% piecewise linear). Addition of an interaction term to model men and women separately did not significantly improve model fit or performance (deviance difference 0.11, df=1, p=1.0) but it did improve binary performance non-significantly (piecewise linear by-sex accuracy 73% versus 71% linear or 72% piecewise linear, p>0.1).
Figure 2.
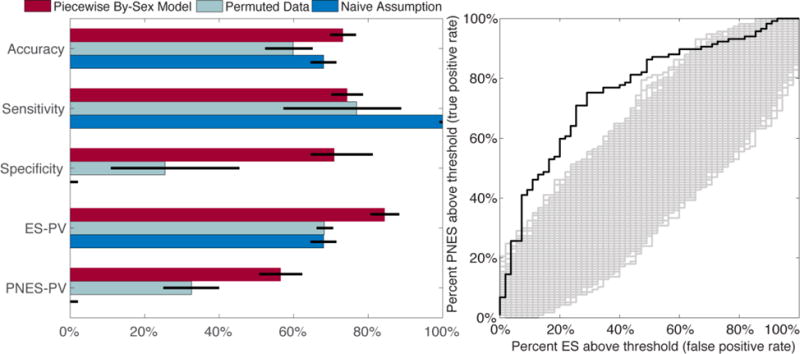
Performance statistics of our retrospectively trained model applied to our prospective dataset. 2A) Binary summary statistics of the performance of our piecewise by-sex model and other naïve models. Exact values for performance of all models is summarized in Supplemental Table 2. Error bars reflect standard error. Abbreviations: predictive value (PV). 2B) Receiver-operating curve for the prediction combined across imputed datasets. Our predicted model shown in black whereas the performance on permuted datasets shown in gray.
The prospective ES and PNES predictive values of our models were significantly higher than both that of the permuted data and the naïve assumption ( <0.013). The overall accuracy of our model was higher than the permuted models ( <10−3), but not significantly higher than that of the naïve assumption (73% vs 71%, Fisher exact test, p=0.28). The sensitivity and specificity of each of our models were comparable (approximate sensitivity 74%, specificity 71%), whereas the permuted data and naïve assumptions had substantially higher sensitivity than specificity (permuted sensitivity 77% vs specificity 25%). The sensitivity of our models were not significantly different from the permuted models ( =0.85), but was lower than that of the naïve assumption that, by definition, has 100% sensitivity (Fisher exact test, p<10−70).
The weighted score that combines all historical factors that contributed to our piecewise linear by-sex model is displayed in Figure 3 and Supplemental Table 3. Factors positively associated with PNES were female gender ( =0.015), concussion ( <10−4), younger age of onset in either sex below 31 years in men and 40 years in women ( =0.0026 male, 0.00076 female), and significant psychological stressors ( =0.002). Sexual abuse provided an additional association with PNES ( =0.003) on top of the association with psychological stressors. Factors positively associated with ES included history of febrile seizures ( =0.040), current employment or student status ( =0.010), traumatic brain injury ( =0.007), longer time from onset of seizure disorder to presentation up to 20 years ( <10−4).
Figure 3.
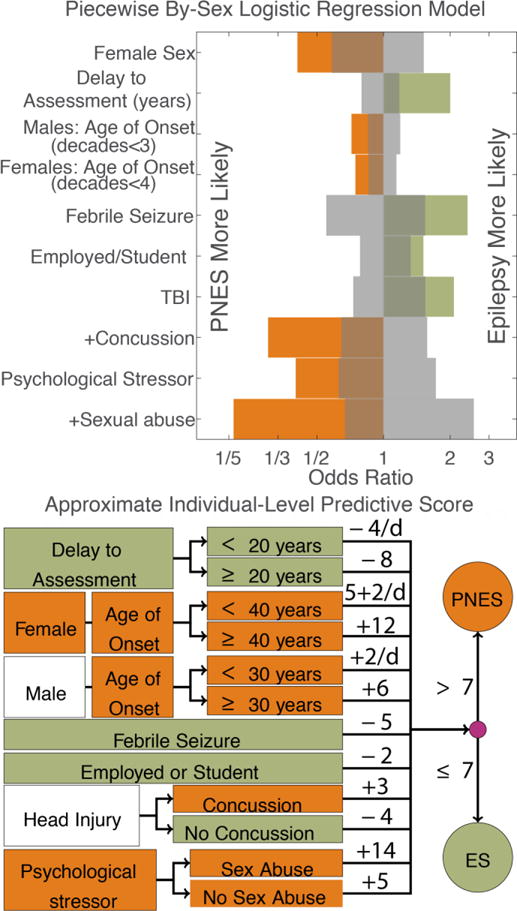
Individual-level predictive model trained on the retrospective dataset. Color reflects direction of effect. A) Odds ratios of all significant factors (permutation tests, <0.05). Gray reflects 95% empirical confidence intervals of chance. The “+” indicates that the effect of concussion and sexual abuse are in addition to the effect of TBI and psychological stressors, respectively. See Supplemental Table 3 for an exact summary of all models. B) Translation of log-odds ratios to a rounded score for implementation. The sum of all components should be compared to the overall threshold of 7. Arrows reflect conditional statements, for example a male patient with an age of onset of 20 years, should add +4 to his score. Abbreviations: per decade (/d).
There was insufficient evidence to suggest that any of the other studied factors had a conditionally independent influence on the likelihood of PNES versus ES ( >0.1). Exact log odds and statistics for all models are available in Supplemental Table 3.
Combining the inconclusive patients from the retrospective and prospective groups, our piecewise-linear sex-split model predicts that 69% (95% CI 62-76%) would go on to be diagnosed with epilepsy. Similarly, when applied to the patients with mixed PNES plus ES, the model predicted 68% (95% CI 54-80%) had ES, as compared to PNES.
3.3 Population-Level Statistics
Exact model statistics including overall model fits are summarized in Supplemental Table 4. The following text summarizes selected population-level differences.
There were substantial sex differences between the populations, with patients with ES being slightly more frequently female than male (log odds versus 50% female=0.32, SE 0.15, p=0.03). Patients with PNES were significantly more likely to be female (log odds difference versus ES=0.88, SE 0.14, p<10−9), as were patients with PSLE (log odds difference versus ES=0.87, SE 0.37, p=0.02). Patients with inconclusive monitoring were less likely to be female than patients with PNES (log odds difference versus PNES=0.44, SE 0.15, p=0.04).
As visualized partially in Figure 1 (trends for all groups displayed in Supplemental Figure 3), there were substantial differences between all of the populations in the age of onset and delay to diagnosis. The order of diagnostic groups from youngest average onset to oldest onset and the longest average delay to shortest delay to diagnosis were the same: ES only, mixed, inconclusive monitoring, PNES only, PSLE. Only two pairs of populations did not differ significantly: mixed versus ES (p>0.93), and PNES versus PSLE (p>0.52). Because of these offsetting factors, the differences in age at assessment were more similar, but the many of the groups still differed significantly: patients with ES were younger on average than patients with inconclusive monitoring, PNES and PSLE (p<0.02), whereas patients with PSLE were older on average than all other patient groups (p<0.0002). Other than these demographic differences, patients with PSLE did not differ significantly from any other population in our studied historical factors (p>0.13).
In addition to the factors that significantly predicted a change in the likelihood of ES compared to PNES, population-level statistics revealed other significant differences that did not contribute to the score because they did not add conditionally independent information beyond the factors included already. In specific, physical abuse and substance abuse both were more common in patients with PNES than ES (log odds>0.7, p<0.02). Without controlling for concussions, there was no significant difference between the rate of TBIs in patients with PNES compared to ES (p>0.1).
Patients with mixed PNES plus ES only differed significantly from other populations in a few factors. Patients with mixed disease more frequently had febrile seizures than those with PNES (p=0.04). Relative to the patients with ES, patients with mixed disease had more sexual abuse (p=0.03), substance abuse (p=0.01), premature birth (p=0.006) and history of remote seizures (p=0.04).
Patients with inconclusive monitoring differed from the other populations based on numerous factors. They reported more significant social stressors than all other patients (p<0.03), but did not report sexual abuse, physical abuse or substance abuse more often than other populations (p>0.07). They reported fewer febrile seizures than patients with ES (p=0.02) and fewer concussions than patients with PNES (p=0.02).
4. Discussion
Distinct from seizure descriptions, patients report valuable information that can be used to differentiate PNES from ES. Our large, unselected database suggests that a combination of nine key historical factors are important for this differentiation: sex, age of onset, delay from seizure onset to referral, head injury, concussion, febrile seizures, employment or student status, significant psychological stressors and sexual abuse. While the other 14 factors we studied may be important in understanding the patient’s story and the context for their seizures, there was insufficient evidence to suggest that these should play a major role in identifying patients with PNES alone.
4.1 Predictive Performance
As shown by the significant predictive values, a simple weighted combination of these nine historical factors provides useful input that objectively raises or lowers the predicted likelihood of ES or PNES. The epilepsy predictive value of 84% suggests that a score indicative of epilepsy effectively reduces the likelihood of PNES well but does not exclude it completely. Unfortunately, the PNES predictive value of 57% suggests that a substantial minority of patients identified as likely PNES do not, in fact, have PNES. However, about 58% of patients admitted for VEM for differential diagnosis of seizure-like events were found to have NES [35]. Therefore, we expect that if PNES-identified patients were triaged towards VEM based on this score, the diagnostic yield may mirror current practice.
The good, but imperfect, overall accuracy of our score highlights the challenge in early and effective diagnosis of PNES and the importance of using VEM to evaluate patients at risk more definitively [36]. Given the imperfect performance of our tool, we anticipate its primary function may be to triage patients more quickly towards VEM. To generate a more comprehensive but qualitative assessment of each patient’s likelihood of PNES, the results of our scores based on patient history should be considered in combination with clinicians’ evaluation of the patient’s ictal behavior [11, 37], comorbidities and other diagnostic work-up [30, 36]. The population of patients with PNES is heterogeneous, and consideration of these other factors may more effectively identify the patients not accurately represented by our history-based score.
4.2 Associated Factors
In addition to establishing a potential early screening tool for PNES, these analyses help establish a list of key factors that can guide the understanding and treatment of PNES.
First, our data and analysis reproduced well-known risk factors for epilepsy including febrile seizures [38, 39] and traumatic brain injury [40]. Febrile seizures, especially febrile status epilepticus, has been associated with injury of the mesial temporal lobe that increase the risk of temporal lobe epilepsy [24]. While febrile status epilepticus clearly confers more risk than a single febrile seizure, patients were unable to provide a sufficiently detailed history of these events to differentiate between the them reliably. Similarly, the mechanism for post-traumatic epilepsy (PTE) has been well established in animal models and human studies [40]. We interpret the positive association of head injury, separate from concussion, to represent the more severe head injuries that are associated with PTE, which is consistent with prior literature in combat veterans [41, 42].
Separate from these epilepsy-associated factors, our analysis provides additional insight into factors associated with PNES. The association of PNES with significant psychologically stressful life events is consistent with the understanding of PNES as a component of conversion disorder [1]. Building upon that knowledge, our data suggest that sexual abuse is unique in its strong association with PNES, and that physical abuse in addition to sexual abuse was not associated more strongly with PNES. The unique role of sexual abuse builds upon the literature that the psychological impact of sexual trauma is quite different than that of other traumatic events, and therefore may confer special considerations for treatment [43–46]. This insight questions whether sexual and physical abuse should be grouped together, as is done frequently [47–49], potentially due to the high incidence of coincident sexual and physical abuse.
Additionally, we found that psychological stressors including sexual and physical abuse were much more prevalent in all groups when discussed specifically in a standardized interview with a non-physician staff member whom they had never met. This suggests that when the interview is conducted in a sensitive and respectful manner, these important historical details can be obtained during a first interview. Further, because sexual abuse was reported more often when asked uniformly, if only patients who present with seizures concerning for PNES are asked about sexual abuse, there may be a tendency for selection bias to underestimate the frequency of sexual abuse in ES and other groups, and thereby to inflate the perceived diagnostic utility of sexual abuse.
Our observation that the general category of TBI was associated with epileptic seizures reaffirms existing literature [50]. However, our finding that the subcategory of concussions, or mild TBI, were more associated with PNES parallels the building body of data suggesting that even mild TBIs have long-lasting effects on patients [48, 51–53]. Due to the high prevalence of unrecognized concussions, patients with PNES may be more likely to report, or even attribute their seizures to, a head injury with mild, transient neurologic symptoms than a patient with ES, who may believe that the same injury is unrelated or not significant enough to report. Patients with PNES do report generally more symptoms on review of systems than patients with ES [3, 54, 55]. Therefore we cannot rule out this difference in rate of reporting or hypervigilance. However, in all diagnostic groups the reported frequency of TBI and concussions increased when the question was asked systematically in all patients, suggesting that the conventional clinical interview may underestimate the frequency of head-trauma associated epilepsies and mild TBI.
This general context of increased overall symptom burden and hypervigilance likely explains why we observed that fewer patients with PNES were employed or pursuing education than patients with ES, when controlling for other factors. This is consistent with the cost literature demonstrating high indirect costs of PNES due to lost work productivity, in addition to the high direct cost through healthcare utilization and emergency room visits [9, 10, 17, 28, 56, 57]. This underscores the importance of addressing both the seizures and the impact of the seizures on the patient’s quality of life and function. Therefore, the goal of treatment for PNES is both seizure freedom and re-integration of the patient into the workforce, when appropriate [8, 58, 59].
In addition to these associated factors, the unique power and diversity of our large dataset demonstrated a novel contribution of patient age and age of onset, particularly in older patients. In interpreting our findings, we highlight that the differences we discuss are in relative prevalence between ES and PNES. This differs substantially from the overall prevalence of each disorder.
While the relative likelihood of PNES did not vary with age at referral except above the age of 75, there were illuminating trends in patient-reported age of onset. Compared to adult PNES, pediatric PNES is rare [60, 61] and the substantial female predominance of PNES is present only after puberty [62–69]. Therefore, it was expected that the likelihood of PNES was low when seizures started prior to age 10 [70, 71]. However, it was unexpected that the likelihood of PNES increased steadily with age for women only. Previous literature suggests that late-onset PNES also is rare [72, 73], so it was surprising that the relative likelihood of PNES plateaued around age 40 because we expected the likelihood to decrease. Compared to younger-onset PNES, late-onset PNES was rare in our dataset, but when compared to late-onset ES, late-onset PNES was more common. The specific age cutoff of 40 slightly proceeds the transition into pre-menopause and menopause for most women. This trend was distinct from the pattern seen in men, where risk of PNES steadily increased at a slower rate until age 31, when it plateaued.
This steady incline during the reproductive years, however, is difficult to disentangle from the patterns in delay to referral. With the average time to diagnosis of PNES being 8.4 years in our retrospective dataset [3], the average time to referral to our center for patients with epilepsy was 16.7 years. Although delay to referral is variable across centers and was distributed exponentially, these averages and difference between PNES and ES were consistent with what has been shown in other large databases [74] and, unfortunately, have not changed since they were assessed by our group using data up to 2008 [75].
More data is needed in patients with later onset epilepsy and PNES to better understand these phenomena that may comprise between 3-10% of all PNES. Our results suggest that after 40 years of age, the relative prevalence of PNES plateaus. Therefore, the nature of PNES before and after 40 may differ. Previous literature regarding later-onset PNES, defined as after the age of 55 or 60, suggests that the female predominance may or may not be as prevalent [76–78] . In comparison to focusing on sexual abuse and psychological stressors, patients more frequently discuss health-related stressors and severe comorbidities. When patients discussed severe health-related trauma, we included it as a psychological stressor. Previously, we showed an increased comorbidity burden in PNES in the entire adult population was conditionally independent of the expected age-related increase in medical comorbidities [30]. While our analysis provides some information regarding late-onset PNES, future detailed analysis of other populations and our dataset is needed to further describe the important population of late-onset PNES that may or may not differ from earlier-onset PNES. We have made a deidentified version of our dataset available for the interested reader.
In addition to late-onset PNES, pediatric PNES differs from adult PNES in many important ways [79] but the threshold for what defines pediatric PNES is unclear. In our work, the relative frequency of PNES was low under the age of 10 to 12. This was consistent with the findings of other literature that suggest that after puberty the prevalence of PNES and the female predominance of PNES increases [80, 81]. The psychological stressors described in pediatric populations focus more on common childhood stressors including family discord, bullying, and school-related difficulties [79]. As our category of psychological stressors was general, these stressors were included in our analysis. Fortunately, the delay to diagnosis in pediatric PNES is much shorter than adult PNES [82]. We do not expect the remainder of our studied factors to differ substantially between the adult and pediatric population. Separate from our studied factors, it is important to note that the ictal behavior and psychiatric comorbidities of pediatric PNES differs from that of adult PNES [62, 81–83].
The differentiation of PNES from ES is complicated by the presence of mixed ES plus PNES. The trends of increased rates of sexual abuse and febrile seizures suggest that patients with mixed seizures have aspects of both ES only and PNES only, but the differences in substance abuse, premature birth, and history of remote seizures suggest that patients with mixed seizures may be a distinct population. Consequentially, our scores predicted that 68% of patients with mixed disease had ES only, which matches the rate of ES only prior to applying our score, 71%. Our score, therefore, does not reliably identify this difficult population with mixed ES plus PNES. The previous history of seizures may serve to be a model for later developing PNES after experiencing epileptic seizures, but more work needs to be done to establish this link.
4.3 Future Directions & Limitations
Our patient population was referred for VEM monitoring, suggesting that the patients’ seizures were sufficiently atypical or medication resistant to warrant definitive diagnostic testing. The outpatient population of patients with seizures likely differs substantially from our sample in that PNES is less prevalent and ES is more prevalent [71, 84–86]. This would result in an increased ES predictive value and reduced PNES predictive value without considering if our observed associations generalize. Therefore, similar studies are necessary to determine if our observed associations are replicated across centers and in an outpatient population.
PNES is uncommon in patients with a pediatric age of onset and, consequentially, PNES is rare in pediatric populations. This low pre-test probability and the potential difference in associated factors suggest that our approach likely should not be extrapolated directly to pediatric patients. Additionally, the clinical interview of an adolescent and child differs from that of an adult. Therefore the information that parents and patients report may need to be interpreted differently. Further, the adolescent and child response to psychological stress differs from adults, therefore the relationship between patient-reported school status, psychological stressors and other factors associated with PNES may vary between adolescents and adults in a complex way. While our approach included a minority of patients who presented initially as adolescents and children, more data are needed to objectively evaluate the factors associated with adolescent PNES.
We also highlight that the method of data collection differed between our retrospective patients and prospective patients. In both cases, our information was primarily patient-reported. While the associated factors established from chart review were consistent enough for our predictions to accurately identify PNES from patient interviews, the difference in prevalence of sexual abuse, TBI and other factors suggests that the method of data collection impacts the findings and resulting conclusions. Therefore, this work demonstrates the potential that an objective screening score may be useful, but any standardized objective score or structured patient interview should be validated further its intended setting.
4.4 Conclusion
The good performance of our objective, evidence-based score to identify PNES using historical factors suggests that probing for a history of TBI, psychological stressors and sexual abuse contributed meaningfully to the diagnostic assessment of patients with seizures. Our prospectively validated score may assist in quantitatively assessing if PNES is suspected in a patient with seizures. While our accuracy was only 73%, the rate of PNES in patients we identified as likely PNES (57%) mirrored the rate of PNES in patients referred to VEM for suspicion of PNES (58%). Therefore, when validated further and utilized early in the diagnostic assessment, our combination of patient-reported historical factors has the potential to identify patients with PNES objectively and triage them towards VEM. Earlier triage may reduce the time to diagnosis, reduce seizure burden and improve quality of life.
Supplementary Material
Highlights.
-
–
Objective screening tools to identify PNES are needed
-
–
Patients with PNES reported more mild traumatic brain injuries than ES
-
–
Older age of onset before the age of 40 increased the likelihood of PNES
-
–
Patients with PNES reported more stressful events of all types than ES
-
–
Sexual abuse, in particular, was more associated with PNES
Acknowledgments
The authors thank Kirk Shattuck, Marc Nuwer, and Edward P. Lau for organization support, access to the data, and technical support. This work was supported by the UCLA-California Institute of Technology Medical Scientist Training Program (NIH T32 GM08042), the Neuroimaging Training Program (NIH T90 DA022768, R90 DA022768 & R90 DA023422 to MSC), the William M. Keck Foundation, research grants to JE (NS03310 & NS080181), the UCLA Departments of Psychiatry & Biobehavioral Sciences and Biomathematics, and the Eisenhower Medical Center Department of Internal Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts & Ethical Publication:
Drs. Engel, Stern and Kerr have clinical responsibilities that include the diagnosis and treatment of patients with epilepsy and non-epileptic seizures. The remaining authors have no declared conflicts of interest. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Dickinson P, Looper KJ. Psychogenic nonepileptic seizures: a current overview. Epilepsia. 2012;53:1679–89. doi: 10.1111/j.1528-1167.2012.03606.x. [DOI] [PubMed] [Google Scholar]
- 2.Alessi R, Valente KD. Psychogenic nonepileptic seizures: should we use response to AEDS as a red flag for the diagnosis? Seizure. 2014;23:906–8. doi: 10.1016/j.seizure.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 3.Kerr WT, Janio EA, Le JM, Hori JM, Patel AB, Gallardo NL, Bauirjan J, Chau AM, D’Ambrosio SR, Cho AY, Engel J, Jr, Cohen MS, Stern JM. Diagnostic delay in psychogenic seizures and the association with anti-seizure medication trials. Seizure. 2016 doi: 10.1016/j.seizure.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razvi S, Mulhern S, Duncan R. Newly diagnosed psychogenic nonepileptic seizures: health care demand prior to and following diagnosis at a first seizure clinic. Epilepsy Behav. 2012;23:7–9. doi: 10.1016/j.yebeh.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Walczak TS, Papacostas S, Williams DT, Scheuer ML, Lebowitz N, Notarfrancesco A. Outcome after diagnosis of psychogenic nonepileptic seizures. Epilepsia. 1995;36:1131–7. doi: 10.1111/j.1528-1157.1995.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 6.Jones SG, TJ OB, Adams SJ, Mocellin R, Kilpatrick CJ, Yerra R, Lloyd JH, Velakoulis D. Clinical characteristics and outcome in patients with psychogenic nonepileptic seizures. Psychosom Med. 2010;72:487–97. doi: 10.1097/PSY.0b013e3181d96550. [DOI] [PubMed] [Google Scholar]
- 7.LaFrance WC, Jr, Benbadis SR. Avoiding the costs of unrecognized psychological nonepileptic seizures. Neurology. 2006;66:1620–1. doi: 10.1212/01.wnl.0000224953.94807.be. [DOI] [PubMed] [Google Scholar]
- 8.Perez DL, LaFrance WC. Nonepileptic seizures: an updated review. CNS Spectr. 2016;21:239–46. doi: 10.1017/S109285291600002X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Begley CE, Famulari M, Annegers JF, Lairson DR, Reynolds TF, Coan S, Dubinsky S, Newmark ME, Leibson C, So EL, Rocca WA. The cost of epilepsy in the United States: an estimate from population-based clinical and survey data. Epilepsia. 2000;41:342–51. doi: 10.1111/j.1528-1157.2000.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 10.Magee JA, Burke T, Delanty N, Pender N, Fortune GM. The economic cost of nonepileptic attack disorder in Ireland. Epilepsy Behav. 2014;33:45–8. doi: 10.1016/j.yebeh.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Syed TU, LaFrance WC, Jr, Kahriman ES, Hasan SN, Rajasekaran V, Gulati D, Borad S, Shahid A, Fernandez-Baca G, Garcia N, Pawlowski M, Loddenkemper T, Amina S, Koubeissi MZ. Can semiology predict psychogenic nonepileptic seizures? A prospective study. Ann Neurol. 2011;69:997–1004. doi: 10.1002/ana.22345. [DOI] [PubMed] [Google Scholar]
- 12.Syed TU, Arozullah AM, Suciu GP, Toub J, Kim H, Dougherty ML, Wehner T, Stojic A, Syed I, Alexopoulos AV. Do observer and self-reports of ictal eye closure predict psychogenic nonepileptic seizures? Epilepsia. 2008;49:898–904. doi: 10.1111/j.1528-1167.2007.01456.x. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert DL, Sethuraman G, Kotagal U, Buncher R. Meta-analysis of EEG test performance shows wide variation among studies. Neurology. 2003;60:564–570. doi: 10.1212/01.wnl.0000044152.79316.27. [DOI] [PubMed] [Google Scholar]
- 14.Asadi-Pooya AA. Psychogenic nonepileptic seizures: a concise review. Neurol Sci. 2017 doi: 10.1007/s10072-017-2887-8. [DOI] [PubMed] [Google Scholar]
- 15.Westbrook LE, Devinsky O, Geocadin R. Nonepileptic seizures after head injury. Epilepsia. 1998;39:978–82. doi: 10.1111/j.1528-1157.1998.tb01447.x. [DOI] [PubMed] [Google Scholar]
- 16.Pakalnis A, Paolicchi J. Psychogenic seizures after head injury in children. J Child Neurol. 2000;15:78–80. doi: 10.1177/088307380001500202. [DOI] [PubMed] [Google Scholar]
- 17.Dworetzky BA, Strahonja-Packard A, Shanahan CW, Paz J, Schauble B, Bromfield EB. Characteristics of male veterans with psychogenic nonepileptic seizures. Epilepsia. 2005;46:1418–22. doi: 10.1111/j.1528-1167.2005.13004.x. [DOI] [PubMed] [Google Scholar]
- 18.van Merode T, de Krom MC, Knottnerus JA. Gender-related differences in non-epileptic attacks: a study of patients' cases in the literature. Seizure. 1997;6:311–6. doi: 10.1016/s1059-1311(97)80079-9. [DOI] [PubMed] [Google Scholar]
- 19.Holmes MD, Dodrill CB, Bachtler S, Wilensky AJ, Ojemann LM, Miller JW. Evidence That Emotional Maladjustment Is Worse in Men Than in Women with Psychogenic Nonepileptic Seizures. Epilepsy Behav. 2001;2:568–573. doi: 10.1006/ebeh.2001.0268. [DOI] [PubMed] [Google Scholar]
- 20.Wilkus RJ, Dodrill CB, Thompson PM. Intensive EEG monitoring and psychological studies of patients with pseudoepileptic seizures. Epilepsia. 1984;25:100–7. doi: 10.1111/j.1528-1157.1984.tb04162.x. [DOI] [PubMed] [Google Scholar]
- 21.Vezzani A, Lang B, Aronica E. Immunity and Inflammation in Epilepsy. Cold Spring Harb Perspect Med. 2015;6:a022699. doi: 10.1101/cshperspect.a022699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams SJ, O’Brien TJ, Lloyd J, Kilpatrick CJ, Salzberg MR, Velakoulis D. Neuropsychiatric morbidity in focal epilepsy. British Journal of Psychiatry. 2008;192:464–469. doi: 10.1192/bjp.bp.107.046664. [DOI] [PubMed] [Google Scholar]
- 23.Jefferys JG, Borck C, Mellanby J. Chronic focal epilepsy induced by intracerebral tetanus toxin. Ital J Neurol Sci. 1995;16:27–32. doi: 10.1007/BF02229071. [DOI] [PubMed] [Google Scholar]
- 24.Hesdorffer DC, Shinnar S, Lewis DV, Moshe SL, Nordli DR, Jr, Pellock JM, MacFall J, Shinnar RC, Masur D, Frank LM, Epstein LG, Litherland C, Seinfeld S, Bello JA, Chan S, Bagiella E, Sun S, team Fs Design and phenomenology of the FEBSTAT study. Epilepsia. 2012;53:1471–80. doi: 10.1111/j.1528-1167.2012.03567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kallen B. The risk of neurodisability and other long-term outcomes for infants born following ART. Semin Fetal Neonatal Med. 2014;19:239–44. doi: 10.1016/j.siny.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Marin-Valencia I, Guerrini R, Gleeson JG. Pathogenetic mechanisms of focal cortical dysplasia. Epilepsia. 2014;55:970–8. doi: 10.1111/epi.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin P, Xu H, Laursen TM, Vestergaard M, Mortensen PB. Risk for schizophrenia and schizophrenia-like psychosis among patients with epilepsy: population based cohort study. BMJ. 2005;331:23. doi: 10.1136/bmj.38488.462037.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baroni G, Piccinini V, Martins WA, de Paola L, Paglioli E, Margis R, Palmini A. Variables associated with co-existing epileptic and psychogenic nonepileptic seizures: a systematic review. Seizure. 2016;37:35–40. doi: 10.1016/j.seizure.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 29.St Louis EK, Cascino GD. Diagnosis of Epilepsy and Related Episodic Disorders. Continuum (Minneap Minn) 2016;22:15–37. doi: 10.1212/CON.0000000000000284. [DOI] [PubMed] [Google Scholar]
- 30.Kerr WT, Janio EA, Braesch CT, Le JM, Hori JM, Patel AB, Gallardo NL, Bauirjan J, D’Ambrosio SR, Chau AM, Hwang ES, Davis EC, Buchard A, Torres-Barba D, Al Banna A, Barritt SE, Cho AY, Engel J, Jr, Cohen MS, Stern JM. Identifying psychogenic seizures through comorbidities and medication history. Epilepsia. 2017 doi: 10.1111/epi.13888. (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giza CC, Kutcher JS, Ashwal S, Barth J, Getchius TS, Gioia GA, Gronseth GS, Guskiewicz K, Mandel S, Manley G, McKeag DB, Thurman DJ, Zafonte R. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250–7. doi: 10.1212/WNL.0b013e31828d57dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller ME, Hui SL, Tierney WM. Validation techniques for logistic regression models. Stat Med. 1991;10:1213–26. doi: 10.1002/sim.4780100805. [DOI] [PubMed] [Google Scholar]
- 33.Rubin DB. Multiple imputation for non-response in surveys. New York: John Wiley & Sons; 1987. [Google Scholar]
- 34.Rubin DB. Multiple imputation after 18+ years (with discussion) JASA. 1996;91:473–489. [Google Scholar]
- 35.Kerr WT, Anderson A, Lau EP, Cho AY, Xia H, Bramen J, Douglas PK, Braun ES, Stern JM, Cohen MS. Automated diagnosis of epilepsy using EEG power spectrum. Epilepsia. 2012;53:e189–92. doi: 10.1111/j.1528-1167.2012.03653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaFrance WC, Jr, Baker GA, Duncan R, Goldstein LH, Reuber M. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach: a report from the International League Against Epilepsy Nonepileptic Seizures Task Force. Epilepsia. 2013;54:2005–18. doi: 10.1111/epi.12356. [DOI] [PubMed] [Google Scholar]
- 37.Seneviratne U, Rajendran D, Brusco M, Phan TG. How good are we at diagnosing seizures based on semiology? Epilepsia. 2012 doi: 10.1111/j.1528-1167.2011.03382.x. [DOI] [PubMed] [Google Scholar]
- 38.Bernasconi N, Natsume J, Bernasconi A. Progression in temporal lobe epilepsy: differential atrophy in mesial temporal structures. Neurology. 2005;65:223–8. doi: 10.1212/01.wnl.0000169066.46912.fa. [DOI] [PubMed] [Google Scholar]
- 39.Pittau F, Bisulli F, Mai R, Fares JE, Vignatelli L, Labate A, Naldi I, Avoni P, Parmeggiani A, Santucci M, Capannelli D, Di Vito L, Gambardella A, Baruzzi A, Tinuper P. Prognostic factors in patients with mesial temporal lobe epilepsy. Epilepsia. 2009;50(Suppl 1):41–4. doi: 10.1111/j.1528-1167.2008.01969.x. [DOI] [PubMed] [Google Scholar]
- 40.Bruns J, Jr, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44(Suppl 10):2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- 41.Ritter AC, Wagner AK, Szaflarski JP, Brooks MM, Zafonte RD, Pugh MJ, Fabio A, Hammond FM, Dreer LE, Bushnik T, Walker WC, Brown AW, Johnson-Greene D, Shea T, Krellman JW, Rosenthal JA. Prognostic models for predicting posttraumatic seizures during acute hospitalization, and at 1 and 2 years following traumatic brain injury. Epilepsia. 2016;57:1503–14. doi: 10.1111/epi.13470. [DOI] [PubMed] [Google Scholar]
- 42.Raymont V, Salazar AM, Lipsky R, Goldman D, Tasick G, Grafman J. Correlates of posttraumatic epilepsy 35 years following combat brain injury. Neurology. 2010;75:224–9. doi: 10.1212/WNL.0b013e3181e8e6d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norris FH. Epidemiology of trauma: frequency and impact of different potentially traumatic events on different demographic groups. J Consult Clin Psychol. 1992;60:409–18. doi: 10.1037//0022-006x.60.3.409. [DOI] [PubMed] [Google Scholar]
- 44.Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry. 1991;48:216–22. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- 45.Scioli-Salter ER, Johnides BD, Mitchell KS, Smith BN, Resick PA, Rasmusson AM. Depression and dissociation as predictors of physical health symptoms among female rape survivors with posttraumatic stress disorder. Psychol Trauma. 2016;8:585–91. doi: 10.1037/tra0000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Badour CL, Feldner MT, Babson KA, Blumenthal H, Dutton CE. Disgust, mental contamination, and posttraumatic stress: unique relations following sexual versus non-sexual assault. J Anxiety Disord. 2013;27:155–62. doi: 10.1016/j.janxdis.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alper K, Devinsky O, Perrine K, Vazquez B, Luciano D. Nonepileptic seizures and childhood sexual and physical abuse. Neurology. 1993;43:1950–3. doi: 10.1212/wnl.43.10.1950. [DOI] [PubMed] [Google Scholar]
- 48.Diseth TH. Dissociation in children and adolescents as reaction to trauma–an overview of conceptual issues and neurobiological factors. Nord J Psychiatry. 2005;59:79–91. doi: 10.1080/08039480510022963. [DOI] [PubMed] [Google Scholar]
- 49.Isaac M, Chand PK. Dissociative and conversion disorders: defining boundaries. Curr Opin Psychiatry. 2006;19:61–6. doi: 10.1097/01.yco.0000194811.83720.69. [DOI] [PubMed] [Google Scholar]
- 50.Frey LC. Epidemiology of posttraumatic epilepsy: a critical review. Epilepsia. 2003;44(Suppl 10):11–7. doi: 10.1046/j.1528-1157.44.s10.4.x. [DOI] [PubMed] [Google Scholar]
- 51.Barry E, Krumholz A, Bergey GK, Chatha H, Alemayehu S, Grattan L. Nonepileptic posttraumatic seizures. Epilepsia. 1998;39:427–31. doi: 10.1111/j.1528-1157.1998.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 52.Taylor B, Hagel Campbell E, Nugent S, Bidelspach D, Kehle-Forbes S, Scholten J, Stroupe K, Sayer N. Three Year Trends in VHA Utilization and Costs Following Traumatic Brain Injury Screening among Veterans with Mild Traumatic Brain Injury. J Neurotrauma. 2017 doi: 10.1089/neu.2016.4910. [DOI] [PubMed] [Google Scholar]
- 53.McInnes K, Friesen CL, MacKenzie DE, Westwood DA, Boe SG. Mild Traumatic Brain Injury (mTBI) and chronic cognitive impairment: A scoping review. PLoS One. 2017;12:e0174847. doi: 10.1371/journal.pone.0174847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robles L, Chiang S, Haneef Z. Review-of-systems questionnaire as a predictive tool for psychogenic nonepileptic seizures. Epilepsy Behav. 2015 doi: 10.1016/j.yebeh.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asadi-Pooya AA, Rabiei AH, Tinker J, Tracy J. Review of systems questionnaire helps differentiate psychogenic nonepileptic seizures from epilepsy. J Clin Neurosci. 2016 doi: 10.1016/j.jocn.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 56.Dixit R, Popeschu A, Bagic A, Ghearing G, Henrdrickson R. Medical comorbidities in patients with psychogenic nonepileptic spells (PNES) referred for video-EEG monitoring. Epilepsy & Behavior. 2013;28:137–140. doi: 10.1016/j.yebeh.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 57.Gazzola DM, Carlson C, Rugino A, Hirsch S, Starner K, Devinsky O. Psychogenic nonepileptic seizures and chronic pain: a retrospective case-controlled study. Epilepsy Behav. 2012;25:662–5. doi: 10.1016/j.yebeh.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 58.LaFrance WC, Jr, Devinsky O. The treatment of nonepileptic seizures: historical perspectives and future directions. Epilepsia. 2004;45(Suppl 2):15–21. doi: 10.1111/j.0013-9580.2004.452002.x. [DOI] [PubMed] [Google Scholar]
- 59.LaFrance WC, Jr, Reuber M, Goldstein LH. Management of psychogenic nonepileptic seizures. Epilepsia. 2013;54(Suppl 1):53–67. doi: 10.1111/epi.12106. [DOI] [PubMed] [Google Scholar]
- 60.Nagamitsu S, Yamashita Y, Ohya T, Shibuya I, Komatsu H, Matsuoka M, Ohzono S, Matsuishi T. Pitfalls in diagnosing psychogenic nonepileptic seizures in a sexually abused child. Brain Dev. 2011;33:601–3. doi: 10.1016/j.braindev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 61.Patel H, Scott E, Dunn D, Garg B. Nonepileptic seizures in children. Epilepsia. 2007;48:2086–92. doi: 10.1111/j.1528-1167.2007.01200.x. [DOI] [PubMed] [Google Scholar]
- 62.Szabo L, Siegler Z, Zubek L, Liptai Z, Korhegyi I, Bansagi B, Fogarasi A. A detailed semiologic analysis of childhood psychogenic nonepileptic seizures. Epilepsia. 2012;53:565–70. doi: 10.1111/j.1528-1167.2012.03404.x. [DOI] [PubMed] [Google Scholar]
- 63.Lancman ME, Asconape JJ, Graves S, Gibson PA. Psychogenic seizures in children: long-term analysis of 43 cases. J Child Neurol. 1994;9:404–7. doi: 10.1177/088307389400900413. [DOI] [PubMed] [Google Scholar]
- 64.Kerr WT, Nguyen ST, Cho AY, Lau EP, Silverman DH, Douglas PK, Reddy NM, Anderson A, Bramen J, Salamon N, Stern JM, Cohen MS. Computer aided diagnosis and localization of lateralized temporal lobe epilepsy using interictal FDG-PET. Front Neurol. 2013 doi: 10.3389/fneur.2013.00031. (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bowman ES, Markand ON. Psychodynamics and psychiatric diagnoses of pseudoseizure subjects. Am J Psychiatry. 1996;153:57–63. doi: 10.1176/ajp.153.1.57. [DOI] [PubMed] [Google Scholar]
- 66.Cragar DE, Berry DT, Fakhoury TA, Cibula JE, Schmitt FA. A review of diagnostic techniques in the differential diagnosis of epileptic and nonepileptic seizures. Neuropsychol Rev. 2002;12:31–64. doi: 10.1023/a:1015491123070. [DOI] [PubMed] [Google Scholar]
- 67.Hubsch C, Baumann C, Maillard L. Psychogenic non-epileptic seizures: clinical classification based on the video-EEG analysis of 145 seizures. J Neurol. 2010;257:S176–S176. doi: 10.1136/jnnp.2010.235424. [DOI] [PubMed] [Google Scholar]
- 68.Oto M, Conway P, McGonigal A, Russell AJ, Duncan R. Gender differences in psychogenic non-epileptic seizures. Seizure. 2005;14:33–9. doi: 10.1016/j.seizure.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 69.Gates JR, Ramani V, Whalen S, Loewenson R. Ictal Characteristics of Pseudoseizures. Arch Neurol. 1985;42:1183–1187. doi: 10.1001/archneur.1985.04060110065017. [DOI] [PubMed] [Google Scholar]
- 70.Meierkord H, Will B, Fish D, Shorvon S. The clinical features and prognosis of pseudoseizures diagnosed using video-EEG telemetry. Neurology. 1991;41:1643–6. doi: 10.1212/wnl.41.10.1643. [DOI] [PubMed] [Google Scholar]
- 71.Krumholz A, Niedermeyer E. Psychogenic seizures: a clinical study with follow-up data. Neurology. 1983;33:498–502. doi: 10.1212/wnl.33.4.498. [DOI] [PubMed] [Google Scholar]
- 72.Duncan R. The withdrawal of antiepileptic drugs in patients with non-epileptic seizures: safety considerations. Expert Opin Drug Saf. 2006;5:609–13. doi: 10.1517/14740338.5.5.609. [DOI] [PubMed] [Google Scholar]
- 73.Seneviratne U, Reutens D, D’Souza W. Stereotypy of psychogenic nonepileptic seizures: insights from video-EEG monitoring. Epilepsia. 2010;51:1159–68. doi: 10.1111/j.1528-1167.2010.02560.x. [DOI] [PubMed] [Google Scholar]
- 74.Reuber M, Fernandez G, Bauer J, Helmstaedter C, Elger CE. Diagnostic delay in psychogenic nonepileptic seizures. Neurology. 2002;58:493–5. doi: 10.1212/wnl.58.3.493. [DOI] [PubMed] [Google Scholar]
- 75.Haneef Z, Stern J, Dewar S, Engel J., Jr Referral pattern for epilepsy surgery after evidence-based recommendations: a retrospective study. Neurology. 2010;75:699–704. doi: 10.1212/WNL.0b013e3181eee457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kellinghaus C, Loddenkemper T, Dinner DS, Lachhwani D, Luders HO. Non-epileptic seizures of the elderly. J Neurol. 2004;251:704–9. doi: 10.1007/s00415-004-0406-3. [DOI] [PubMed] [Google Scholar]
- 77.Duncan R, Oto M, Martin E, Pelosi A. Late onset psychogenic nonepileptic attacks. Neurology. 2006;66:1644–7. doi: 10.1212/01.wnl.0000223320.94812.7a. [DOI] [PubMed] [Google Scholar]
- 78.Behrouz R, Heriaud L, Benbadis SR. Late-onset psychogenic nonepileptic seizures. Epilepsy Behav. 2006;8:649–50. doi: 10.1016/j.yebeh.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 79.Vincentiis S, Valente KD, Thome-Souza S, Kuczinsky E, Fiore LA, Negrao N. Risk factors for psychogenic nonepileptic seizures in children and adolescents with epilepsy. Epilepsy Behav. 2006;8:294–8. doi: 10.1016/j.yebeh.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 80.Tojek TM, Lumley M, Barkley G, Mahr G, Thomas A. Stress and other psychosocial characteristics of patients with psychogenic nonepileptic seizures. Psychosomatics. 2000;41:221–6. doi: 10.1176/appi.psy.41.3.221. [DOI] [PubMed] [Google Scholar]
- 81.Wyllie E, Glazer JP, Benbadis S, Kotagal P, Wolgamuth B. Psychiatric features of children and adolescents with pseudoseizures. Arch Pediatr Adolesc Med. 1999;153:244–8. doi: 10.1001/archpedi.153.3.244. [DOI] [PubMed] [Google Scholar]
- 82.Valente KD, Alessi R, Vincentiis S, Santos BD, Rzezak P. Risk Factors for Diagnostic Delay in Psychogenic Nonepileptic Seizures Among Children and Adolescents. Pediatr Neurol. 2017;67:71–77. doi: 10.1016/j.pediatrneurol.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 83.Alessi R, Vincentiis S, Rzezak P, Valente KD. Semiology of psychogenic nonepileptic seizures: age-related differences. Epilepsy Behav. 2013;27:292–5. doi: 10.1016/j.yebeh.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 84.Scott DF. Recognition and diagnostic aspects of nonepileptic seizures. In: Riley TL, Roy A, editors. Pseudoseizures. Baltimore: Williams & Wilkins Co; 1982. pp. 21–24. [Google Scholar]
- 85.Lesser RP. Psychogenic seizures. Neurology. 1996;46:1499–507. doi: 10.1212/wnl.46.6.1499. [DOI] [PubMed] [Google Scholar]
- 86.Gumnit RJ, Walczak TS, National Association of Epilepsy C Guidelines for essential services, personnel, and facilities in specialized epilepsy centers in the United States. Epilepsia. 2001;42:804–14. doi: 10.1046/j.1528-1157.2001.08701.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


