Abstract
Background
Pentavalent rotavirus vaccine (RV5) was recommended for routine use in 2006 followed by monovalent rotavirus vaccine (RV1) in 2008.
Purpose
To describe, among a U.S. sample of pediatricians (n=289 respondents) and family medicine physicians (n=243 respondents), (1) current practices regarding rotavirus vaccine (RV) and barriers to use with comparison to a 2007 survey and (2) knowledge of recent safety concerns regarding RV1 and their impact on its use.
Methods
A mail and Internet survey was conducted with the physicians, from November 2010 to January 2011; analyses were conducted March–September 2011.
Results
Response rates were 70% (289/410) for pediatricians and 61% (243/401) for family medicine physicians; routine administration of RV was reported by 95% of pediatricians and 65% of family medicine physicians (2007: 85% and 45%). Almost all barriers to use of RV had decreased compared to 2007. For pediatricians and family medicine physicians, respectively, 94% and 70% were aware of the temporary suspension of RV1 due to presence of porcine circovirus; 49% and 45%, respectively, were aware of the addition to RV1 labeling regarding a possible increased risk of intussusception. Among physicians aware of the safety issues, <5% reported stopping giving RV as a result. After reading information about porcine circovirus, 35% of pediatricians and 59% of family medicine physicians reported it had increased their own concerns about the safety of RV; and 31% and 60%, respectively, reported this regarding intussusception.
Conclusions
The acceptance of RV has increased, and barriers to use have decreased. Among physicians, recent safety questions about RV1 have not affected use of RV, although they have raised safety concerns.
Introduction
The first rotavirus vaccine (RotaShield, Wyeth-Lederle) was licensed and recommended for routine use by the U.S. Advisory Committee on Immunization Practices (ACIP) and the American Academy of Pediatrics, Committee on Infectious Diseases (AAP-COID), in 1998 but was withdrawn from the market within 1 year after it was associated with an increased risk of intussusception.1,2 A study in 2003 suggested that pediatricians would use a new rotavirus vaccine if it were safer than RotaShield and was recommended routinely by the AAP and the ACIP.3 Two other rotavirus vaccines—a pentavalent rotavirus vaccine (RV5, RotaTeq, Merck and Co.) and a monovalent rotavirus vaccine (RV1; Rotarix, GSK Biologicals)—were in development at the time, and each underwent extensive prelicensure testing looking specifically for an increased risk of intussusception. None was found.4,5
The ACIP made a formal recommendation to include RV5 as part of the routine infant series in February 2006,6 and updated the recommendation in June 2008 to include RV1.7 Since the recommendation of RV5 and RV1, their use in routine practice occurred relatively rapidly among pediatricians and to a lesser extent among family physicians.8 The impact of the vaccines in reducing hospitalizations and outpatient visits in the U.S. has been substantial.9–15
However, two recent findings have raised potential concerns about the safety of current rotavirus vaccines. In March 2010, the U.S Food and Drug Administration (FDA) recommended temporarily suspending use of RV1 because of the detection of porcine circovirus, a nonhuman pathogen, in the vaccine.16 Genetic material of porcine circovirus later was identified also in RV5. A subsequent FDA review concluded that the presence of porcine circovirus likely posed no threat to human health, and the temporary suspension of RV1 use was lifted in May 2010. Shortly thereafter, in August 2010, the Global Advisory Committee on Vaccine Safety of the WHO reviewed preliminary data from postmarketing studies that showed a possible increased risk of intussusception with RV1 in certain populations.17 On September 22, 2010, the FDA recommended a label change for RV1 advising providers of the new data.18
Given that the observed health benefits of rotavirus vaccination far exceed the potential risks, the ACIP and other international health bodies have made no changes to the recommendations to routinely vaccinate against rotavirus. However, the extent to which these safety concerns have affected physicians’ opinions and use of rotavirus vaccine is unknown. The objectives of the current study were to assess, through a national survey of pediatricians and family medicine physicians, (1) trends in attitudes and practices regarding rotavirus vaccine and barriers to its use through comparison with a survey conducted prior to the new safety concerns; (2) physicians’ perceptions of rotavirus vaccine effectiveness; (3) physicians’ knowledge and perception of parents’ knowledge about the recent safety concerns and its impact on their attitudes and practice regarding rotavirus vaccine.
Methods
Study Setting
From November 2010 to January 2011, a mail and Internet survey was conducted regarding the rotavirus vaccines in a nationwide sample of pediatricians (n=289 respondents) and family medicine physicians (n=243 respondents). The present study was reviewed and approved for exempt status by the Colorado Multiple IRB, and informed consent was not required.
Population
As part of the Vaccine Policy Collaborative Initiative,19 a national network of primary care physicians was developed through recruitment of family medicine physicians and pediatricians from the American Academy of Family Physicians (AAFP) and the American Academy of Pediatrics (AAP), respectively. Quota sampling19,20 was performed to make sure that physicians in the networks were similar to AAFP and AAP memberships with respect to the region of the country, urban versus rural location, and practice type. Physicians in the network were compared to physicians in the same specialty randomly sampled from the American Medical Association Physician Master File and were generally similar with regard to demographic characteristics, practice attributes, and reported attitudes regarding a range of vaccination issues.19 In addition, this national network of primary care physicians allows documentation of demographic data of both respondents and nonrespondents.
Survey Design
The survey was pretested with a panel of six pediatricians and six family medicine physicians and then piloted among 23 pediatricians and 21 family medicine physicians from different regions of the country. Questions regarding practices and barriers to the use of rotavirus vaccine were based on a prior survey by the current authors’ group from 20078; respondents used 4-point Likert-type scales for their answers. New questions using 4-point Likert-type scales assessed physician knowledge of rotavirus vaccine effectiveness, physician and perceived parental knowledge of the new information regarding porcine circovirus and intussusception, and the impact of a temporary suspension and a change in labeling on providers’ use of rotavirus vaccines.
The Likert-type scales regarding practices and attitudes were classified from strongly agree to strongly disagree. The Likert scales regarding barriers were classified from definitely a barrier to not a barrier at all. For the Results section and for Figures 1 and 2, responses were collapsed as strongly or somewhat agree and somewhat or definitely a barrier.
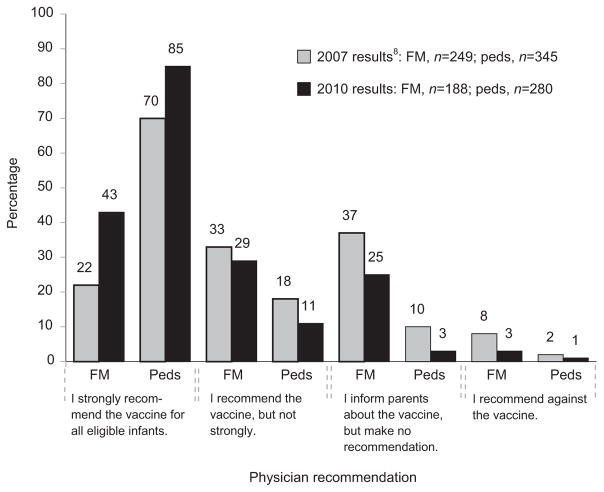
Figure 1.
Current practice with respect to recommending rotavirus vaccination among pediatricians and family medicine physicians
Note: p<0.0001 for KS test for comparison of distributions of responses between Peds and FM in both 2007 and 2010 results; p<0.001 for FM and p=0.002 for Peds for KS test comparison of distributions of responses between 2007 and 2010.
FM, family medicine physicians; KS, Kolmogorov-Smirnov; Peds, pediatricians
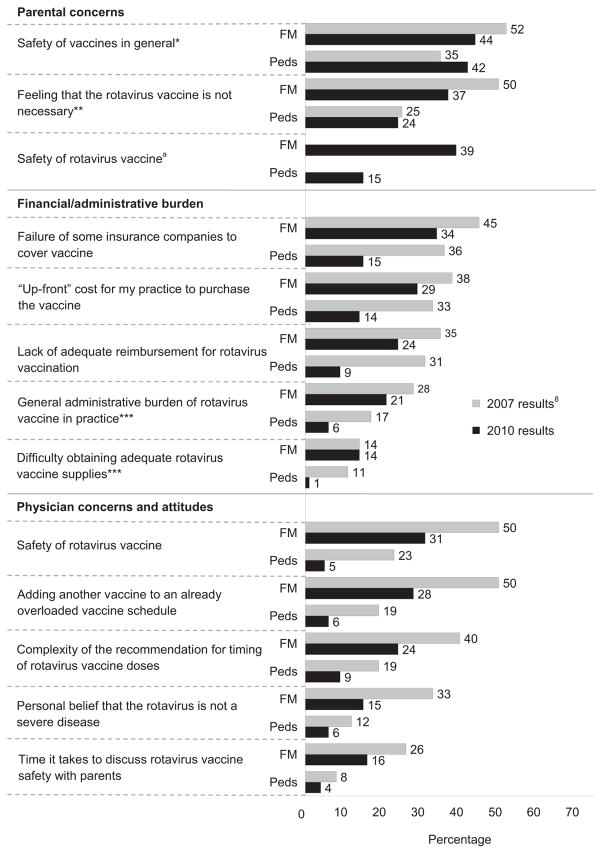
Figure 2.
Reported barriers to administering rotavirus vaccine, percentage of physicians reporting somewhat of a barrier or definitely a barrier
Note: p<0.05 for McNemar’s test for differences between 2007 and 2010 for both specialties, unless otherwise noted.
aThis question was not asked in the 2007 survey.
*p=NS for McNemar’s test for difference between 2007 and 2010 for both specialties.
**p=NS for McNemar’s test for difference between 2007 and 2010 for Peds, p<0.05 for FM.
***p=NS for McNemar’s test for difference between 2007 and 2010 for FM, p<0.05 for Peds.
FM, family medicine physicians; Peds, pediatricians
Survey Administration
Physicians were surveyed through the Internet or by mail depending on preference. The Internet survey was administered using Vovici, a web-based program. The Internet respondent group received an initial e-mail and up to nine e-mail reminders to complete the survey, and the mail group received up to three surveys by mail. Subjects in the Internet group received two final paper surveys by mail if they had not responded to e-mail reminders.
Data Analysis
Internet and mail surveys were pooled for all analyses, as physician attitudes assessed with the two methods have been found to be comparable.21 Chi-squared and Wilcoxon rank-sum tests were used for comparisons of characteristics of respondents and nonrespondents and Kolmogorov–Smirnov (KS) tests for comparisons of overall distributions of responses between respondents in the two specialties. All analyses were conducted March through September 2011 and were performed using SAS, version 9.2.
Results
Response Rates and Study Sample
Response rates were 70% (289/410) for pediatricians and 61% (243/401) for family medicine physicians. Fifty-one family medicine physicians and four pediatricians were excluded because they reported not seeing infants aged <6 months, leaving a final analysis cohort of 477. Respondents were similar to nonrespondents, except for differences among family medicine physicians in practice setting and region of the country (Table 1). Also shown in Table 1 are other characteristics of respondents’ practices.
Table 1.
Comparison of survey respondents versus nonrespondents and additional characteristics of respondents, % (n) unless otherwise indicated
| Characteristics | Pediatricians | p-value* | Family medicine physicians | p-value* | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Respondent (n=289) | Nonrespondent (n=121) | Respondent (n=243) | Nonrespondent (n=158) | |||
| Median age (years) | 49 | 46 | 0.20 | 52 | 50 | 0.11 |
|
| ||||||
| Male | 41 (118) | 45 (55) | 0.39 | 57 (137) | 60 (95) | 0.49 |
|
| ||||||
| Region | ||||||
|
| ||||||
| Midwest | 22 (65) | 15 (18) | 34 (83) | 24 (38) | ||
|
| ||||||
| Northeast | 22 (63) | 30 (36) | 18 (43) | 12 (19) | ||
|
| ||||||
| South | 35 (100) | 35 (42) | 28 (69) | 39 (61) | ||
|
| ||||||
| West | 21 (61) | 21 (25) | 0.20 | 20 (48) | 25 (40) | 0.02 |
|
| ||||||
| Location of practice | ||||||
|
| ||||||
| Urban | 42 (120) | 44 (53) | 30 (72) | 28 (45) | ||
|
| ||||||
| Suburban | 48 (140) | 42 (51) | 40 (96) | 55 (87) | ||
|
| ||||||
| Rural | 10 (29) | 14 (17) | 0.36 | 31 (75) | 16 (26) | 0.002 |
|
| ||||||
| Settinga | ||||||
|
| ||||||
| Private practice | 81 (235) | 83 (100) | 77 (186) | 78 (123) | ||
|
| ||||||
| Hospital/clinic | 14 (41) | 13 (16) | 19 (47) | 18 (29) | ||
|
| ||||||
| HMO | 5 (13) | 4 (5) | 0.95 | 4 (10) | 4 (6) | 0.95 |
|
| ||||||
| Patients with Medicaid or SCHIP (%) | ||||||
|
| ||||||
| <10 | 24 (66) | NA | 39 (91) | NA | ||
|
| ||||||
| 10–24 | 27 (75) | NA | 30 (70) | NA | ||
|
| ||||||
| 25–49 | 24 (66) | NA | 19 (45) | NA | ||
|
| ||||||
| ≥50 | 25 (68) | NA | 12 (28) | NA | ||
|
| ||||||
| Patients of Hispanic race/ethnicity (%) | ||||||
|
| ||||||
| <10 | 49 (138) | NA | 68 (158) | NA | ||
|
| ||||||
| 10–24 | 29 (83) | NA | 21 (48) | NA | ||
|
| ||||||
| ≥25 | 22 (63) | NA | 12 (28) | NA | ||
|
| ||||||
| Patients of black race/ethnicity (%) | ||||||
|
| ||||||
| <10 | 49 (140) | NA | 69 (162) | NA | ||
|
| ||||||
| 10–24 | 31 (89) | NA | 22 (51) | NA | ||
|
| ||||||
| ≥25 | 19 (55) | NA | 10 (23) | NA | ||
Setting where respondents reported providing most of their outpatient care
p-value represents comparison between respondents and nonrespondents within each specialty; statistical analyses used: chi-square, Wilcoxon rank-sum test.
HMO, group or staff model HMO or MCO; NA, not available; SCHIP, State Childhood Insurance Program
Trends in Use of, Attitudes Regarding, and Perceived Barriers to Use of Rotavirus Vaccine
Pediatricians more often reported strongly recommending the vaccine, whereas family medicine physicians were more likely to report informing parents about the vaccine but making no recommendation (Figure 1). Few physicians reported recommending against the vaccine. For both specialties, more providers were strongly recommending the vaccine in 2010 than in 2007. Ninety-five percent of pediatricians and 65% of family medicine physicians reported that they routinely administer rotavirus vaccine to all eligible infants whose parents consent (85% and 45% in 2007), whereas 27% of family physicians and 4% of pediatricians reported that they do not administer rotavirus vaccine (42% and 11% in 2007) (p<0.001 for both differences between specialties and years).
Compared to 2007, more physicians in both specialties endorsed positive attitudes toward rotavirus vaccine in 2010 (Appendix A, available online at www.ajpmonline.org). Most physicians strongly or somewhat agreed that “because rotavirus infections are common and potentially severe in the U.S., there is a need for a safe and effective rotavirus vaccine” and also with the ACIP’s recommendation that rotavirus vaccine should be routinely recommended for all eligible infants.
Statements related to perceived parental concern about safety were the most commonly identified barriers by all physicians (Figure 2). For all barriers presented, the percentage of physicians reporting definitely or somewhat of a barrier decreased from 2007 to 2010 with one exception: 42% of pediatricians in 2010 reported that “Parents’ concern about the safety of vaccines in general” was definitely or somewhat of a barrier compared to 35% in 2007 (p=NS).
Perception of Rotavirus Vaccine Effectiveness
Compared to family medicine physicians, pediatricians more often strongly or somewhat agreed with the statement, “Rotavirus vaccine has resulted in marked decreases in hospitalizations and emergency room visits for diarrhea in the United States” (95% vs 66%, p<0.001). In bivariate analysis among family medicine physicians, among those who reported administering rotavirus vaccine, 75% strongly or somewhat agreed with the preceding statement compared to 40% who reported not administering the vaccine (p<0.001).
Physician Knowledge of U.S. Food and Drug Administration Recommendations
Among physicians who reported administering any rotavirus vaccine as of March 2010 (n=397), 94% of pediatricians and 70% of family medicine physicians reported having heard about the FDA’s recommendation to temporarily suspend the use of RV1 because of the presence of porcine circovirus (p<0.001). Among physicians who reported administering any rotavirus vaccine and who reported that they had heard about the FDA’s recommendation to temporarily suspend the use of RV1 because of the presence of porcine circovirus (n=337), 68% of pediatricians and 53% of family medicine physicians reported that they did not alter their practice, as they were already using only RV5; 28% of pediatricians and 25% of family physicians reporting that they stopped using RV1 and gave only RV5; and 4% of pediatricians and 19% of family physicians reported that they temporarily stopped giving any rotavirus vaccine (p<0.001). Among the same group of physicians who reported having heard about the suspension of RV1 (n=337), at least 6 months after the FDA’s announcement that it was appropriate to resume use of both rotavirus vaccines, 78% of pediatricians and 67% of family physicians reported that they were administering RV5 only, although 1% and 6%, respectively, reported no longer administering any rotavirus vaccine (p=0.002).
Physician Knowledge of Labeling Change
A total of 49% of pediatricians and 45% of family physicians reported having heard about the FDA’s addition to the warnings and precautions section of the RV1 labeling regarding a possible increased risk of intussusception (p=0.51). After reading an explanation about these labeling changes, all physicians were asked to respond to a question assessing the impact of these changes. As with the questions regarding porcine circovirus, most physicians who reported that they were administering any rotavirus vaccine were administering RV5 and would not alter their practice (pediatricians, 72%; family physicians, 45%; p<0.001). A total of 4% from each speciality reported that they had stopped or would stop giving RV1 and give only RV5, and 2% of family medicine physicians and 1% of pediatricians reported that they had stopped or would stop giving any rotavirus vaccine based on the information about intussusception.
Physicians’ Attitudes and Perceptions of Parental Knowledge
The majority of both specialties agreed that most parents were unaware of either the FDA’s recommendation to temporarily suspend the use of RV1 because of the presence of porcine circovirus or the possible association of RV1 with intussusception (Appendix B, available online at www.ajpmonline.org). The majority of both specialties reported that the information about a possible association of RV1 and intussusception had increased their own concerns about the safety of RV1, and 60% of family medicine physicians and 30% of pediatricians reported that it had increased their own concerns about the safety of both rotavirus vaccines.
Discussion
This nationally representative survey of pediatricians and family medicine physicians found that for most physicians, the recent information about porcine circovirus and a possible increased risk of intussusception and the subsequent FDA actions had little impact on their practice, and most physicians report that questions from parents regarding these issues were uncommon. However, the new information appears to have increased physicians’ concerns about the safety of rotavirus vaccine, particularly family medicine physicians.
The study also shows that acceptance and use of rotavirus vaccines has increased during the 5 years since the ACIP recommendation, with 95% of pediatricians reporting routinely administering rotavirus vaccine to all eligible infants. These changing attitudes and acceptance of the vaccine have translated into relatively rapid increases in RV coverage.22 However, less than half of family medicine physicians report strongly recommending the vaccine, and only 65% routinely administer the vaccine; thus, efforts to increase acceptance and use of rotavirus vaccine must continue.
Barriers to use of rotavirus vaccine have decreased in both specialties. The decreases in financial barriers are particularly encouraging, as these were among the most commonly endorsed in 2007. This decrease is likely due to virtually all insurance companies now covering the vaccine. Physician attitudes regarding rotavirus vaccine also have decreased as reported barriers, a finding that probably is related both to experience with the vaccine and knowledge of its impact. Physicians’ report of a continuing high level of parental concern regarding vaccine safety in general is consistent with other studies,23 although it is encouraging that few physicians reported parental concern specifically about rotavirus vaccine.
As with other studies on the adoption of new vaccines,24–26 there were important differences between family medicine physicians and pediatricians in practices and attitudes regarding rotavirus vaccine. Although both specialties are more likely to administer rotavirus vaccine now than they were 5 years ago, pediatricians are substantially more likely to use the vaccine, report a higher level of confidence in the effectiveness of rotavirus vaccine, and feel favorable about the vaccine. Reasons for differences in adoption between the specialties are likely fueled by a variety of issues that have been discussed previously in the literature.8 The current data also suggest that, in the case of rotavirus, lack of awareness of the data demonstrating effectiveness of the vaccine also appears to be a factor associated with lower use by family medicine physicians.
Limitations
The current study has some important limitations. First, survey respondents may have differed from nonrespondents. In addition, this survey was based on sentinel physician networks from the AAP and AAFP, and members of these organizations may have had different experiences or perceptions than nonmembers. Also, the survey took place at the end of 2010, 3 months and 6 months after the actions by the FDA regarding intussusception and porcine circovirus, respectively, so parents and physicians may not yet have heard about these actions. In addition, because many of the respondents had not heard of the recent actions by the FDA, their responses were based on reading an informational statement provided in the survey. Their responses might have differed if they had received the information from other sources. Finally, the survey relied on self-report rather than observation of practice.
Conclusion
The acceptance and use of rotavirus vaccines have increased since the ACIP recommendation and barriers to use, particularly financial ones, have decreased greatly. The recent publicity about potential safety concerns does not appear to have had a negative impact on physician acceptance of rotavirus vaccine, or vaccine administration practices of physicians, although it has raised safety concerns among some physicians, especially those in family medicine. With providers reporting increasing parental concern with vaccine safety,23 physicians’ confidence in the safety and importance of vaccines assumes even greater importance than in the past. There will be an ongoing need to assess the impact of increased safety concerns among providers on vaccine use.
Supplementary Material
Acknowledgments
This investigation was funded by a grant from the CDC PEP (MM-1040-08/08) through the Association of American Medical Colleges, Washington DC. The authors thank Lynn Olson, PhD, and Karen O’Connor from the Department of Research, AAP, Herbert Young, MD, and Bellinda Schoof, MHA, at the AAFP, and the leaders of the AAP and AAFP for collaborating in the establishment of the sentinel networks in pediatrics and family medicine. The authors also thank all pediatricians and family medicine physicians in the networks for participating in and responding to this survey.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Appendix. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.amepre.2012.10.001.
Footnotes
No financial disclosures were reported by the authors of this paper.
References
- 1.CDC. Withdrawal of rotavirus vaccine recommendation. MMWR Morb Mortal Wkly Rep. 1999;48(43):1007. [PubMed] [Google Scholar]
- 2.Murphy TV, Gargiullo PM, Massoudi MS, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344(8):564–72. doi: 10.1056/NEJM200102223440804. [DOI] [PubMed] [Google Scholar]
- 3.Iwamoto M, Saari TN, McMahon SR, et al. A survey of pediatricians on the reintroduction of a rotavirus vaccine. Pediatrics. 2003;112(1 Pt 1):e6–e10. doi: 10.1542/peds.112.1.e6. [DOI] [PubMed] [Google Scholar]
- 4.Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 6.CDC. Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR–12):1–13. [PubMed] [Google Scholar]
- 7.Cortese MM, Parashar UD. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2009;58(RR–2):1–25. [PubMed] [Google Scholar]
- 8.Kempe A, Patel MM, Daley MF, et al. Adoption of rotavirus vaccination by pediatricians and family medicine physicians in the U. S Pediatrics. 2009;124(5):e809–e816. doi: 10.1542/peds.2008-3832. [DOI] [PubMed] [Google Scholar]
- 9.CDC. Reduction in rotavirus after vaccine introduction—U.S., 2000–2009. MMWR Morb Mortal Wkly Rep. 2009;58(41):1146–9. [PubMed] [Google Scholar]
- 10.Begue RE, Perrin K. Reduction in gastroenteritis with the use of pentavalent rotavirus vaccine in a primary practice. Pediatrics. 2010;126(1):e40–e45. doi: 10.1542/peds.2009-2069. [DOI] [PubMed] [Google Scholar]
- 11.Cortese MM, Tate JE, Simonsen L, Edelman L, Parashar UD. Reduction in gastroenteritis in U.S. children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatr Infect Dis J. 2010;29(6):489–94. doi: 10.1097/INF.0b013e3181d95b53. [DOI] [PubMed] [Google Scholar]
- 12.Wang FT, Mast TC, Glass RJ, Loughlin J, Seeger JD. Effectiveness of the pentavalent rotavirus vaccine in preventing gastroenteritis in the U. S Pediatrics. 2010;125(2):e208–e213. doi: 10.1542/peds.2009-1246. [DOI] [PubMed] [Google Scholar]
- 13.Tate JE, Mutuc JD, Panozzo CA, et al. Sustained decline in rotavirus detections in the U.S. following the introduction of rotavirus vaccine in 2006. Pediatr Infect Dis J. 2011;30(1S):S30–S34. doi: 10.1097/INF.0b013e3181ffe3eb. [DOI] [PubMed] [Google Scholar]
- 14.Curns AT, Steiner CA, Barrett M, Hunter K, Wilson E, Parashar UD. Reduction in acute gastroenteritis hospitalizations among U.S. children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 U.S. states. J Infect Dis. 2010;201(11):1617–24. doi: 10.1086/652403. [DOI] [PubMed] [Google Scholar]
- 15.Boom JA, Tate JE, Sahni LC, et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the U. S Pediatrics. 2010;125(2):e199–e207. doi: 10.1542/peds.2009-1021. [DOI] [PubMed] [Google Scholar]
- 16.Victoria JG, Wang C, Jones MS, et al. Viral nucleic acids in live-attenuated vaccines: detection of minority variants and an adventitious virus. J Virol. 2010;84(12):6033–40. doi: 10.1128/JVI.02690-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meeting of the Global Advisory Committee on Vaccine Safety, December 2010. Wkly Epidemiol Rec. 2011;86(5):38–43. [PubMed] [Google Scholar]
- 18.Food and Drug Administration. Information on Rotarix—labeling revision pertainingtointussusception. www.fda.gov/biologicsbloodvaccines/vaccines/approvedproducts/ucm226690.htm.
- 19.Crane LA, Daley MF, Barrow J, et al. Sentinel physician networks as a technique for rapid immunization policy surveys. Eval Health Prof. 2008;31(1):43–64. doi: 10.1177/0163278707311872. [DOI] [PubMed] [Google Scholar]
- 20.Dillman DA, Smyth J, Christian LM. Internet, mail and mixed-mode surveys: the tailored desgin method. 3. New York NY: Wiley; 2009. [Google Scholar]
- 21.McMahon SR, Iwamoto M, Massoudi MS, et al. Comparison of e-mail, fax, and postal surveys of pediatricians. Pediatrics. 2003;111(4 Pt 1):e299–e303. doi: 10.1542/peds.111.4.e299. [DOI] [PubMed] [Google Scholar]
- 22.National and state vaccination coverage among children aged 19–35 months—U.S., 2010. MMWR Morb Mortal Wkly Rep. 2011;60(34):1157–63. [PubMed] [Google Scholar]
- 23.Kempe A, Daley MF, McCauley MM, et al. Prevalence of parental concerns about childhood vaccines: the experience of primary care physicians. Am J Prev Med. 2011;40(5):548–55. doi: 10.1016/j.amepre.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 24.Davis MM, Ndiaye SM, Freed GL, Clark SJ. One-year uptake of pneumococcal conjugate vaccine: a national survey of family physicians and pediatricians. J Am Board Fam Pract. 2003;16(5):363–71. doi: 10.3122/jabfm.16.5.363. [DOI] [PubMed] [Google Scholar]
- 25.Freed GL, Freeman VA, Clark SJ, Konrad TR, Pathman DE. Pediatrician and family physician agreement with and adoption of universal hepatitis B immunization. J Fam Pract. 1996;42(6):587–92. [PubMed] [Google Scholar]
- 26.Schaffer SJ, Szilagyi PG, Shone LP, et al. Physician perspectives regarding pneumococcal conjugate vaccine. Pediatrics. 2002;110(6):e68. doi: 10.1542/peds.110.6.e68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.