Abstract
A series of pyrano[3,2-c]quinoline based structural analogues was synthesized using one-pot multicomponent condensation between 2,4-dihydroxy-1-methylquinoline, malononitrile, and diverse un(substituted) aromatic aldehydes. The synthesized compounds were evaluated for their anti-inflammatory and cytotoxicity activity. Initially, all the compounds were evaluated for the percent inhibition of cytokine release, and cytotoxicity activity and 50% inhibitory concentrations (IC50) were also determined. Based on the primary results, it was further studied for their ability to inhibit TNF-α production in the human peripheral blood mononuclear cells (hPBMC) assay. The screening results revealed that compound 4c, 4f, 4i, and 4j were found most active candidates of the series against both anti-inflammatory and anticancer activity. The structure–activity relationship is discussed and suggested that 3-substitution on the aryl ring at C4 position of the pyrano[3,2-c]quinolone structural motif seems to be an important position for both TNF-α and IL-6 inhibition and anticancer activity as well. However, structural diversity with electron withdrawing, electron donating, sterically hindered, and heteroaryl substitution sincerely affected both the inflammation and anticancer activities.
Keywords: Pyrano[3,2-c]quinoline; TNF-α; IL-6; anti-inflammatory; anticancer
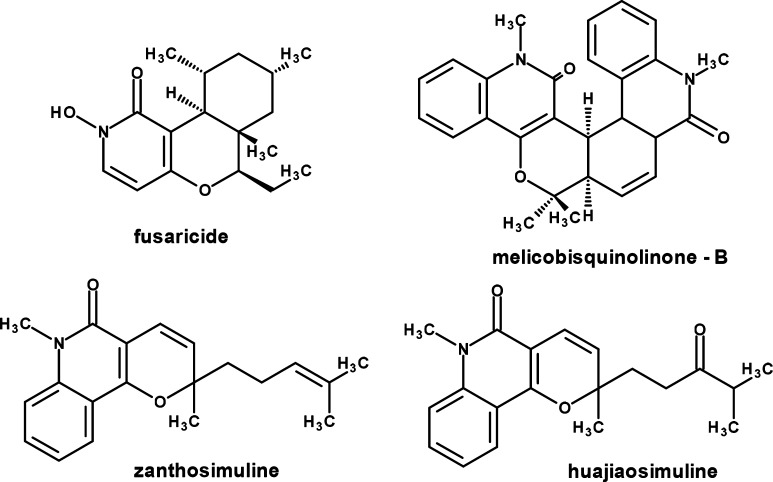
Chronic inflammation is associated with many types of cancers, where most of solid tumors were shown inflammatory indication. Immune cells play an important role in initiation, growth and progression of tumors. A number of these effects are regulated by proinflammatory cytokines like tumor necrosis factor (TNF)-α and interleukin 6 (IL-6). As master regulators of tumor-related inflammation and tumorigenesis, TNF-α and IL-6 become attractive targets for adjuvant treatment in cancer.1 In therapeutic context, quinoline analogues exhibit diversified biological activities depending on the structure type.2 As a part of the number of natural products 2,4-dihydroxy quinolines are important compounds and also exhibit a variety of interesting pharmacological properties.3 Alkaloids with pyrano[3,2-c]pyridone and pyrano[3,2-c]quinolone moieties reveal broad spectrum of biological activities.4 Many of these alkaloids (Figure 1) like fusaricide,5 melicobisquinolinone-B,6 zanthosimuline, and huajiaosimuline7 exhibit cytotoxicity against cancer cells and referred as potential anticancer agents.
Figure 1.
Pyrano[3,2-c]pyridine- and pyrano[3,2-c]quinolone-based anticancer alkaloids.
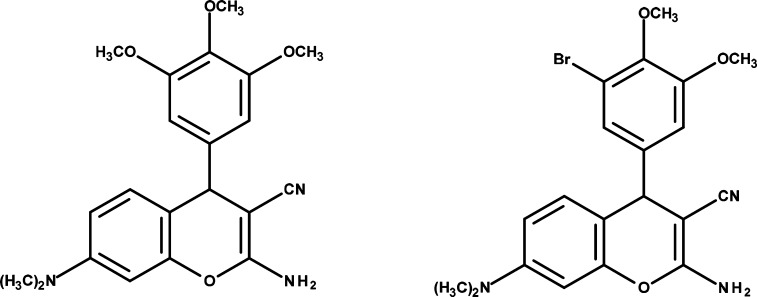
Additionally promising anticancer activity of structurally diverse chromene scaffolds (Figure 2) were described in a number of recent publications and patents.8−18 These agents exhibit high potency against taxoland vinblastine-resistant P-glycoprotein overexpressing cell types and inhibit tubulin polymerization and induce apoptosis in cancer cells.11 Moreover, they interrupt tumor vasculature in various human solid tumor xenografts and currently they are in development as anticancer agents9,10
Figure 2.
Chromenes investigated as anticancer agents.
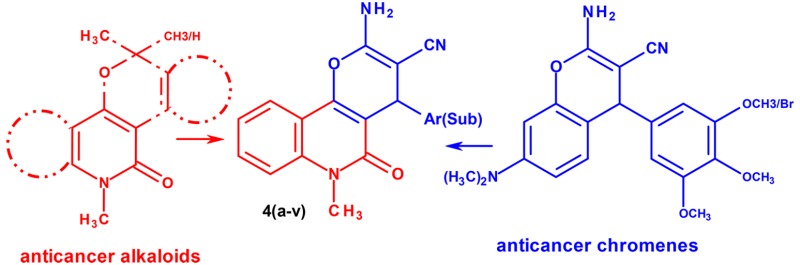
A series of pyrano[3,2-c]pyridine and related fused heterocyclic scaffolds were patented for their promising activity against apoptosis, antiproliferation, and vascular disruption in an animal study19,20 With this background and on the basis of the pre-established correlation between inflammation with cancer cell growth, we have set a hypothesis that whether the fusion of the potent quinoline alkaloids with structurally diverse bioactive chromene scaffolds can lead to the identification of the potent new chemical entities, which can be targeted for both inflammatory and cytotoxicity indications. Consequently, we have designed and synthesized a series of pyrano[3,2-c]quinolone derivatives using multicomponent reaction. All candidates of this heterocyclic privilege were screened for their anti-inflammatory and cytotoxicity activity. The potential candidates of the series shown promising results against TNF-α and IL-6 inhibition and anticancer activity.
Results and Discussion
Chemistry
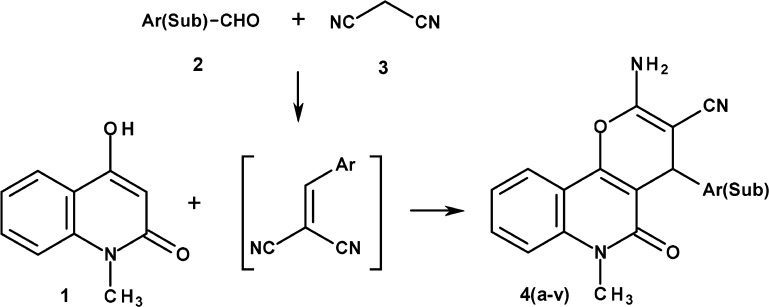
The synthesis of the 2-amino-6-methyl-5-oxo-4-sub(aryl)-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile derivatives 4(a–v) is shown in Scheme 1. 2,4-Dihydroxy-1-methylquinoline (1, Scheme 1) was prepared by thermal cyclization between N-methyl aniline and diethyl malonate as a literature procedure.21 In the next step, a reaction mixture of an equal molar proportion of 2,4-dihydroxy-1-methylquinolin (1, Scheme 1), aromatic aldehyde (2, Scheme 1), malononitrile (3, Scheme 1), and catalytic amount of triethylamine was refluxed in absolute ethyl alcohol to give the final product. The proposed mechanism of reaction involves a base-catalyzed Knoevenagel condensation between un(substituted) aromatic aldehydes (2, Scheme 1) and malononitrile (3, Scheme 1) resulting into cinnamic nitrile derivative in situ. The latter, on reaction with 2,4-dihydroxy-1-methylquinolin (1, Scheme 1), undergoes Michael addition followed by cyclization to end up with the final product.22 The final product is precipitated directly from the reaction mixtures, and further purification is not required. However, the purity of the final product was confirmed by thin layer chromatography.
Scheme 1. Reaction Scheme for Synthesis of 2-Amino-6-methyl-5-oxo-4-sub(aryl)-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile (4a–v).
The yield of the final products depends on the nature of substituents present in hetero(aromatic) aldehyde, varying from 67 to 93% summarized in Table 1. The structures of the pyrano[3,2-c]quinolone were characterized by 1H and 13C NMR, IR, and mass spectral analysis.
Table 1. Anti-inflammatory and Antiproliferative Activity Data.
| anti-inflammatory activity |
%cytotoxicity/antiproliferative activity |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| comp ID | subn. (Ar) | % yield | conc | % TNF- α inh | % IL-6 inh | % tox | A-549 | ACHN | MDA-MB-231 | MIA-PACA-2 |
| 4a | phenyl | 87 | 1 | 0 | 0 | 0 | 34 | 61 | 0 | 45 |
| 10 | 0 | 66 | 13 | 73 | 82 | 61 | 41 | |||
| 4b | 2-Cl phenyl | 85 | 1 | 0 | 0 | 0 | 12 | 48 | 0 | 26 |
| 10 | 12 | 0 | 23 | 81 | 82 | 51 | 69 | |||
| 4c | 3-Cl phenyl | 86 | 1 | 0 | 0 | 3 | 71 | 81 | 53 | 63 |
| 10 | 44 | 74 | 23 | 52 | 68 | 18 | 38 | |||
| 4d | 4-Cl phenyl | 84 | 1 | 0 | 0 | 0 | 19 | 45 | 0 | 21 |
| 10 | 42 | 75 | 37 | 44 | 69 | 12 | 63 | |||
| 4e | 2-NO2 phenyl | 67 | 1 | 0 | 0 | 0 | 21 | 49 | 0 | 35 |
| 10 | 36 | 78 | 6 | 36 | 68 | 18 | 51 | |||
| 4f | 3-NO2 phenyl | 67 | 1 | 11 | 0 | 17 | 74 | 79 | 53 | 65 |
| 10 | 43 | 79 | 30 | 71 | 83 | 65 | 70 | |||
| 4g | 4-NO2 phenyl | 77 | 1 | 0 | 0 | 4 | 0 | 9 | 0 | 15 |
| 10 | 27 | 0 | 28 | 77 | 75 | 50 | 64 | |||
| 4h | 4-F phenyl | 82 | 1 | 0 | 0 | 0 | 0 | 27 | 0 | 16 |
| 10 | 0 | 66 | 6 | 22 | 50 | 24 | 45 | |||
| 4i | 3-Br phenyl | 87 | 1 | 2 | 0 | 8 | 59 | 73 | 45 | 61 |
| 10 | 38 | 82 | 24 | 78 | 75 | 54 | 70 | |||
| 4j | 3-OC6H5 phenyl | 93 | 1 | 18 | 0 | 16 | 70 | 69 | 47 | 66 |
| 10 | 34 | 52 | 28 | 67 | 71 | 51 | 72 | |||
| 4k | 4-SCH3 phenyl | 88 | 1 | 0 | 0 | 9 | 0 | 0 | 0 | 16 |
| 10 | 28 | 37 | 21 | 5 | 2 | 10 | 17 | |||
| 4l | 3-OH phenyl | 74 | 1 | 5 | 0 | 0 | 0 | 0 | 0 | 17 |
| 10 | 4 | 11 | 0 | 5 | 18 | 12 | 29 | |||
| 4m | 4-OH phenyl | 78 | 1 | 0 | 0 | 0 | 13 | 55 | 23 | 55 |
| 10 | 0 | 0 | 23 | 76 | 83 | 77 | 83 | |||
| 4n | 4-N(CH3)2 phenyl | 88 | 1 | 0 | 0 | 0 | 10 | 41 | 20 | 34 |
| 10 | 0 | 0 | 5 | 16 | 39 | 22 | 31 | |||
| 4o | 2-OCH3 phenyl | 91 | 1 | 0 | 0 | 0 | 14 | 37 | 22 | 24 |
| 10 | 0 | 32 | 14 | 16 | 37 | 18 | 21 | |||
| 4p | 4-OCH3 phenyl | 93 | 1 | 0 | 0 | 18 | 73 | 78 | 70 | 80 |
| 10 | 0 | 77 | 3 | 79 | 85 | 81 | 84 | |||
| 4q | 3,4-DiOCH3 phenyl | 93 | 1 | 0 | 0 | 0 | 23 | 38 | 20 | 40 |
| 10 | 0 | 43 | 13 | 24 | 38 | 24 | 50 | |||
| 4r | 9-anthracyl | 90 | 1 | 0 | 0 | 8 | 10 | 25 | 19 | 40 |
| 10 | 0 | 0 | 29 | 58 | 65 | 69 | 66 | |||
| 4s | 3-OC2O5, 4-OH phenyl | 83 | 1 | 0 | 0 | 0 | 0 | 6 | 12 | 27 |
| 10 | 0 | 0 | 67 | 78 | 81 | 88 | 68 | |||
| 4t | 3-indolyl | 82 | 1 | 0 | 0 | 6 | 8 | 27 | 16 | 18 |
| 10 | 0 | 0 | 7 | 69 | 71 | 67 | 77 | |||
| 4u | 3-OCH3 phenyl | 93 | 1 | 0 | 0 | –6 | 70 | 67 | 66 | 76 |
| 10 | 0 | 33 | 23 | 69 | 73 | 75 | 75 | |||
Biology
Percent inhibition of cytokine release and percent cytotoxicity results were summarized in Table 1, whereas 50% inhibitory concentration (IC50) values were summarized in Table 2. Dexomethasone was used as a reference standard for anti-inflammatory assay;23 while flavopiridol and gemcitabine were used as reference standards for cytotoxicity assay.24 The screening results revealed that compound 4c with 3-chloro substituent was found to be the most potent candidate of the series. It revealed 44 and 74% inhibition at 10 μM concentration as well as 0.5 and 0.2 μM IC50 value for TNF-α and IL-6 cytokine inhibition, respectively, and not toxic at IC50 > 10 μM ; moreover, it showed 81 to 53% antiproliferative inhibition at 1 μM concentration with IC50 value between 0.1 to 1.0 μM against all cancer cell lines. 3-Bromo (4i) analogue seems to be equally potent with 32 and 82% inhibition at 10 μM concentration as well as 0.5 and 0.08 μM IC50 value for TNF-α and IL-6 cytokine inhibition, respectively, but toxic at IC50 value 10 μM; whereas it exerted 75 to 54% antiproliferative inhibition at 10 μM concentration with IC50 value between 0.8 to 0.9 μM against all cancer cell lines. 4-Fluoro (4h) analogue seems to be poorly active against both inflammatory and proliferative inhibition. Besides this, insertion of electronegative nitro group at 3-position of aryl ring linked on C4 of pyrano[3,2-c]quinolone skeleton influences potency against both inflammatory cytokines and proliferative inhibition. As a result, compound 4f exerted 43 and 79% inhibition at 10 μM concentration as well as 0.3 and 0.25 μM IC50 value for TNF-α and IL-6 inhibition, respectively, and not toxic at IC50 value >10 μM; while it revealed 83 to 65% cytotoxicity at 10 μM concentration with IC50 value between 1.1 to 6.6 μM against all cancer cell lines. In contrast, electron-donating 3-methoxy analogue was neither active against inflammatory cytokine nor against proliferative inhibition. Beyond this insertion of sterically hindered 3-phenoxy substitution at C4 position does not alter the orientation of bioactive conformer of pyrano[3,2-c]quinolone. Compound 4j showed 34% and 52% inhibition at 10 μM concentration as well as IC50 value of 0.2 μM for both TNF-α and IL-6 inhibition and toxicity at IC50 value 11 μM; whereas it exerts 72 to 51% antiproliferative inhibition at 10 μM concentration along with IC50 value between 1.4 to 5.1 μM against all cancer cell lines. Introduction of hetero aryl 3-indolyl ring (compound 4t) imparts loss of potency for TNF-α inhibition with 11 μM but sustain IL-6 inhibition along with 1.4 μM IC50 value; whereas it showed 77 to 66% cytotoxicity at 10 μM concentration against all cancer cell lines. However, unsubstituted phenyl ring at C4 position of phenyl ring compound 4a exhibited moderate potency with 66% inhibition at 10 μM concentration against IL-6 as well as 4.9 and <0.03 μM IC50 value for TNF-α and IL-6 inhibition, respectively, and not toxic at IC50 value >10 μM along with 82 to 41% cytotoxicity at 10 μM concentration with IC50 value between 1.0 to 2.8 μM against all cancer cell lines. 4-Hydroxy phenyl and 4-methoxy phenyl analogues with toxicity IC50 value of 30 μM seem to be moderately active against TNF-α and IL-6 cytokine inhibition with 10 and 4 μM as well as 12.8 and 1.7 μM IC50 values, respectively; while neither of the candidates exerted significant antiproliferative activity against any of the used cell lines. The rest of the structural diversity along with combination of other substituents of the present series did not exert remarkable activity against both inflammatory cytokine or antiproliferative inhibition. Based on these primary screenings, these compounds were further evaluated for their ability to inhibit TNF-α production in the human peripheral blood mononuclear cells (hPBMC) assay.25 Compounds 4f and 4j (Figure 3) significantly inhibited the production of TNF-α in a concentration-dependent manner with IC50 value of 0.2 μM. Compound 4i inhibited the production of TNF-α, albeit at higher concentrations with IC50 value of 0.5 μM. Compound 4c was the least active of the four with an IC50 value of 1.8 μM; whereas compounds 4a, 4m, 4p, and 4t were found not active in hPBMC assay. Of note, the IC50 values for TNF-a inhibition for all four compounds were far lower than the IC50 values for cytotoxicity.
Table 2. Anti-inflammatory and Anticancer Activity Dataa.
| anti-inflammatory activity (IC50, μM) |
anticancer activity
(IC50, μM) |
|||||||
|---|---|---|---|---|---|---|---|---|
| comp ID | TNF-α | IL-6 | toxicity | ACHN | Panc-1 | HCT-116 | H-460 | Calu-1 |
| 4a | 4.9 | <0.03 | >10 | 2.8 | 1.5 | 1.0 | 1.3 | 1.2 |
| 4c | 0.5 | 0.2 | >10 | 0.1 | 1.0 | 0.3 | 0.3 | 0.5 |
| 4f | 0.3 | 0.25 | >10 | 1.6 | 2.5 | 6.6 | 1.9 | 1.1 |
| 4i | 0.5 | 0.08 | 10 | 0.9 | 0.8 | 0.9 | 0.8 | 0.8 |
| 4j | 0.2 | 0.2 | 11 | 3.4 | 4.7 | 5.1 | 1.4 | 1.8 |
| 4m | 10 | 4 | 30 | 1.8 | 1.9 | 0.6 | 1.2 | 1.1 |
| 4p | 12.8 | 1.7 | 30 | >20 | >20 | >20 | >20 | >20 |
| 4t | 11 | 1.4 | 6.8 | >20 | >20 | >20 | >20 | >20 |
| 7 HF (1 μM) | 44 | 58 | 0 | |||||
| dexamethasone 1 μM | 40 | 55 | 0 | |||||
| flavopiridol 700 nM | 71 | 78 | 71 | 88 | 74 | |||
| gemcitabine 500 nM | 73 | 74 | 73 | 71 | 79 | |||
ACHN, renal cancer cell line; Panc1, pancreas cancer cell line; HCT-116, colon cancer cell line; H-460, nonsmall cell lung carcinoma cell line; Calu-1, lung cancer cell line.
Figure 3.
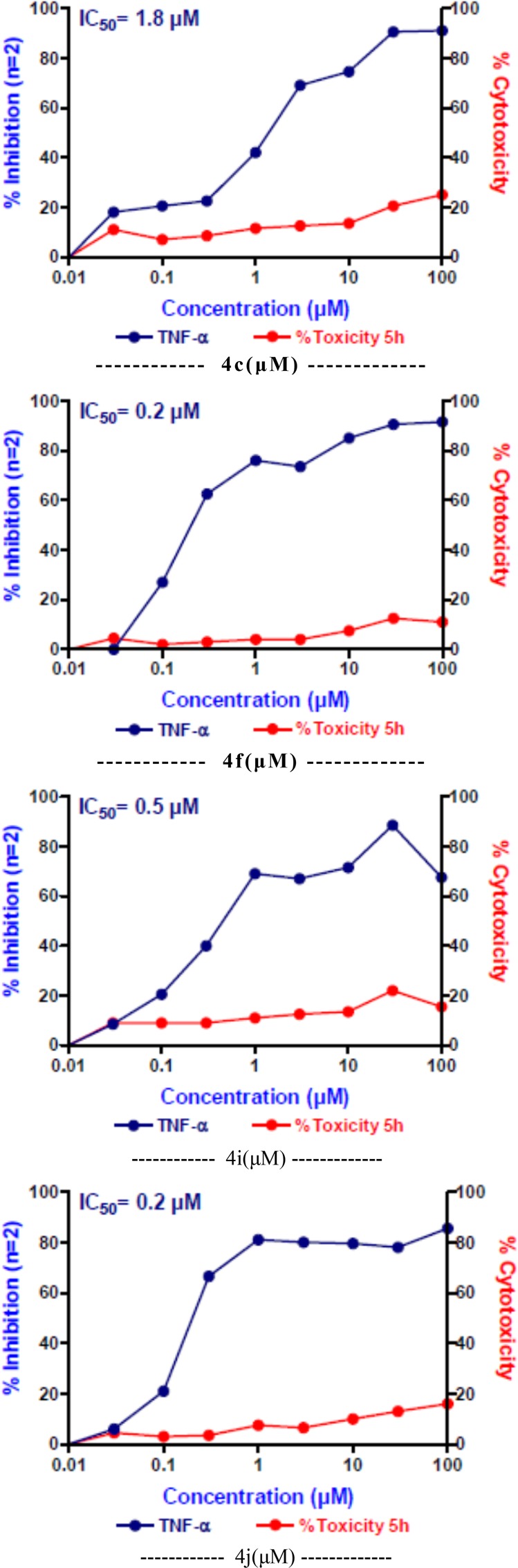
Effect of 4c, 4f, 4i, and 4j on the production of TNF-α by LPS-stimulated cells in hPBMCs in vitro assay.
Conclusion
A new series of the pyrano[3,2-c]quinolonine analogues was synthesized using multicomponent reaction and evaluated for its anti-inflammatory and anticancer activity. The screening results revealed that compounds 4c, 4f, 4i, and 4j were found as most active candidates of the series against both anti-inflammatory and anticancer activity. The structure–activity relationship is discussed and suggested that 3-substitution on the aryl ring at C4 position of the pyrano[3,2-c]quinolone structural motif seems to be an important position for both TNF-α and IL-6 inhibition and anticancer activity as well. However, structural diversity with electron withdrawing, electron donating, sterically hindered, and heteroaryl substitution sincerely affected both the inflammation and anticancer activities. The advance research in the same line may identify a lead molecule, which can be developed for the clinical trial for its therapeutic use.
Acknowledgments
The authors are sincerely thankful to Dr. Somesh Sharma, Dr.(Mrs.) Asha Almeida, Dr. R. D. Gupta, Mrs. Sapna Parikh, Ms. Kalpna Joshi, and Mr. Anagha Damre, Piramal Life Science, Mumbai, for anti-inflammatory and anticancer activities. The authors genuinely acknowledge financial support of the DST (India) under National Facility for Drug Discovery (NFDD), Saurashtra University, Rajkot for spectral and instrumental analysis.
Glossary
ABBREVIATIONS
- TNF
tumor nacrosis factor-α
- IL
interleukin
- IC50
inhibitory concentration at 50% level
- TLC
thin layer chromatography
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.7b00545.
Experimental details of synthesis, anti-inflammatory and cytotoxicity activity assay, and characterization data of all the synthesized compounds (PDF)
Author Contributions
The manuscript was written through contributions of all authors.
The authors declare no competing financial interest.
Supplementary Material
References
- Grivennikov S. I.; Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann. Rheum. Dis. 2011, 70 (Suppl 1), i104. 10.1136/ard.2010.140145. [DOI] [PubMed] [Google Scholar]
- Kulagowski J. J.; Baker R.; Curtis N. R.; Leeson P. D.; Mawer I. M.; Moseley A. M.; et al. 3′-(Arylmethyl)- and 3′-(aryloxy)-3-phenyl-4-hydroxyquinolin-2(1H)-ones: orally active antagonists of the glycine site on the NMDA receptor. J. Med. Chem. 1994, 37 (10), 1402–5. 10.1021/jm00036a002. [DOI] [PubMed] [Google Scholar]
- Sharp J. Antifungal methods employing certain carbostyrils. Patent US3836657A, 1974.
- Magedov IV; Manpadi M.; Ogasawara M. A.; Dhawan A. S.; Rogelj S.; Van Slambrouck S.; et al. Structural simplification of bioactive natural products with multicomponent synthesis. 2. antiproliferative and antitubulin activities of pyrano[3,2-c]pyridones and pyrano[3,2-c]quinolones. J. Med. Chem. 2008, 51 (8), 2561–70. 10.1021/jm701499n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBrien K. D.; Gao Q.; Huang S.; Klohr S. E.; Wang R. R.; Pirnik D. M.; et al. Fusaricide, a new cytotoxic N-hydroxypyridone from Fusarium sp. J. Nat. Prod. 1996, 59 (12), 1151–3. 10.1021/np960521t. [DOI] [PubMed] [Google Scholar]
- Kamperdick C.; Van N. H.; Van Sung T.; Adam G. Bisquinolinone alkaloids from Melicope ptelefolia. Phytochemistry 1999, 50 (1), 177–81. 10.1016/S0031-9422(98)00500-7. [DOI] [Google Scholar]
- Chen I. S.; Wu S. J.; Tsai I. L. Chemical and bioactive constituents from Zanthoxylum simulans. J. Nat. Prod. 1994, 57 (9), 1206–11. 10.1021/np50111a003. [DOI] [PubMed] [Google Scholar]
- Kemnitzer W.; Kasibhatla S.; Jiang S.; Zhang H.; Zhao J.; Jia S.; et al. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. 2. Structure-activity relationships of the 7- and 5-, 6-, 8-positions. Bioorg. Med. Chem. Lett. 2005, 15 (21), 4745–51. 10.1016/j.bmcl.2005.07.066. [DOI] [PubMed] [Google Scholar]
- Gourdeau H.; Leblond L.; Hamelin B.; Desputeau C.; Dong K.; Kianicka I.; et al. Antivascular and antitumor evaluation of 2-amino-4-(3-bromo-4,5-dimethoxy-phenyl)-3-cyano-4H-chromenes, a novel series of anticancer agents. Mol. Cancer Ther. 2004, 3 (11), 1375–84. [PubMed] [Google Scholar]
- Kasibhatla S.; Gourdeau H.; Meerovitch K.; Drewe J.; Reddy S.; Qiu L.; et al. Discovery and mechanism of action of a novel series of apoptosis inducers with potential vascular targeting activity. Mol. Cancer Ther. 2004, 3 (11), 1365–74. [PubMed] [Google Scholar]
- Kemnitzer W.; Drewe J.; Jiang S.; Zhang H.; Wang Y.; Zhao J.; et al. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. 1. Structure-activity relationships of the 4-aryl group. J. Med. Chem. 2004, 47 (25), 6299–310. 10.1021/jm049640t. [DOI] [PubMed] [Google Scholar]
- Kemnitzer W.; Drewe J.; Jiang S.; Zhang H.; Zhao J.; Crogan-Grundy C.; et al. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. 3. Structure-activity relationships of fused rings at the 7,8-positions. J. Med. Chem. 2007, 50 (12), 2858–64. 10.1021/jm070216c. [DOI] [PubMed] [Google Scholar]
- Cai S. X.; Jiang S.; Kemnitzer W. E.; Zhang H.; Attardo G.; Denis R.. Substituted 4-aryl-4H-pyrrolo[2,3-h]chromenes and analogs as activators of caspases and inducers of apoptosis and the use thereof. Patent WO2003097806A2, 2003. Nov 27.
- Cai S. X.; Jiang S.; Attardo G.; Denis R.; Storer R.; Rej R.. 4H-Chromenes, 2H-chromenes substitues, chromans et analogues utilises comme activateurs de caspases et inducteurs de l′apoptose, et utilisation de ces composes. Patent WO2003096982A2, 2003. Nov 27.
- Cai S. X.; Zhang H.; Jiang S.; Storer R.. Substituted 4H-chromenes and analogs as activators of caspases and inducers of apoptosis and the use thereof. Patent WO2002092594A1, 2002. Nov 21.
- Cai S. X.; Xu L.; Storer R.; Attardo G.. 7,8-Fused 4H-chromene and analogs as activators of caspases and inducers of apoptosis and the use thereof. Patent WO2002092083A1, 2002. Nov 21.
- Cai S. X.; Zhang H.; Kemmitzer W. E.; Jiang S.; Drewe J. A.; Storer R.. Substituted coumarins and quinolines as caspases activators. Patent WO2002092076A1, 2002. Nov 21.
- Drewe J. A.; Cai S. X.; Wang Y.. 4H-Chromene substitue et ses analogues en tant qu’activateurs de caspases et qu’inducteurs d’apoptose ainsi que leur utilisation. Patent WO2001034591A2, 2001. May 17.
- Kornienko A.; Magedov I. V.; Rogelj S.. Pyrano [3,2-C] pyridones and related heterocyclic compounds as pharmaceutical agents for treating disorders responsive to apoptosis, antiproliferation or vascular disruption, and the use thereof. Patent US8349864B2, 2013. Jan 8.
- Kornienko A.; Magedov I. V.; Rogelj S.. Pyrano [3,2-C] Pyridones and Related Heterocyclic Compounds as Pharmaceutical Agents for Treating Disorders Responsive to Apoptosis, Antiproliferation or Vascular Disruption, and the Use Thereof. Patent US20090247566A1, 2009. Oct 1.
- Glasnov T. N.; Stadlbauer W.; Kappe C. O. Microwave-Assisted Multistep Synthesis of Functionalized 4-Arylquinolin-2(1H)-ones Using Palladium-Catalyzed Cross-Coupling Chemistry. J. Org. Chem. 2005, 70 (10), 3864–70. 10.1021/jo0502549. [DOI] [PubMed] [Google Scholar]
- Chaniyara R.; Thakrar S.; Kakadiya R.; Marvania B.; Detroja D.; Vekariya N.; et al. DBU-catalyzed Multicomponent Synthesis: Facile Access of 4,5,6,9-Tetrahydro-pyrido[3,2-c]quinolines. J. Heterocycl Chem. 2014, 51 (2), 466–74. 10.1002/jhet.1662. [DOI] [Google Scholar]
- Hwang C.; Gatanaga M.; Granger G. A.; Gatanaga T. Mechanism of release of soluble forms of tumor necrosis factor/lymphotoxin receptors by phorbol myristate acetate-stimulated human THP-1 cells in vitro. J. Immunol. 1993, 151 (10), 5631. [PubMed] [Google Scholar]
- Dengler W. A.; Schulte J.; Berger D. P.; Mertelsmann R.; Fiebig H. H. Development of a propidium iodide fluorescence assay for proliferation and cytotoxicity assays. Anti-Cancer Drugs 1995, 6 (4), 522–32. 10.1097/00001813-199508000-00005. [DOI] [PubMed] [Google Scholar]
- Henry J. R.; Rupert K. C.; Dodd J. H.; Turchi I. J.; Wadsworth S. A.; Cavender D. E.; et al. Potent inhibitors of the MAP kinase p38. Bioorg. Med. Chem. Lett. 1998, 8 (23), 3335–40. 10.1016/S0960-894X(98)00589-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.