Abstract
Macrophages play critical roles in homeostatic maintenance of the myocardium under normal conditions and in tissue repair after injury. In the steady-state heart, resident cardiac macrophages remove senescent and dying cells and facilitate electrical conduction. In the aging heart, the shift in macrophage phenotype to a proinflammatory subtype leads to inflammaging. Following myocardial infarction (MI), macrophages recruited to the infarct produce both proinflammatory and anti-inflammatory mediators (cytokines, chemokines, matrix metalloproteinases, and growth factors), phagocytize dead cells, and promote angiogenesis and scar formation. These diverse properties are attributed to distinct macrophage subtypes and polarization status. Infarct macrophages exhibit a proinflammatory M1 phenotype early and become polarized toward an anti-inflammatory M2 phenotype later post- MI. Although this classification system is oversimplified and needs to be refined to accommodate the multiple different macrophage subtypes that have been recently identified, general concepts on macrophage roles are independent of subtype classification. This review summarizes current knowledge about cardiac macrophage origins, roles, and phenotypes in the steady state, with aging, and after MI, as well as highlights outstanding areas of investigation.
INTRODUCTION
Macrophages were first identified by Ilya Ilyich Mechnikov in 1882 and belong to the vertebrate first-line defense system against infection and injury.1 With the advent of genetic fate mapping and tracing techniques (cell reporter lines, parabiosis, bone marrow transplant, and intravital microscopy), our understanding of macrophage physiology has been revolutionized over the past decade. We now know that macrophages reside in all organs in the steady state.2 Tissue resident macrophages persist from embryogenesis into adulthood and minimally rely on monocyte infiltration for renewal, with the exception of skin and gut macrophages that depend on monocyte entry to maintain numbers.3,4 In response to infection or injury, circulating monocytes are mobilized to inflamed tissue and differentiate into macrophages, which constitute the majority of the macrophage population during the acute inflammatory phase.5
In the steady state, tissue resident macrophages exert homeostatic functions, including defending against infection and removing senescent or damaged cells. Moreover, macrophages exhibit distinct organ and tissue-specific physiological functions. For instance, skin macrophages participate in regulating salt-dependent extracellular volume and blood pressure homeostasis.6 In adipose tissue, macrophages generate catecholamines to sustain adaptive thermogenesis and promote insulin resistance by nuclear receptor co-repressor–dependent mechanisms.7,8 Peritoneal macrophages orchestrate migration of immunoglobulin A-producing B cells to the intestine, where they play a key role in the early response to pathogens.9 Macrophages are also involved in erythrocyte removal and iron recycling in the liver, synaptic pruning and normal brain development, and hematopoietic control in the bone marrow and spleen.10–12
In addition to maintaining equilibrium, the macrophage plays an indispensable role in response to injury, including myocardial infarction (MI) both in the presence and absence of reperfusion. The importance of macrophages during post-MI remodeling has been high-lighted by studies in which depletion of macrophages by clodronate liposomes compromises cardiac repair in mouse MI models.13,14 Following MI, macrophages can secrete proinflammatory, anti-inflammatory, proangiogenic, or proreparative factors; can phagocytize dying cells; and can directly interact with other cell types to orchestrate the repair response.15,16 The diverse functions of macrophages are partially attributed to their different phenotypes and polarization status. Macrophage polarization is a process by which macrophages exhibit vastly different gene expression profiles and functions in response to extremes in environmental signals. Post-MI macrophages show a proinflammatory M1 phenotype early and an anti-inflammatory M2 phenotype later, with these phenotypes playing distinct and even opposite roles.17,18 In this review, we will discuss current literature regarding cardiac macrophage origins, roles, and phenotypes in the steady state, the aging heart, and post-MI, as well as emphasize outstanding areas of investigation to complete our understanding of macrophage polarization in the heart.
MONOCYTE/MACROPHAGE MARKERS
Monocytes and macrophages have been assessed by multiple approaches using a variety of markers to label cells and cell subtypes. Table I provides a comprehensive list of monocyte and macrophage markers that have been used. Ly6C/Gr-1 is expressed in rodents, but not in humans, whereas all other markers in Table I are expressed in both rodents and humans. Single-marker labeling is commonly used in experiments with immunohistochemistry, immunoblotting, or immunofluorescence approaches. One underappreciated concept is the fact that the marker used to identify cell type by itself has biological functions. For instance, the most commonly used macrophage marker F4/80 has proinflammatory properties and can induce antigen-specific regulatory T cells (Tregs).33 Distinct gating strategies using flow cytometry can delineate monocyte and macrophage origin and subset types based on marker expression patterns. Table II summarizes current gating strategies to discriminate distinct blood and cardiac monocyte and macrophage phenotypes under steady state and after injury. In addition, the Macrophage Community Website (www.macrophages.com)51 and the Immunological Genome Project (www.immgen.org) provide excellent macrophage database resources.
Table I.
A list of known monocyte and macrophage markers
| Marker | Location | Expressed by | Cell physiological functions | References |
|---|---|---|---|---|
| CCR2/CD192 | Cell surface | Monocytes, macrophages | Mediates Ly6Chigh monocyte recruitment and migration | 19,20 |
| CD11 b/ITGAM | Cell surface | Monocytes, macrophages, neutrophils, NK cells | Couples with CD18 to form integrin αMβ2 (also named Mac1 or complement receptor 3) to initiate immune responses | 21,22 |
| CD14 | Cell surface | Human monocytes | Mediates toll-like receptor 4 activation and production of IFN-β | 23,24 |
| CD16/FCGR3 | Cell surface | Human monocytes | Binds to the Fc portion of IgG antibodies, antigen presentation, anti-inflammatory cytokine production | 25 |
| CD64/FcγR1 | Cell surface | Monocytes, macrophages | Antibody-dependent phagocytosis, recognizes the Fc region of IgG | 19,26,27 |
| CD68/macrosialin | Endosomal/lysosomal compartment, cell surface | Monocytes, macrophages | Antigen processing and presentation, binds to oxidized low-density lipoprotein | 28 |
| CD163 | Cell surface, secreted (soluble) | Macrophages, neutrophils | Hemoglobin/haptoglobin scavenger receptor, anti-inflammatory | 29,30 |
| CX3CR1 | Cell surface | Monocytes, macrophages | Mediates Ly6Clow monocyte recruitment, inhibits proliferation of local macrophages | 31,32 |
| F4/80/EMR1 | Cell surface | Macrophages | Promotes proinflammatory factor production, induces antigen-specific efferent Treg cells | 33 |
| Galectin 3/Mac2 | Cell surface, secreted | Macrophages | Induces monocyte-macrophage differentiation, interferes with dendritic cell fate decision, regulates T cell apoptosis, inhibits B-lymphocyte differentiation into plasma cells | 34 |
| Ly6C/Gr-1* | Cell surface | Monocytes | A specific marker for proinflammatory monocytes | 19 |
| Mac3 | Cell surface | Macrophages | A glycoprotein | 35,36 |
| MERTK | Cell surface | Macrophages, phagocytes | Mediates phagocytosis, increases migration | 19,37 |
| MHCII | Cell surface | Macrophages, dendritic cells, B cells | Mediates antigen presentation | 38 |
Ly6C/Gr-1 is expressed in rodents, but not in humans, whereas all other markers in Table I are expressed in both rodents and humans.
Table II.
Gating strategies to label blood and cardiac monocytes and macrophages
| Gating strategy | Cells labeled | Species | References |
|---|---|---|---|
| Monocytes | |||
| Ly6ChighCCR2highCX3CR1lowCD62 L+ | Classical monocytes | Mouse | 15 |
| Ly6ClowCCR2lowCX3CR1highCD62L− | Nonclassical monocytes | Mouse | 15 |
| CCR2+Ly6Chigh | Inflammatory blood monocytes | Mouse | 39 |
| CD14+CD16−, CD14+CD16+ | Blood monocytes | Human | 40 |
| B220−F4/80+CD115+Ly6C−, B220−F4/80+CD115+Ly6C+ | Blood monocytes | Mouse | 40 |
| MHCIIlowCCR2+ | Cardiac monocytes | Mouse | 41 |
| CD11 b+F4/80−Ly6G−Ly6Chigh, CD11 b+F4/80−Ly6G−Ly6Clow | Monocytes | Mouse | 42 |
| Lineage−CD11 b+F4/80lowLy6C+ | Cardiac monocytes | Mouse | 43 |
| CD11 b+CD11C−MHCII−CD68−Ly6Clow, CD11 b+CD11C−MHCII−CD68−Ly6Chigh | Blood and cardiac monocytes | Mouse | 44 |
| Macrophages | |||
| CD45+CD11 b+F4/80+CD206− | M1 macrophages | Mouse | 45 |
| CD45+CD11 b+F4/80+CD206+ | M2 macrophages | Mouse | 45 |
| CD45+CD11 b+F4/80+Ly6Clow | Resident cardiac macrophages | Mouse | 43 |
| CD11 b+F4/80+CD206+ | Alternatively activated macrophages | Mouse | 46 |
| CD11 b+F4/80+CD64+Ly6C+MHCII+/− | M1 like macrophages | Mouse | 42 |
| CD11 b+F4/80+CD64+Ly6C−MHCII+/− | M2 like macrophages | Mouse | 42 |
| F4/80+CD86+ | M1 macrophages | Mouse | 14 |
| F4/80+CD206+ | M2 macrophages | Mouse | 14,47 |
| F4/80+CD206− | M1 macrophages | Mouse | 47 |
| CD45+CD68+ | Cardiac, blood, and spleen macrophages | Rat | 48 |
| CD11 b+F4/80+CD68+Ly6Clow, CD11 b+F4/80+CD68+Ly6Chigh | Monocyte-derived cardiac macrophages | Mouse | 44 |
| CD14+CD64+MERTK+F4/80+CX3CR1+MHCII−, CD14+CD64+MERTK+F4/80+CX3CR1+MHCII+, CD14+CD64+MERTK+F4/80+CX3CR1−MHCII−, CD14+CD64+MERTK+F4/80+CX3CR1−MHCII+ | Resident cardiac macrophages | Mouse | 31 |
| F4/80+CD11 b+Ly6Clow, F4/80+CD11 b+Ly6Cmedium, F4/80+CD11 b+Ly6Clhigh | Alternatively activated macrophages | Mouse | 49 |
| CD45+CD11 b+F4/80+Ly6C−MHCIIhigh, CD45+CD11 b+F4/80+Ly6C−MHCIIlow, CD45+ CD11 b+F4/80+Ly6C+MERTK+CD206+, CD45+ CD11 b+F4/80+Ly6C+MERTK−CD206− | Cardiac resident macrophages | Mouse | 19 |
| CD45+F4/80+MHC-IIlowCCR2−, CD45+F4/80+MHC-IIhighCCR2− | Cardiac resident macrophages | Mouse | 41 |
| CD45+F4/80+MHCIIhighCCR2+ | Monocyte-derived cardiac macrophages | Mouse | 41 |
| CD14+CD16+CD163+CD204+CD206+CD209− | Anti-inflammatory M2c macrophages | Human | 50 |
MACROPHAGES IN THE STEADY STATE HEART
Macrophage origins
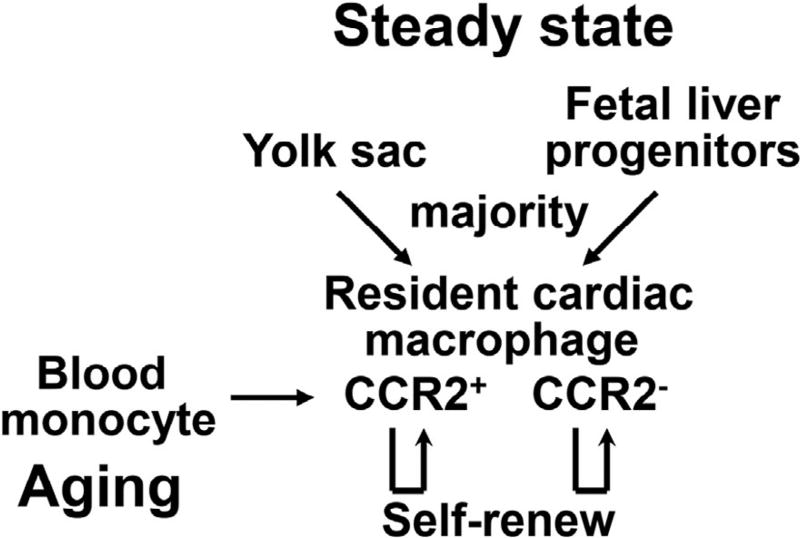
The earlier dogma that macrophages are exclusively derived from circulating monocytes generated by the bone marrow and spleen has been challenged.52 In the past decade, a growing body of literature demonstrates that tissue resident macrophages, in the brain, spleen, liver, lung, bone marrow, kidney, pancreas, peritoneum, and heart are established prenatally, persist throughout the life span, and self-renew locally.53,54 In the steady state, resident cardiac macrophages in mice are reported to account for approximately 5%–10% of nonmyocytes in the heart.43,55 Resident macrophages adopt a spindle-like shape and intermingle closely with myocytes, endothelial cells, and fibroblasts.43 Genetic fate mapping and lineage-tracing studies reveal that the vast majority of resident cardiac macrophages originate from embryonic yolk sac and fetal liver progenitors (Fig 1).19 Replenishment occurs at the rate of about once per month via proliferation.19,43 In terms of subpopulations, CCR2+ macrophages are replenished by blood monocyte recruitment and local proliferation, whereas CCR2− macrophages are repopulated largely by local proliferation (Fig 1).19
Fig 1.
Macrophage origins in the steady-state heart and the aging heart. In the steady state, the vast majority of resident cardiac macrophages originate from the yolk sac and fetal liver progenitors, with minimal dependence on blood monocytes as a source. In terms of subpopulations, CCR2+ macrophages are replenished by blood monocyte recruitment and local proliferation, whereas CCR2− macrophages are repopulated largely by local proliferation. With age, self-renewal of resident cardiac macrophage declines, and blood monocytes increasingly contribute to the cardiac macrophage population.
Macrophage roles
Cardiac macrophages in the healthy state closely resemble alternatively activated anti-inflammatory M2 macrophages, expressing a plethora of M2-designated markers.5,56 This is logical, as M2 macrophages promote tissue rebuilding after injury and thus help to re-establish homeostasis. In terms of cell physiology, resident macrophages can engulf fluorescently labeled bacteria, indicating the capacity to phagocytose dying cells.43 The Nahrendorf et al. recently revealed a novel function for macrophages in the healthy mouse heart. Using specific macrophage reporter lines in combination with optical clearing techniques and confocal microscopy, they for the first time demonstrated that macrophages are abundant in the atrioventricular (AV) node, and these AV nodal macrophages intervene with cardiomyocytes through connexin-43-containing gap junctions to accelerate myocyte repolarization and electrical conduction.57 This is supported by the observation that deleting connexin-43 in macrophages delays AV conduction, and macrophage deletion induces AV block.57 Therefore, the macrophage is involved in myocardial conduction, representing a novel target to treat cardiac arrhythmias. Although this study only evaluated the steady state, it raises interesting questions as to whether macrophages are involved in arrhythmia generation after injury and whether macrophages help to regulate myocyte contraction under normal conditions.
Macrophage phenotypes
In the steady state, resident cardiac macrophages are heterogeneous in origin. Different laboratories have divided macrophages into subpopulations using different markers. Four populations expressing varying levels of Ly6C and major histocompatibility complex class II (MHCII) have been identified in the mouse heart.58 Of these populations, the Ly6C−CCR2− population comprises the vast majority, which originates from the yolk sac and contains MHCIIhigh and MHCIIlow subsets. The third (Ly6C+CCR2−) and the fourth (Ly6C+CCR2+) subsets are both derived from hematopoiesis.19,58,59 The exact roles for these macrophage subsets are incompletely understood. MHCIIhigh cardiac macrophages more efficiently present antigen to T lymphocytes, whereas MHCIIlow cells have higher phagocytic capability. CCR2+ macrophages express high levels of NLRP3-inflammasome associated genes, implying a proinflammatory role for this subtype.19
Cardiac macrophages in mice can also be divided into 4 populations based on CX3CR1 and MHCII expression: CX3CR1−MHCII−, CX3CR1−MHCII+, CX3CR1+MHCII−, and CX3CR1+MHCII+.31 Molawi et al. demonstrated that almost all macrophages at birth were CX3CR1+MHCII−, and with age, there was a progressive increase in MHCII+ cells and a decrease of CX3CR1+ population, leading to a more even distribution of these cell populations by adulthood.31 Combined, the above studies showed the existence of MHCII− and MHCII+ macrophages. However, the question about the relationship between CCR2 and CX3CR1 lineages remains to be addressed.
MACROPHAGES IN THE AGING HEART
Macrophage origins
Aging is a major risk factor for cardiac morbidity and mortality. Cardiac aging is characterized by myocardial sarcopenia, hypertrophy, vascular hyperpermeability, inflammation, fibrosis, and mild cardiac physiology impairment.60–62 In mice, blood pressure does not increase with age, and thus changes in the heart due to age can be attributed to direct changes on the myocardium rather than alterations in ventricular pre-load or after-load.63 In mouse studies, young (<9 months), middle-aged (12–15 months), old (18– 24 months), and senescent (>26 months) mice are commonly used to define different age groups.47,63 We have previously reported that the number of cardiac macrophages in mice increases beginning at about 18 months, and numbers positively correlate with age.47,63,64 By genetic fate mapping and parabiotic approaches, Molawi et al. have reported that with age, self-renewal of resident cardiac macrophages in mice declines, and blood monocytes increasingly contribute to the cardiac macrophage population.31 Although this study used young mice (2- to 9-month old),31 it is likely that in the aging heart, macrophages derive from both mechanisms (self-renewal and blood monocyte differentiation) (Fig 1).
Macrophage roles
Aging involves an upregulation in the basal inflammatory response, a process termed inflammaging.65,66 The macrophage is a key contributor, evidenced by increased numbers of cardiac macrophages and enhanced levels of proinflammatory molecules such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, matrix metalloproteinases (MMPs), and chemokine C-C motif ligand-2 (CCL2)/monocyte chemoattractant protein-1 during cardiac aging.67 Macrophages produce MMP-9 and CCL2, both of which positively correlate with the increase in LV dimensions, indicating a role for macrophages in cardiac aging.67
Immunosenescence refers to the gradual deterioration of the immune system with age, with concomitant higher incidences of infection, neoplasia, autoimmune, and cardiovascular diseases, as well as a worse prognosis after infection or injury.68–70 Compared to young controls (10–12 week old), splenic macrophages from 18–20 month old mice exhibit reduced responses to a variety of proinflammatory or anti-inflammatory stimuli, indicative of age-induced desensitization.71 Old macrophages also display impaired phagocytic capacity and reduced production of nitric oxide and hydrogen peroxide.72,73 Macrophages from old mice produce higher levels of immunosuppressive prostaglandin E2, which contributes to dysregulated immune function.74 Taken together, aging induces immune senescence to increase the susceptibility to and poor prognosis after cardiovascular disease.75
Macrophage phenotypes
How the different subpopulations of resident cardiac macrophages in the steady state change with age in mice has been evaluated. Using flow cytometry, our laboratory has shown that there is a linear increase in cardiac proinflammatory M1 (F4/80+CD206−) macrophages and a decrease in anti-inflammatory M2 macrophages (F4/80+CD206+) with age.47 This is prevented by MMP-9 deletion, indicating that MMP-9 modifies aging related macrophage polarization.47 The fact that in vitro MMP-9 alone activates young macrophages to an M1/M2 mid-transition phase implies that other unknown factors contribute to in vivo age-induced macrophage M1 polarization.47 Increased inflammatory macrophages during cardiac aging may be a result of exaggerated monocyte recruitment, alterations in monocyte fate specification, or changes in resident macrophage behavior. The precise functions of aging associated M1 and M2 macrophages and how many additional macrophage phenotypes there are need to be determined.
MACROPHAGES IN THE MI HEART
Macrophage origins
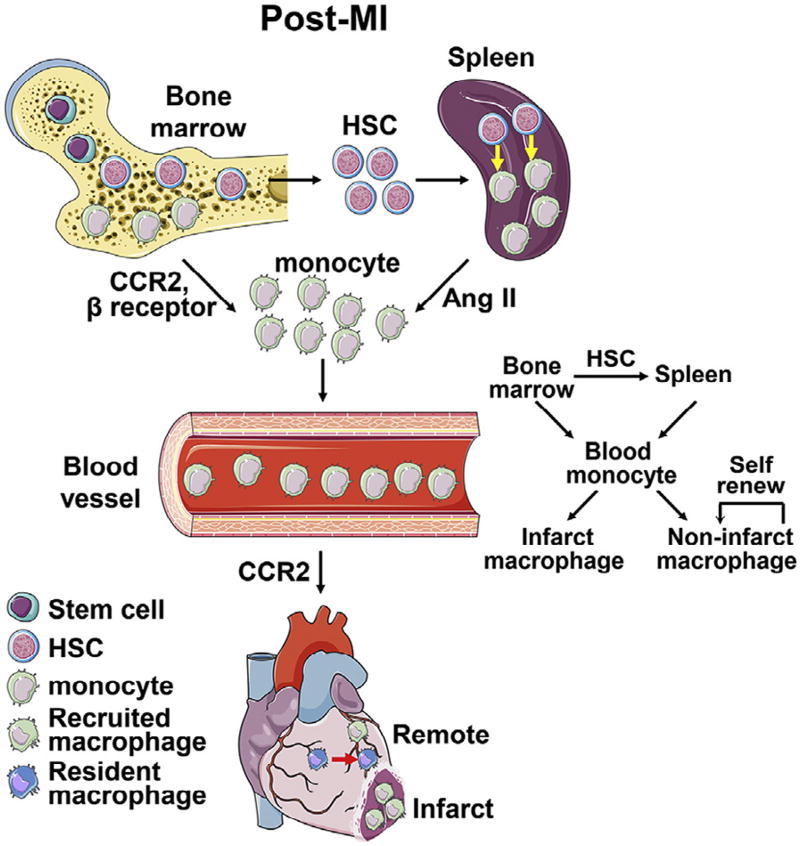
Following MI, abundant blood monocytes infiltrate the ischemic and border regions and differentiate into macrophages, which is the major source of infarct macrophages during the first 7 days post-MI (Fig 2).43 Immediately on exposure to ischemia, resident cardiac macrophages begin to die and are almost completely removed by 24 h post-MI in mice; and their numbers begin to recover by day 4 post-MI. After the acute phase of monocyte recruitment in the first 2 weeks post-MI, resident macrophages in the infarct regain independence from blood monocytes and can proliferate locally.43 This is perhaps caused by the differentiation of local progenitor or stem cells into tissue resident macrophages.
Fig 2.
Macrophage origins in the postmyocardial infarction (MI) heart. Following MI, bone marrow hematopoiesis and extramedullary hematopoiesis by the spleen produce abundant numbers of monocytes, which translocate to the circulation and are recruited to the ischemic heart. In the infarct area, the vast majority of macrophages in the first 3 days are derived from recruitment of blood monocytes, and the renewal of resident macrophages is trivial; in contrast, in the nonischemic remote myocardium, macrophages arise from both local renewal of resident macrophages and recruitment of blood monocytes. The images of cells and organs were obtained from Servier Medical Art (www.servier.com).
Remote nonischemic myocardium also exhibits alterations in inflammation and macrophage numbers after MI, albeit with lower and delayed changes compared to the infarct area. We have previously shown that the remote region has more inflammation than the infarct region at day 28 post-MI, indicating a secondary inflammatory response that occurs at a late time point and distant location.76 Sager et al. recently reported that macrophage numbers in the mouse remote myocardium increased 2.9-fold at 8 weeks after MI, which resulted from both local macrophage renewal and blood monocyte recruitment (Fig 2).77 More importantly, inhibition of monocyte extravasation into the cardiac tissue by silencing 5 endothelial cell adhesion molecules (Icam1, Icam2, Vcam1, E-selectin, and P-selectin) decreased macrophage numbers and improved cardiac physiology, suggesting that macrophages contribute to adverse remodeling of the remote myocardium.77 In this study, the authors also revealed that mechanical strain, the deformation of the heart, could elicit macrophage proliferation.77
During the acute inflammatory phase, the majority of macrophages recruited to the ischemic area derive from the differentiation of peripheral blood monocytes, which stem from 2 sources: bone marrow and spleen. The following paragraphs provide detailed information on these sources.
Peripheral blood monocytes
Mouse blood monocytes are heterogeneous and have 2 subsets based on Ly6C expression. Ly6Chi monocytes are inflammatory and express large amounts of chemokine C-X-C motif receptor (CCR) 2 and low chemokine C-X3-C motif receptor (CX3CR) 1 level; and Ly6Clow monocytes low CCR2 and high CX3CR1.17 In the steady state, 50%–60% of circulating mouse monocytes are Ly6ChiCCR2highCX3CR1lowCD62 L+, and have a relatively short life span.15 Ly6Clow monocytes arise from Ly6Chi cell conversion, instead of from different progenitors.78,79 Likewise, the circulating monocyte pool in humans is heterogeneous and divided into 3 phenotypes based on CD14 and CD16 expression. CD14++CD16− monocytes, which resemble mouse Ly6Chi monocytes, account for 80%–90% of total circulating monocytes and are proinflammatory. CD14+CD16++ cells are anti-inflammatory and resemble mouse Ly-6Clow population in terms of function.40,80 The third population of CD14++CD16+ cells has proinflammatory features and can secrete proinflammatory TNF-α after exposure to lipopolysaccharide (LPS).81
Bone marrow hematopoiesis
Circulating monocytes derive from hematopoietic stem cells (HSCs) residing in the bone marrow. HSCs sequentially differentiate into common myeloid progenitors, granulocyte macrophage progenitors, macrophage monocyte progenitors, common monocyte progenitors, and ultimately monocytes in the bone marrow, a process termed hematopoiesis.82,83 Numerous housekeeping cells, including mesenchymal stem cells, endothelial cells, CD169+ macrophages, nerve cells, and osteoblasts reside in the hematopoietic niche and regulate blood cell production. They produce growth factors and fate-regulating signals such as granulocyte colony-stimulating factor, angiopoietin-1, chemokine C-X-C motif ligand (CXCL) 12, and stem cell factor.83–85 CXCL12 facilitates quiescent HSC retention in the bone marrow.86 Lower CXCL12 levels after MI lead to the liberation of HSCs from the bone marrow niche, which then migrate to the spleen and differentiate into monocytes that are subsequently recruited to the infarct.87 CCR2 and β-adrenergic receptor signaling cascade also mediate monocyte mobilization from the bone marrow to circulation (Fig 2).87,88
Spleen extramedullary hematopoiesis
The spleen is an additional reservoir for monocytes that actually outnumbers their equivalents in circulation and contributes to the blood monocyte pool. The spleen can generate new monocytes by a process called extramedullary hematopoiesis.89,90 Splenic monocytes are located in the subcapsular red pulp of the spleen and resemble circulating counterparts.15 Splenic hematopoiesis occurs during embryogenesis and in disease settings but not in the steady state after birth. Post-MI, the spleen produces sufficient monocytes to enter the blood and infiltrate the ischemic myocardium (Fig 2). This process is at least partially angiotensin II-dependent and can be attenuated by angiotensin converting enzyme inhibitors.91,92 Extramedullary hematopoiesis shrinks the mouse spleen weight by 50% and depletes the number of splenic monocytes by 24 h post-MI.15 IL-1β, IL-3, and granulocyte macrophage colony-stimulating factor (GM-CSF) can modulate the production of splenic monocytes.90 More importantly, abrogation of extramedullary monocytopoiesis in mice exacerbates adverse cardiac remodeling and heart failure progression, indicating that spleen-derived monocytes are essential for post-MI cardiac repair.90
Macrophage roles
As early as 30 min following MI, blood monocytes infiltrate the infarct, initially outnumbering neutrophils.93,94 Recruitment of monocytes is dependent on activation of the CCL2/CCR2 signaling pathway.95,96 On arrival at the tissue, these monocytes begin to differentiate into macrophages. Some monocytes, however, do not undergo differentiation; these monocytes serve similar tissue roles as their macrophage counterparts.97 Delineation between monocytes and macrophages in mice has been shown based on the expression of F4/80/I-Ab/CD11c. Monocytes are (F4/80/I-Ab/CD11c)low, whereas macrophages are (F4/80/I-Ab/CD11c)high.97 CD64 and myeloid epithelial reproductive tyrosine kinase (MERTK) can also distinguish cardiac monocytes from cardiac macrophages, with macrophages expressing both CD64 and MERTK and monocytes expressing CD64 and not MERTK.19,98 In the mouse MI model, macrophages are in the infarct peak at days 5–7 after MI.35,45,99 MI patients show similar but delayed kinetics of macrophage infiltration compared to rodents. Timely reperfusion reduces leukocyte numbers accumulated in the infarct, shifts the peak of the innate immune response earlier, and blunts the adaptive immune response.45
Although inflammation is essential for orchestrating post-MI cardiac repair, timely resolution is necessary for favorable cardiac repair. Following MI, there is a burst of acute inflammation over the first 5 days. After this period, inflammation gradually wanes. Targeting monocyte recruitment to attenuate inflammation is protective by enhancing myocardial repair.77,100 Phagocytosis of apoptotic myocytes and neutrophils by macrophages is a prerequisite for the resolution of inflammation. Impaired macrophage phagocytic capacity prolongs inflammation and impedes post-MI cardiac repair.101
Macrophages play pivotal roles in the post-MI wound healing response. Macrophage depletion compromises wound healing and accelerates adverse remodeling, and adoptive transfer of activated macrophages improves cardiac repair.13,14,102,103 Similarly, clinical findings demonstrate that patients with high inflammatory CD14+CD16− blood monocyte counts at the onset of MI have larger cardiac dilation at follow-up, and the peak levels of CD14+CD16− monocytes negatively correlate with the extent of myocardial salvage.104,105 The macrophage coordinates each phase of the remodeling process, including the acute inflammatory, reparative, and maturation phases. Macrophage roles include: (1) secreting an extensive array of inflammatory cytokines, chemokines, growth factors, and MMPs to regulate inflammation and degrade the extracellular matrix; (2) phagocytizing dead cell and tissue debris to clean up the wound; (3) producing proangiogenic and proreparative factors (eg, vascular endothelial growth factor and transforming growth factor [TGF]-β1) to facilitate neoangiogenesis and scar building; and (4) presenting antigen to lymphocytes to induce an adaptive immune response.58 Neonate mice depleted of macrophages lose their myocardial regenerative capacity,106 and these reparative macrophages are embryonic and not monocyte-derived.41 These findings indicate that macrophages may mediate myocardial regeneration in the neonatal heart. This field is controversial, and the results need to be further validated. The multi-functional capacity of macrophages is, at least partially, attributed to different cell polarization phenotypes.
Macrophage phenotypes
Macrophages demonstrate high plasticity and adaptability, both in vitro and in vivo. They can adopt differential phenotypes in response to varying stimuli or when residing in varying environments. Macrophages have been classified into classically activated (M1) and alternatively activated (M2) subsets.107 Macrophage subsets are further divided based on the in vitro stimuli to which they are exposed. For instance, M1 macrophages can be divided into M1a if stimulated with toll-like receptors or M1b subsets if stimulated with high-mobility group protein B1.58 Subsets also have distinct cell physiology; for example, M1b is less phagocytic than M1a.
M2 macrophages are further subdivided into M2a if stimulated with IL-4 or IL-13 and M2b if stimulated with immune complexes in combination with IL-1β and M2c if stimulated with IL-10, TGF-β, or glucocorticoids.17,108 M2a and M2c macrophages are primarily responsible for coordinating adaptive immune response, whereas M2b macrophages suppress inflammation.58,109 More recently, an M4 phenotype has been proposed to describe monocytes exposed to CXCL4.110 Moreover, different phenotypes can mutually convert under in vitro conditions. For example, M1 macrophages could switch to the M2 phenotype after stimulation with pro-M2 factors, and vice versa.111 Although macrophage classification and conversion concepts are based on in vitro stimulation responses, the current literature borrows from this nomenclature to define in vivo stimulated macrophages. This complicates communication in the field, as the M1 in vivo stimulated macrophage is much different than macrophages stimulated in vitro by one or a few stimuli.
Macrophages in the infarcted heart are heterogeneous. M1 macrophages dominate at days 1–3 post-MI, whereas M2 macrophages are the major cell at days 5–7 post-MI in the mouse heart.45 Proinflammatory M1 macrophages secrete cytokines, chemokines, growth factors, and MMPs to help clear the cell debris and degrade extracellular matrix (Table III).18 However, the prolonged presence of M1 macrophages can lead to expansion of infarct size and impede the resolution of inflammation and scar formation.100 In contrast, anti-inflammatory M2 macrophages are proreparative.18 M2 macrophages can produce anti-inflammatory, proangiogenic, and proreparative factors (eg, IL-10, vascular endothelial growth factor, and TGF-β1) and engulf apoptotic cells to facilitate neoangiogenesis and scar repair (Table III). Shifting the balance from M1 to M2 macrophages improves myocardial repair and function post-MI.118–121 Likewise, our laboratory has shown that MMP-9, MMP-28, and IL-10 regulate post-MI cardiac remodeling by affecting the M1/M2 balance.35,112,122–125
Table III.
Characteristics of MI-associated proinflammatory and anti-inflammatory macrophages17,45,58,107,112–117
| Proinflammatory | Proinflammatory | |
|---|---|---|
| Stimuli | GM-CSF, IFN-γ, TNF-α, IL-1β | Hydrogen sulfide, IL-4, IL-10, IL-13, IL-33, TGF-β1, M-CSF |
| Transcription factors | AP-1, HIF-1α, IRF3, IRF5, NF-κB, STAT1 | c-Maf, c-Myc, IRF4, JMJD3, KLF4, PPAR-γ, STAT3, STAT6 |
| Markers | CCL2 (MCP1), CCL3 (MIP1a), CCL4 (MIP1b), CCL5 (RANTES), CCL7, CCL8, CCR2, CD80, CD86, CXCL1, CXCL2, CXCL6, CXCL8 (IL-8), CXCL9, CXCL10, CXCL11, CXCL16, IL-1β, IL-6, IL-12, IL-23, iNOS, MHCII, RNS, ROS, S100a8, S100a9, TNF-α | Arg1, CCL1, CCL16, CCL17, CCL18, CCL22, CCL24, CXCL13, CXCL17, CXCL22, CXCL24, CXCR1, CXCR2, CD163, CD206 (MRC1), CD280 (MRC2), Cd301a (Clec10a, Mgl1), Cd301 b (Mgl2), Dectin-1, Fizz1 (Retnla, Relmα), IL-10, PGE2, Spp1 (osteopontin), Stabilin1, TGF-β1, VEGF, Ym1 (Chi3l3) |
| Cell physiology | Proinflammation; proteolysis; phagocytosis of debris; antigen presentation to lymphocytes | Anti-inflammation and resolution of inflammation; phagocytosis of apoptotic cells; pro-angiogenesis; ECM production and scar formation |
Abbreviations: AP-1, activator protein 1; HIF-1α, hypoxia-inducible factor-1α; IRF, interferon-regulatory factor; NF-κB, nuclear factor-κB; STAT,signal transducer and activator of transcription; KLF4, Kruppel-like factor 4; PPAR-γ, peroxisome proliferator-activated receptor-γ; iNOS, induciblenitric oxide synthase; RNS, reactive nitrogen species; ROS, reactive oxygen species; M-CSF, macrophage colony-stimulating factor; Arg1,arginase 1; CXCR, C-X-C chemokine receptor; Fizz1, found in inflammatory zone1; VEGF, vascular endothelial growth factor.
Macrophage polarization mechanisms
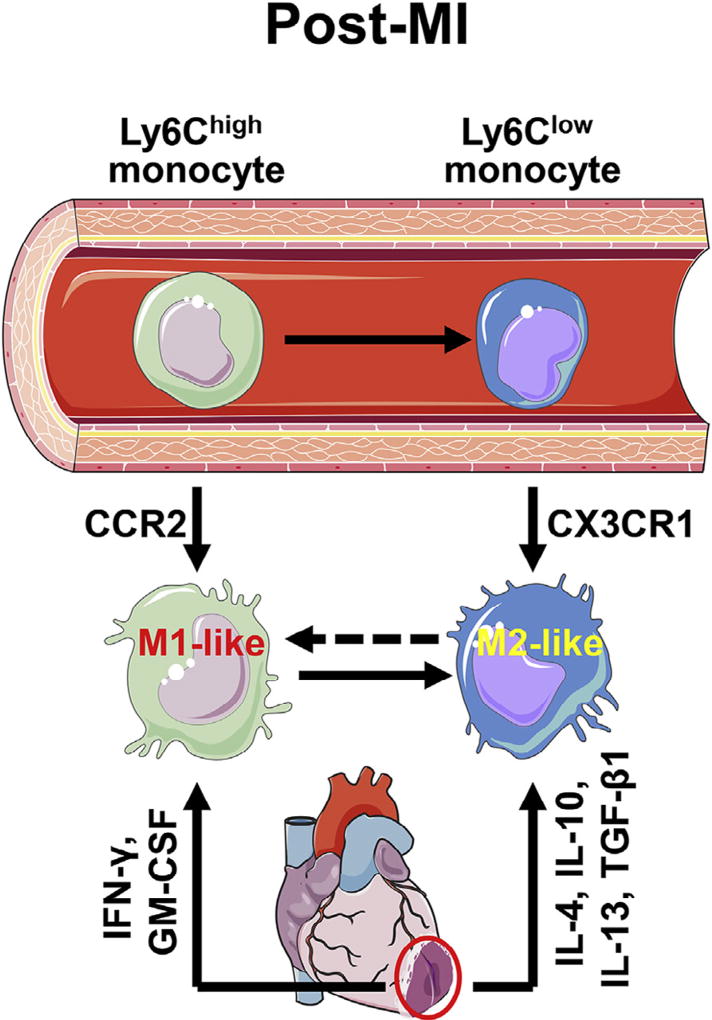
The exact mechanisms regarding post-MI in vivo macrophage polarization remain poorly understood. Nahrendorf et al. previously identified sequential infiltration of 2 distinct monocyte subsets into the ischemic heart.97 Ly6Chigh monocytes predominate at days 1–4 post-MI due to selective expansion, whereas cardiac Ly6Clow cells dominate from days 5 onward due to increased migration capacity. Using genetically modified mice, they also showed that early Ly6Chigh subset recruitment relies on CCR2, whereas later Ly6Clow accumulation depends on CX3CR1 (Fig 3). In addition, Ly6Chigh monocytes can differentiate into Ly6Clow monocytes during the reparative phase and proliferate locally.44,81 Ly6Chigh monocytes are proinflammatory, phagocytic, and proteolytic; in contrast, Ly6Clow monocytes are anti-inflammatory, proangiogenic, and proreparative.97 The monocyte time course reported by Nahrendorf et al. is completely consistent with the time course of macrophages reported by Yan et al.45 As both Ly6Chigh and Ly6Clow cells circulate in the blood, and recruitment is the major source of infarct macrophages, it is reasonable to conclude that infarct proinflammatory M1-like and anti-inflammatory M2-like macrophages are derived from blood Ly6Chigh and Ly6Clow monocytes, respectively. This hypothesis, however, ignores the impact of the local microenvironment on macrophage polarization. Inflammatory monocytes can switch their phenotype to an anti-inflammatory subset and further differentiate into M2-like macrophages in models of skeletal muscle injury and allergic skin.126,127 Peritoneal macrophages acquire features of pulmonary macrophages after adoptive transfer to the lung.128 These findings highlight a deterministic role for the microenvironment in guiding polarization of monocytes and macrophages. The infarct microenvironment is filled with early pro-M1 mediators (eg, IFN-γ and GM-CSF) and later pro-M2 factors (eg, IL-10 and TGF-β1), which likely direct macrophage polarization.112,129 However, the short life span of post-MI monocytes (~20 h) suggests that phenotypic conversion has to be very rapid if it occurs in the MI setting. Alternatively, conversion may account for a small percentage (~20%) of the macrophage pool. Although it is likely that the 2 mechanisms combined determine macrophage polarization (Fig 3), these ideas need to be validated in future experiments. Table III lists known characteristics of MI-associated M1 and M2 macrophages. Additional studies are warranted to systematically identify pathway networks that coordinate post-MI macrophage polarization.
Fig 3.
Proposed post-MI macrophage polarization mechanisms. The integration of 2 mechanisms determines the polarization status of macrophages in the MI heart. (1) M1 and M2 macrophages originate from circulating Ly6Chigh and Ly6Clow monocytes, respectively; Recruitment of Ly6Chigh monocytes depends on CCR2 signaling, whereas recruitment of Ly6Clow monocytes is CX3CR1 dependent; and (2) the mix of pro-M1 and pro-M2 factors existing in the MI myocardium orchestrates macrophage polarization status. MI, myocardial infarction. The images of cells and organs were obtained from Servier Medical Art (www.servier.com).
FUTURE DIRECTIONS IN OUR UNDERSTANDING OF POST-MI MACROPHAGE POLARIZATION
The M1 and M2 nomenclature has been helpful for appreciating the heterogeneity of macrophages. The M1/M2 paradigm, however, was originally based on the in vitro stimuli used, surface marker expression, and production of inflammatory associated factors. For instance, LPS + IFN-γ induce macrophage production of proinflammatory Ccl3, IL-1β, IL-6, and TNF-α; and thus, this macrophage is termed M1. IL-4 elicits macrophages to produce anti-inflammatory Cd206, Arg1, Fizz1, and Ym1, namely M2 macrophage markers.35 The main limitation with this nomenclature system is that the simple in vitro setting does not reflect the complex in vivo microenvironment. In the ischemic heart, there is a complex mixture of both pro-M1 and pro-M2 stimuli. Defining M1 or M2 phenotype based only on 1 marker (eg, CD206), or even a combination of several M1/M2 markers, does not reflect the in vivo situation. One simple example is that CD206 could not distinguish pre-MI resident vs post-MI M2 macrophages, as both express high levels of CD206.46 Second, it is arbitrary to force in vivo data onto an in vitro M1/M2 spectrum.130 Frequently, there is a mixture of M1 or M2 markers that may not follow the simplified in vitro pattern. Macrophages overall may have more total M1 markers, while displaying divergence in particular markers (eg, less TNF-α or IL-10). If only TNF-α and IL-10 are measured and are lower in the comparison group, one might conclude these cells were M2, which would not be accurate based on the other M1 markers. Third, one assumption this classification system makes is that all stimuli induce macrophages to the same phenotype. For example, although LPS + IFN-γ and GM-CSF both trigger an M1 phenotype, transcriptional profiles induced by these 2 stimuli vary, indicating that M1 does not equal M1.131,132 In view of the limitations stated previously about the current M1/M2 polarization paradigm, we discuss here 3 outstanding areas of investigation needed to better understand macrophage polarization in the post-MI LV.109
(1) The polarization phenotypes of cardiac macrophages at day 0 (before MI) and at varying time points post-MI (eg days 1, 3, and 7) need to be systematically mapped. We propose that there are likely differences in cell phenotypes along the post-MI continuum that span beyond the simple M1/M2 paradigm. As mentioned previously, although day 1 and day 3 macrophages have similar M1 phenotypes in terms of some markers, they are likely different in terms of transcriptional programs and cell physiology. In addition, individual cell phenotypes at a given time may be different. At day 3 post-MI, for example, M1 macrophages may also be heterogeneous, reflecting the exact cytokine and chemokine environments they are exposed to on entry into the infarct region, an environment that is in rapid flux over the first days post-MI. Therefore, we need to know the continuous phenotypes across the time course of MI and the variability across individual cell phenotypes at the same time. The first thing we need to know is what markers distinguish phenotypes. This could be addressed by globally examining transcriptional profiles of macrophages isolated from different time point post-MI using RNA sequencing. Flow cytometry could further distinguish individual cell phenotypes.
The use of a novel nomenclature system on the basis of post-MI time when macrophages are activated may be a better way to define macrophage phenotypes. For instance, cM(MI-D1) could be used to denote cardiac macrophages at day 1 post-MI. This system could be used for in vitro macrophages stimulated by different factors as well. cM(IL-4) represents resident cardiac macrophages stimulated with IL-4. The advantage of this classification system is that we can more clearly distinguish what cell type is under examination.
(2) Computational models mimicking post-MI macrophage polarization have not been established.109 Mathematical algorithms can provide a means to predict outcomes that integrate complex in vivo factors at molecular, cellular, organ, and systemic levels as well as reduce complexity.133 Algorithms for macrophage physiology have recently been established for some biological processes, such as the acute inflammatory response, chronic wound inflammation, cholesterol efflux, tumor, and iron release.134–139 These models do not incorporate macrophage polarization nor have models been developed to describe macrophages in the infarcted heart. Our team has previously developed cellular models of macrophage polarization and myocardial remodeling on a limited scale.140,141 A more complete computational map that includes macrophage activation factors, signaling network, and phenotypic information is warranted. Building these algorithms requires the building of an initial framework to ensure the establishment of optimum models.142
Macrophage polarization has been defined for the most part by single-stimulus responses; we need to examine how macrophages respond to mixed stimuli. Initial models could focus on short-term in vitro treatment of macrophages with different factors known to regulate macrophage polarization and post-MI remodeling. The structure of the computational algorithm could be based on previously known pathways in conjunction with bridging these inputs to specific downstream genes, secreted proteins, and cell physiology outputs.109,142 Conversion of these initial models into logic-based distinct equations will provide a window for simulations with other key players that may be identified to build on the existing framework.142 The in silico integration of complex data sets can help define key trigger point responses, combined with bioinformatics analysis to provide a more comprehensive evaluation. Subsequent model iterations could incorporate comprehensive evaluations of transcriptome and secretome profiles, allowing inference of novel players in these processes. Computational models generated for post-MI macrophage polarization could be used in the future to understand cardiac remodeling patterns, which would allow predictions of new therapeutic interventions to be tested, validated, and refined.
(3) There is a need to know how to modulate endogenous and exogenous targets to generate predictable macrophage polarization subsets.109 Interfering with endogenous signaling cascades will tell us whether the developed models have successfully defined the key drivers of macrophage polarization and accurately dissected their roles in cardiac remodeling. Similarly, modifying exogenous pathways will tell us whether modifying phenotypes could affect outcomes in a predictable manner. Imitating effects of individual components and combinations on macrophage polarization will provide a systematic picture of the in vivo complexity.
CONCLUSIONS
Our understanding of macrophage ontogeny, polarization, and cell physiology has greatly expanded over the past decade. Basic and pre-clinical studies have shown the promising potential of targeting macrophages to prevent adverse cardiac remodeling and physiological deterioration in the post-MI LV. With emerging knowledge of the beneficial and detrimental functions of macrophages, future studies can be aimed at targeting specific detrimental functions while preserving beneficial roles. Establishing the progression of post-MI macrophage polarization and signaling patterns will provide mechanistic insight into how macrophages coordinate cardiac repair and help us identify novel intervention targets. Developing predictable computational models that incorporate the macrophage phenotype continuum will help to achieve this goal.
Acknowledgments
The authors acknowledge funding from the American Heart Association under award number 15SDG22930009, from the National Institutes of Health under award numbers GM104357, GM114833, and GM115428, HL051971, HL075360, HL105324, HL129823, and from the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development under award number 5I01BX000505.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the American Heart Association, the National Institutes of Health, or the Veterans Administration.
All authors have reviewed and approved the article.
Abbreviations
- AV
atrioventricular
- CCL
chemokine C-C motif ligand
- CCR
chemokine C-C motif receptor
- CXCL
chemokine C-X-C motif ligand
- CX3CR
chemokine C-X3-C motif receptor
- ECM
extracellular matrix
- GM-CSF
granulocyte macrophage colony-stimulating factor
- HSC
hematopoietic stem cell
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- MERTK
myeloid epithelial reproductive tyrosine kinase
- MHCII
major histocompatibility complex class II
- MI
myocardial infarction
- MMPs
matrix metalloproteinases
- TGF-β1
transforming growth factor-β1
- TNF
tumor necrosis factor
Footnotes
Conflicts of Interest: All authors have read the journal authorship agreement and policy on disclosure of potential conflicts of interest and have nothing to disclose.
References
- 1.Gordon S. The macrophage: past, present and future. Eur J Immunol. 2007;37:S9–17. doi: 10.1002/eji.200737638. [DOI] [PubMed] [Google Scholar]
- 2.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamoutounour S, Guilliams M, Montanana Sanchis F, et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity. 2013;39:925–38. doi: 10.1016/j.immuni.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Zigmond E, Varol C, Farache J, et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37:1076–90. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Swirski FK, Robbins CS, Nahrendorf M. Development and function of arterial and cardiac macrophages. Trends Immunol. 2016;37:32–40. doi: 10.1016/j.it.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Machnik A, Neuhofer W, Jantsch J, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15:545–52. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen KD, Qiu Y, Cui X, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–8. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li P, Spann NJ, Kaikkonen MU, et al. NCoR repression of LXRs restricts macrophage biosynthesis of insulin-sensitizing omega 3 fatty acids. Cell. 2013;155:200–14. doi: 10.1016/j.cell.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–44. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theurl I, Hilgendorf I, Nairz M, et al. On-demand erythrocyte disposal and iron recycling requires transient macrophages in the liver. Nat Med. 2016;22:945–51. doi: 10.1038/nm.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paolicelli RC, Bolasco G, Pagani F, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–8. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 12.Dutta P, Hoyer FF, Grigoryeva LS, et al. Macrophages retain hematopoietic stem cells in the spleen via VCAM-1. J Exp Med. 2015;212:497–512. doi: 10.1084/jem.20141642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frantz S, Hofmann U, Fraccarollo D, et al. Monocytes/macrophages prevent healing defects and left ventricular thrombus formation after myocardial infarction. FASEB J. 2013;27:871–81. doi: 10.1096/fj.12-214049. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Mordechai T, Holbova R, Landa-Rouben N, et al. Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J Am Coll Cardiol. 2013;62:1890–901. doi: 10.1016/j.jacc.2013.07.057. [DOI] [PubMed] [Google Scholar]
- 15.Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res. 2013;112:1624–33. doi: 10.1161/CIRCRESAHA.113.300890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swirski FK, Nahrendorf M. Macrophage-stem cell crosstalk after myocardial infarction. J Am Coll Cardiol. 2013;62:1902–4. doi: 10.1016/j.jacc.2013.07.058. [DOI] [PubMed] [Google Scholar]
- 17.Gombozhapova A, Rogovskaya Y, Shurupov V, et al. Macrophage activation and polarization in post-infarction cardiac remodeling. J Biomed Sci. 2017;24:13. doi: 10.1186/s12929-017-0322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ter Horst EN, Hakimzadeh N, van der Laan AM, Krijnen PA, Niessen HW, Piek JJ. Modulators of macrophage polarization Influence healing of the infarcted myocardium. Int J Mol Sci. 2015;16:29583–91. doi: 10.3390/ijms161226187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epelman S, Lavine KJ, Beaudin AE, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsou CL, Peters W, Si Y, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–9. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Todd RF., 3rd The continuing saga of complement receptor type 3 (CR3) J Clin Invest. 1996;98:1–2. doi: 10.1172/JCI118752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou H, Liao J, Aloor J, et al. CD11b/CD18 (Mac-1) is a novel surface receptor for extracellular double-stranded RNA to mediate cellular inflammatory responses. J Immunol. 2013;190:115–25. doi: 10.4049/jimmunol.1202136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma CY, Chang WE, Shi GY, et al. Recombinant thrombomodulin inhibits lipopolysaccharide-induced inflammatory response by blocking the functions of CD14. J Immunol. 2015;194:1905–15. doi: 10.4049/jimmunol.1400923. [DOI] [PubMed] [Google Scholar]
- 24.Guillou C, Freret M, Fondard E, et al. Soluble alpha-enolase activates monocytes by CD14-dependent TLR4 signalling pathway and exhibits a dual function. Sci Rep. 2016;6:23796. doi: 10.1038/srep23796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcgamma receptors in dendritic cells and macrophages. Nat Rev Immunol. 2014;14:94–108. doi: 10.1038/nri3582. [DOI] [PubMed] [Google Scholar]
- 26.De Calisto J, Villablanca EJ, Mora JR. FcgammaRI (CD64): an identity card for intestinal macrophages. Eur J Immunol. 2012;42:3136–40. doi: 10.1002/eji.201243061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mittal R, Sukumaran SK, Selvaraj SK, et al. Fcgamma receptor I alpha chain (CD64) expression in macrophages is critical for the onset of meningitis by Escherichia coli K1. PLoS Pathog. 2010;6:e1001203. doi: 10.1371/journal.ppat.1001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chistiakov DA, Killingsworth MC, Myasoedova VA, Orekhov AN, Bobryshev YV. CD68/macrosialin: not just a histochemical marker. Lab Invest. 2017;97:4–13. doi: 10.1038/labinvest.2016.116. [DOI] [PubMed] [Google Scholar]
- 29.Etzerodt A, Moestrup SK. CD163 and inflammation: biological, diagnostic, and therapeutic aspects. Antioxid Redox Signal. 2013;18:2352–63. doi: 10.1089/ars.2012.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rafatian N, Westcott KV, White RA, Leenen FH. Cardiac macrophages and apoptosis after myocardial infarction: effects of central MR blockade. Am J Physiol Regul Integr Comp Physiol. 2014;307:R879–87. doi: 10.1152/ajpregu.00075.2014. [DOI] [PubMed] [Google Scholar]
- 31.Molawi K, Wolf Y, Kandalla PK, et al. Progressive replacement of embryo-derived cardiac macrophages with age. J Exp Med. 2014;211:2151–8. doi: 10.1084/jem.20140639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engel DR, Krause TA, Snelgrove SL, et al. CX3CR1 reduces kidney fibrosis by inhibiting local proliferation of profibrotic macrophages. J Immunol. 2015;194:1628–38. doi: 10.4049/jimmunol.1402149. [DOI] [PubMed] [Google Scholar]
- 33.Lin HH, Faunce DE, Stacey M, et al. The macrophage F4/80 receptor is required for the induction of antigen-specific efferent regulatory T cells in peripheral tolerance. J Exp Med. 2005;201:1615–25. doi: 10.1084/jem.20042307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Oliveira FL, Gatto M, Bassi N, et al. Galectin-3 in autoimmunity and autoimmune diseases. Exp Biol Med. 2015;240:1019–28. doi: 10.1177/1535370215593826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma Y, Halade GV, Zhang J, et al. Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circ Res. 2013;112:675–88. doi: 10.1161/CIRCRESAHA.111.300502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho MK, Springer TA. Tissue distribution, structural characterization, and biosynthesis of Mac-3, a macrophage surface glycoprotein exhibiting molecular weight heterogeneity. J Biol Chem. 1983;258:636–42. [PubMed] [Google Scholar]
- 37.Tang Y, Wu S, Liu Q, et al. Mertk deficiency affects macrophage directional migration via disruption of cytoskeletal organization. PLoS One. 2015;10:e0117787. doi: 10.1371/journal.pone.0117787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ting JP, Trowsdale J. Genetic control of MHC class II expression. Cell. 2002;109:S21–33. doi: 10.1016/s0092-8674(02)00696-7. [DOI] [PubMed] [Google Scholar]
- 39.Motomura Y, Kanno S, Asano K, et al. Identification of pathogenic cardiac CD11c+ macrophages in Nod1-mediated acute Coronary Arteritis. Arterioscler Thromb Vasc Biol. 2015;35:1423–33. doi: 10.1161/ATVBAHA.114.304846. [DOI] [PubMed] [Google Scholar]
- 40.Ingersoll MA, Spanbroek R, Lottaz C, et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:e10–9. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavine KJ, Epelman S, Uchida K, et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci U S A. 2014;111:16029–34. doi: 10.1073/pnas.1406508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zlatanova I, Pinto C, Silvestre JS. Immune modulation of cardiac repair and regeneration: the Art of Mending broken hearts. Front Cardiovasc Med. 2016;3:40. doi: 10.3389/fcvm.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heidt T, Courties G, Dutta P, et al. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res. 2014;115:284–95. doi: 10.1161/CIRCRESAHA.115.303567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hilgendorf I, Gerhardt LM, Tan TC, et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res. 2014;114:1611–22. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan X, Anzai A, Katsumata Y, et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol. 2013;62:24–35. doi: 10.1016/j.yjmcc.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 46.Shiraishi M, Shintani Y, Shintani Y, et al. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J Clin Invest. 2016;126:2151–66. doi: 10.1172/JCI85782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma Y, Chiao YA, Clark R, et al. Deriving a cardiac ageing signature to reveal MMP-9-dependent inflammatory signalling in senescence. Cardiovasc Res. 2015;106:421–31. doi: 10.1093/cvr/cvv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Couto G, Liu W, Tseliou E, et al. Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction. J Clin Invest. 2015;125:3147–62. doi: 10.1172/JCI81321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Falkenham A, de Antueno R, Rosin N, et al. Nonclassical resident macrophages are important determinants in the development of myocardial fibrosis. Am J Pathol. 2015;185:927–42. doi: 10.1016/j.ajpath.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 50.Zizzo G, Hilliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol. 2012;189:3508–20. doi: 10.4049/jimmunol.1200662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robert C, Lu X, Law A, Freeman TC, Hume DA. Macrophages.com: an on-line community resource for innate immunity research. Immunobiology. 2011;216:1203–11. doi: 10.1016/j.imbio.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 52.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128:415–35. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nahrendorf M, Swirski FK. Innate immune cells in ischaemic heart disease: does myocardial infarction beget myocardial infarction? Eur Heart J. 2016;37:868–72. doi: 10.1093/eurheartj/ehv453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 55.Pinto AR, Ilinykh A, Ivey MJ, et al. Revisiting cardiac cellular composition. Circ Res. 2016;118:400–9. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pinto AR, Paolicelli R, Salimova E, et al. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS One. 2012;7:e36814. doi: 10.1371/journal.pone.0036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hulsmans M, Clauss S, Xiao L, et al. Macrophages facilitate electrical conduction in the heart. Cell. 2017;169:510–22. e20. doi: 10.1016/j.cell.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ben-Mordechai T, Palevski D, Glucksam-Galnoy Y, Elron-Gross I, Margalit R, Leor J. Targeting macrophage subsets for infarct repair. J Cardiovasc Pharmacol Ther. 2015;20:36–51. doi: 10.1177/1074248414534916. [DOI] [PubMed] [Google Scholar]
- 59.Cohen HB, Mosser DM. Cardiac macrophages: how to mend a broken heart. Immunity. 2014;40:3–5. doi: 10.1016/j.immuni.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 60.Lin J, Lopez EF, Jin Y, et al. Age-related cardiac muscle sarcopenia: combining experimental and mathematical modeling to identify mechanisms. Exp Gerontol. 2008;43:296–306. doi: 10.1016/j.exger.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yabluchanskiy A, Ma Y, Chiao YA, et al. Cardiac aging is initiated by matrix metalloproteinase-9-mediated endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2014;306:H1398–407. doi: 10.1152/ajpheart.00090.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lindsey ML, Goshorn DK, Squires CE, et al. Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovasc Res. 2005;66:410–9. doi: 10.1016/j.cardiores.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 63.Chiao YA, Ramirez TA, Zamilpa R, et al. Matrix metalloproteinase-9 deletion attenuates myocardial fibrosis and diastolic dysfunction in ageing mice. Cardiovasc Res. 2012;96:444–55. doi: 10.1093/cvr/cvs275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma Y, Chiao YA, Zhang J, Manicone AM, Jin YF, Lindsey ML. Matrix metalloproteinase-28 deletion amplifies inflammatory and extracellular matrix responses to cardiac aging. Microsc Microanal. 2012;18:81–90. doi: 10.1017/S1431927611012220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baylis D, Bartlett DB, Patel HP, Roberts HC. Understanding how we age: insights into inflammaging. Longev Healthspan. 2013;2:8. doi: 10.1186/2046-2395-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang M, Shah AM. Age-associated pro-inflammatory remodeling and functional phenotype in the heart and large arteries. J Mol Cell Cardiol. 2015;83:101–11. doi: 10.1016/j.yjmcc.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chiao YA, Dai Q, Zhang J, et al. Multi-analyte profiling reveals matrix metalloproteinase-9 and monocyte chemotactic protein-1 as plasma biomarkers of cardiac aging. Circ Cardiovasc Genet. 2011;4:455–62. doi: 10.1161/CIRCGENETICS.111.959981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Linton PJ, Thoman ML. Immunosenescence in monocytes, macrophages, and dendritic cells: lessons learned from the lung and heart. Immunol Lett. 2014;162:290–7. doi: 10.1016/j.imlet.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120:435–46. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pawelec G. Immunosenescence: impact in the young as well as the old? Mech Ageing Dev. 1999;108:1–7. doi: 10.1016/s0047-6374(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 71.Mahbub S, Deburghgraeve CR, Kovacs EJ. Advanced age impairs macrophage polarization. J Interferon Cytokine Res. 2012;32:18–26. doi: 10.1089/jir.2011.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khare V, Sodhi A, Singh SM. Effect of aging on the tumoricidal functions of murine peritoneal macrophages. Nat Immun. 1996;15:285–94. [PubMed] [Google Scholar]
- 73.Ding A, Hwang S, Schwab R. Effect of aging on murine macrophages. Diminished response to IFN-gamma for enhanced oxidative metabolism. J Immunol. 1994;153:2146–52. [PubMed] [Google Scholar]
- 74.Beharka AA, Wu D, Han SN, Meydani SN. Macrophage prostaglandin production contributes to the age-associated decrease in T cell function which is reversed by the dietary antioxidant vitamin E. Mech Ageing Dev. 1997;93:59–77. doi: 10.1016/s0047-6374(96)01819-2. [DOI] [PubMed] [Google Scholar]
- 75.Dace DS, Apte RS. Effect of senescence on macrophage polarization and angiogenesis. Rejuvenation Res. 2008;11:177–85. doi: 10.1089/rej.2007.0614. [DOI] [PubMed] [Google Scholar]
- 76.Ramirez TA, Iyer RP, Ghasemi O, et al. Aliskiren and valsartan mediate left ventricular remodeling post-myocardial infarction in mice through MMP-9 effects. J Mol Cell Cardiol. 2014;72:326–35. doi: 10.1016/j.yjmcc.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sager HB, Hulsmans M, Lavine KJ, et al. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res. 2016;119:853–64. doi: 10.1161/CIRCRESAHA.116.309001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yona S, Kim KW, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sunderkotter C, Nikolic T, Dillon MJ, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–7. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 80.Cros J, Cagnard N, Woollard K, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–86. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dutta P, Nahrendorf M. Monocytes in myocardial infarction. Arterioscler Thromb Vasc Biol. 2015;35:1066–70. doi: 10.1161/ATVBAHA.114.304652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–61. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Frantz S, Nahrendorf M. Cardiac macrophages and their role in ischaemic heart disease. Cardiovasc Res. 2014;102:240–8. doi: 10.1093/cvr/cvu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med. 2011;208:421–8. doi: 10.1084/jem.20110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–9. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 86.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–88. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 87.Dutta P, Courties G, Wei Y, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–9. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–7. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 89.Robbins CS, Chudnovskiy A, Rauch PJ, et al. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–74. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leuschner F, Rauch PJ, Ueno T, et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–37. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leuschner F, Panizzi P, Chico-Calero I, et al. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res. 2010;107:1364–73. doi: 10.1161/CIRCRESAHA.110.227454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–6. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jung K, Kim P, Leuschner F, et al. Endoscopic time-lapse imaging of immune cells in infarcted mouse hearts. Circ Res. 2013;112:891–9. doi: 10.1161/CIRCRESAHA.111.300484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ma Y, Yabluchanskiy A, Iyer RP, et al. Temporal neutrophil polarization following myocardial infarction. Cardiovasc Res. 2016;110:51–61. doi: 10.1093/cvr/cvw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dewald O, Zymek P, Winkelmann K, et al. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–9. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 96.Kaikita K, Hayasaki T, Okuma T, Kuziel WA, Ogawa H, Takeya M. Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. Am J Pathol. 2004;165:439–47. doi: 10.1016/S0002-9440(10)63309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–47. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gautier EL, Shay T, Miller J, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–28. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Troidl C, Mollmann H, Nef H, et al. Classically and alternatively activated macrophages contribute to tissue remodelling after myocardial infarction. J Cell Mol Med. 2009;13:3485–96. doi: 10.1111/j.1582-4934.2009.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leuschner F, Dutta P, Gorbatov R, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–10. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wan E, Yeap XY, Dehn S, et al. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res. 2013;113:1004–12. doi: 10.1161/CIRCRESAHA.113.301198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJ. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol. 2007;170:818–29. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leor J, Rozen L, Zuloff-Shani A, et al. Ex vivo activated human macrophages improve healing, remodeling, and function of the infarcted heart. Circulation. 2006;114:I94–100. doi: 10.1161/CIRCULATIONAHA.105.000331. [DOI] [PubMed] [Google Scholar]
- 104.Tsujioka H, Imanishi T, Ikejima H, et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol. 2009;54:130–8. doi: 10.1016/j.jacc.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 105.Maekawa Y, Anzai T, Yoshikawa T, et al. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction:a possible role for left ventricular remodeling. J Am Coll Cardiol. 2002;39:241–6. doi: 10.1016/s0735-1097(01)01721-1. [DOI] [PubMed] [Google Scholar]
- 106.Aurora AB, Porrello ER, Tan W, et al. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014;124:1382–92. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–61. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 109.Lindsey ML, Saucerman JJ, DeLeon-Pennell KY. Knowledge gaps to understanding cardiac macrophage polarization following myocardial infarction. Biochim Biophys Acta. 2016;1862:2288–92. doi: 10.1016/j.bbadis.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gleissner CA, Shaked I, Little KM, Ley K. CXC chemokine ligand 4 induces a unique transcriptome in monocyte-derived macrophages. J Immunol. 2010;184:4810–8. doi: 10.4049/jimmunol.0901368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pelegrin P, Surprenant A. Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1beta release through pyrophosphates. EMBO J. 2009;28:2114–27. doi: 10.1038/emboj.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jung M, Ma Y, Iyer RP, et al. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol. 2017;112:33. doi: 10.1007/s00395-017-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maekawa Y, Anzai T, Yoshikawa T, et al. Effect of granulocyte-macrophage colony-stimulating factor inducer on left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol. 2004;44:1510–20. doi: 10.1016/j.jacc.2004.05.083. [DOI] [PubMed] [Google Scholar]
- 114.Miao L, Shen X, Whiteman M, et al. Hydrogen Sulfide Mitigates myocardial infarction via promotion of Mitochondrial Biogenesis-dependent M2 polarization of macrophages. Antioxid Redox Signal. 2016;25:268–81. doi: 10.1089/ars.2015.6577. [DOI] [PubMed] [Google Scholar]
- 115.Yin H, Li P, Hu F, Wang Y, Chai X, Zhang Y. IL-33 attenuates cardiac remodeling following myocardial infarction via inhibition of the p38 MAPK and NF-kappaB pathways. Mol Med Rep. 2014;9:1834–8. doi: 10.3892/mmr.2014.2051. [DOI] [PubMed] [Google Scholar]
- 116.Leblond AL, Klinkert K, Martin K, et al. Systemic and cardiac depletion ofM2 macrophage through CSF-1R signaling inhibition Alters cardiac function post myocardial infarction. PLoS One. 2015;10:e0137515. doi: 10.1371/journal.pone.0137515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tugal D, Liao X, Jain MK. Transcriptional control of macrophage polarization. Arterioscler Thromb Vasc Biol. 2013;33:1135–44. doi: 10.1161/ATVBAHA.113.301453. [DOI] [PubMed] [Google Scholar]
- 118.Harel-Adar T, Ben Mordechai T, Amsalem Y, Feinberg MS, Leor J, Cohen S. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc Natl Acad Sci U S A. 2011;108:1827–32. doi: 10.1073/pnas.1015623108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Courties G, Heidt T, Sebas M, et al. In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. J Am Coll Cardiol. 2014;63:1556–66. doi: 10.1016/j.jacc.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou LS, Zhao GL, Liu Q, Jiang SC, Wang Y, Zhang DM. Silencing collapsin response mediator protein-2 reprograms macrophage phenotype and improves infarct healing in experimental myocardial infarction model. J Inflamm (Lond) 2015;12:11. doi: 10.1186/s12950-015-0053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Weirather J, Hofmann UD, Beyersdorf N, et al. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res. 2014;115:55–67. doi: 10.1161/CIRCRESAHA.115.303895. [DOI] [PubMed] [Google Scholar]
- 122.Yabluchanskiy A, Ma Y, DeLeon-Pennell KY, et al. Myocardial infarction Superimposed on aging: MMP-9 deletion promotes M2 macrophage polarization. J Gerontol A Biol Sci Med Sci. 2016;71:475–83. doi: 10.1093/gerona/glv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.DeLeon-Pennell KY, de Castro Bras LE, Iyer RP, et al. P. gingivalis lipopolysaccharide intensifies inflammation postmyocardial infarction through matrix metalloproteinase-9. J Mol Cell Cardiol. 2014;76:218–26. doi: 10.1016/j.yjmcc.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zamilpa R, Ibarra J, de Castro Bras LE, et al. Transgenic overexpression of matrix metalloproteinase-9 in macrophages attenuates the inflammatory response and improves left ventricular function post-myocardial infarction. J Mol Cell Cardiol. 2012;53:599–608. doi: 10.1016/j.yjmcc.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zamilpa R, Kanakia R, Cigarroa J, 4th, et al. CC chemokine receptor 5 deletion impairs macrophage activation and induces adverse remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol. 2011;300:H1418–26. doi: 10.1152/ajpheart.01002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–69. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Egawa M, Mukai K, Yoshikawa S, et al. Inflammatory monocytes recruited to allergic skin acquire an anti-inflammatory M2 phenotype via basophil-derived interleukin-4. Immunity. 2013;38:570–80. doi: 10.1016/j.immuni.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 128.Lavin Y, Winter D, Blecher-Gonen R, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–26. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Christia P, Bujak M, Gonzalez-Quesada C, et al. Systematic characterization of myocardial inflammation, repair, and remodeling in a mouse model of reperfused myocardial infarction. J Histochem Cytochem. 2013;61:555–70. doi: 10.1369/0022155413493912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nahrendorf M, Swirski FK. Abandoning M1/M2 for a network model of macrophage function. Circ Res. 2016;119:414–7. doi: 10.1161/CIRCRESAHA.116.309194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 132.Lehtonen A, Ahlfors H, Veckman V, Miettinen M, Lahesmaa R, Julkunen I. Gene expression profiling during differentiation of human monocytes to macrophages or dendritic cells. J Leukoc Biol. 2007;82:710–20. doi: 10.1189/jlb.0307194. [DOI] [PubMed] [Google Scholar]
- 133.Yang JH, Saucerman JJ. Computational models reduce complexity and accelerate insight into cardiac signaling networks. Circ Res. 2011;108:85–97. doi: 10.1161/CIRCRESAHA.110.223602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vodovotz Y, Clermont G, Chow C, An G. Mathematical models of the acute inflammatory response. Curr Opin Crit Care. 2004;10:383–90. doi: 10.1097/01.ccx.0000139360.30327.69. [DOI] [PubMed] [Google Scholar]
- 135.Day J, Friedman A, Schlesinger LS. Modeling the immune rheostat of macrophages in the lung in response to infection. Proc Natl Acad Sci U S A. 2009;106:11246–51. doi: 10.1073/pnas.0904846106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nagaraja S, Wallqvist A, Reifman J, Mitrophanov AY. Computational approach to characterize causative factors and molecular indicators of chronic wound inflammation. J Immunol. 2014;192:1824–34. doi: 10.4049/jimmunol.1302481. [DOI] [PubMed] [Google Scholar]
- 137.Gaus K, Gooding JJ, Dean RT, Kritharides L, Jessup W. Akinetic model to evaluate cholesterol efflux from THP-1 macrophages to apolipoprotein A-1. Biochemistry. 2001;40:9363–73. doi: 10.1021/bi010323n. [DOI] [PubMed] [Google Scholar]
- 138.Owen MR, Sherratt JA. Modelling the macrophage invasion of tumours: effects on growth and composition. IMA J Math Appl Med Biol. 1998;15:165–85. [PubMed] [Google Scholar]
- 139.Potdar AA, Sarkar J, Das NK, et al. Computational modeling and analysis of iron release from macrophages. PLoS Comput Biol. 2014;10:e1003701. doi: 10.1371/journal.pcbi.1003701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jin YF, Han HC, Berger J, Dai Q, Lindsey ML. Combining experimental and mathematical modeling to reveal mechanisms of macrophage-dependent left ventricular remodeling. BMC Syst Biol. 2011;5:60. doi: 10.1186/1752-0509-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang Y, Yang T, Ma Y, et al. Mathematical modeling and stability analysis of macrophage activation in left ventricular remodeling post-myocardial infarction. BMC Genomics. 2012;13:S21. doi: 10.1186/1471-2164-13-S6-S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ma Y, Iyer RP, Jung M, Czubryt MP, Lindsey ML. Cardiac fibroblast activation post-myocardial infarction: current knowledge gaps. Trends Pharmacol Sci. 2017;38:448–58. doi: 10.1016/j.tips.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]