Capsule Summary
NLRC4-inflammasome hyperactivity causes infantile-onset Macrophage Activation Syndrome and enterocolitis with extraordinary serum IL-18 elevation (NLRC4-MAS). Herein, we report a critically ill infant with severe, refractory NLRC4-MAS who showed sustained response to treatment with experimental IL-18 inhibition.
Keywords: Inflammasome, IL-18, Macrophage Activation Syndrome, investigational therapy, personalized medicine
To the Editor
Clinical application of several rapidly-evolving technologies: next-generation DNA sequencing, biomarker discovery, and targeted cytokine blockade, has been particularly beneficial to understanding an expanding spectrum of genetically-defined autoinflammatory diseases(1). Our understanding of the pathways that cause hemophagocytic disorders, like Macrophage Activation Syndrome (MAS) and Hemophagocytic Lymphohistiocytosis (HLH), is evolving similarly. MAS and HLH are life-threatening sepsis-like conditions notable for hyperferritinemia, acute cytopenias, and hepatitis. If not promptly recognized and treated, they can progress to consumptive coagulopathy, hemophagocytosis, multi-organ failure, and high mortality. HLH is classically associated with genetic defects in cytotoxicity, whereas MAS is observed as a complication of rheumatic diseases(1).
We recently implicated gain-of-function mutations in NLRC4, a protein that activates an inflammasome, in a syndrome of recurrent MAS with early-onset enterocolitis (NLRC4-MAS, OMIM#616050)(2, 3). Inflammasomes are large innate immune complexes that quickly and exponentially catalyze the activation of pro-IL-1β and pro-IL-18. Although IL-1β blockade is effective in many “inflammasomopathies”(1, 2), the role of IL-1β in MAS is controversial. IL-1 blockade is effective in treating MAS-prone diseases, but was not protective against the development of MAS. The effects of blocking IL-18 are unknown. NLRC4-MAS patients have extraordinary and chronic elevation of serum IL-18(2, 3); and although extraordinary IL-18 levels are a feature of MAS more generally, IL-18 is only modestly elevated in other genetic inflammasomopathies.
Classically, myeloid cell-derived IL-18 enhances IL-12 driven Interferon-γ (IFNγ) production; but IL-18 can also synergize to promote a variety of diverse immunological effects. Notably, IFNγ is the cytokine most implicated in driving familial forms of HLH(4), although it’s role in MAS is more controversial. IL-18 is also highly expressed in intestinal (and other) epithelial cells and has complex roles in intestinal homeostasis and colitis(5). IL-18 Binding Protein (IL-18BP) is an endogenous protein that binds tightly to IL-18, preventing signaling but not serologic detection(6).
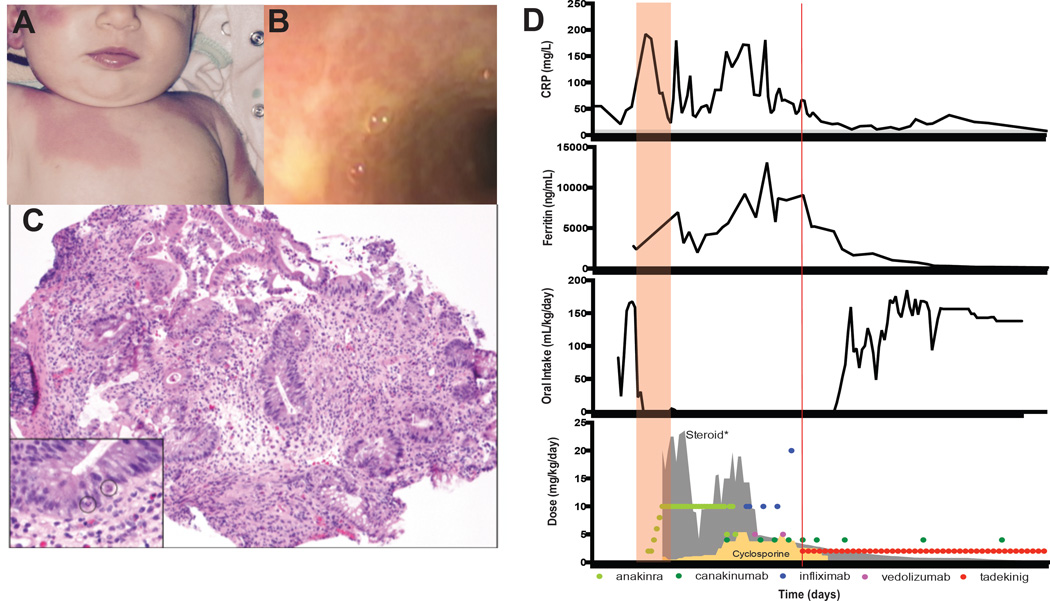
Herein, we report a previously healthy Caucasian female who developed a parainfluenza upper respiratory infection at 6 weeks of age. Cough, coryza, and viral RNA cleared, but hectic fevers and erythrodermic rash (Figs 1A & D) persisted for several weeks. After three weeks of fever, she acutely developed oral intolerance and severe secretory diarrhea that persisted despite cessation of oral intake. Her condition worsened with acute rise in inflammatory markers, relative thrombocytopenia, and rising ferritin, consistent with an MAS-like syndrome. Thorough infection and malignancy work-ups were unrevealing, and there were no signs of immunodeficiency. Endoscopy showed severe mucosal ulcerations and inflammation extending from stomach through large intestine, with normal staining for intestinal regulatory T-cells (Figs 1B & C, E1A). Functional assessment of cytotoxicity was normal. Clinical whole exome sequencing returned a de novo heterozygous mutation in NLRC4 (c.1022T>C, p.Val341Ala) within two weeks. This same mutation had previously been associated with a very similar, but dominantly-inherited syndrome in a small kindred, including an infant who succumbed soon after birth(3).
Fig 1. Rash, enterocolitis, and clinical course in NLRC4-related autoinflammation.
(A) erythrodermal skin lesions. (B) Ulcerative duodenal plaques. (C) Edematous sigmoid colon with flattened villi, crypt drop-out, and apoptotic crypt epithelial cells (inset circles); 20×, H&E. (D) C-reactive protein (CRP), ferritin, enteral intake, and anti-inflammatory medications. Red box: MAS onset. Red line: start of rhIL-18bp. *Steroid (gray outline): running seven-day average of daily prednisone-equivalent dose.
The patient was treated aggressively with corticosteroids (including 11 pulses of 30 mg/kg in one month) and IL-1 blockade (10 mg/kg/day anakinra) with minimal response (Fig 1D). The addition of maximal doses of TNFα-blockade (infliximab, up to 20 mg/kg), cyclosporine, and α4β7-integrin inhibition (vedolizumab) resolved her fever and coagulopathy but did not improve anemia, hyperferritinemia, or enterocolitis. We assessed serum for total IL-18, as well as free IL-18 using a novel assay(6), and found both to be extraordinarily elevated (Fig 2A). Under an emergency compassionate-use Investigational New Drug authorization from the Food and Drug Administration, the patient was given recombinant human IL-18BP (rhIL-18BP) at a dose of 2 mg/kg subcutaneously every 48 hours. Within the first two doses, the patient’s overall demeanor improved rapidly in correlation with an acute drop in ferritin (Fig 1D). C-reactive protein and enterocolitis also improved, and the patient successfully restarted enteral feeding 11 days after rhIL-18BP initiation. While on combined IL-18 and IL-1β blockade, the patient also weaned from all other forms of immunosuppression (Fig 1D). Given the severe presentation, persistent total IL-18 elevation, and the chronic course associated with this mutation(3), further attempts at weaning were gradual. The patient remains well after 11 months of combined IL-1β and IL-18 blockade, having weathered vaccination (except live-virus vaccines), typical infections, and partial weaning of IL-1β blockade without flare.
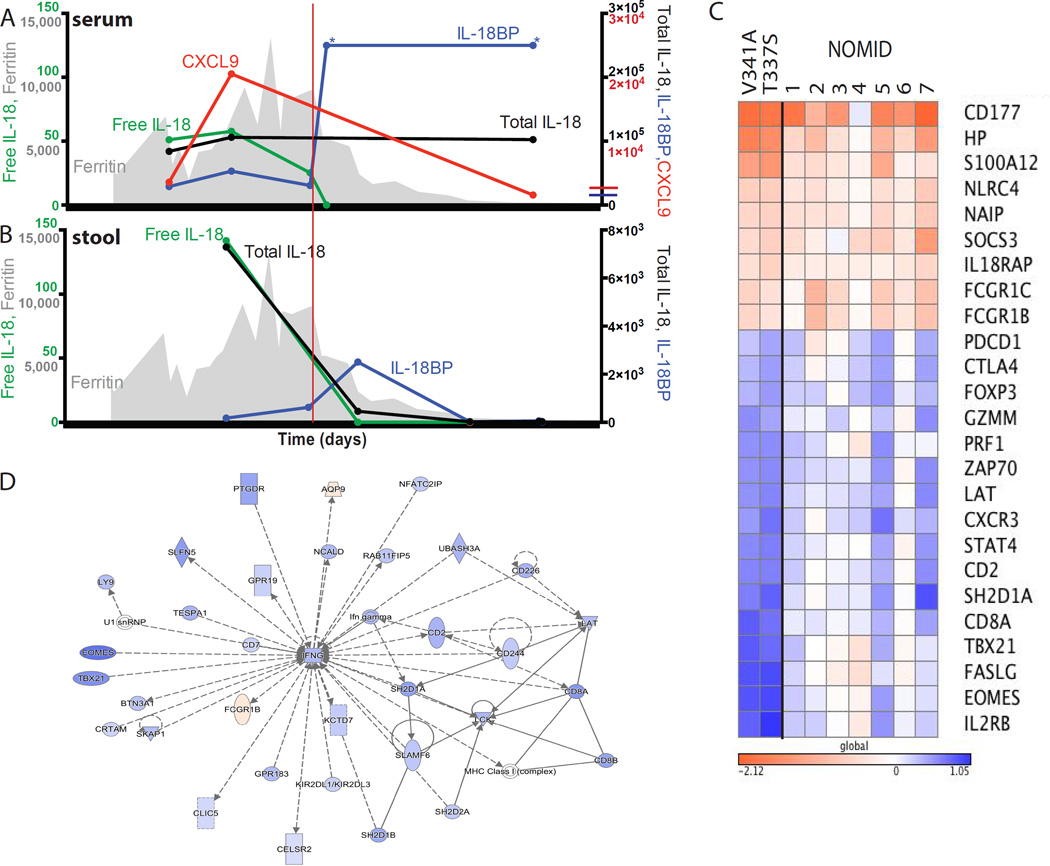
Fig 2. Serum, Stool, and Transcriptional Analyses.
Serum (A) and stool (B) cytokines (in pg/mL) and serum ferritin (gray, ng/mL). Upper limit of healthy controls, if visible, crosses y-axis. (C) and (D) Whole blood RNA-seq comparing flare versus post-treatment in this patient (V341A), another NLRC4-MAS patient (T337S)(2), and seven NOMID patients. Log10 fold-change for genes higher (red) or lower (blue) during flare in both NLRC4-MAS patients. See also Supplemental Table 1. (D) Most-enriched transcriptional network generated by the differentially-expressed gene list. *Upper Limit of detection.
To better understand the pathogenic mechanisms at work, we measured IL-18 and related cytokines in serum and stool, and obtained whole blood transcriptional profiles. Serum analysis demonstrated massive, chronic, and stable serum total IL-18 elevation, consistent with our original reports (Fig 2A)(2, 3). Serum levels of CXCL9, a chemokine induced by IFNγ (and other inflammatory mediators) and associated with HLH activity(7), remained high despite high-dose steroids and IL-1 inhibition, but fell after initiation of rhIL-18BP (Figs 1 & 2A). Free IL-18 fell slightly with immunosuppression, but quickly fell to normal with rhIL-18BP. In stool, we measured almost 15-fold less total IL-18 than in serum, but minimal IL-18BP, resulting in nearly three times more free IL-18 (Fig 2B). rhIL-18BP treatment correlated with a brief spike in stool IL-18BP levels. All cytokines decreased with clinical improvement of gut disease.
Whole blood transcriptional analysis from flare and after clinical improvement was compared with similar profiles in a previously described NLRC4-MAS patient(2), and several Neonatal-Onset Multisystem Inflammatory Disease (NOMID, OMIM 607115) patients with NLRP3 mutations). Transcripts encoding neutrophil-associated genes (CD177, HP), components of the IL-18 receptor (IL18RAP), NLRC4 itself, and NAIP (known to promote NLRC4 inflammasome assembly) were elevated during flare in both NLRC4-MAS and NOMID samples (Fig 2C). Elevated levels of NLRC4 transcripts have also been observed in patients with active sJIA(8). However, there were prominent decreases in transcripts associated with cytotoxic T and natural killer cells (EOMES, TBX21, CD8A, PRF1), as well as regulatory T cells (FOXP3, CTLA4), during disease flare most pronounced in NLRC4-MAS (Fig 2C). Network analysis of genes up- or down-regulated in NLRC4-MAS patients identified an IFNγ-associated network as the most enriched (Fig 2D and Table E1). Taken together, these transcriptional findings support myeloid cell activation and cytotoxic and regulatory T cell dysfunction as potential pathomechanisms.
Rational choice of immunomodulatory therapies in monogenic inflammatory diseases can be challenging. Although our recent reports reinforced IL-18 as an enticing target for this patient, our prior measurements suggested possibly more IL-18 than could be reasonably neutralized (often more than 100,000 pg/mL). However, the free IL-18 assay demonstrated levels of unbound IL-18 consistent with the potential for pharmacologic inhibition(6), and enabled us to verify free IL-18 normalization soon after treatment initiation (Fig 2A). Importantly, rhIL-18BP was administered with and after multiple other immunosuppressive drugs (Figure 1D). Its beneficial effects may have been mediated by improving other drugs’ efficacy or pharmacodynamics (intestinal protein losses, in particular).
Interestingly, IL-18 remains chronically elevated long after NLRC4-MAS patients have clinically improved ((2) & Fig 2A), demonstrating that IL-18 alone is not sufficient to drive MAS. Likewise, NK cells from sJIA patients may become chronically insensitive to IL-18(9). The rapidity of our patient’s response to rhIL-18BP suggests inhibition of ongoing IL-18 signaling rather than release of IL-18 insensitivity. Her durability of response suggests disruption of one of the amplification loops (possibly involving IFNγ) that often underlie autoinflammatory diseases(1). The chronicity of serum (but not blood transcriptional) IL-18 elevation in NLRC4-MAS suggests a possible non-hematopoietic source, and measurements in stool suggest minimal baseline luminal secretion and complex tissue-specific regulation.
Gene expression analysis identified potentially generalizable patterns of myeloid cell activation and Treg suppression shared between NLRC4-MAS and NOMID, but also NLRC4-specific depression of cytotoxicity-related transcripts reminiscent of HLH. Importantly, these transcriptional findings could represent shifts in cellular composition caused by steroid use or acute inflammation rather than underlying pathophysiology.
This report raises hope for more general benefit treating diseases marked by excessive free IL-18 and also preventing progression to MAS in susceptible patients. After nearly a year of chronic IL-18 blockade, there are no signs of significant immunosuppression or dysregulated mucosal homeostasis (normal growth, and lung and intestinal function). In conclusion, this patient’s life-threatening autoinflammatory condition improved due to rapid clinical and genetic diagnosis, identification of a novel biomarker, and successful deployment of an experimental, targeted therapy. The patient’s improvement, coupled with ongoing studies in HLH and sepsis, help define relevant roles for IL-18, IL-1β, and IFNγ in sepsis-like systemic inflammation.
Supplementary Material
Acknowledgments
Support:
SC, LM, AD, and RG were supported by the Intramural Research Program of the National Institute of Arthritis, Musculoskeletal, and Skin Diseases. EB was supported by NIH R01 HL112836-A1, The Nancy Taylor Foundation, and Sean Fischel Connect. CGa is supported by Swiss National Science Foundation grant 10030_152638, The Rheumasearch Foundation and the Institute of Arthritis Research.
Abbreviations Used
- MAS
Macrophage Activation Syndrome
- HLH
Hemophagocytic Lymphohistiocytosis
- IFNγ
Interferon gamma
- IL-18BP
Interleukin-18 Binding Protein
- NOMID
Neonatal-Onset Multisystem Inflammatory Disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Jesus AA, Canna SW, Liu Y, Goldbach-Mansky R. Molecular mechanisms in genetically defined autoinflammatory diseases: disorders of amplified danger signaling. Annu Rev Immunol. 2015 Mar 21;33:823–874. doi: 10.1146/annurev-immunol-032414-112227. PubMed PMID: 25706096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canna SW, de Jesus AA, Gouni S, Brooks SR, Marrero B, Liu Y, et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet. 2014 Oct;46(10):1140–1146. doi: 10.1038/ng.3089. PubMed PMID: 25217959. Pubmed Central PMCID: 4177369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romberg N, Al Moussawi K, Nelson-Williams C, Stiegler AL, Loring E, Choi M, et al. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat Genet. 2014 Sep 14; doi: 10.1038/ng.3066. PubMed PMID: 25217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004 Aug 1;104(3):735–743. doi: 10.1182/blood-2003-10-3413. PubMed PMID: 15069016. Epub 2004/04/08. eng. [DOI] [PubMed] [Google Scholar]
- 5.Nowarski R, Jackson R, Gagliani N, de Zoete MR, Palm NW, Bailis W, et al. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell. 2015 Dec 3;163(6):1444–1456. doi: 10.1016/j.cell.2015.10.072. PubMed PMID: 26638073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girard C, Rech J, Brown M, Allali D, Roux-Lombard P, Spertini F, et al. Elevated serum levels of free interleukin-18 in adult-onset Still's disease. Rheumatology (Oxford) 2016 Sep 10; doi: 10.1093/rheumatology/kew300. PubMed PMID: 27616144. [DOI] [PubMed] [Google Scholar]
- 7.Takada H, Takahata Y, Nomura A, Ohga S, Mizuno Y, Hara T. Increased serum levels of interferon-gamma-inducible protein 10 and monokine induced by gamma interferon in patients with haemophagocytic lymphohistiocytosis. Clin Exp Immunol. 2003 Sep;133(3):448–453. doi: 10.1046/j.1365-2249.2003.02237.x. PubMed PMID: 12930373. Pubmed Central PMCID: 1808805. Epub 2003/08/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quartier P, Allantaz F, Cimaz R, Pillet P, Messiaen C, Bardin C, et al. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial) Ann Rheum Dis. 2011 May;70(5):747–754. doi: 10.1136/ard.2010.134254. PubMed PMID: 21173013. Pubmed Central PMCID: 3070271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jager W, Vastert SJ, Beekman JM, Wulffraat NM, Kuis W, Coffer PJ, et al. Defective phosphorylation of interleukin-18 receptor beta causes impaired natural killer cell function in systemic-onset juvenile idiopathic arthritis. Arthritis and rheumatism. 2009 Sep;60(9):2782–2793. doi: 10.1002/art.24750. PubMed PMID: 19714583. Epub 2009/08/29. eng. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.