Abstract
Background:
Although the standard treatment for the patients with recurrent and metastatic prostate cancer (CaP) is androgen deprivation therapy, castration-resistant prostate cancer (CRPC) eventually emerges. Our previous report indicated that bone morphogenetic protein 6 (BMP6) induced CRPC via tumour-infiltrating macrophages. In a separate line of study, we have observed that the WNT5A/BMP6 loop in CaP bone metastasis mediates resistance to androgen deprivation in tissue culture. Simultaneously, we have reported that BMP6 induced castration resistance in CaP cells via tumour-infiltrating macrophages. Therefore, our present study aims to investigate the mechanism of WNT5A and its interaction with macrophages on CRPC.
Methods:
Doxycycline inducible WNT5A overexpression prostate cancer cell line was used for detailed mechanical study.
Results:
WNT5A was associated with increased expression of chemokine ligand 2 (CCL2) in the human CaP cell line, LNCaP. Mechanistically, this induction of CCL2 by WNT5A is likely to be mediated via the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) signalling pathway. Our in vivo experiments demonstrated that the overexpression of WNT5A in LNCaP cells promoted castration resistance. Conversely, this resistance was inhibited with the removal of macrophages via clodronate liposomes. When patient-derived CaP LuCaP xenografts were analysed, high levels of WNT5A were correlated with increased levels of CCL2 and BMP6. In addition, higher levels of CCL2 and BMP6 were more commonly observed in intra-femoral transplanted tumours as compared to subcutaneous-transplanted tumours in the patient-derived PCSD1 bone-niche model.
Conclusions:
These findings collectively suggest that WNT5A may be a key gene that induces CRPC in the bone niche by recruiting and regulating macrophages through CCL2 and BMP6, respectively.
Keywords: WNT5A, BMP6, CCL2, macrophages, castration-resistant prostate cancer, ROR2
Prostate cancer (CaP) is the second leading cause of cancer death in men in the United States (Siegel et al, 2017). Since the androgen receptor (AR) signalling is critical for growth of both normal and malignant prostate epithelial cells, androgen deprivation therapy (ADT) is the standard therapy for recurrent and metastatic CaP. Although an overwhelming majority of the patients are responsive to ADT initially, castration-resistant prostate cancer (CRPC) inevitably develops. Currently, there are several mechanisms for the induction of CRPC, including intracellular androgen synthesis, crosstalk among molecules in AR and other signalling pathways, and amplification and/or overexpression of AR (Egan et al, 2014). In addition, it is also suggested that tumour microenvironment plays an important role in androgen sensitivity and progression of CaP via pro-inflammatory cytokine, IL-6 (Chun et al, 2009). In particular, it was reported that the interaction between tumour-infiltrating macrophages and CaP cells mediates androgen resistance (Zhu et al, 2006). Similarly, our previous reports also suggested that tumour microenvironments, including infiltration of macrophages into the bone stromal environment, are critical mediators for inducing castration resistance via WNT5A and its interaction with BMP6 (Lee et al, 2013a, 2014).
WNT signalling is comprised of 19 secreted glycoproteins identified as regulators of embryogenesis, development, cell proliferation, and differentiation. WNT transduces a signal through two different manners: (1) canonical β-catenin dependent and (2) non-canonical β-catenin independent pathways. In the canonical β-catenin dependent pathway, WNT1, 3a, and 7 bind receptor complex between FZD family and WNT co-receptors. This ligand-receptor complex inhibits GSK-3β and β-catenin from translocating into the nucleus for regulation of gene expression (Baron and Kneissel, 2013). On the other hand, in the non-canonical pathway, WNT5A, 5b, and 11 are responsible for activating the signalling cascade (Minami et al, 2010; Sandsmark et al, 2017). In particular, it was reported that WNT5A binds to ROR2 a member of Ror receptor tyrosine kinase family and activates JNK/c-Jun, Src and Ca++ signalling for regulating gene expression (Schambony and Wedlich, 2007; Nomachi et al, 2008; O'Connell et al, 2010; Hasegawa et al, 2017; Stricker et al, 2017).
Aberrant expression of WNT5A has been reported with several cancers (Weeraratna et al, 2002; Kurayoshi et al, 2006; Pukrop et al, 2006; Miyamoto et al, 2015); however, discrepancies exist with regards to the role of WNT5A in cancers affecting different organ system. For example, WNT5A functions as a tumour suppressor in colon cancer, breast cancer, neuroblastoma, and leukaemia (Liang et al, 2003; Blanc et al, 2005; Dejmek et al, 2005; Leris et al, 2005). On the other hand, high expression of WNT5A was associated with tumour progression in several cancers (Kurayoshi et al, 2006; Da Forno et al, 2008; Bellon et al, 2013). In CaP, it has been observed that elevated level of WNT5A is highly correlated with high Gleason score and biochemical relapse (Yamamoto et al, 2010). Mechanistically, there is evidence of WNT5A regulating metalloproteinase via Ror2 and Jun-N-terminal kinase for acquiring tumour aggressiveness (Yamamoto et al, 2010). In addition, the tumourigenic characteristics of WNT5A is further supported as it stimulates production of inflammatory cytokines and chemokines in endothelial cells, monocytes, bone stromal cells, and cancer cells (Rauner et al, 2012; Jung et al, 2013; Li et al, 2014) via mitogen-activated protein kinase (MAPKs), Akt, and NF-κB signalling pathways.
During the process of tissue remodeling, inflammation, and immunologic response, macrophages are intricately involved as phagocytic cells clearing apoptotic cells. Recent reports demonstrated that high infiltration of macrophages within tumours, called tumour associated macrophages (TAM), showed poor prognosis in CaP (Lissbrant et al, 2000). Accumulation of CC chemokine ligand 2 (CCL2), CC chemokine ligand 3 (CCL3), CC chemokine ligand 4 (CCL4), CC chemokine ligand 5 (CCL5), and CC chemokine ligand 8 (CCL8) in the tumour microenvironment was also associated with high levels of macrophage infiltration in various types of malignancies, including CaP (Negus et al, 1997; Azenshtein et al, 2002; Loberg et al, 2007; Szebeni et al, 2017). Among many chemokines, CCL2, a polypeptide composed with 76-amino acid and a member of C–C chemokine family, is known to recruit monocytes, macrophages, and other immune cells to inflammatory sites. Functionally, CCL2 is involved in regulating tumour cell growth by regulating infiltration of macrophages into tumours, promoting osteoclast maturation, and suppressing cytotoxic lymphocytes in CaP (Loberg et al, 2007; Zhang et al, 2010; Wu et al, 2017).
In this study, we have investigated the role of WNT5A in the induction of CRPC. We report that the overexpression of WNT5A induces CRPC with the mediating effects of CCL2 and infiltration of macrophages.
Materials and methods
Mice and cell culture
Athymic nude mice were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA). The study was approved by the Institutional Animal Care and Use Committee at the Rutgers Robert Wood Johnson Medical School. Tumour size was measured using calipers and tumour volume was calculated using the formula: tumour volume=length × width2/2. WNT5A/LNCaP cells were subcutaneously injected into five mice per group. The mice were fed with water containing 2 mg/ml of doxycycline (Sigma-Aldrich, Saint Louis, MI, USA) in 5% sucrose to induce WNT5A. For systemic removal of macrophages, the mice were injected with 0.1 ml clodronate liposomes or equal volume of PBS liposomes i.p. (final dose of 25 mg/Kg) every 4 days. Human CaP cell lines, 22RV1 and LNCaP, were purchased from the American Type Culture Collection and were cultured in RPMI 1640 contained 10% FBS.
WNT5A-inducible LNCaP cell lines
WNT5A STOP was a gift from Marian Waterman (Addgene plasmid #35874) (Najdi et al, 2012). WNT5A gene was cloned into pLenti4/V5-Dest expression vector. After making pLenti4/V5-Dest/WNT5A STOP viral supernatant with the Optimized Packaging Mix, the supernatant was added to the Virapower T-Rex LNCaP cell line. After selection with Zeocin and Blasticidin, WNT5A-inducible LNCaP cells (WNT5A/LNCaP) were selected and the expression of WNT5A was detected using PCR. The selected clones were cultured in RPMI 10% FBS media containing 100 mg/ml of Blasticidin and Zeocin (Life Technologies, Carlsbad, CA, USA).
Quantitative RT-PCR and PCR
Total RNA was isolated with TRIzol LS reagent (Thermo Fisher Scientific, Waltham, MA, USA), and 1–2 μg of total RNA was used for synthesizing cDNA with High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA). The cDNA was then used for quantitative PCR in a StepOnePlus (Applied Biosystems, Foster City, CA, USA) with SYBR Green ROX qPCR Mastermix (QIAGEN, Valencia, CA, USA) and semi-quantitative PCR. All PCR primers were shown as follows: human Qpcr CCL2 (forward: 5′-CCCCAGTCACCTGCTGTTAT-3′, reverse: 5′-TGGAATCCTGAACCCACTTC-3′); human Qpcr β-actin (forward: 5′-AGAGCTACGAGCTGCCTGAC-3′, reverse: 5′-AGCACTGTGTTGGCGTACAG-3′); human Qpcr BMP6 (forward: 5′-CATGAGCTTTGTGAACCTGG-3′, reverse: 5′-CACCTCACCCTCAGGAATCT-3′); human Qpcr WNT5A (forward: 5′-ATTCTTGGTGGTCGCTAGGT-3′, reverse: 5′-TCCTTGAGAAAGTCCTGCCA-3′); human Qpcr ROR2 (forward: 5′-TTTGTGCGGCTGGGTCCAA-3′, reverse: 5′-GTAAGGCTGGCAGAACCCAT-3′); human CCL2 (forward: 5′-CCGAGAGGCTGAGACTAACC-3′, reverse: 5′-AGGGTGTCTGGGGAAAGCTA-3′); human β-actin (forward: 5′-GGCATCGTGATGGACTCC-3′, reverse: 5′-GCTGGAAGGTGGACAGCG-3′); human WNT5A (forward: 5′-GTTGTAATTGAAGCCAATTCTTGGTGGTC-3′, reverse: 5′-GCCCGGCTCATGGCGTTCACCAC-3′); human BMP6 (forward: 5′-ACAGCATAACATGGGGCTTC-3′, reverse: 5′-CTCGGGGTTCATAAGGTGAA-3′); and human ROR2 (forward: 5′-GGACCGGTTTGGGAAAGTCT-3′, reverse: 5′-TAATCGGCGGCATACACCTC-3′).
Immunoblot and enzyme-linked immunosorbent assay
The CaP cells were collected and lysed with lysis buffer (20 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1 mm Na2 EDTA, 1 mm EGTA, 1% Triton, 2.5 mm sodium pyrophosphate, 1 mm beta-glycerophosphate, 1 mm Na3VO4, 1 μg/ml leupeptin) with 1 mm phenylmethylsulfonyl fluoride (PMSF). The cell lysates were centrifuged and supernatant was used as proteins. After separating of 25–50 μg protein in SDS-PAGE, samples were incubated with CCL2 (Thermo Fisher Scientific, Waltham, MA, USA), β-actin antibody (Sigma-Aldrich, St. Louis, MO, USA), p-ERK and ERK (Cell Signaling Technology, MA, USA). For CCL2, p-ERK, ERK immunoblots, 1:1000 diluted antibody solutions in 5% skim milk was used. For the β-actin immunoblot, 1:10 000 diluted antibody solutions were used. All antibody solutions were incubated overnight at 4 °C. Following the incubation with appropriate secondary antibody, immunoblot was analysed using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, Waltham, MA, USA).
For ELISA, LNCaP cells were treated with recombinant WNT5A and culture medium was collected and centrifuged after 48 h. The level of CCL2 was measured with Human MCP-1/CCL2 ELISA MAX Deluxe Set (Biolegend, San Diego, CA, USA).
Overexpression and knockdown of ROR2
Flag-ROR2 construct (Gao et al, 2011) was given from Dr. Yingzi Yang (Harvard School of Dental Medicine, Boston, MA, USA) as a gift. ROR2 MISSION shRNA was purchased from Sigma-Aldrich (St. Louis, MO, USA). ROR2 shRNA lentiviral supernatant was generated with ViraPower Lentiviral Packaging Mix (Thermo Fisher Scientific, Waltham, MA, USA), and this was used to infect LNCaP cells. The expression of ROR2 was analysed using quantitative RT-PCR and immunoblot.
Immunofluorescence for human CaP tissue microarray and mouse tumour
After receiving Institutional Review Board (IRB) approval, human tissue microarray (TMA) was generated at the Department of Pathology at the Rutgers Cancer Institute of New Jersey. Prostate specimens from patients with localized CaP who underwent radical prostatectomy at the Rutgers Robert Wood Johnson University Hospital between study period between 2011 and 2013 were considered for the construction of a TMA. After performing cylindrical core biopsies, 100 prostate tissues that met the quality control standards were embedded in paraffin blocks and were subsequently stained with haematoxylin and eosin. Cases were dichotomized into two groups as follows: Gleason 3+4 or lower vs Gleason 4+3 or higher (Epstein et al, 2016). For Immunofluorescence (IF), all collected mouse tumours were embedded in paraffin, and the tissues were stained with haematoxylin and eosin. The paraffin-embedded sections were stained with anti-WNT5A antibody (Abcam, Cambridge, MA, USA), CCL2 antibody (Thermo Fisher Scientific, Waltham, MA, USA), human BMP6 antibody (Minneapolis, MN, USA), anti-mouse F4/80 Antigen Purified (Affymetrix eBioscience, San Diego, CA, USA), and PE mouse anti-human CD68 (BD Bioscience, Franklin Lakes, NJ, USA). The secondary antibodies used in this study were Anti-Rabbit Alexa Fluor 488, Anti-goat Alexa Fluor 594, Anti-Rat Alexa Fluor 488, and Anti-mouse Alexa Fluor 594 purchased from Thermo Fisher Scientific (Waltham, MA, USA).
PCSD1 bone-niche model and LuCaP patient-derived xenograft models
cDNA samples of the PCSD1 bone-niche model were received from Dr Christina Jamieson (University of California, San Diego, CA, USA). The total RNA samples of LuCaP patient-derived xenografts were provided by Dr. Colm Morrissey (University of Washington, Seattle, WA, USA) and reverse transcribed for synthesis of cDNA. All cDNA was analysed with quantitative PCR.
Quantification of human TMA and mouse tumour specimens
The TMA and mouse tumour slides were scanned using Olympus VS120 Florescence/Bright-field whole slide scanner (Olympus Scientific Solutions Americas Corp., Waltham, MA, USA) after staining with the appropriate antibody. All scanned cores or slides were individually quantified using NIH ImageJ V1.50i (NIH, Bethesda, MD, USA). Values were represented as mean fluorescence intensity (MFI).
Statistical analysis
Student’s t-test was used for analysing continuous variables while χ2-test was used for analysing categorical variables. All statistical analyses were done with SPSS, version 21 SAS, version 9.4 with 2-sided P<0.05 considered significant. All in vitro experiment was performed at least three times.
Results
WNT5A induces castration resistance in prostate cancer cells
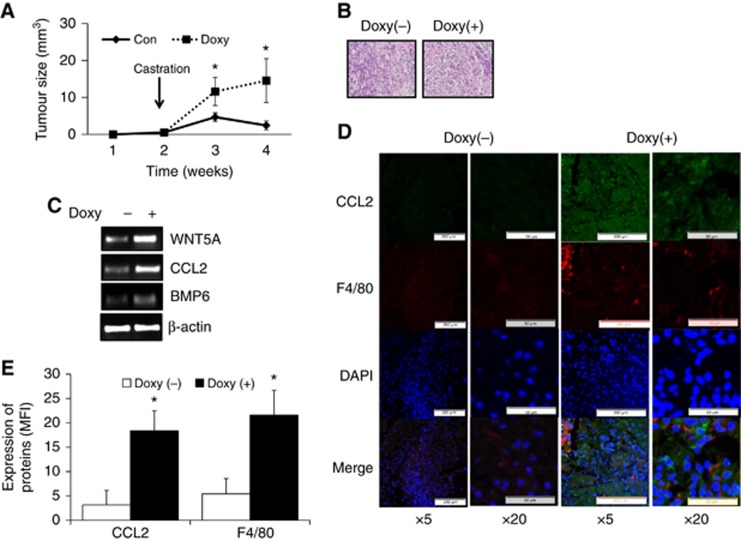
To investigate the role of WNT5A in castration resistance, WNT5A/LNCaP cells were generated and the expression of WNT5A was confirmed with tetracycline (Tet) treatment (Supplementary Figure S1A and B). When the clones were cultured with tetracycline, there were no significant differences in cell growth between Tet (−) and Tet (+) groups (Supplementary Figure S1C). Due to most high induction by WNT5A by tetracyclin, the selected cells (clone 3) were subcutaneously injected into each nude mouse. When WNT5A was overexpressed in tumours by administering doxycycline via the drinking water, LNCaP cells became resistant to castration (Figure 1A). No significant histopathological difference in tumour was noted between Doxy (−) and Doxy (+) groups (Figure 1B). Since our previous report suggested that BMP6 leads to CRPC via macrophages, the expression of BMP6 and CCL2 were also analysed. The mRNA levels of both BMP6 and CCL2 were increased in WNT5A overexpressing tumours (Figure 1C). WNT5A overexpressing tumours had greater numbers of infiltrating macrophages and high levels of CCL2, BMP6, and WNT5A (Figures 1D and E, Supplementary Figure S2) by IF.
Figure 1.
Role of WNT5A in castrated mice (N=5 mice per group). (A) LNCaP cells with inducible WNT5A were subcutaneously injected into nude mice. All animals were castrated and divided to two groups. When WNT5A was induced by administering doxycycline via drinking water, growth of tumour was increased significantly. Error bars represent average±SE. N=5 per group. (B) There is no significant difference between Doxy (−) and Doxy (+) tumours in haematoxylin staining. (C) WNT5A, CCL2, and BMP6 mRNA were analysed from tissues using RT-PCR. When WNT5A was overexpressed, expression of CCL2 and BMP6 were also increased. (D) A single-picture representative of IF for CCL2 and F4/80. (E) Quantification of IF demonstrated that CCL2 levels in WNT5A overexpressing tumours are increased and macrophages (F4/80) are more infiltrated. Mean fluorescence intensity (MFI) was measured by Image J. Values are means+SE. N=5 per group. *P < 0.05. BMP6=bone morphogenetic protein 6; CCL2=chemokine ligand 2; MFI=mean fluorescence intensity.
WNT5A promotes CCL2 expression in hormone sensitive cells
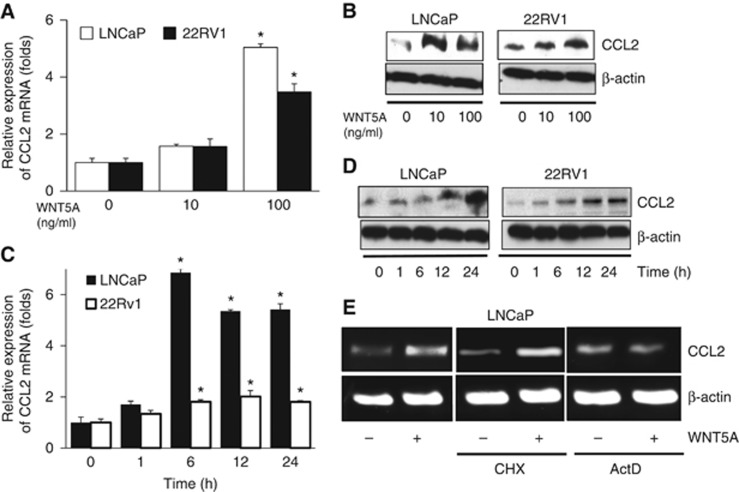
On the basis of the previous report that WNT5A promotes the expression of cytokines and chemokines in human cells (Rauner et al, 2012; Li et al, 2014; Zhao et al, 2014b), androgen-sensitive human CaP cells were treated with recombinant WNT5A protein. In 22Rv1 and LNCaP cells, Wnt5A induced CCL2 mRNA and protein in a concentration-dependent (Figure 2A and B) and time-dependent manner (Figure 2C and D). This finding was replicated in an additional hormone sensitive human CaP cell line, LAPC4 (Supplementary Figure S3). Secreted CCL2 in culture medium was 319 pg/ml by ELISA after treating LNCaP cells with 100 ng/ml of recombinant WNT5A (Supplementary Figure S4). In addition, when WNT5A was overexpressed in WNT5A/LNCaP cells, a threefold increase in CCL2 mRNA from baseline was observed (data not shown). To elucidate the mechanism of CCL2 induction by WNT5A, WNT5A/LNCaP cells were treated with cycloheximide and actinomycin D. Semi-quantitative RT-PCR revealed that CCL2 induction was inhibited by actinomycin D (Figure 2E). These results suggest that WNT5A regulates the expression of CCL2 at the transcript level.
Figure 2.
Induction of CCL2 expression in hormone sensitive CaP cells by WNT5A. (A) LNCaP and 22RV1 cells were treated with 0–100 ng/ml of WNT5A for 24 h. mRNA of CCL2 was increased. (B) The level of CCL2 protein was also increased by WNT5A. Upon treatment of WNT5A (100 ng/ml), CCL2 induction was increased at the (C) mRNA level and (D) protein level in a time-dependent manner. (E) A semi-quantitative RT-PCR revealed that cycloheximide (CHX) not actinomycin D (ActD) inhibited the expression of CCL2 by WNT5A in LNCaP cells. All study was repeated at least three times. *P < 0.05. CCL2=chemokine ligand 2.
Receptor tyrosine kinase like orphan receptor 2 (ROR2) mediates ERK activation and regulates CCL2 expression
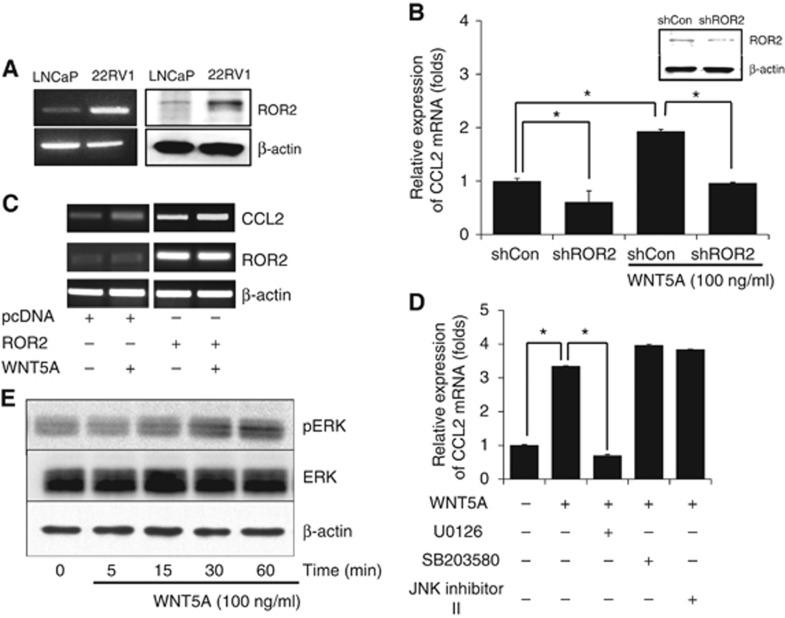
It has been reported that the expression of WNT5A and ROR2 promotes invasion by regulating matrix metalloproteinase-13 expression (Enomoto et al, 2009), which is involved with cytokine production in dendritic cells (Sato et al, 2015). To understand the induction mechanism of CCL2 by WNT5A in CaP cells, the expression of ROR2 in hormone sensitive cells was analysed. ROR2 mRNA and protein were detected in both LNCaP and 22RV1 cells (Figure 3A). When the expression of ROR2 was inhibited with shRNA (Figure 3B), CCL2 expression was decreased in LNCaP cells. Conversely, the overexpression of ROR2 promoted the expression of CCL2 (Figure 3C). Several studies suggested that WNT5A induced the expression of CCL2 in various types of cells via the MAPK signalling pathway (Rauner et al, 2012; Jung et al, 2013; Zhao et al, 2014a, 2014b). MAPK inhibitors were used to analyse CCL2 expression from WNT5A-treated LNCaP cells. Our results demonstrated that U0126, an inhibitor of extracellular signal-regulated kinase (ERK), significantly inhibited mRNA expression of CCL2 in LNCaP cells (Figure 3D). SB203580, p38 inhibitor, and JNK inhibitor II had no effect of CCL2 mRNA expression (Figure 3D). Recombinant WNT5A treatment increased the level of ERK phosphorylation in LNCaP cells (Figure 3E). These observations suggest that WNT5A induces CCL2 expression in LNCaP cells via the ERK signalling pathway.
Figure 3.
Induction of CCL2 by WNT5A signalling. (A) LNCaP and 22RV1 cells expressed ROR2 mRNA (left) and protein (right). (B) When ROR2 was blocked with ROR2 shRNA, CCL2 expression was decreased. (C) When ROR2 was overexpressed with flag-ROR2 expression construct, CCL2 expression was significantly increased. (D) ERK inhibitor (U0126) significantly decreased CCL2 expression in LNCaP cells after treating WNT5A. (E) When WNT5A was treated on LNCaP cells, immunoblot of ERK demonstrated that ERK was phosphorylated. All study was repeated three times. *P < 0.05. CCL2=chemokine ligand 2; ERK=extracellular signal-regulated kinase.
Removal of macrophages attenuates WNT5A-mediated CRPC
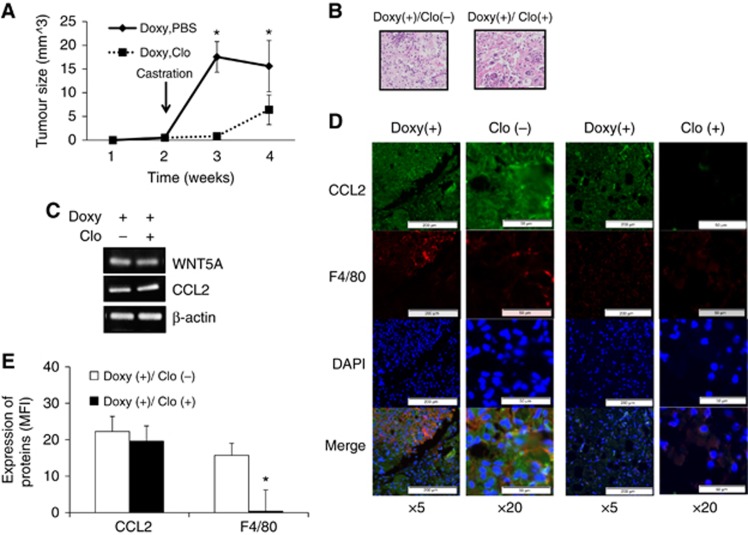
Because CCL2 is an important mediating factor of CaP growth via macrophage infiltration (Loberg et al, 2007), the role of macrophages in WNT5A-mediated CRPC development was investigated using macrophage depleting agent (Supplementary Figure S5), clodronate liposomes (Weisser et al, 2012; Lee et al, 2013a, 2013b). When macrophages were depleted, the induction of CRPC was significantly attenuated (Figure 4A). Histopathologically, however, no significant differences between PBS injected and clodronate injected tumours were found (Figure 4B). The mRNA expression of WNT5A and CCL2 were confirmed using semi-quantitative PCR (Figure 4C). In IF, a decreased level of macrophage infiltration was detected in the clodronate liposomes injected group when compared to the PBS liposome injected group (Figures 4D and E). In addition, a decreased level of CCL2 in the clodronate liposome injected group was likely due to decreased number of macrophage responsible for producing CCL2.
Figure 4.
WNT5A induced CRPC via macrophages. (A) Tumour growth rate is significantly decreased in clodronate liposomes injected group. After tumour injection, when the palpable tumours found, all mice got surgical castration. Then clodronate or control liposome was injected. (B) H&E staining showed that no significant difference between clodronate and PBS group. Error bars indicate average±SE. N=5 per group. (C)The expression of WNT5A and CCL2 in isolated tumours from both groups of mice were confirmed with semi-quantitative RT-PCR. (D) Representative pictures of IF for WNT5A, BMP6, CCL2 and F4/80. (E) Quantification of IF demonstrated that clodronate liposomes injected group showed decreased level of macrophage infiltration in tumour. MFI was measured by Image J. Values indicate means+SE. N=5 per group. *P < 0.05. CCL2=chemokine ligand 2; MFI=mean fluorescence intensity.
Analysis of patient-derived CaP model and human TMA
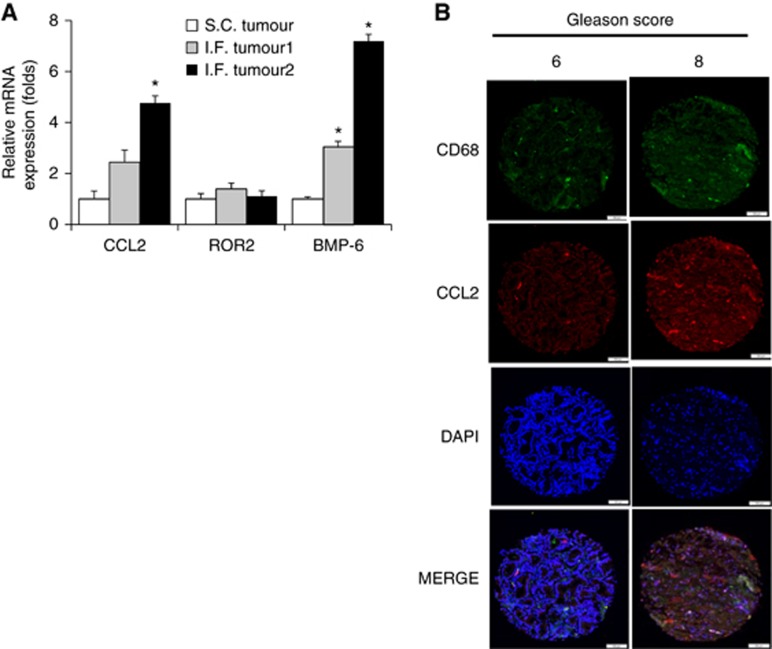
To find a clinical relevance of WNT5A in CaP, samples from different patient-derived CaP models, namely LuCaP and PCSD1 (Morrissey et al, 2010; Godebu et al, 2014), were evaluated. The levels of CCL2, BMP6, and WNT5A were higher in LuCaP 81 (Right pelvic metastasis CaP) and LuCaP 35 (Lymph node metastasis CaP) when compared to the levels in LuCaP 141 (Localized CaP), indicating that these genes may play a role in CaP progression (Supplementary Figure S6). Moreover, in the patient-derived PCSD1 model, CCL2, ROR2, and BMP6 were highly expressed in intra-femoral transplanted tumours as compared to subcutaneous transplanted tumours (Figure 5A) at the mRNA level. This suggests possible involvement of these genes in bone metastasis in CaP. Moreover, the expression of WNT5A, BMP6, and CCL2 were examined in a TMA by IHC (N=100) (Figure 5B and Supplementary Figure S7). Comparing tumours with high Gleason scores (4+3 or higher) to low-Gleason score (3+4 or lower), the expression of WNT5A, BMP6, and CCL2 were higher in the high Gleason group (27.0 vs 37.2; 47.4 vs 60.0; 25.0 vs 43.3, respectively; all P<0.05) (Table 1). Collectively, these results support our hypothesis that WNT5A expression is associated with aggressive disease (Supplementary Figure S8).
Figure 5.
Analysis of patient-derived CaP xenograft samples. (A) The expression of BMP6, ROR2, and CCL2 was analysed in tumours from PCSD1 bone-niche model. PCR was repeated at least three times. (B) and a single-picture representative of IF demonstrated that high expression of CCL2 with high-Gleason tumour as compared with low-Gleason tumour. Macrophage infiltration also little bit higher in high-Gleason tumour than low-Gleason tumour. *P < 0.05. BMP6=bone morphogenetic protein 6; CCL2=chemokine ligand 2.
Table 1. Clinicodemographic chracteristics and the genes of interest in tissue microarray.
| Gleason=<3+4 | Gleason>=4+3 | ||
|---|---|---|---|
| N=74 | N=26 | P-value | |
|
Demographics | |||
| Age, years, mean (SD) | 63.8 (6.38) | 64.6 (7.12) | 0.62 |
|
Race,
N
(%) | |||
| Caucasians | 55 (74.3) | 21 (80.8) | 0.44 |
| African | 13 (17.6) | 2 (7.7) | |
| Others | 6 (8.1) | 3 (11.5) | |
|
Pathologic staging,
N
(%) | |||
| pT2 | 52 (70.3) | 15 (57.7) | 0.33 |
| pT3 | 22 (29.7) | 11 (42.3) | |
|
Genes | |||
| WNT5A, MFI, mean (SD) | 27.0 (19.4) | 37.2 (23.2) | 0.03 |
| BMP6, MFI, mean (SD) | 47.4 (31.3) | 60.0 (25.2) | 0.066 |
| CCL2, MFI, mean (SD) | 25.0 (19.3) | 43.3 (41.2) | 0.003 |
| CD68, MFI, mean (SD) | 31.1 (19.7) | 33.8 (21.3) | 0.56 |
Abbreviations: BMP6=bone morphogenetic protein 6; CCL2=chemokine ligand 2; MFI=mean fluorescence intensity.
Discussion
With the growing body of evidence demonstrating a strong association between WNT5A expression and aggressive CaP, we investigated the role of WNT5A in CaP in vivo. The overexpression of WNT5A resulted in the development of CRPC (Supplementary Figures S9 and S10), but the resistance was inhibited by the removal of macrophages. Our in vitro study revealed that WNT5A promoted the expression of CCL2 in androgen-sensitive cells in the ERK-MAPKs signalling pathway via Ror2.
We previously reported that macrophages and bone stromal environment modulate androgen sensitivity, but the role of WNT5A in CRPC remained to be determined (Lee et al, 2014). Our present in vivo study result suggests that WNT5A contributes to the induction of lethal CRPC. To further demonstrate clinical implications of this finding, higher expression levels of WNT5A, BMP6, and CCL2 were observed in CaP tissues with high Gleason score (⩾4+3) as compared to CaP tissues with low-Gleason score (⩽3+4). Moreover, higher levels of CCL2, ROR2, and BMP6 were observed in the intra-femoral transplanted PCSD1 tumours than subcutaneous-transplanted tumours, indicating the potential association of these genes with bone metastasis. These results along with the previously published literature provide evidence for our hypothesis that high levels of WNT5A may promote castration resistance in CaP.
Although our results suggest pro-tumourigenic role of WNT5A in CaP, a recent publication reported that WNT5A has anti-CaP effects in vivo (Thiele et al, 2015). There are two potential explanations for this observed discrepancy. First, different experimental systems were used. Thiele and colleagues used a transient WNT5A overexpression system, which showed expression levels peaking at 2 days, followed by a gradual reduction (Thiele et al, 2015). In our study, the tetracycline inducible overexpression system allowed a constant level of WNT5A in tumours. Second, similar to TGF-β, WNT5A may behave as a bifunctional gene that functions differently based on the tumour stage. It has been well established that TGF-β functions as a tumour suppressor in the early stages of tumour, but as an oncogene in the advanced stages by promoting immune suppression, angiogenesis, and epithelial mesenchymal transition (Meulmeester and Ten Dijke, 2011). Similarly, in the more advanced form of CaP, it is possible that WNT5A is likely an oncogene based on our study. Nonetheless, additional studies are underway to understand the precise role of WNT5A in CaP.
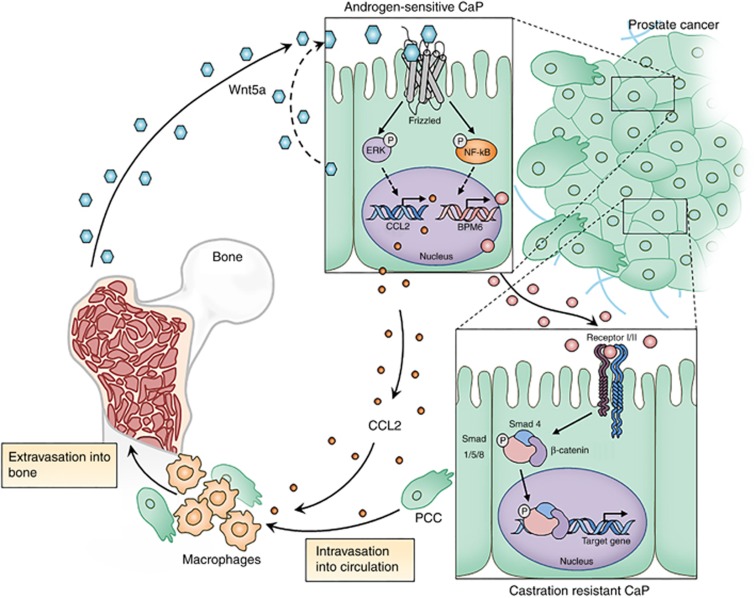
Bone metastasis is frequently observed among patients who suffer from advanced CaP, but the exact mechanism is yet to be elucidated. Our present and previous studies provide some insight into the mechanism by introducing potential genes of interest in bone metastasis in CaP. Our previous report suggested that bone-tumour interactions function as a critical mediator for CRPC via WNT5A and BMP6 expression in the bone stromal cells and CaP cells, respectively (Lee et al, 2014). In addition, it has been demonstrated that BMP6 induced CRPC via the infiltration of macrophages (Lee et al, 2013a). In this study, the PCSD1 bone-niche xenograft model was established from CRPC patients with bone metastasis (Godebu et al, 2014). Intra-femoral transplanted PCSD1 tumours produced higher levels of CCL2, ROR2, and BMP6 compared to subcutaneous-transplanted tumours, further supporting the association between bone metastasis and the interplay of the above genes. Once CaP cells migrate to the bone, CaP cells are stimulated by WNT5A to produce CCL2 and BMP6 by WNT5A. While BMP6 induces CRPC (Lee et al, 2013a, 2014) in cancer cells, CCL2 recruits macrophages on tumour sites and promotes CRPC induction. Through this array of events, it is proposed that WNT5A promotes bone metastasis in CRPC (Figure 6).
Figure 6.
Proposed mechanism of WNT5A role in CRPC and bone metastasis. Bone stromal cells and CaP cells produced WNT5A which can induce BMP6 and CCL2 from CaP cells. Secreted CCL2 recruits macrophages into tumours and BMP6 induces CRPC in CaP cells. In turn, interaction between tumour cells and bone stromal cells would promote bone metastasis of CaP.
It should be noted that several limitations exist in testing the proposed mechanism in this study. Since hormone sensitive LNCaP does not metastasize to the bone in mice via orthotopic, intracardiac, or tail vein injection (Pettaway et al, 1996), an appropriate in vivo model for testing our proposed mechanism could not be used. Since athymic nude mice were used in this study, it did not consider the role of adaptive immunity on CaP. Despite these limitations, our present study not only confirms the previously reported correlation between WNT5A and BMP6, but also established other associations with CRPC and bone metastasis.
In conclusion, this study suggests that WNT5A induces CRPC by mediating macrophage infiltration. A mechanistic study revealed that WNT5A induced the expression of CCL2 in androgen-sensitive CaP cells by the activation of ERK signalling pathway. In addition, the hypothesis of this study that WNT5A is associated with induction of CRPC the most aggressive type of CaP was further supported from patient-derived CaP xenograft models and our tissue microarray.
Acknowledgments
We thank Colm Morrissey and Eva Corey for providing LuCaP models and editing the manuscript. This work was supported in part by the Tanzman Foundation, Mr Jeffries Shein, and Mr Mal Wernik.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
The authors declare no conflict of interest.
Supplementary Material
References
- Azenshtein E, Luboshits G, Shina S, Neumark E, Shahbazian D, Weil M, Wigler N, Keydar I, Ben-Baruch A (2002) The CC chemokine RANTES in breast carcinoma progression: regulation of expression and potential mechanisms of promalignant activity. Cancer Res 62(4): 1093–2102. [PubMed] [Google Scholar]
- Baron R, Kneissel M (2013) WNT signalling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19(2): 179–192. [DOI] [PubMed] [Google Scholar]
- Bellon M, Ko NL, Lee MJ, Yao Y, Waldmann TA, Trepel JB, Nicot C (2013) Adult T-cell leukemia cells overexpress Wnt5a and promote osteoclast differentiation. Blood 121(25): 5045–5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc E, Roux GL, Benard J, Raguenez G (2005) Low expression of Wnt-5a gene is associated with high-risk neuroblastoma. Oncogene 24(7): 1277–1283. [DOI] [PubMed] [Google Scholar]
- Chun JY, Nadiminty N, Dutt S, Lou W, Yang JC, Kung HJ, Evans CP, Gao AC (2009) Interleukin-6 regulates androgen synthesis in prostate cancer cells. Clin Cancer Res 15(15): 4815–4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Forno PD, Pringle JH, Hutchinson P, Osborn J, Huang Q, Potter L, Hancox RA, Fletcher A, Saldanha GS (2008) WNT5A expression increases during melanoma progression and correlates with outcome. Clin Cancer Res 14(18): 5825–5832. [DOI] [PubMed] [Google Scholar]
- Dejmek J, Dejmek A, Safholm A, Sjolander A, Andersson T (2005) Wnt-5a protein expression in primary dukes B colon cancers identifies a subgroup of patients with good prognosis. Cancer Res 65(20): 9142–9146. [DOI] [PubMed] [Google Scholar]
- Egan A, Dong Y, Zhang H, Qi Y, Balk SP, Sartor O (2014) Castration-resistant prostate cancer: adaptive responses in the androgen axis. Cancer Treat Rev 40(3): 426–433. [DOI] [PubMed] [Google Scholar]
- Enomoto M, Hayakawa S, Itsukushima S, Ren DY, Matsuo M, Tamada K, Oneyama C, Okada M, Takumi T, Nishita M, Minami Y (2009) Autonomous regulation of osteosarcoma cell invasiveness by Wnt5a/Ror2 signalling. Oncogene 28(36): 3197–3208. [DOI] [PubMed] [Google Scholar]
- Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, Vickers AJ, Parwani AV, Reuter VE, Fine SW, Eastham JA, Wiklund P, Han M, Reddy CA, Ciezki JP, Nyberg T, Klein EA (2016) A contemporary prostate cancer grading system: a validated alternative to the gleason score. Eur Urol 69(3): 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, Andre P, Robinson J, Sood R, Minami Y, Economides AN, Yang Y (2011) Wnt signalling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell 20(2): 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godebu E, Muldong M, Strasner A, Wu CN, Park SC, Woo JR, Ma W, Liss MA, Hirata T, Raheem O, Cacalano NA, Kulidjian AA, Jamieson CA (2014) PCSD1, a new patient-derived model of bone metastatic prostate cancer, is castrate-resistant in the bone-niche. J Transl Med 12: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa D, Wada N, Yoshida S, Mitarai H, Arima M, Tomokiyo A, Hamano S, Sugii H, Maeda H (2017) Wnt5a suppresses osteoblastic differentiation of human periodontal ligament stem cell-like cells via Ror2/JNK signalling. J Cell Physiol 233(2): 1752–1762. [DOI] [PubMed] [Google Scholar]
- Jung YS, Lee HY, Kim SD, Park JS, Kim JK, Suh PG, Bae YS (2013) Wnt5a stimulates chemotactic migration and chemokine production in human neutrophils. Exp Mol Med 45: e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, Yasui W, Kikuchi A (2006) Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res 66(21): 10439–10448. [DOI] [PubMed] [Google Scholar]
- Lee GT, Jung YS, Ha YS, Kim JH, Kim WJ, Kim IY (2013. a) Bone morphogenetic protein-6 induces castration resistance in prostate cancer cells through tumour infiltrating macrophages. Cancer Sci 104(8): 1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GT, Kang DI, Ha YS, Jung YS, Chung J, Min K, Kim TH, Moon KH, Chung JM, Lee DH, Kim WJ, Kim IY (2014) Prostate cancer bone metastases acquire resistance to androgen deprivation via WNT5A-mediated BMP-6 induction. Br J Cancer 110(6): 1634–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lee GT, Woo SH, Ha YS, Kwon SJ, Kim WJ, Kim IY (2013. b) BMP-6 in renal cell carcinoma promotes tumour proliferation through IL-10-dependent M2 polarization of tumour-associated macrophages. Cancer Res 73(12): 3604–3614. [DOI] [PubMed] [Google Scholar]
- Leris AC, Roberts TR, Jiang WG, Newbold RF, Mokbel K (2005) WNT5A expression in human breast cancer. Anticancer Res 25(2A): 731–734. [PubMed] [Google Scholar]
- Li S, Wang W, Zhang N, Ma T, Zhao C (2014) IL-1beta mediates MCP-1 induction by Wnt5a in gastric cancer cells. BMC Cancer 14: 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Chen Q, Coles AH, Anderson SJ, Pihan G, Bradley A, Gerstein R, Jurecic R, Jones SN (2003) Wnt5a inhibits B cell proliferation and functions as a tumour suppressor in hematopoietic tissue. Cancer Cell 4(5): 349–360. [DOI] [PubMed] [Google Scholar]
- Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A (2000) Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol 17(3): 445–451. [DOI] [PubMed] [Google Scholar]
- Loberg RD, Ying C, Craig M, Yan L, Snyder LA, Pienta KJ (2007) CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia 9(7): 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulmeester E, Ten Dijke P (2011) The dynamic roles of TGF-beta in cancer. J Pathol 223(2): 205–218. [DOI] [PubMed] [Google Scholar]
- Minami Y, Oishi I, Endo M, Nishita M (2010) Ror-family receptor tyrosine kinases in noncanonical Wnt signalling: their implications in developmental morphogenesis and human diseases. Dev Dyn 239(1): 1–15. [DOI] [PubMed] [Google Scholar]
- Miyamoto DT, Zheng Y, Wittner BS, Lee RJ, Zhu H, Broderick KT, Desai R, Fox DB, Brannigan BW, Trautwein J, Arora KS, Desai N, Dahl DM, Sequist LV, Smith MR, Kapur R, Wu CL, Shioda T, Ramaswamy S, Ting DT, Toner M, Maheswaran S, Haber DA (2015) RNA-Seq of single prostate CTCs implicates noncanonical Wnt signalling in antiandrogen resistance. Science 349(6254): 1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey C, Dowell A, Koreckij TD, Nguyen H, Lakely B, Fanslow WC, True LD, Corey E, Vessella RL (2010) Inhibition of angiopoietin-2 in LuCaP 23.1 prostate cancer tumours decreases tumour growth and viability. Prostate 70(16): 1799–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najdi R, Proffitt K, Sprowl S, Kaur S, Yu J, Covey TM, Virshup DM, Waterman ML (2012) A uniform human Wnt expression library reveals a shared secretory pathway and unique signalling activities. Differentiation 84(2): 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus RP, Stamp GW, Hadley J, Balkwill FR (1997) Quantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokines. Am J Pathol 150(5): 1723–1734. [PMC free article] [PubMed] [Google Scholar]
- Nomachi A, Nishita M, Inaba D, Enomoto M, Hamasaki M, Minami Y (2008) Receptor tyrosine kinase Ror2 mediates Wnt5a-induced polarized cell migration by activating c-Jun N-terminal kinase via actin-binding protein filamin A. J Biol Chem 283(41): 27973–27981. [DOI] [PubMed] [Google Scholar]
- O'Connell MP, Fiori JL, Xu M, Carter AD, Frank BP, Camilli TC, French AD, Dissanayake SK, Indig FE, Bernier M, Taub DD, Hewitt SM, Weeraratna AT (2010) The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signalling in metastatic melanoma. Oncogene 29(1): 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettaway CA, Pathak S, Greene G, Ramirez E, Wilson MR, Killion JJ, Fidler IJ (1996) Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin Cancer Res 2(9): 1627–1636. [PubMed] [Google Scholar]
- Pukrop T, Klemm F, Hagemann T, Gradl D, Schulz M, Siemes S, Trumper L, Binder C (2006) Wnt 5a signalling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci USA 103(14): 5454–5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauner M, Stein N, Winzer M, Goettsch C, Zwerina J, Schett G, Distler JH, Albers J, Schulze J, Schinke T, Bornhauser M, Platzbecker U, Hofbauer LC (2012) WNT5A is induced by inflammatory mediators in bone marrow stromal cells and regulates cytokine and chemokine production. J Bone Miner Res 27(3): 575–585. [DOI] [PubMed] [Google Scholar]
- Sandsmark E, Hansen AF, Selnaes KM, Bertilsson H, Bofin AM, Wright AJ, Viset T, Richardsen E, Drablos F, Bathen TF, Tessem MB, Rye MB (2017) A novel non-canonical Wnt signature for prostate cancer aggressiveness. Oncotarget 8(6): 9572–9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Kayama H, Shojima K, Matsumoto S, Koyama H, Minami Y, Nojima S, Morii E, Honda H, Takeda K, Kikuchi A (2015) The Wnt5a-Ror2 axis promotes the signalling circuit between interleukin-12 and interferon-gamma in colitis. Sci Rep 5: 10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambony A, Wedlich D (2007) Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signalling pathway. Dev Cell 12(5): 779–792. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A (2017) Cancer Statistics, 2017. Cancer J Clin 67(1): 7–30. [DOI] [PubMed] [Google Scholar]
- Stricker S, Rauschenberger V, Schambony A (2017) ROR-family receptor tyrosine kinases. Curr Top Dev Biol 123: 105–142. [DOI] [PubMed] [Google Scholar]
- Szebeni GJ, Vizler C, Kitajka K, Puskas LG (2017) Inflammation and cancer: extra- and intracellular determinants of tumour-associated macrophages as tumour promoters. Mediators Inflamm 2017: 9294018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele S, Gobel A, Rachner TD, Fuessel S, Froehner M, Muders MH, Baretton GB, Bernhardt R, Jakob F, Gluer CC, Bornhauser M, Rauner M, Hofbauer LC (2015) WNT5A has anti-prostate cancer effects in vitro and reduces tumour growth in the skeleton in vivo. J Bone Miner Res 30(3): 471–480. [DOI] [PubMed] [Google Scholar]
- Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM (2002) Wnt5a signalling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 1(3): 279–288. [DOI] [PubMed] [Google Scholar]
- Weisser SB, van Rooijen N, Sly LM (2012) Depletion and reconstitution of macrophages in mice. J Vis Exp 66: 4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CY, Yang YH, Lin YY, Kuan FC, Lin YS, Lin WY, Tsai MY, Yang JJ, Cheng YC, Shu LH, Lu MC, Chen YJ, Lee KD, Kang HY (2017) Anti-cancer effect of danshen and dihydroisotanshinone I on prostate cancer: targeting the crosstalk between macrophages and cancer cells via inhibition of the STAT3/CCL2 signalling pathway. Oncotarget 8(25): 40246–40263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Oue N, Sato A, Hasegawa Y, Yamamoto H, Matsubara A, Yasui W, Kikuchi A (2010) Wnt5a signalling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene 29(14): 2036–2046. [DOI] [PubMed] [Google Scholar]
- Zhang J, Patel L, Pienta KJ (2010) CC chemokine ligand 2 (CCL2) promotes prostate cancer tumourigenesis and metastasis. Cytokine Growth Factor Rev 21(1): 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Bu X, Wang W, Ma T, Ma H (2014. a) GEC-derived SFRP5 inhibits Wnt5a-induced macrophage chemotaxis and activation. PLoS One 9(1): e85058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wang CL, Li RM, Hui TQ, Su YY, Yuan Q, Zhou XD, Ye L (2014. b) Wnt5a promotes inflammatory responses via nuclear factor kappaB (NF-kappaB) and mitogen-activated protein kinase (MAPK) pathways in human dental pulp cells. J Biol Chem 289(30): 21028–21039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Baek SH, Bourk EM, Ohgi KA, Garcia-Bassets I, Sanjo H, Akira S, Kotol PF, Glass CK, Rosenfeld MG, Rose DW (2006) Macrophage/cancer cell interactions mediate hormone resistance by a nuclear receptor derepression pathway. Cell 124(3): 615–629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.