Abstract
BACKGROUND
Both balanced crystalloids and saline are used for intravenous fluid administration in critically ill adults, but it is not known which results in better clinical outcomes.
METHODS
In a pragmatic, cluster-randomized, multiple-crossover trial conducted in five intensive care units at an academic center, we assigned 15,802 adults to receive saline (0.9% sodium chloride) or balanced crystalloids (lactated Ringer’s solution or Plasma-Lyte A) according to the randomization of the unit to which they were admitted. The primary outcome was a major adverse kidney event within 30 days — a composite of death from any cause, new renal-replacement therapy, or persistent renal dysfunction (defined as an elevation of the creatinine level to ≥200% of baseline) — all censored at hospital discharge or 30 days, whichever occurred first.
RESULTS
Among the 7942 patients in the balanced-crystalloids group, 1139 (14.3%) had a major adverse kidney event, as compared with 1211 of 7860 patients (15.4%) in the saline group (marginal odds ratio, 0.91; 95% confidence interval [CI], 0.84 to 0.99; conditional odds ratio, 0.90; 95% CI, 0.82 to 0.99; P = 0.04). In-hospital mortality at 30 days was 10.3% in the balanced-crystalloids group and 11.1% in the saline group (P = 0.06). The incidence of new renal-replacement therapy was 2.5% and 2.9%, respectively (P = 0.08), and the incidence of persistent renal dysfunction was 6.4% and 6.6%, respectively (P = 0.60).
CONCLUSIONS
Among critically ill adults, the use of balanced crystalloids for intravenous fluid administration resulted in a lower rate of the composite outcome of death from any cause, new renal-replacement therapy, or persistent renal dysfunction than the use of saline. (Funded by the Vanderbilt Institute for Clinical and Translational Research and others; SMART-MED and SMART-SURG ClinicalTrials.gov numbers, NCT02444988 and NCT02547779.)
Intravenous crystalloid solutions are commonly administered in critical care, yet the question of whether crystalloid composition affects patient outcomes remains unanswered.1 Historically, 0.9% sodium chloride (saline) has been the most commonly administered intravenous fluid.2,3 Data suggest that intravenous saline may be associated with hyperchloremic metabolic acidosis,4 acute kidney injury,5 and death.6,7 Crystalloid solutions with electrolyte compositions closer to that of plasma (balanced crystalloids, such as lactated Ringer’s solution or Plasma-Lyte A) represent an increasingly used alternative to saline.8 Several observational studies6,9,10 and a before-and-after trial5 suggested that the use of balanced crystalloids is associated with lower rates of acute kidney injury, renal-replacement therapy, and death. However, in two pilot trials,11,12 no significant difference in any patient outcome was reported between those who received balanced crystalloids and those who received saline.
To determine the effect of isotonic crystalloid composition on clinical outcomes in critically ill adults, we conducted the Isotonic Solutions and Major Adverse Renal Events Trial (SMART), which compared the use of balanced crystalloids with the use of saline in patients in medical (SMART-MED) and nonmedical (SMART-SURG) intensive care units (ICUs). We hypothesized that the use of balanced crystalloids would result in a lower overall incidence of death, new renal-replacement therapy, and persistent renal dysfunction than saline.
METHODS
TRIAL DESIGN AND OVERSIGHT
We conducted a pragmatic, unblinded, cluster-randomized, multiple-crossover trial in which the use of balanced crystalloids was compared with saline for intravenous fluid administration among critically ill adults admitted to five ICUs at Vanderbilt University Medical Center between June 1, 2015, and April 30, 2017. The trial was approved by the institutional review board at Vanderbilt University with a waiver of informed consent (see the Supplementary Appendix, available with the full text of this article at NEJM.org), was registered online before initiation, and was overseen by an independent data and safety monitoring board. The protocol, available at NEJM.org, and the statistical analysis plan were published before the conclusion of enrollment.13 All authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.
TRIAL SITES AND PATIENT POPULATION
All adults (18 years of age or older) who were admitted to a participating ICU during the trial period were enrolled at the time of ICU admission (site characteristics are described in the Supplementary Appendix). Enrolled patients who were discharged from the hospital were eligible to participate again if they were readmitted to a participating ICU. We assessed the effect of repeat hospitalizations in individual patients in sensitivity analyses. Patients who were admitted to a non-ICU ward from the emergency department were enrolled in a separate trial (Saline against Lactated Ringer’s or Plasma-Lyte in the Emergency Department [SALT-ED]) in which balanced crystalloids and saline were compared among adults who were not critically ill. The results of that trial are also reported in this issue of the Journal.14
RANDOMIZATION
For each month of the trial, participating ICUs were assigned to use either balanced crystalloids or saline for any intravenous administration of isotonic crystalloid. ICUs were randomly assigned to use saline during even-numbered months and balanced crystalloids during odd-numbered months, or vice versa (Fig. S1 in the Supplementary Appendix). To allow coordination of crystalloid use between ICUs and the emergency department and operating rooms, the three ICUs that admit the majority of patients from the emergency department underwent randomization together, as did the two ICUs that admit the majority of patients from operating rooms.13 Patients, clinicians, and investigators were aware of group assignments.
TREATMENTS
Patients in the saline group received 0.9% sodium chloride when intravenous isotonic crystalloid was administered, whereas patients in the balanced-crystalloids group received either lactated Ringer’s solution or Plasma-Lyte A, according to the preference of the treating clinician (Table S1 in the Supplementary Appendix). An electronic advisor within the electronic order-entry system informed providers about the trial, asked about relative contraindications to the assigned crystalloid, and, if none were present, guided providers to order the assigned crystalloid. Relative contra-indications to the use of balanced crystalloids included hyperkalemia and brain injury. The treating clinician determined the severity of hyperkalemia or brain injury at which saline rather than balanced crystalloids would be used. The unassigned crystalloid was also available from the pharmacy when clinicians believed it to be required for the safe treatment of any patient.
The trial was coordinated with the emergency department and operating rooms so that when feasible, patients being admitted to a participating ICU or receiving a surgical intervention during ICU admission would receive the crystalloid assigned to that ICU.15 The need for access to an intravenous crystalloid at all times precluded the use of washout periods, and patients who remained in the ICU from the end of one calendar month to the start of another may have been exposed to both types of crystalloid. The effect of dual exposure was evaluated in prespecified sensitivity analyses.
DATA COLLECTION
We used data collected in routine care and electronically extracted from electronic health records.12,16 These data included information on pre-enrollment renal function, demographic characteristics, diagnoses, predicted risk of inhospital death, orders for intravenous fluids and blood products, plasma electrolyte and creatinine values, receipt of renal-replacement therapy, and vital status at hospital discharge. Trial personnel who were unaware of group assignment performed manual chart reviews to confirm receipt of renal-replacement therapy and identify indications for new renal-replacement therapy.
OUTCOMES
The primary outcome was the proportion of patients who met one or more criteria for a major adverse kidney event within 30 days16–20 — the composite of death, new receipt of renal-replacement therapy, or persistent renal dysfunction (defined as a final inpatient creatinine value ≥200% of the baseline value) — all censored at hospital discharge or 30 days after enrollment, whichever came first. The National Institute of Diabetes and Digestive and Kidney Diseases work group on clinical trials in acute kidney injury recommends the use of a major adverse kidney event within 30 days as a patient-centered outcome for phase 3 trials.16,18 We determined a value for baseline creatinine level using a previously described hierarchical approach in which creatinine values obtained during the year before hospitalization were given priority over in-hospital measurements obtained before ICU admission. The baseline creatinine level was estimated with a previously described three-variable formula when no pre-enrollment measurements were available (for details, see the Supplementary Appendix).16,21 Patients who had received renal-replacement therapy before enrollment were ineligible to meet the criteria for new renal- replacement therapy or persistent renal dysfunction but could qualify for the primary outcome if they died in the hospital.
Secondary clinical outcomes included in-hospital death before ICU discharge or at 30 days or 60 days, as well as ICU-free days, ventilator-free days, vasopressor-free days, and days alive and free of renal-replacement therapy during the 28 days after enrollment.13 Secondary renal outcomes included new receipt of renal-replacement therapy, persistent renal dysfunction, acute kidney injury of stage 2 or higher as defined in the Kidney Disease: Improving Global Outcomes criteria for creatinine level,22 the highest creatinine level during the hospital stay, the change from baseline to the highest creatinine level, and the final creatinine level before hospital discharge.13
STATISTICAL ANALYSIS
Complete details regarding the sample-size justification have been reported previously.13 Initially, we planned to enroll 8000 patients during 60 unit-months (12 months in five ICUs) to detect a 12% relative between-group difference11,12 in the primary outcome of a major adverse kidney event within 30 days, assuming a 22.0% incidence of the outcome in the saline group on the basis of the findings in a previous report.19 We subsequently obtained observational data for patients admitted to the ICUs involved in the trial in the year before the trial began. These data suggested that the incidence of the outcome in the saline group would be approximately 15.0%. To retain adequate power to detect the targeted difference in relative risk, in collaboration with the data and safety monitoring board, the duration of the trial was increased to 82 unit-months. Enrolling approximately 14,000 patients during 82 unit-months would provide power of 90% at a type I error rate of 0.05 to detect a relative difference of 12% (an absolute difference of 1.9 percentage points) in the primary outcome between groups.13 The data and safety monitoring board conducted two interim analyses; details are provided in the Supplementary Appendix.
Analyses were conducted at the level of each patient’s hospitalization in an intention-to-treat fashion. Continuous variables are reported as means and standard deviations or as medians and interquartile ranges; categorical variables are reported as frequencies and proportions.
The primary analysis compared the incidence of the primary outcome in the balanced-crystalloids and saline groups with a generalized, linear, mixed-effects model that included fixed effects (group assignment, age, sex, race, source of admission, mechanical-ventilation status, vasopressor receipt, diagnosis of sepsis, and diagnosis of traumatic brain injury) and random effects (ICU to which the patient was admitted) (for details, see the Supplementary Appendix).23,24 Both conditional (ICU-level) and marginal (population-level) effects are reported.
Prespecified secondary analyses involved a similar approach. First, we compared secondary outcomes between trial groups. Second, we performed subgroup analyses according to type of ICU, source of admission, receipt of mechanical ventilation, receipt of vasopressors, diagnosis of sepsis or traumatic brain injury (for details, see the Supplementary Appendix), baseline renal function, predicted in-hospital mortality, and total volume of isotonic crystalloid administered through day 30. Third, we conducted sensitivity analyses using alternative approaches to addressing the issue of missing data on baseline creatinine level (for details, see the Supplementary Appendix). Fourth, we performed sensitivity analyses according to the volume of crystalloid administered, accounting for crossover and limiting the analyses to each patient’s first ICU admission.13 Other between-group comparisons were made with the Mann–Whitney rank-sum test for continuous variables and the chi-square test for categorical variables.
A two-sided P value of less than 0.048 indicated statistical significance for the primary outcome after accounting for interim analyses. All other analyses were considered to be hypothesis-generating.13 With 14 secondary outcomes, the likelihood of observing a P value of less than 0.05 for at least one secondary outcome by chance alone was 51.2%. All analyses were performed with the statistical software R, version 3.3.0, with a prespecified analysis code published before the conclusion of enrollment.13
RESULTS
BASELINE CHARACTERISTICS
In all, 15,802 patients from five ICUs were enrolled in the trial (Fig. S2 in the Supplementary Appendix). The median age was 58 years, and 57.6% of patients were men. More than one third of patients were receiving mechanical ventilation and one quarter were receiving vasopressors at enrollment. There were no significant differences in baseline characteristics between the patients assigned to receive balanced crystalloids (7942 patients) and those assigned to receive saline (7860 patients) (Table 1, and Tables S2 and S3 in the Supplementary Appendix).
Table 1.
Participant Characteristics at Baseline.*
| Characteristic | Balanced Crystalloids (N = 7942) | Saline (N = 7860) |
|---|---|---|
| Age — yr | ||
| Median | 58 | 58 |
| Interquartile range | 44–69 | 44–69 |
| Male sex — no. (%) | 4540 (57.2) | 4557 (58.0) |
| White race — no. (%)† | 6384 (80.4) | 6322 (80.4) |
| Weight — kg‡ | ||
| Median | 80 | 79 |
| Interquartile range | 69–96 | 68–95 |
| Coexisting renal conditions — no. (%) | ||
| Chronic kidney disease of stage 3 or higher§ | 1388 (17.5) | 1360 (17.3) |
| Previous receipt of renal-replacement therapy — no. (%) | 384 (4.8) | 402 (5.1) |
| Source of admission to ICU — no. (%) | ||
| Emergency department | 3975 (50.1) | 3997 (50.9) |
| Operating room | 1732 (21.8) | 1649 (21.0) |
| Transfer from another hospital | 1038 (13.1) | 1018 (13.0) |
| Hospital ward | 788 (9.9) | 780 (9.9) |
| Outpatient | 363 (4.6) | 359 (4.6) |
| Another ICU within hospital | 46 (0.6) | 57 (0.7) |
| Diagnosis on ICU admission — no. (%) | ||
| Sepsis or septic shock | 1167 (14.7) | 1169 (14.9) |
| Traumatic brain injury | 698 (8.8) | 665 (8.5) |
| Mechanical ventilation — no. (%) | 2723 (34.3) | 2731 (34.7) |
| Vasopressors — no. (%) | 2094 (26.4) | 2058 (26.2) |
| Mean predicted risk of in-hospital death — % (95% CI)¶ | 9.4 (9.0–9.9) | 9.6 (9.2–10.0) |
| Baseline creatinine level — mg/dl|| | ||
| Median | 0.89 | 0.89 |
| Interquartile range | 0.74–1.10 | 0.74–1.10 |
| Acute kidney injury of stage 2 or higher — no. (%)** | 681 (8.6) | 643 (8.2) |
There were no significant differences in baseline characteristics between the two study groups (P values range from 0.12 to 0.94). To convert the values for creatinine to micromoles per liter, multiply by 88.4. ICU denotes intensive care unit.
Race was reported by patients or their surrogates and recorded in the electronic health record as a part of routine clinical care.
Information on weight at enrollment was missing for 698 patients.
Chronic kidney disease of stage 3 or higher is defined as a glomerular filtration rate less than 60 ml per minute per 1.73 m2, as calculated with the equation developed by the Chronic Kidney Disease Epidemiology Collaboration25 with the patient’s baseline creatinine value.
Predicted risk of in-hospital death is an estimated probability of death before hospital discharge generated through the Vizient database (formerly known as the University HealthSystem Consortium).26 Information on the predicted risk of in-hospital death was missing for 126 patients.
For the purposes of the trial, the baseline creatinine level was defined as the lowest plasma creatinine level measured in the 12 months preceding hospitalization, unless not available, in which case the lowest plasma creatinine level measured between hospitalization and admission to the ICU was used. An estimated creatinine level was used for patients for whom there was no level available from the 12 months before hospitalization to the time of admission to the ICU. Baseline creatinine levels were estimated for a total of 863 patients (10.9%) in the balanced-crystalloids group and 826 patients (10.5%) in the saline group (Table S3 in the Supplementary Appendix).
Acute kidney injury of stage 2 or higher is defined according to the Kidney Disease: Improving Global Outcomes creatinine criteria22 as a first plasma creatinine value after enrollment of at least 200% of the baseline value or both a value greater than 4.0 mg per deciliter (350 μmol per liter) and an increase of at least 0.3 mg per deciliter (27 μmol per liter) from the baseline value.
FLUID THERAPY AND ELECTROLYTES
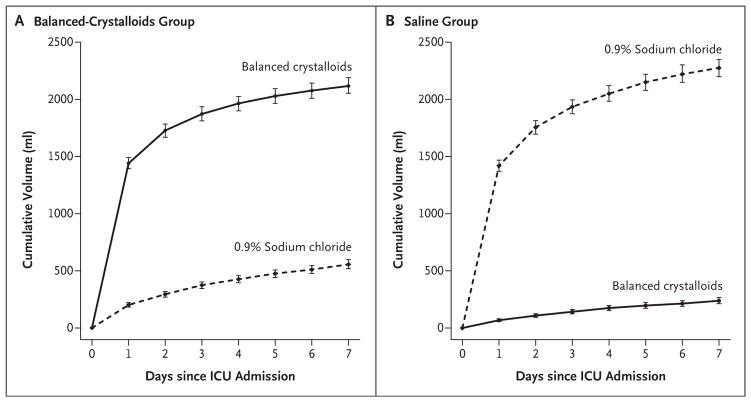
Because the fluid therapy provided in the emergency department and operating room was coordinated with that provided in the ICU to which patients were being admitted, the majority of pre-ICU fluid that patients received was consistent with trial-group assignment (Table S4 in the Supplementary Appendix). The median volume of balanced crystalloids administered to patients in the balanced-crystalloids group between ICU admission and hospital discharge or 30 days (whichever occurred first) was 1000 ml (inter-quartile range, 0 to 3210), and the median volume of 0.9% sodium chloride administered to patients in the saline group was 1020 ml (inter-quartile range, 0 to 3500) (Fig. 1, and Tables S5 and S6 in the Supplementary Appendix). Only 426 patients (5.4%) in the balanced-crystalloids group and 343 patients (4.4%) in the saline group received any volume of unassigned crystalloid as a result of remaining in the ICU from one calendar month to the next (Table S5 in the Supplementary Appendix). There was no significant between-group difference in the median volume of nonisotonic intravenous fluid, blood products, or medications administered (Table S7 in the Supplementary Appendix).
Figure 1. Volume of Intravenous Isotonic Crystalloid Administered According to Group.
The cumulative volume of intravenous balanced crystalloids (solid line) and 0.9% sodium chloride (dotted line) between admission to the intensive care unit (ICU) and hospital discharge is shown for patients in the balanced-crystalloids group (Panel A) and the saline group (Panel B). I bars indicate 95% confidence intervals.
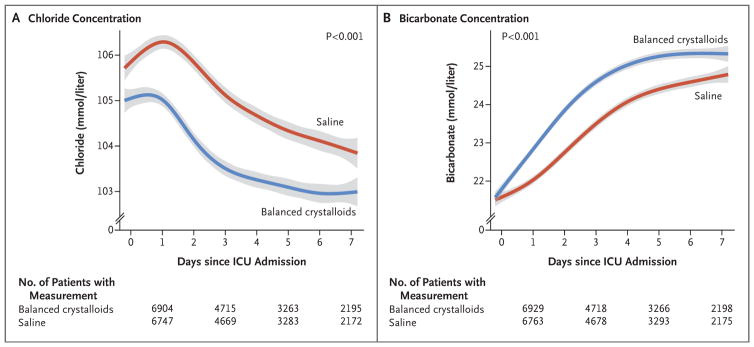
Fewer patients in the balanced-crystalloids group than in the saline group had a measured plasma chloride concentration greater than 110 mmol per liter (24.5% vs. 35.6%, P<0.001) or a plasma bicarbonate concentration less than 20 mmol per liter (35.2% vs. 42.1%, P<0.001) (Fig. 2, and Fig. S3 and Table S8 in the Supplementary Appendix). Differences between groups in chloride and bicarbonate concentration were greater for patients who received larger volumes of isotonic crystalloid (Figs. S4 and S5 in the Supplementary Appendix).
Figure 2. Plasma Chloride and Bicarbonate Concentration According to Group.
The mean and 95% confidence interval (denoted by gray shading) for the first measurement of plasma chloride concentration (Panel A) or bicarbonate concentration (Panel B) on the first 7 days since admission to the intensive care unit (ICU) are shown for patients in the balanced-crystalloids group and in the saline group with locally weighted scatterplot smoothing. Plasma chloride and bicarbonate concentrations were similar between groups at presentation (Table S3 in the Supplementary Appendix), but because fluid therapy in the emergency department and operating room was coordinated with the ICU to which patients were being admitted, plasma chloride concentration differed between the balanced-crystalloids and saline groups at the time of ICU admission.
PRIMARY OUTCOME
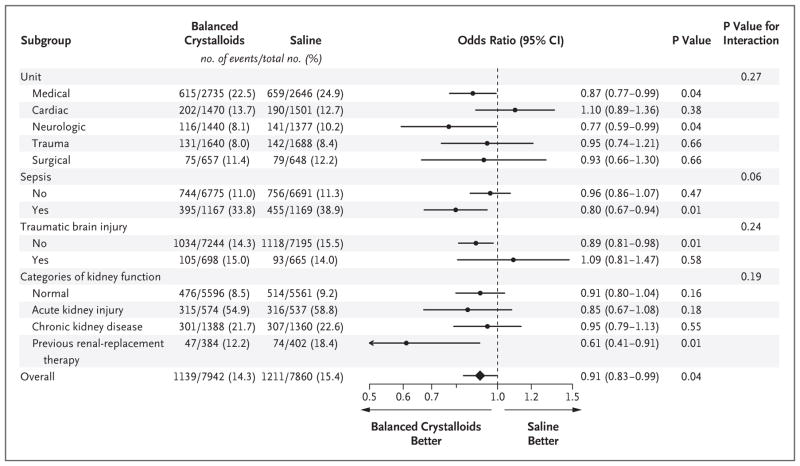
A total of 1139 patients (14.3%) in the balanced-crystalloids group and 1211 patients (15.4%) in the saline group had a major adverse kidney event (marginal odds ratio, 0.91; 95% confidence interval [CI], 0.84 to 0.99; conditional odds ratio, 0.90; 95% CI, 0.82 to 0.99; P = 0.04) (Table 2, and Table S9 and Fig. S6 in the Supplementary Appendix). The results were similar in six pre-specified sensitivity analyses: one was restricted to patients who received 500 ml or more of isotonic crystalloid in the 72 hours after enrollment, a second excluded patients admitted in the week preceding a crossover in the fluid assigned to the ICU, a third excluded patients who transferred between ICUs or remained in the ICU through a crossover, a fourth included only the first ICU admission for each patient, a fifth addressed the issue of missing values for baseline creatinine levels, and a sixth used alternative modeling approaches (odds ratios between 0.87 and 0.93 for all sensitivity analyses; see Table S10 in the Supplementary Appendix). In prespecified subgroup analyses, the difference in the rate of the primary outcome between the balanced-crystalloids group and the saline group was greater among patients who received larger volumes of isotonic crystalloid and among patients with sepsis (Fig. 3, and Fig. S7 in the Supplementary Appendix). Among patients with sepsis, 30-day inhospital mortality was 25.2% with balanced crystalloids and 29.4% with saline (adjusted odds ratio, 0.80; 95% CI, 0.67 to 0.97; P = 0.02).
Table 2.
Clinical Outcomes.*
| Outcome | Balanced Crystalloids (N = 7942) | Saline (N = 7860) | Adjusted Odds Ratio (95% CI)† | P Value† |
|---|---|---|---|---|
| Primary outcome | ||||
| Major adverse kidney event within 30 days — no. (%)‡ | 1139 (14.3) | 1211 (15.4) | 0.90 (0.82 to 0.99) | 0.04 |
| Components of primary outcome | ||||
| In-hospital death before 30 days — no. (%) | 818 (10.3) | 875 (11.1) | 0.90 (0.80 to 1.01) | 0.06 |
| Receipt of new renal-replacement therapy — no./total no. (%)§ | 189/7558 (2.5) | 220/7458 (2.9) | 0.84 (0.68 to 1.02) | 0.08 |
| Among survivors | 106/6787 (1.6) | 117/6657 (1.8) | ||
| Final creatinine level ≥200% of baseline — no./total no. (%)§ | 487/7558 (6.4) | 494/7458 (6.6) | 0.96 (0.84 to 1.11) | 0.60 |
| Among survivors | 259/6787 (3.8) | 273/6657 (4.1) | ||
| Among survivors without new renal-replacement therapy | 215/6681 (3.2) | 219/6540 (3.3) | ||
| Secondary outcomes | ||||
| In-hospital death — no. (%) | ||||
| Before ICU discharge | 528 (6.6) | 572 (7.3) | 0.89 (0.78 to 1.02) | 0.08 |
| Before 60 days | 928 (11.7) | 975 (12.4) | 0.92 (0.83 to 1.02) | 0.13 |
| ICU-free days¶ | 0.94 | |||
| Median | 25.3 | 25.3 | 1.00 (0.89 to 1.13) | |
| Interquartile range | 22.1 to 26.6 | 22.2 to 26.6 | ||
| Mean | 21.8±8.3 | 21.7±8.6 | ||
| Ventilator-free days¶ | 1.06 (0.97 to 1.16) | 0.22 | ||
| Median | 28.0 | 28.0 | ||
| Interquartile range | 26.0 to 28.0 | 26.0 to 28.0 | ||
| Mean | 24.2±8.6 | 23.9±8.9 | ||
| Vasopressor-free days¶ | 1.05 (0.97 to 1.14) | 0.26 | ||
| Median | 28.0 | 28.0 | ||
| Interquartile range | 27.0 to 28.0 | 27.0 to 28.0 | ||
| Mean | 24.7±8.5 | 24.4±8.8 | ||
| Renal-replacement therapy–free days¶ | 1.11 (1.02 to 1.20) | 0.01 | ||
| Median | 28.0 | 28.0 | ||
| Interquartile range | 28.0 to 28.0 | 28.0 to 28.0 | ||
| Mean | 25.0±8.6 | 24.8±8.9 | ||
| Secondary renal outcomes§ | ||||
| Stage 2 or higher AKI developing after enrollment — no./total no. (%)|| | 807/7558 (10.7) | 858/7458 (11.5) | 0.91 (0.82 to 1.01) | 0.09 |
| Creatinine — mg/dl** | ||||
| Highest before discharge or day 30 | 1.01 (0.97 to 1.05) | 0.58 | ||
| Median | 0.99 | 0.99 | ||
| Interquartile range | 0.78 to 1.53 | 0.78 to 1.52 | ||
| Change from baseline to highest value | 0.98 (0.94 to 1.02) | 0.35 | ||
| Median | 0.04 | 0.04 | ||
| Interquartile range | −0.08 to 0.31 | −0.08 to 0.32 | ||
| Final value before discharge or 30 days | 1.02 (0.97 to 1.06) | 0.51 | ||
| Median | 0.83 | 0.83 | ||
| Interquartile range | 0.70 to 1.11 | 0.70 to 1.11 | ||
Plus–minus values are means ±SD. To convert the values for creatinine to micromoles per liter, multiply by 88.4. ICU denotes intensive care unit.
Categorical outcomes were compared with a generalized, linear, mixed-effects model, with adjustment for the ICU to which the patient was admitted as a random effect and prespecified covariates as fixed effects.13 Continuous outcomes were compared between groups with a proportional-odds model, with adjustment for the same variables.
A major adverse kidney event within 30 days is the composite of death, receipt of new renal-replacement therapy, or final creatinine level that was at least 200% of the baseline level, with all events censored at hospital discharge or at 30 days after admission to the ICU, whichever occurred first. The effect of study group on major adverse kidney events within 30 days is the conditional effect. The marginal effect yielded an odds ratio of 0.91 and a 95% confidence interval of 0.84 to 0.99.
Data on receipt of new renal-replacement therapy, final creatinine level that was at least 200% of the baseline level, and secondary renal outcomes are provided for the 15,016 patients not known to have received renal-replacement therapy before ICU admission.
ICU-free, ventilator-free, vasopressor-free, and renal-replacement-therapy–free days refer to the number of days on which a patient was alive and free from the specified therapy in the first 28 days after enrollment. Odds ratios of higher than 1.0 indicate a better outcome (i.e., more days alive and free from the specified therapy) with balanced crystalloids than with saline.
The development of acute kidney injury (AKI) of stage 2 or higher after enrollment was defined in accordance with the Kidney Disease: Improving Global Outcomes plasma creatinine criteria22 as any creatinine level between enrollment and discharge or 30 days that increased by at least 0.3 mg per deciliter (27 μmol per liter) from a preceding post-enrollment value and was at least 200% of the baseline value, at least 200% of a preceding post-enrollment value, or at least 4.0 mg per deciliter (350 μmol per liter) or as new receipt of renal-replacement therapy.
Among patients who had not received previous renal-replacement therapy, the plasma creatinine level was measured a mean of 8.0 times between enrollment and the first of discharge or 30 days in each group; the plasma creatinine level was not measured between enrollment and the first of discharge or 30 days for 418 of 7558 patients (5.5%) in the balanced-crystalloids group and 443 of 7458 patients (5.9%) in the saline group.
Figure 3. Subgroup Analysis of Rates for the Composite Outcome of Death, New Receipt of Renal-Replacement Therapy, or Persistent Renal Dysfunction.
The odds ratio and 95% confidence interval are shown overall and according to subgroup for the percentage of patients in the balanced-crystalloids group and the saline group who met the criteria for the composite outcome of death from any cause, new renal-replacement therapy, or persistent renal dysfunction. Normal kidney function refers to patients who had no acute kidney injury, chronic kidney disease, or renal-replacement therapy before enrollment. Acute kidney injury refers to patients without chronic kidney disease whose first creatinine level after enrollment was at least 200% of the baseline value or was both greater than 4.0 mg per deciliter (350 μmol per liter) and had increased at least 0.3 mg per deciliter (27 μmol per liter) from the value at baseline.22 Chronic kidney disease refers to patients with a glomerular filtration rate less than 60 ml per minute per 1.73 m2 as calculated according to the Chronic Kidney Disease Epidemiology Collaboration equation with the value for the patient’s baseline creatinine level.25 Previous renal-replacement therapy refers to patients known to have received any form of renal-replacement therapy before enrollment.
SECONDARY OUTCOMES
A total of 818 patients (10.3%) in the balanced-crystalloids group died before hospital discharge and within 30 days of ICU admission as compared with 875 patients (11.1%) in the saline group (P = 0.06) (Table 2, and Figs. S8 and S9 in the Supplementary Appendix). A total of 189 patients (2.5%) in the balanced-crystalloids group and 220 patients (2.9%) in the saline group received new renal-replacement therapy (P = 0.08) (Table S11 in the Supplementary Appendix). The highest stage of acute kidney injury and the incidence of persistent renal dysfunction did not differ significantly between groups (Table 2, and Table S12 in the Supplementary Appendix).
DISCUSSION
Although both saline and balanced crystalloids have been administered to patients in clinical practice for decades,3 few trials have addressed the effects of crystalloid composition on clinical outcomes.1 In preclinical models, the high chloride content of saline has been reported to cause hyperchloremia,27 acidosis,27 inflammation,28 renal vasoconstriction,29 acute kidney injury,30 hypotension,31 and death.32 Studies involving healthy volunteers suggest saline may decrease renal perfusion through chloride-mediated renal vaso-constriction.33 Observational studies involving critically ill adults have shown higher rates of acute kidney injury,34 renal-replacement therapy,5,10 and death6,7,9,35 with saline than with balanced crystalloids, although results have been inconsistent.36 Although underpowered for clinical outcomes, two recent pilot trials involving critically ill adults showed an absolute difference of 1 percentage point in mortality in favor of balanced crystalloids.11,12
In the current trial, the use of balanced crystalloids rather than saline resulted in an absolute difference of 1.1 percentage points in favor of balanced crystalloids in the primary outcome. This finding is consistent with the results of the SALT-ED trial conducted concurrently in noncritically ill adults.14 Although the effect size achieved in the current trial was modest in terms of percentages, if our data on the use of balanced crystalloids were applied to the care of the more than 5 million patients admitted to ICUs each year, the reduction in death, new renal-replacement therapy, or persistent renal dysfunction could be substantial.37 Our results suggest that the use of balanced crystalloids rather than saline might prevent 1 patient among every 94 patients admitted to an ICU from the need for new renal-replacement therapy, from persistent renal dysfunction, or from death. Moreover, the difference in outcomes between balanced crystalloids and saline appeared to be greater for patients with sepsis and patients who received larger volumes of isotonic crystalloid.
The appropriate composition of a fluid may depend on the indication for its use and the condition of the individual patient. Concern that the relative hypotonicity of balanced crystalloids could increase intracranial pressure in patients with brain injury led us to systematically present clinicians with the option of administering 0.9% sodium chloride to patients with brain injury, regardless of trial group. Thus, our results cannot be used to provide guidance as to whether balanced crystalloids should be used in patients with traumatic brain injury.
Our trial has several strengths. The large sample size provided statistical power to detect small differences in patient outcomes. As was the case in each of the previous trials that compared balanced crystalloids with saline in critically ill adults,5,11,12 group assignment in our trial occurred at the level of the ICU. This trial design allowed delivery of the assigned crystalloid early in each patient’s critical illness. Enrolling all adults admitted to participating ICUs and allowing clinical providers to deliver the assigned crystalloid during clinical care minimized selection bias and improved generalizability.
The trial also has several limitations. Conduct at a single academic center limits generalizability. Treating clinicians were aware of the composition of the assigned crystalloid and of the group-assignment sequence of their ICU. The outcomes of death and creatinine level are objective, but a clinician’s decision to initiate renal-replacement therapy may be susceptible to treatment bias. Censoring data collection at hospital discharge may underestimate the true incidence of death at 30 days and may overestimate the true incidence of persistent renal dysfunction at 30 days.16 On the basis of the hypothesized mechanism of chloride-induced organ injury or acidosis,29,33 we evaluated lactated Ringer’s solution and Plasma-Lyte A together, and this trial does not inform the choice between the two.
In conclusion, in this trial involving critically ill adults, intravenous administration of balanced crystalloids rather than saline had a favorable effect on the composite outcome of death, new renal-replacement therapy, or persistent renal dysfunction.
Supplementary Material
Acknowledgments
Supported by the Vanderbilt Institute for Clinical and Translational Research (through grants UL1 TR000445 and UL1TR002243 from the National Center for Advancing Translational Sciences). Dr. Semler was supported in part by grants from the National Heart, Lung, and Blood Institute (NHLBI) (HL087738-09 and K12HL133117). Dr. Self was supported in part by a grant from the National Institute of General Medical Sciences (NIGMS) (K23GM110469). Dr. Hughes was supported by an American Geriatrics Society Jahnigen Career Development Award and by grants from the National Institutes of Health (NIH) (HL111111, AG045085, and GM120484). Dr. May was supported in part by grants from the NIGMS (1R01GM115353-01) and the Department of Defense (12277261). Dr. Casey was supported in part by a grant from the NHLBI (HL087738-09). Dr. Siew was supported by the Vanderbilt Center for Kidney Disease and the Department of Veterans Affairs’ Health Services Research and Development Service. Dr. Rice was supported in part by a grant from the NIH (R34HL105869).
We thank the patients, nurses, nurse practitioners, pharmacists, residents, fellows, and attending physicians in the Vanderbilt Learning Healthcare System for making this trial possible and, in particular, recognize the mentorship of Arthur P. Wheeler, M.D.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. 2013;369:1243–51. doi: 10.1056/NEJMra1208627. [DOI] [PubMed] [Google Scholar]
- 2.Finfer S, Liu B, Taylor C, et al. Resuscitation fluid use in critically ill adults: an international cross-sectional study in 391 intensive care units. Crit Care. 2010;14:R185. doi: 10.1186/cc9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awad S, Allison SP, Lobo DN. The history of 0. 9% saline. Clin Nutr. 2008;27:179–88. doi: 10.1016/j.clnu.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Yunos NM, Kim IB, Bellomo R, et al. The biochemical effects of restricting chloride-rich fluids in intensive care. Crit Care Med. 2011;39:2419–24. doi: 10.1097/CCM.0b013e31822571e5. [DOI] [PubMed] [Google Scholar]
- 5.Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566–72. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 6.Raghunathan K, Shaw A, Nathanson B, et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis. Crit Care Med. 2014;42:1585–91. doi: 10.1097/CCM.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 7.Rochwerg B, Alhazzani W, Sindi A, et al. Fluid resuscitation in sepsis: a systematic review and network meta-analysis. Ann Intern Med. 2014;161:347–55. doi: 10.7326/M14-0178. [DOI] [PubMed] [Google Scholar]
- 8.Hammond NE, Taylor C, Finfer S, et al. Patterns of intravenous fluid resuscitation use in adult intensive care patients between 2007 and 2014: an international cross-sectional study. PLoS One. 2017;12(5):e0176292. doi: 10.1371/journal.pone.0176292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw AD, Raghunathan K, Peyerl FW, Munson SH, Paluszkiewicz SM, Schermer CR. Association between intravenous chloride load during resuscitation and inhospital mortality among patients with SIRS. Intensive Care Med. 2014;40:1897–905. doi: 10.1007/s00134-014-3505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw AD, Bagshaw SM, Goldstein SL, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0. 9% saline compared to Plasma-Lyte. Ann Surg. 2012;255:821–9. doi: 10.1097/SLA.0b013e31825074f5. [DOI] [PubMed] [Google Scholar]
- 11.Young P, Bailey M, Beasley R, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA. 2015;314:1701–10. doi: 10.1001/jama.2015.12334. [DOI] [PubMed] [Google Scholar]
- 12.Semler MW, Wanderer JP, Ehrenfeld JM, et al. Balanced crystalloids versus saline in the intensive care unit: the SALT randomized trial. Am J Respir Crit Care Med. 2017;195:1362–72. doi: 10.1164/rccm.201607-1345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semler MW, Self WH, Wang L, et al. Balanced crystalloids versus saline in the intensive care unit: study protocol for a cluster-randomized, multiple-crossover trial. Trials. 2017;18:129. doi: 10.1186/s13063-017-1871-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378:819–28. doi: 10.1056/NEJMoa1711586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Self WH, Semler MW, Wanderer JP, et al. Saline versus balanced crystalloids for intravenous fluid therapy in the emergency department: study protocol for a cluster-randomized, multiple-crossover trial. Trials. 2017;18:178. doi: 10.1186/s13063-017-1923-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semler MW, Rice TW, Shaw AD, et al. Identification of major adverse kidney events within the electronic health record. J Med Syst. 2016;40:167. doi: 10.1007/s10916-016-0528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw A. Models of preventable disease: contrast-induced nephropathy and cardiac surgery-associated acute kidney injury. Contrib Nephrol. 2011;174:156–62. doi: 10.1159/000329387. [DOI] [PubMed] [Google Scholar]
- 18.Palevsky PM, Molitoris BA, Okusa MD, et al. Design of clinical trials in acute kidney injury: report from an NIDDK workshop on trial methodology. Clin J Am Soc Nephrol. 2012;7:844–50. doi: 10.2215/CJN.12791211. [DOI] [PubMed] [Google Scholar]
- 19.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kellum JA, Zarbock A, Nadim MK. What endpoints should be used for clinical studies in acute kidney injury? Intensive Care Med. 2017;43:901–3. doi: 10.1007/s00134-017-4732-1. [DOI] [PubMed] [Google Scholar]
- 21.Závada J, Hoste E, Cartin-Ceba R, et al. A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol Dial Transplant. 2010;25:3911–8. doi: 10.1093/ndt/gfp766. [DOI] [PubMed] [Google Scholar]
- 22.Kidney Disease Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2(Suppl):1–138. [Google Scholar]
- 23.Parienti J-J, Kuss O. Cluster-crossover design: a method for limiting clusters level effect in community-intervention studies. Contemp Clin Trials. 2007;28:316–23. doi: 10.1016/j.cct.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Turner RM, White IR, Croudace T. Analysis of cluster randomized cross-over trial data: a comparison of methods. Stat Med. 2007;26:274–89. doi: 10.1002/sim.2537. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahian DM, Wolf RE, Iezzoni LI, Kirle L, Normand S-LT. Variability in the measurement of hospital-wide mortality rates. N Engl J Med. 2010;363:2530–9. doi: 10.1056/NEJMsa1006396. [DOI] [PubMed] [Google Scholar]
- 27.Kellum JA, Bellomo R, Kramer DJ, Pinsky MR. Etiology of metabolic acidosis during saline resuscitation in endotoxemia. Shock. 1998;9:364–8. doi: 10.1097/00024382-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Kellum JA, Song M, Almasri E. Hyper-chloremic acidosis increases circulating inflammatory molecules in experimental sepsis. Chest. 2006;130:962–7. doi: 10.1378/chest.130.4.962. [DOI] [PubMed] [Google Scholar]
- 29.Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–35. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou F, Peng Z-Y, Bishop JV, Cove ME, Singbartl K, Kellum JA. Effects of fluid resuscitation with 0. 9% saline versus a balanced electrolyte solution on acute kidney injury in a rat model of sepsis. Crit Care Med. 2014;42(4):e270–e278. doi: 10.1097/CCM.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kellum JA, Song M, Venkataraman R. Effects of hyperchloremic acidosis on arterial pressure and circulating inflammatory molecules in experimental sepsis. Chest. 2004;125:243–8. doi: 10.1378/chest.125.1.243. [DOI] [PubMed] [Google Scholar]
- 32.Kellum JA. Fluid resuscitation and hyperchloremic acidosis in experimental sepsis: improved short-term survival and acid-base balance with Hextend compared with saline. Crit Care Med. 2002;30:300–5. doi: 10.1097/00003246-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0. 9% saline and Plasma-Lyte 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24. doi: 10.1097/SLA.0b013e318256be72. [DOI] [PubMed] [Google Scholar]
- 34.Krajewski ML, Raghunathan K, Paluszkiewicz SM, Schermer CR, Shaw AD. Meta-analysis of high- versus low-chloride content in perioperative and critical care fluid resuscitation. Br J Surg. 2015;102:24–36. doi: 10.1002/bjs.9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sen A, Keener CM, Sileanu FE, et al. Chloride content of fluids used for large-volume resuscitation is associated with reduced survival. Crit Care Med. 2017;45(2):e146–e153. doi: 10.1097/CCM.0000000000002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rochwerg B, Alhazzani W, Gibson A, et al. Fluid type and the use of renal replacement therapy in sepsis: a systematic review and network meta-analysis. Intensive Care Med. 2015;41:1561–71. doi: 10.1007/s00134-015-3794-1. [DOI] [PubMed] [Google Scholar]
- 37.Wunsch H, Angus DC, Harrison DA, et al. Variation in critical care services across North America and Western Europe. Crit Care Med. 2008;36(10):2787–93. e1–9. doi: 10.1097/CCM.0b013e318186aec8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.