Abstract
Background:
Breast cancer can negatively influence working life, but it is unclear how many working years women with breast cancer can expect to lose.
Methods:
Women diagnosed with breast cancer between 1997 and 2012 were identified in the Breast Cancer Data Base Sweden (N=19 661), together with breast cancer-free comparison women (N=81 303). Using flexible parametric survival modelling, the loss in working years was calculated as the difference in the remaining years in the work force between women with and without breast cancer.
Results:
Women aged 50 years at diagnosis with stage I disease lost on average 0.5 years (95% CI, 0.2–0.7) of their remaining working time; the corresponding estimates were 0.9 years (0.5–1.2) in stage II, 2.5 years (1.9–3.1) in stage III and 8.1 years (6.5–9.7) in stage IV. Women with in situ breast cancer did not lose any working years. The strongest treatment determinant was axillary lymph node dissection.
Conclusions:
We found a loss in working years not only in late but also in early-stage breast cancer. Although it is reassuring that some groups had no or only a modest work loss, the economic consequences for society are considerable given the large number of women annually diagnosed with breast cancer.
Keywords: breast cancer, employment, disability pension, retirement, sick leave, Sweden, survival
More than half of all women with breast cancer are diagnosed during working age. In Europe, around 250 000 women under age 65 years are annually diagnosed with breast cancer (Ferlay et al, 2013). Survival rates have increased, and in many European countries, the 5-year relative survival in women aged 45–64 years today exceeds 90% (Sant et al, 2015), resulting in an increasing number of breast cancer survivors of working age. Owing to recurrent disease or side effects, many women face challenges in subsequent working life. Results from earlier studies show that breast cancer survivors are at an increased risk of losing paid employment (Carlsen et al, 2008a; Paalman et al, 2016), receiving sickness benefits (Eaker et al, 2011; Lundh et al, 2014; Kvillemo et al, 2017) and disability pension (Carlsen et al, 2008b; Eaker et al, 2011; Hauglann et al, 2012; Lundh et al, 2014; Kvillemo et al, 2017) despite a strong desire and an economic need to re-engage in paid work after completed treatment.
Most previous studies on the work situation after a breast cancer diagnosis have used a relative measure such as the hazard ratio to describe the association. Although such a measure has its advantages, it can be difficult to interpret. In this study, we present a new measure estimating the difference between the remaining years in the work force among women with and without breast cancer. The concept of this measure builds upon a recently presented method estimating the loss in expectation of life due to cancer (Andersson et al, 2013; Dehbi et al, 2017), and answers the question ‘On average, how many working years can a woman expect to lose as a consequence of her breast cancer?’.
Using a large population-based cohort, we estimated the loss in working years following a breast cancer diagnosis. We hypothesised that women with breast cancer lose more working years than women without breast cancer, irrespective of disease stage. We also examined the influence of different treatment modalities.
Methods
Data sources and study population
We included women with breast cancer identified in the Breast Cancer Data Base Sweden (BCBaSe). BCBaSe is based upon three Breast Cancer Quality Registers (from the Stockholm-Gotland, the Uppsala-Örebro and the Northern regions), which together cover a source population of approximately 60% of the Swedish population. The registers contain detailed clinical data on patient and tumour characteristics, clinical stage and treatment. In a comparison with the National Swedish Cancer Register to which reporting is mandated by law, the completeness of the registers exceeds 99% (National Breast Cancer Quality Register of Sweden, 2014). In BCBaSe, the breast cancer quality registers have been cross-linked to several registers: the Longitudinal Integration Database for Health Insurance and Labour Market Studies managed by Statistics Sweden, which provided data on education, labour force participation and old-age pension; the MiDAS database administered by the Swedish Social Insurance Agency, which was used to retrieve information on sick leave and disability pension; the Patient Register, held by the National Board of Health and Welfare, from which we obtained data regarding comorbidities, further classified into Charlson’s Comorbidity Index (Charlson et al, 1987); the Total Population Register that provided data on vital status and was used to randomly select a comparison cohort of breast cancer-free women, matched on birth year and county of residency in a ratio of 1:5.
In the present study, data in BCBaSe was analysed for women aged up to 60 years at diagnosis. To allow for a sufficiently long follow-up until the official age of retirement in Sweden at age 65 years, women aged ⩽55 years diagnosed between 1 January 1997 and 31 December 2012, and women aged 56–60 years diagnosed between 1 January 2003 and 31 December 2012 were chosen as the study population. We restricted the analysis to women working before diagnosis (defined as having an income from work in the calendar year before diagnosis, no disability pension (⩾75%) at the time of diagnosis and no old-age retirement in the year of diagnosis), resulting in a final study population of 19 661 women with breast cancer and 81 303 breast cancer-free women.
Outcomes
All women were followed from the year of diagnosis until permanent exit from the labour market or censoring (reaching age 65 years, emigration or end of follow-up 31 December 2012), whichever came first. Permanent exit from the labour market was defined as receipt of disability pension of at least 75%, old-age retirement or death. Disability pension is granted by the Swedish Social Insurance Agency in case of permanent reduction of working ability due to disease or injury and can be given full or part time. As <1% return to work after being granted disability pension (Swedish Social Insurance Agency, 2007), we considered receipt of disability pension as a permanent exit from the labour market. As previously proposed (Svensson et al, 2015), old-age retirement was defined as the year when the annual income from old-age pension exceeded the annual income from pensionable earnings. Although the official retirement age is 65 years, earnings-related pensions can be obtained from age 61 years. For a women working at the age of 47 years, the average age at exit from the labour market in 2004 was 62.6 years (Swedish Social Insurance Agency, 2006).
To also include other potential reasons for permanent exit from the labour market, we performed a sensitivity analysis in which we defined permanent exit as the year when the annual income from labour earnings decreased to zero. With this approach, only individuals with potential follow-up until age 65 years could be included.
In a separate analysis, we also estimated the additional number of days lost from work because of sick leave (paid by the Swedish Social Insurance Agency).
Statistical analysis
We applied a period survival approach, which has been found to be superior in describing survival experiences of more recently diagnosed subjects, using the most recent data to describe short-term survival with older data only contributing to long-term estimates (Brenner and Hakulinen, 2009). The period was set to 1 January 2008–31 December 2012.
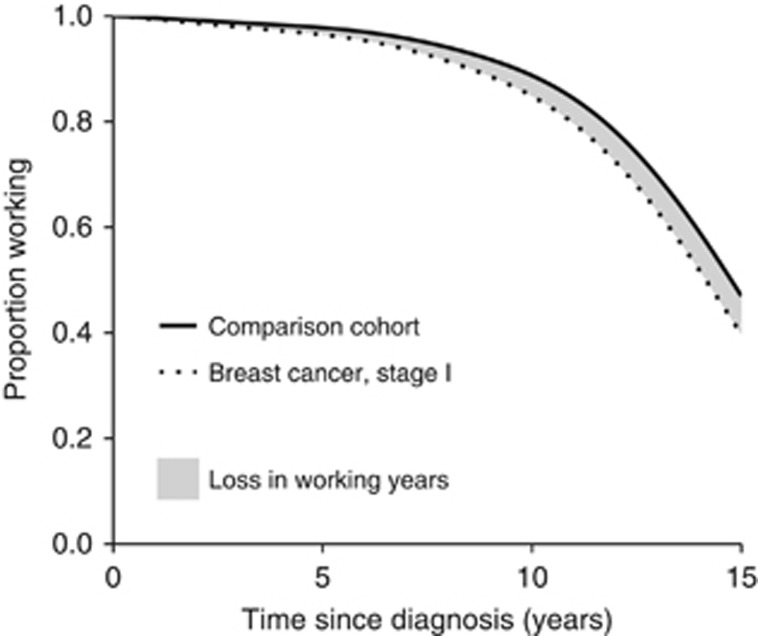
The outcome measure of interest was the average working time until age 65 years, referred to as the restricted mean survival time (RMST), which is a measure of the average survival time between two specified time points (Royston and Parmar, 2013). In this study, RMST was calculated between the date of diagnosis and date of turning 65 years (for specific ages). We calculated the difference in RMST between women with and without breast cancer, corresponding to the area between the two survival curves (Figure 1).
Figure 1.
Graphical illustration of the concept loss in working years comparing women with breast cancer (stage I) aged 50 years at diagnosis with breast cancer-free women of the same age.
Flexible parametric survival models were used to predict the RMST while taking time-dependent effects into account (Royston, 2015). We included age at diagnosis, stage at diagnosis (classified according to the UICC TNM classification Seventh edition) (Sobin et al, 2009) and highest level of education (low (⩽9 years), middle (10–12 years) and high (⩾13 years)) in the models, together with interactions between age and stage and between age and education. In a separate analysis, treatment modality was also added to the model, with interactions between endocrine therapy and chemotherapy, and between type of surgery and axillary lymph node dissection. We modelled age continuously using restricted cubic splines and included time-varying effects of age and stage at diagnosis, using 5 degrees of freedom for the baseline function and 2 degrees of freedom for time-varying effects. All models were parameterised so that different combinations of stage and treatment always were compared with breast cancer-free women (McKnight et al, 1999). From the models, we predicted estimates of RMST and difference in RMST for each stage at age 50, 55 and 60 years. Estimates from models including treatment modalities were predicted for age 55 years. All analyses were complete-case analyses.
Using a competing risk approach, we also examined the primary reasons for exit from the labour market. A flexible parametric survival model that incorporated the three outcomes simultaneously was fitted and the cumulative incidence function for each cause was calculated.
To estimate the additional days lost because of sick leave, we calculated the mean difference in days lost in the first 3 years after diagnosis between women with and without breast cancer using multivariable linear regression with non-parametric bootstrapping.
As the matching was conditioned on breast cancer-free control women being alive at the end of the calendar year the corresponding women with breast cancer were diagnosed, we also checked the expected mortality among control women in this period using national mortality statistics. Less than 0.5% of control women were expected to die in this period, making this a negligible issue.
Data preparations were performed using R version 3.2.4 and statistical analysis using Stata version 14.1 (StataCorp, College Station, TX, USA). The stpm2 package was used for fitting flexible parametric survival models (Lambert and Royston, 2009).
The study was approved by the Ethical Review Board at Karolinska Institutet, Stockholm. The need for individual consent was waived as the study is based on routinely collected data.
Results
Of the 19 661 women with breast cancer, the majority was diagnosed with stage I (37%) or stage II (33%) breast cancer (Table 1). The baseline characteristics of women with breast cancer were similar to breast cancer-free women, except for a higher proportion of women in the highest education level among women with breast cancer (44 vs 40%).
Table 1. Baseline characteristics of women with breast cancer and breast cancer-free comparison women aged ⩽60 years at diagnosis in the Breast Cancer Data Base Sweden.
| Characteristic | Women with breast cancer, N (%) | Breast cancer-free women, N (%) |
|---|---|---|
| Total | 19 661 (100) | 81 303 (100) |
| Age at diagnosis (years) | ||
| Median (IQR) | 50 (45–54) | 50 (45–54) |
| Year of diagnosisa | ||
| 1997–1999 | 3019 (15) | 12 847 (16) |
| 2000–2004 | 5783 (29) | 24 253 (30) |
| 2005–2009 | 6717 (34) | 27 298 (34) |
| 2010–2012 | 4142 (21) | 16 905 (21) |
| Education | ||
| Low (0–9 years) | 2164 (11) | 10 071 (12) |
| Middle (10–12 years) | 8874 (45) | 38 382 (47) |
| High (>12 years) | 8585 (44) | 32 660 (40) |
| Missing | 38 (0) | 190 (0) |
| Unemployment record during the 5 years before diagnosis | ||
| No | 15 447 (79) | 62 737 (77) |
| Yes | 4214 (21) | 18 566 (23) |
| Sick days 1-3 years before diagnosisb | ||
| 0 | 14 651 (75) | 60 442 (74) |
| >0–30 | 1309 (7) | 5570 (7) |
| >30–90 | 1551 (8) | 6189 (8) |
| >90–180 | 675 (3) | 2830 (3) |
| >180–360 | 663 (3) | 2854 (4) |
| >360 | 812 (4) | 3418 (4) |
| Comorbidity during the 10 years before diagnosis | ||
| CCI 0 | 18 856 (96) | 78 198 (96) |
| CCI 1 | 433 (2) | 1761 (2) |
| CCI 2+ | 372 (2) | 1344 (2) |
| Pathological TNM stage | ||
| 0 (in situ) | 2218 (11) | |
| I | 7301 (37) | |
| II | 6535 (33) | |
| III | 2197 (11) | |
| IV | 340 (2) | |
| Missing | 1070 (5) |
Abbreviations: CCI=Charlson’s comorbidity index; IQR=interquartile range; TNM=Tumour, Node, Metastasis.
Index year for breast cancer-free women.
Sick days include days on sick leave or disability pension compensated by the Swedish Social Insurance Agency.
Table 2 shows the estimated remaining working time until age 65 years and the loss in working years by age at diagnosis and stage of disease. The loss in working years was most pronounced in women of younger ages and in women with advanced disease. For example, for a woman aged 50 years at diagnosis the estimated loss in working years compared with breast cancer-free women was 0.5 years (95% confidence interval (CI), 0.2–0.7 years) in stage I and 8.1 years in stage IV disease (95% CI, 6.5–9.7). Corresponding hazard ratios are presented in Supplementary Figure 1. In a sensitivity analysis defining permanent exit as the year when the annual income from labour earnings decreased to zero, the results were similar to the main analysis (Supplementary Table 1).
Table 2. Average working time until age 65 years and average loss in working years by age and stage at diagnosis.
|
Age 50 years |
Age 55 years |
Age 60 years |
||||
|---|---|---|---|---|---|---|
| Average working time until age 65 years (years) | Loss in working yearsa (95% CI) | Average working time until age 65 years (years) | Loss in working yearsa (95% CI) | Average working time until age 65 years (years) | Loss in working yearsa (95% CI) | |
| No BCa | 13.3 | Ref. | 8.5 | Ref. | 3.9 | Ref. |
| In situ | 13.3 | −0.1 (−0.5 to 0.4) | 8.2 | 0.3 (0.0 to 0.6) | 3.9 | 0.0 (−0.3 to 0.3) |
| Stage I | 12.8 | 0.5 (0.2 to 0.7) | 8.3 | 0.2 (0.0 to 0.4) | 3.8 | 0.1 (−0.1 to 0.2) |
| Stage II | 12.4 | 0.9 (0.5 to 1.2) | 8.1 | 0.4 (0.2 to 0.6) | 3.6 | 0.3 (0.1 to 0.4) |
| Stage III | 10.8 | 2.5 (1.9 to 3.1) | 7.3 | 1.2 (0.9 to 1.6) | 3.6 | 0.3 (0.0 to 0.6) |
| Stage IV | 5.1 | 8.1 (6.5 to 9.7) | 3.9 | 4.6 (3.8 to 5.4) | 1.4 | 2.5 (1.9 to 3.1) |
Abbreviations: BCa=breast cancer; CI=confidence interval.
Measured as the difference in average working time until age 65 years between women with breast cancer and without breast cancer.
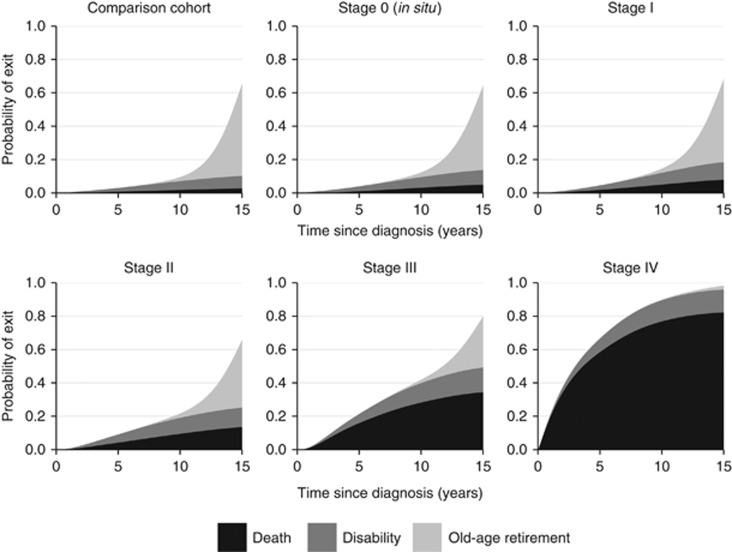
The probability of leaving the labour market by primary reason for exit in women aged 50 years at diagnosis is presented in Figure 2. Fifteen years after diagnosis, corresponding to age 65 years, the percentage of breast cancer-free women who had left the labour market was 65%, compared with 64% (in situ breast cancer), 68% (stage I), 66% (stage II), 80% (stage III) and 98% (stage IV). Women with breast cancer had a higher probability of exit due to disability pension and death. For example, 7% of breast cancer-free women had left the labour market due to disability, compared with 9% (in situ), 11% (stage I), 12% (stage II), 15% (stage III) and 14% (stage IV).
Figure 2.
Probability of exit from the labour market by primary reason for exit and stage at diagnosis for women aged 50 years at diagnosis.
We also examined additional days lost from work because of sick leave and found that women aged 50 years at diagnosis lost between 159 (stage I) and 609 (stage IV) days because of sick leave in the first 3 years after diagnosis (Supplementary Table 2).
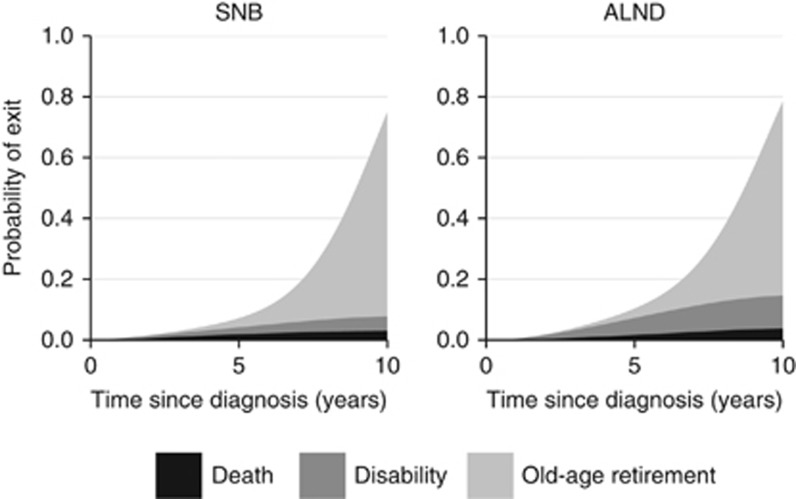
We further investigated the impact of different treatment modalities on the loss in working years in women aged 55 years at diagnosis with stage I (Table 3) and stage II breast cancer (Supplementary Table 3). Axillary lymph node dissection had the largest influence on the estimates, with up to on average 7 months increased loss of the remaining working time compared with women treated with sentinel node biopsy only. When comparing primary reasons for exit from the labour market by type of axillary surgery, we found that women treated with axillary lymph node dissection had an increased probability of leaving the labour market due to disability (Figure 3).
Table 3. Average working time until age 65 years and average loss in working years in women aged 55 years at diagnosis with stage I breast cancer by type of treatment.
|
With SNB only |
With ALND |
|||||
|---|---|---|---|---|---|---|
| Percentage with treatment | Average working time until age 65 years (years) | Loss in working yearsa (95% CI) | Percentage with treatment | Average working time until age 65 years (years) | Loss in working yearsa (95% CI) | |
| No BCa | 8.5 | Ref. | 8.5 | Ref. | ||
| Breast-conserving surgery | ||||||
| +RT | 4 | 8.4 | 0.1 (−0.2 to 0.4) | 13 | 8.3 | 0.2 (0.0 to 0.5) |
| +RT, ET | 22 | 8.4 | 0.1 (−0.1 to 0.3) | 21 | 8.3 | 0.2 (0.0 to 0.4) |
| +RT, CHEMO, ET | 4 | 8.5 | 0.0 (−0.4 to 0.4) | 3 | 8.4 | 0.1 (−0.3 to 0.5) |
| +RT, CHEMO | 3 | 8.0 | 0.5 (0.1 to 1.0) | 4 | 7.8 | 0.7 (0.3 to 1.1) |
| Mastectomy | ||||||
| +ET | 3 | 8.5 | 0.0 (−0.6 to 0.5) | 6 | 8.0 | 0.5 (0.2 to 0.8) |
| +CHEMO, ET | 1 | 8.7 | −0.2 (−0.7 to 0.4) | 1 | 8.2 | 0.3 (−0.1 to 0.8) |
| +CHEMO | 1 | 8.1 | 0.4 (−0.3 to 1.1) | 1 | 7.5 | 1.0 (0.5 to 1.4) |
Abbreviations: ALND=axillary lymph node dissection; BCa=breast cancer; CHEMO=chemotherapy; CI=confidence interval; ET=endocrine therapy; RT=radiotherapy; SNB=sentinel node biopsy.
Measured as the difference in average working time until age 65 years between women with breast cancer and without breast cancer.
Figure 3.
Probability of exit from the labour market by primary reason for exit and type of axillary surgery for women aged 55 years at diagnosis with stage I breast cancer. ALND=axillary lymph node dissection; SNB=sentinel node biopsy.
Discussion
In this large population-based study, we estimated the loss in working years after breast cancer in women of working age. The loss in working years varied according to age and stage at diagnosis and was most substantial in women with advanced-stage disease. In women aged 50 years at diagnosis, the estimated loss in working years was on average 0.5 years (stage I), 0.9 years (stage II), 2.5 years (stage III) and 8.1 years (stage IV).
Two common exit pathways from the labour market include disability pension and old-age retirement. In previous studies, women with breast cancer were found to have a 2.7-fold increased risk of disability pension (Hauglann et al, 2012) and a 1.2-fold increased risk of early old-age retirement (Taskila-Abrandt et al, 2005) compared with cancer-free controls. However, the meaning of this increased risk is difficult to interpret. To our knowledge, our study is the first to quantify the work loss in a unit that is directly interpretable. Our measure provides additional information to traditional survival statistics and is of special importance for young and middle-aged women with early-stage disease who have many years left in the work force.
Corroborating previous research (Eaker et al, 2011; Hauglann et al, 2012; Hoyer et al, 2012; Paalman et al, 2016), treatment modality was a determinant of loss in working years, with axillary lymph node dissection having the largest influence on our estimates. The most common treatment in women with stage I tumours, consisting of breast-conserving surgery, sentinel node biopsy, radiotherapy and endocrine therapy, did not result in any significant loss in working years among women aged 55 years at diagnosis, which is reassuring. However, women treated with axillary lymph node dissection did on average lose between 1 and 7 months more of their remaining working time compared with women treated with sentinel node biopsy only, possibly explained by the known side effects of axillary dissection such as lymphedema (Shih et al, 2009) and chronic pain (Mejdahl et al, 2013). In women treated with mastectomy, the effect of axillary lymph node dissection was larger than among women treated with breast-conserving surgery, indicating that a more extensive surgery increases the risk of work loss. During the calendar period under study, axillary dissection was replaced by sentinel node biopsy as the standard method for evaluating nodal status. Our findings confirm that axillary lymph node dissection in node-negative early-stage breast cancer can lead to an unnecessary physical, psychosocial and financial burden (Lyman et al, 2014). Whereas chemotherapy had no large effect on the estimates of loss in working years among women receiving endocrine therapy, women receiving chemotherapy but not endocrine therapy had a more substantial loss in working years. This suggests that it is not the receipt of chemotherapy that causes the work loss but rather underlying disease characteristics affecting prognosis.
The measure of focus in our study does not include temporary absence from work, such as sick leave or unemployment. Previous studies have found that women are away from work on average for nearly a year after diagnosis (Roelen et al, 2009; Fantoni et al, 2010), a time period that was not covered in our calculation. However, we estimated the additional time away from work due to sick leave in a separate analysis and found that, in the first 3 years after diagnosis, women aged 50 years at diagnosis lost on average between 0.4 (stage I) and 1.7 (stage IV) additional years because of sick leave. The total amount of absence from work due to breast cancer including also temporary absence is thus larger than estimated in our main analysis.
The major strength of our study was the population-based design, allowing inclusion of virtually all women with breast cancer within the capture areas. By means of record linkage, we obtained complete and prospectively collected follow-up data. We also had detailed information on possible confounders and effect modifiers, including treatment modalities. Our study has some limitations. To calculate the loss in working years without following every individual until retirement, we assumed that disability pension, old-age retirement and death were the only pathways out of the labour market and that exit was permanent. These assumptions may not hold for all individuals. However, our estimates of remaining working time until age 65 years in the breast cancer-free control group were similar in a sensitivity analysis with a different definition of exit and correspond well with national figures (Swedish Social Insurance Agency, 2006), confirming our assumptions.
Another issue is the generalisability of our findings to other countries and to other types of cancer. Given similar patterns of treatment, we have no reason to believe that the long-term sequelae after breast cancer differ between countries, but the likelihood to leave the labour market might be different. Our findings are based on data from a setting with a generous social insurance system covering all citizens and are likely to be generalisable to countries with similar systems but not necessarily to all countries. Our findings are also not generalisable to other types of cancer, as they in part reflect sequelae that are specific to treatment for breast cancer (i.e., arm symptoms related to axillary lymph node dissection). The method to estimate loss in working years is, however, applicable for other types of cancer.
In conclusion, by use of a new measure we show a loss in working years not only in late but also in early-stage breast cancer. Although it is reassuring that some groups of women had no or only a modest loss in working years, the economic consequences for society are considerable given the large number of women of working age diagnosed with breast cancer. An increased focus on workplace support may limit the economic loss following a breast cancer diagnosis, both on an individual and on a societal level. Our results should also motivate further efforts to reduce long-term sequelae, for example, by careful consideration of use of axillary lymph node dissection followed by active rehabilitation. Our age- and treatment-specific estimates of loss in working years can also improve the understanding of what women can expect in terms of employment and work after a breast cancer diagnosis.
Acknowledgments
This work was supported by grants from the Swedish Medical Research Council (521-2012-3047), the Swedish Cancer Society (14-0324) and the Swedish Breast Cancer Association (BRO). We also thank the Breast Cancer Quality Register steering groups in Stockholm, Uppsala-Örebro and the Northern region for providing data for this study.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
ML owns Pfizer and AstraZeneca shares. All other authors declare no conflict of interest.
Supplementary Material
References
- Andersson TM, Dickman PW, Eloranta S, Lambe M, Lambert PC (2013) Estimating the loss in expectation of life due to cancer using flexible parametric survival models. Stat Med 32(30): 5286–5300. [DOI] [PubMed] [Google Scholar]
- Brenner H, Hakulinen T (2009) Up-to-date cancer survival: period analysis and beyond. Int J Cancer 124(6): 1384–1390. [DOI] [PubMed] [Google Scholar]
- Carlsen K, Dalton SO, Diderichsen F, Johansen C, Danish Cohort S (2008. a) Risk for unemployment of cancer survivors: a Danish cohort study. Eur J Cancer 44(13): 1866–1874. [DOI] [PubMed] [Google Scholar]
- Carlsen K, Oksbjerg Dalton S, Frederiksen K, Diderichsen F, Johansen C (2008. b) Cancer and the risk for taking early retirement pension: a Danish cohort study. Scand J Public Health 36(2): 117–125. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5): 373–383. [DOI] [PubMed] [Google Scholar]
- Dehbi HM, Royston P, Hackshaw A (2017) Life expectancy difference and life expectancy ratio: two measures of treatment effects in randomised trials with non-proportional hazards. BMJ 357: j2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaker S, Wigertz A, Lambert PC, Bergkvist L, Ahlgren J, Lambe M Uppsala/Orebro Breast Cancer Group (2011) Breast cancer, sickness absence, income and marital status. A study on life situation 1 year prior diagnosis compared to 3 and 5 years after diagnosis. PLos One 6(3): e18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantoni SQ, Peugniez C, Duhamel A, Skrzypczak J, Frimat P, Leroyer A (2010) Factors related to return to work by women with breast cancer in northern France. J Occup Rehabil 20(1): 49–58. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M (2013) GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. International Agency for Research on Cancer: Lyon, France, Available at: http://globocan.iarc.fr (accessed on 3 June 2016). [Google Scholar]
- Hauglann B, Benth JS, Fossa SD, Dahl AA (2012) A cohort study of permanently reduced work ability in breast cancer patients. J Cancer Surviv 6(3): 345–356. [DOI] [PubMed] [Google Scholar]
- Hoyer M, Nordin K, Ahlgren J, Bergkvist L, Lambe M, Johansson B, Lampic C (2012) Change in working time in a population-based cohort of patients with breast cancer. J Clin Oncol 30(23): 2853–2860. [DOI] [PubMed] [Google Scholar]
- Kvillemo P, Mittendorfer-Rutz E, Branstrom R, Nilsson K, Alexanderson K (2017) Sickness absence and disability pension after breast cancer diagnosis: a 5-year nationwide cohort study. J Clin Oncol 35(18): 2044–2052. [DOI] [PubMed] [Google Scholar]
- Lambert PC, Royston P (2009) Further development of flexible parametric models for survival analysis. Stata J 9(2): 265–290. [Google Scholar]
- Lundh MH, Lampic C, Nordin K, Ahlgren J, Bergkvist L, Lambe M, Berglund A, Johansson B (2014) Sickness absence and disability pension following breast cancer - a population-based matched cohort study. Breast 23(6): 844–851. [DOI] [PubMed] [Google Scholar]
- Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, Weaver DL, Benson AB 3rd, Bosserman LD, Burstein HJ, Cody H 3rd, Hayman J, Perkins CL, Podoloff DA, Giuliano AE American Society of Clinical Oncology Clinical Practice (2014) Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 32(13): 1365–1383. [DOI] [PubMed] [Google Scholar]
- McKnight B, Cook LS, Weiss NS (1999) Logistic regression analysis for more than one characteristic of exposure. Am J Epidemiol 149(11): 984–992. [DOI] [PubMed] [Google Scholar]
- Mejdahl MK, Andersen KG, Gartner R, Kroman N, Kehlet H (2013) Persistent pain and sensory disturbances after treatment for breast cancer: six year nationwide follow-up study. BMJ 346: f1865. [DOI] [PubMed] [Google Scholar]
- National Breast Cancer Quality Register of Sweden (2014) Annual report. [In Swedish] Available at: http://www.cancercentrum.se.
- Paalman CH, van Leeuwen FE, Aaronson NK, de Boer AG, van de Poll-Franse L, Oldenburg HS, Schaapveld M (2016) Employment and social benefits up to 10 years after breast cancer diagnosis: a population-based study. Br J Cancer 114(1): 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelen CA, Koopmans PC, de Graaf JH, Balak F, Groothoff JW (2009) Sickness absence and return to work rates in women with breast cancer. Int Arch Occup Environ Health 82(4): 543–546. [DOI] [PubMed] [Google Scholar]
- Royston P (2015) Estimating the treatment effect in a clinical trial using difference in restricted mean survival time. Stata J 15(4): 1098–1117. [Google Scholar]
- Royston P, Parmar MK (2013) Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol 13: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant M, Chirlaque Lopez MD, Agresti R, Sanchez Perez MJ, Holleczek B, Bielska-Lasota M, Dimitrova N, Innos K, Katalinic A, Langseth H, Larranaga N, Rossi S, Siesling S, Minicozzi P Group E-W (2015) Survival of women with cancers of breast and genital organs in Europe 1999-2007: Results of the EUROCARE-5 study. Eur J Cancer 51(15): 2191–2205. [DOI] [PubMed] [Google Scholar]
- Shih YC, Xu Y, Cormier JN, Giordano S, Ridner SH, Buchholz TA, Perkins GH, Elting LS (2009) Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol 27(12): 2007–2014. [DOI] [PubMed] [Google Scholar]
- Sobin L, Gospodarowicz M, Wittekind C (2009) TNM Classification of Malignant Tumours (ed 7). Wiley: Hoboken, NJ, USA. [Google Scholar]
- Swedish Social Insurance Agency (2006) Genomsnittlig Pensionsålder i de Nordiska Länderna: Med Internationell Utblick. Swedish Social Insurance Agency: Stockholm, Sweden, [In Swedish]. Available at: http://www.fk.se/filer/publikationer/pdf/ana0611.pdf. [Google Scholar]
- Swedish Social Insurance Agency (2007) Vägen Tillbaka: En Beskrivande Studie av Flödet ut Från Sjuk- Och Aktivitetsersättning. Swedish Social Insurance Agency: Stockholm, Sweden, [In Swedish]. Available at: http://www.forsakringskassan.se/filer/publikationer/pdf/ana0712.pdf. [Google Scholar]
- Svensson I, Lundholm E, De Luna X, Malmberg G (2015) Family life course and the timing of women’s retirement - a sequence analysis approach. Popul Space Place 21(8): 856–871. [Google Scholar]
- Taskila-Abrandt T, Pukkala E, Martikainen R, Karjalainen A, Hietanen P (2005) Employment status of Finnish cancer patients in 1997. Psychooncology 14(3): 221–226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.