Abstract
Introduction
Radiotherapy outcomes are influenced by treatment delivery geometric accuracy and organ‐at‐risk dose. The location of abdominal structures such as the liver, kidneys and tumour volumes can be strongly influenced by respiratory motion. This increases geometric uncertainty and dose to organs‐at‐risk. One common method of minimising respiratory motion is abdominal compression (AC).
Methods
Fifteen patients being treated for radiotherapy to upper abdominal tumours were analysed. Each patient underwent 2 four‐dimensional computerised tomography (4D‐CT) scans, one with and one without AC with a pneumatic compression belt. Liver and kidney positions were measured on the 4DCT scans at the peak inspiratory and expiratory respiratory phases. The patient received radiation therapy treatment planned on the CT data set with the technique (compression or no compression) that provided the least respiratory motion.
Results
There was no statistically significant motion difference over the sample population with AC for the kidneys or liver. Of the 14 evaluable patients, 4, 6 and 6 saw reduction in superior‐inferior motion for left kidney, right kidney and liver respectively. The remainder either had negligible (<2 mm) or increase in motion with AC. For anterior‐posterior motion, 2, 2 and 1 saw a reduction for left‐kidney, right‐kidney and liver respectively.
Conclusion
AC through the use of a pneumatic compression belt was found to result in inconsistent reduction in kidney and liver respiratory motion. It is recommended that the effect of AC is evaluated on a per‐patient basis.
Keywords: abdominal compression, liver, SBRT, respiratory motion
Introduction
The outcome of radiotherapy for cancer is influenced by many factors among which include both the prescription dose and the accuracy of treatment delivery. To maximise the prescription dose and minimise the dose to critical structures, it is ideal to have the smallest planning target volume (PTV) achievable. Respiratory motion can lead to a larger PTV and consequential reduction in prescription dose to meet critical structure constraints. Respiratory motion can vary between simulation and treatment, and from fraction to fraction, presenting a risk of geographical miss and/or under‐dosing tumour or over‐dosing critical structures, particularly in the era of stereotactic body radiotherapy (SBRT) and image‐guidance.1, 2, 3 A number of techniques have been developed to improve accuracy and they vary according to the anatomical area being treated. The location of structures in the abdomen such as the liver, kidneys, pancreas and tumour volumes can be strongly influenced by respiratory motion. One commonly used method of minimising respiratory motion during radiotherapy is abdominal compression.4, 5, 6
Various abdominal compression devices, both in‐house and commercially available, have been used to reduce organ motion.1, 2, 3, 4, 5, 6 These devices include indexed compression plates, compressed using a screw, dual vacuum compression and belts with inflatable air bladders. In one study, an in‐house developed compression belt with an air bladder was used.7 Tolerable pressure was applied to the abdomen restricting motion of the internal organs. The effectiveness of compression was measured in several ways, but all researchers found a significant reduction in movement of either the tumour or the liver with abdominal compression. Motion analysis using fiducial markers implanted near the tumour concluded that the use of these compression devices led to a significant reduction in motion in the superior inferior (SI) plane.4
This study investigated the use of a commercially available QFix StradivariusTM compression belt, developing an understanding of whether it would significantly limit the respiration‐related motion of internal organs in patients being treated with stereotactic body radiation therapy (SBRT) for upper gastro‐intestinal (GI) tumours.
Method
This retrospective study was approved by the Northern Sydney Local Health District Human Research Ethics Committee as a low risk, single centre project to be conducted at Northern Sydney Cancer Centre.
Patient sample
Our abdominal compression protocol described below was applied to upper gastrointestinal patients receiving SBRT to tumours subject to respiratory motion. All patients treated under this protocol between September 2015 and February 2016 were selected for this analysis. Briefly, the simulation protocol involved performing two sequential 4DCT scans; the first with and the second without abdominal compression. The motion of the liver dome at the location closest to the tumour in both scans was assessed, the scenario resulting in the lowest motion was selected for treatment.
Simulation
Patients were simulated using a Philips Big Bore 16 Slice CT (Philips Healthcare, Cleveland, OH, USA) and the Philips bellows was used to acquire the patient's respiratory cycle. The bellows device is a thin air tube which expands and contracts with breathing motion; the change in air pressure in the tube is a surrogate for breathing phase. Patients were positioned according to the departmental standard for SBRT simulations; supine, in a full body Elekta BodyFix (Elekta, Crawley, UK) bag with arms above their head, neutral headrest and a low knee block. The patient's respiratory cycle was observed prior to placing the bellows. The bellows were positioned on the upper abdomen in the area which had the highest observable motion during respiration. The bellows was tightened until observable motion could be seen on the device. The QFix StradivariusTM compression belt (QFix, Avondale, USA) was positioned mid abdomen inferior to the bellows.
Two factors were used to minimise motion with the compression belt: tightness and the pressure of the air bladder. The compression band strap was tightened to fit securely but comfortably around the patient's abdomen using the Velcro fastener. The left and right scale position of the compression band indicated how tightly the band had been fastened. The superior and inferior aspects for the compression band were marked on the BodyFix for reproducibility of compression band location. The air bladder was inflated to the patient's maximum tolerance with the maximum allowable compression of 100 mmHg (maximum pressure recommended by the manufacturer due to structural integrity of the air bladder). The compression band scales and the pressure of the compression band with the location of the tattoos were all documented for reproducibility at treatment.
The scan range was from the apex of the lungs to 20 mm below the inferior level of the liver with a CT slice thickness of 2 mm. Where indicated for contouring purposes, intravenous contrast was administered prior to the first 4DCT scan (with compression) and the appropriate delay before acquisition was applied before scanning. The second 4DCT scan was completed immediately following the first scan, without the compression belt and without contrast. The intensity projection images (minimum, maximum and average) were created prior to exporting the 4DCT scans to the Eclipse treatment planning system (V. 13.6, Varian Medical Systems, Palo Alto, USA).
Evaluation of abdominal compression
From the 4DCTs, the magnitude of compression was assessed via measuring the circumference of the patient at the mid‐point of the belt superiorly‐inferiorly, the indexing of the band (the circumference of the band) and pressure of the bladder after inflation.
Evaluation of organ motion
Prior to analysis, all 4DCTs were reviewed for the presence of artefacts that may affect measurement of motion. The change in amplitude of organs between the peak inspiration and peak expiration of each patients’ breathing cycle was measured. The kidneys and liver were easily delineated on a CT scan and represent varying proximity to the diaphragm, so all measurements were performed on these structures. Both the left and right kidneys were contoured in exhale phase and rigidly copied to match the inhale phase. The difference in the centre of mass between these two phases was measured in both the compression and non‐compression 4DCT scans. The liver was rigidly registered (translation only) between the maximum inhale and exhale scans of the 4DCT. Matching was prioritised on the liver dome. The translations in each direction were recorded. This was repeated for both compression and non‐compression 4DCT scans. The Wilcoxon signed‐rank test was used to determine statistical significance of the difference in organ excursion between the two 4DCTs (threshold P < 0.05).
Results
A total of 15 participants were included in the study (10 male and 5 female). Thirteen participants were treated with SBRT to the liver and two with conventional radiotherapy to the pancreas. All participants had two 4DCTs; one patient's non‐compression 4DCT was unable to be used due to severe artefacts. Therefore, data from twenty nine 4DCTs informed the findings of this study. The participants ranged in age from 46 to 89 with a mean age of 67.9 (SD: 11.3).
All patients used the medium size belt with the exception of one patient (large belt). For patients with the medium belt there was a directly linear relationship between the band and patient circumference (R 2 = 0.89), indicating a consistent level of tightening of the band around the patients prior to inflation. A large range in compression tolerability was observed, with bladder pressure ranging from 10 to 84 mmHg (median 50 mmHg).
Kidneys
Figure 1 shows the motion of the kidneys in the superior‐inferior and anterior‐posterior directions. Motion in the left‐right direction was less than 1 mm for all cases so is not presented. A motion change of <2 mm was considered negligible. Table 1 shows the number of patients in which a reduction, increase or negligible change in motion was observed for the kidneys. For both kidneys, the majority of patients had a negligible change in motion. Table 2 shows the kidney motion amplitude with and without compression. There was no statistically significant difference in average amplitude of motion for either kidney with abdominal compression. Dominant motion direction for the kidneys was in the superior‐inferior direction with average amplitude of 5–7 mm. There was no relationship between compression pressure and change in amplitude with compression, as shown in Figure 2A and B.
Figure 1.
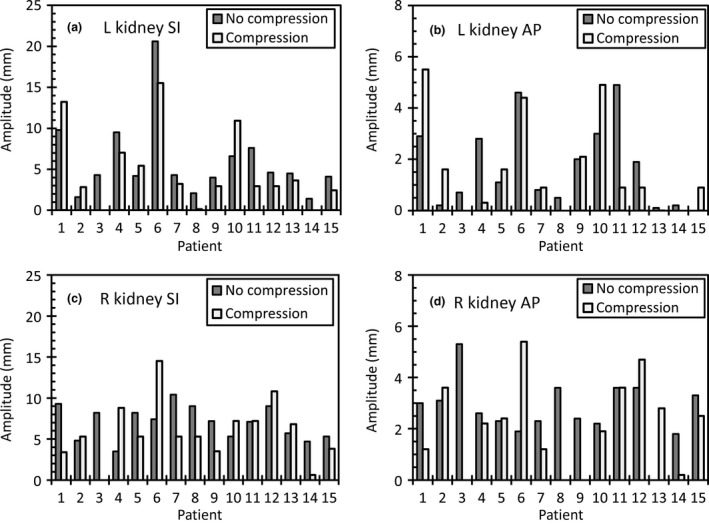
Peak‐to‐peak amplitude of the left kidney in the (A) superior‐inferior and (B) anterior‐posterior (AP) directions and the right kidney in the (C) superior‐inferior (SI) and (D) anterior‐posterior (AP) directions.
Table 1.
Number of patients (out of total 14) that had reductions, increases and negligible changes in motion
| Superior‐inferior | Anterior‐posterior | |||||
|---|---|---|---|---|---|---|
| Reduction | Increase | Negligible | Reduction | Increase | Negligible | |
| Left kidney | 4 | 2 | 8 | 2 | 1 | 11 |
| Right kidney | 6 | 2 | 6 | 2 | 2 | 10 |
| Liver | 6 | 1 | 7 | 1 | 3 | 10 |
Negligible was defined as <2 mm change.
Table 2.
Amplitude of motion (average ± standard deviation) in each direction for the kidneys and liver dome
| No compression | Compression | ||||
|---|---|---|---|---|---|
| LR | AP | SI | LR | AP | SI |
| Left kidney | |||||
| 0.8 ± 0.3 | 1.8 ± 0.8 | 6.1 ± 2.5 | 0.8 ± 0.5 | 1.7 ± 0.9 | 5.2 ± 2.4 |
| Right kidney | |||||
| 0.9 ± 0.3 | 2.5 ± 0.6 | 6.9 ± 1.0 | 0.7 ± 0.5 | 2.3 ± 0.8 | 6.3 ± 1.7 |
| Liver | |||||
| 0.7 ± 1.1 | 4.7 ± 3.8 | 8.7 ± 3.0 | 0.7 ± 1.0 | 5.4 ± 4.2 | 8.0 ± 3.8 |
All measurements are in mm. The comparison does not include patient 3. LR, left‐right; AP, anterior‐posterior; SI, superior‐inferior.
Figure 2.
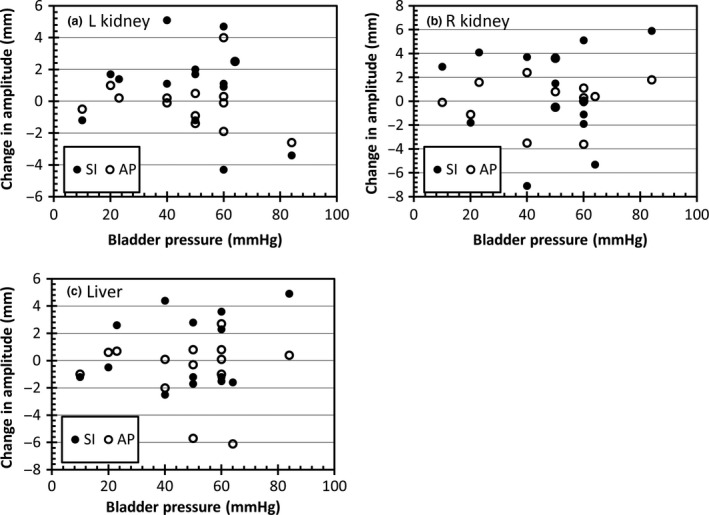
Change in amplitude with compression as a function of the compression pressure for (A) left kidney (B) right kidney and (C) liver dome. Positive values are reduction in motion with compression. LR, left‐right; AP, anterior‐posterior; SI, superior‐inferior.
Liver
The motion in the left‐right direction was typically less than 2 mm so was not included in the analysis. Table 2 and Figure 3 show the liver motion with and without compression. Over the sample there was no statistically significant difference in liver motion with compression. Similar to the kidneys, in the majority of patients there was a negligible difference in the motion in either direction with abdominal compression, as shown in Table 1 and Figure 3. Figure 2C shows no relationship between compression pressure and change in amplitude.
Figure 3.
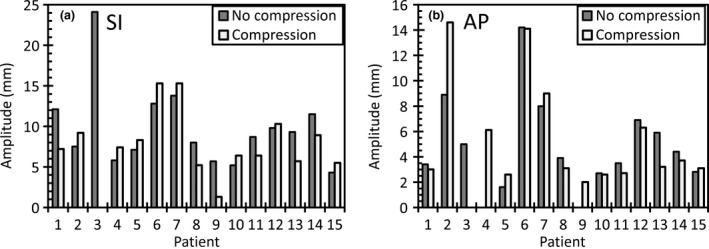
Superior‐inferior peak to peak amplitude of the liver dome for (A) superior‐inferior (S) and (B) anterior‐posterior (AP) directions.
Discussion
SBRT delivers highly conformal doses of large biological effect to both tumour and normal tissue which necessitates tight margins and treatment of as small a volume as possible. When treating with SBRT there is an increased need for more accurate and reproducible immobilisation. The role of abdominal compression is to reduce tumour excursion from respiration thus reducing the margins required to account for respiration, leading to a reduction in treated volume.
A consistent reduction in organ motion with abdominal compression has been reported previously with other types of compression devices. Wunderink et al.5 and Heinzerling et al.4 found significant reduction in tumour motion when using a compression plate when assessing specific organs between two 4DCT scans. These study methods however included patients with tumour motion greater than 10 mm and applied compression until motion was reduced below 10 mm. Therefore, only data on patients that saw a reduction in motion were reported. Lovelock et al7 found a significant reduction in the longitudinal motion when assessing fiducial markers on fluoroscopy using an in‐house built pneumatic compression belt; compression reduced the fiducial marker excursion to less than 5 mm when compared to no compression. Finally, Mampuya et al.8 found a reduction in amplitude of tumour motion, but also found an increase in the inter‐fraction variation in tumour position. Their study used a plate system that incorporated a graduated pressure screw. In contrast, Eccles et al.6 observed limited benefit of compression plate over a population of 60 patients, as measured using cine MRI; 47% of patients saw a reduction in motion of ≥3 mm and 8% saw an increase of ≥3 mm in at least one plane. In this study, left and right kidney SI motion increased by ≥3 mm for two patients, and right kidney and liver AP motion increased by ≥3 mm for two patients for each organ.
In this study, the effect of the pneumatic pressure belt was inconsistent; no statistically significant reduction in liver or kidney motion was observed. Furthermore, the motion reduction was not comparable between the left and the right kidneys. For the left kidney, four patients showed a benefit (patients 4, 6, 8 and 11) and for the right kidney, six patients showed a benefit (patient 1, 5, 7, 8, 9 and 14), with only one patient (#8) having motion reduced for both kidneys. Only six patients had a reduction in liver motion greater than 2 mm, with 5 mm the largest reduction in superior‐inferior liver amplitude. Moreover, compression increased the anterior‐posterior motion in two patients by 6 mm.
Comparison with previous studies is difficult due to a number of reasons. One of the major reasons is the difference in equipment used within each study. In our study we used a commercially available compression belt which allowed for circumferential compression utilising a tightening band on either side of the patient's body in combination with an air bladder to increase the rate of compression anteriorly. Other studies have used a combination of in‐house built compression bands, in‐house built compression plate systems, or commercially available plate compression systems. Another reason is the differences in the placement of the compression devices on a patient's abdomen. Hu et al measured the rate of compression at multiple levels within the abdomen to find the position that best provides a reduction in motion of the liver.9 This study found that the optimal placement of a compression device is in the cephalic area between sub‐xiphoid and umbilicus, with a reduction in the benefit of compression as the compression location moved inferiorly. The placement of the device in our study was between xiphoid and umbilicus; however the effect of the compression was lower in our study.
A major limitation of the compression levels achieved in our study was due to patient comfort. Several patients expressed high levels of discomfort with the compression procedure, although no data was collected on this. Standard department protocol was to tighten the band and increase the pressure in the bladder to as high as tolerable for the patient, but keeping below 100 mmHg. As patients will have varying thresholds of discomfort as well as other comorbidities, that is surgery scars or recurring abdominal pain, we had a large inconsistency of the achievable pressure. This in turn could have affected the compression hence reduction in respiratory motion. We only had a small number of patients with a large pressure, therefore there is not enough data to show whether the compression rate at high pressure (50–100 mmHg) was more effective. However this reflects the clinical scenario in which abdominal compression is used and patient comfort is important to consider. It would not be informative to use a compression pressure specific for the study that was not directly translatable to the real life scenario.
It is important to elicit the degree of benefit of an intervention before implementing it into standard practice. As can be seen from the results, there are patients who experienced no improvement or an increase in organ motion with the use of the device. Overall, the benefits of using abdominal compression in this study did not appear to outweigh the potential disadvantages including the compromise to patient comfort or logistics including the time spent in set up and the potential errors in reproducibility and did not present a strong argument to maintain it as standard of care. However, a need for motion minimisation is acknowledged and alternative methods are currently being investigated.
Conclusion
Abdominal compression through the use of a pneumatic compression belt was found to result in inconsistent reduction in kidney and liver respiratory motion in a small cohort of 15 patients. There was no correlation between compression levels attained and change in liver and kidney motion. It is recommended that if a pneumatic compression belt is used, the effect of the belt on a per‐patient basis is quantified before using for treatment.
Conflict of Interest
The authors declare no conflict of interest.
J Med Radiat Sci 65 (2018) 48–54
References
- 1. Keall PJ, Mageras GS, Balter JM, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med Phys 2006; 33: 3874–900. [DOI] [PubMed] [Google Scholar]
- 2. Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med Phys 2010; 37: 4078–101. [DOI] [PubMed] [Google Scholar]
- 3. Case RB, Moseley DJ, Sonke JJ, et al. Interfraction and intrafraction changes in amplitude of breathing motion in stereotactic liver radiotherapy. Int J Radiat Oncol Biol Phys 2010; 77: 918–25. [DOI] [PubMed] [Google Scholar]
- 4. Heinzerling JH, Anderson JF, Papiez L, et al. Four‐dimensional computed tomography scan analysis of tumor and organ motion at varying levels of abdominal compression during stereotactic treatment of lung and liver. Int J Radiat Oncol Biol Phys 2008; 70: 1571–8. [DOI] [PubMed] [Google Scholar]
- 5. Wunderink W, Mendez Romero A, de Kruijf W, de Boer H, Levendag P, Heijmen B. Reduction of respiratory liver tumor motion by abdominal compression in stereotactic body frame, analyzed by tracking fiducial markers implanted in liver. Int J Radiat Oncol Biol Phys 2008; 71: 907–15. [DOI] [PubMed] [Google Scholar]
- 6. Eccles CL, Patel R, Simeonov AK, Lockwood G, Haider M, Dawson LA. Comparison of liver tumor motion with and without abdominal compression using cine‐magnetic resonance imaging. Int J Radiat Oncol Biol Phys 2011; 79: 602–8. [DOI] [PubMed] [Google Scholar]
- 7. Lovelock DM, Zatcky J, Goodman K, Yamada Y. The effectiveness of a pneumatic compression belt in reducing respiratory motion of abdominal tumors in patients undergoing stereotactic body radiotherapy. Technol Cancer Res Treat 2014; 13: 259–67. [DOI] [PubMed] [Google Scholar]
- 8. Mampuya WA, Nakamura M, Matsuo Y, et al. Interfraction variation in lung tumor position with abdominal compression during stereotactic body radiotherapy. Med Phys 2013; 40: 091718. [DOI] [PubMed] [Google Scholar]
- 9. Hu Y, Zhou YK, Chen YX, Shi SM, Zeng ZC. 4D‐CT scans reveal reduced magnitude of respiratory liver motion achieved by different abdominal compression plate positions in patients with intrahepatic tumors undergoing helical tomotherapy. Med Phys 2016; 43: 4335. [DOI] [PubMed] [Google Scholar]


