Abstract
Background And Objective
Many patients with cannabis use disorder (CUD) do not achieve or do not have abstinence as a goal of treatment, rather they reduce their use. Assessing outcome measures as they relate to functioning and reductions in cannabis use is an important area of study. Quality of life (QoL) shows promise as one such measure. Past studies have demonstrated gender differences in QoL and CUD. We aim to assess (1) the relationship between cannabis use and QoL and (2) gender effects in an outpatient medication treatment study for CUD.
Methods
Data from an 11-weeks, double-blind, placebo-controlled trial of lofexidine and dronabinol for CUD (n = 62) was analyzed. Pearson’s correlations between baseline QoL as measured with the Quality of Life, Enjoyment, and Satisfaction Questionnaire-Short Form (QLES-Q-SF) and cannabis use assessed with modified timeline follow-back (TLFB) were examined. Multiple linear regression models of cannabis use on end of study QLES-Q-SF were analyzed, while adjusting for baseline QLES-Q-SF, study arm, and gender. Moderation effects with gender were also tested.
Results
No significant association between baseline cannabis use and QoL was found. End of study abstinence (F1,47 = 8.34, p = .006) and reduced proportion of using days (F1,47 = 9.48, p = .004) were each significantly associated with end of study QoL. Reduction in grams (F1,27 = 0.25, p = .62) was not associated with QoL at end of study. Gender was not a significant moderator.
Discussion and Conclusions
Abstinence and lower frequency of use are associated with higher QoL, regardless of gender.
Scientific Significance
This is the first time QoL has been demonstrated to change over the course of CUD medication treatment. QoL is an important outcome in CUD treatment.
Trial Registration
NCT01020019. (Am J Addict 2018;27:101–107).
INTRODUCTION
To date, primary outcome measures in cannabis use disorder (CUD) treatment trials have focused on abstinence as measured with biological (urine cannabinoid testing) and self-report measures of cannabis use.1 While abstinence is the ultimate goal, these treatment studies have demonstrated overall low rates of abstinence, though many patients reduce their use.2 One factor may be that it is common for patients with CUD to identify reduction in use as a treatment goal, and not abstinence.3 Further, with increasing state legalization of both recreational and medical marijuana, there is a movement to understand if some amount of cannabis use is “acceptable,” in that it does not negatively impact health in a significant way.4 If reduction in cannabis use that does not equate to abstinence is a reasonable target for treatment assuming some amount of cannabis use is not detrimental to one’s health, a clear consensus on what constitutes a clinically meaningful reduction during treatment is needed. As a start, we need to understand the relationship between changes in cannabis use and other functional outcomes in order to establish endpoints for interventions.
Quality of life (QoL) is a subjective, person-centered, multi-dimensional concept that assesses physical, mental, emotional, and social well-being. The assessment of QoL in cannabis users shows promise as one outcome measure to track alongside changes in cannabis use during treatment.5 Regular and heavy cannabis users have associated poorer subjective ratings on QoL with regards to exercise, activity, sleep, and general health as compared to light or non-users.6,7 A recent systematic review, demonstrated heavy cannabis use or CUD is associated with reduced QoL.8 Notably, in recreational users, regular, weekly cannabis use is associated with lower mental health related QoL whereas occasional use of cannabis, defined as less than weekly use, is not associated with diminished QoL in individuals with co-occurring anxiety disorders or depressive disorders.9 This suggests a dose response relationship of cannabis use in terms of frequency and amount on QoL. Prospective and retrospective studies of cannabis users found events that led to improvement in QoL (eg, marriage, employment, parenthood) were associated with reduction in use or abstinence while continued use was associated with factors that decreased QoL (eg, unemployment, low income, stress, other substance use disorders that decrease functioning).10,11 Large epidemiological data from the NESARC show cannabis users report poorer mental health related QoL (eg, poorer general mental health, lower levels of vitality, and less productivity due to emotional problems) compared to non-users, with these differences being greater in women compared to men.4 This finding was replicated in women with cannabis use disorders and co-occurring depression.12 The relationship between cannabis use and QoL appears to be differentially impacted by the severity of cannabis use, with gender playing a potential moderating role.
The aim of this secondary analysis was to assess whether cannabis use in individuals with CUD undergoing outpatient treatment through a double-blind randomized placebo-controlled trial (see Levin et al.3 combination of lofexidine and dronabinol verses placebo) is associated with self-reported measures of QoL. Additionally, we also examine whether this association is significantly moderated by gender. We hypothesized that (1) more severe cannabis use would be associated with poorer QoL at the start of the clinical trial, and (2) reduction in cannabis use both in terms of quantity in grams and frequency in days and/or abstinence, while accounting for treatment arm, will be associated with higher QoL in women than in men at end of study.
METHODS
Sample and Study Design
Details regarding screening, inclusion, and exclusion criteria of participants, and study design have been previously published.3,13 All participants met criteria for cannabis dependence based on the Structured Clinical Interview (SCID) for Diagnostic and Statistical Manual of Mental Disorders-Axis I disorders DSM-IV and were seeking treatment. Baseline characteristics of participants are described elsewhere in detail.3 Of the 156 participants enrolled in the double-blind study, 122 participants were randomized to receive either placebo or active medication (dronabinol and lofexidine) for 11 weeks in an outpatient clinic setting.3 A total of 62 participants completed 12 weeks of the study and completed QLES-Q-SF scores at week 12. All participants received manualized motivational enhancement and cognitive behavioral/relapse prevention therapy over the course of the trial.
MEASURES
Cannabis Use
The timeline follow-back (TLFB) assessment modified for marijuana14 was used to assess participant cannabis use at twice weekly clinic visits. Details of this procedure are provided in earlier studies developed by our group and allow a better quantification of amount of cannabis use measured in grams in addition to frequency of use measured in days.13,15 While quantitative urine drug screens were collected, dronabinol produces a positive screen. A strong association had previously been found between TLFB and urine drug screens in the placebo arm of a past study from our group using dronabinol, supporting the use of the TLFB as a reliable method to measure cannabis use.13
Quality of Life
The Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form (QLES-Q-SF), a 16-item self-report questionnaire, was used to assess the degree of enjoyment and satisfaction experienced by subjects in different areas of daily functioning, on a 5-point scale.16 Satisfaction with physical functioning, household duties, work, leisure, social support and relationships, subjective feelings, medication, and overall life are assessed. The sum score of the first 14 items is computed, where higher scores on the QLES-Q-SF indicate greater contentment or satisfaction. The minimum raw score on the QLES-Q-SF is 14, and the maximum is 70. Raw scores can be converted into a percentage (raw score-14)/56) of the maximum possible score on the QLES-Q-SF to provide a clearer conceptual framework for interpreting quality of life scores. The QLES-Q-SF has been previously used in a number of treatment trials and in diverse patient populations with mood, anxiety, and psychotic disorders to assess quality of life dimensions, demonstrating a differential improvement for patients on active treatment, including medications17 The summary score has been shown to be a reliable and valid measure of quality of life that is related to but not redundant with severity of illness.16 Previous studies of normative community samples have reported a mean QLES-Q-SF raw score of 57.8 and converted percentage score of 78.3% (SD = 11.3%), where scores within 10 percentage points of this value (QLES-Q-SF ≥ 53.5 or 70.47%) are considered “within-normal.”18 QLES-Q-SF scores less than 2 SD below the community norm scores, that is, QLES-Q-SF scores ≤ 45.2 or 55.7%, are considered “severely-impaired” QoL.
Data Analyses
The relationship between baseline amount of cannabis use (a natural log transformed total dollar amounts and total grams of cannabis 1 month prior to treatment) and baseline QLES-Q-SF raw scores was analyzed using Pearson’s correlations. The primary outcome of QoL at the end of study (QLES-Q-SF raw scores at week 12) was analyzed using separate multiple linear regression models for each of the three predictors of interest: (1) abstinence during the last 2 weeks of the trial (yes or no); (2) frequency of use as measured with the average proportion of using days per week in the last 2 weeks; and (3) quantity of use as measured with the reduction in actual grams used from the weekly average at baseline (past 28 days) to the weekly average during the last 4 weeks of the trial (weeks 9–12). Baseline QLES-Q-SF raw scores, study arm, and gender were treated as covariates in each model. The interaction between gender and each predictor of interest was tested for moderation effects; if the interaction was not found to be significant, the interaction term was omitted and a model with only the main effects was fit.
RESULTS
Participants
Demographic and baseline clinical characteristics of randomized participants who completed week 12 QLES-Q-SF (N = 62) are shown in Table 1. The sample was predominantly male (66.1%), in their mid-thirties (mean age = 36.7), unmarried (77.4%), and white (46.8%). The majority of the sample completed college (36.1%) or graduate school (19.4%), and half (51.6%) were employed with part-time or full-time work.
TABLE 1.
Demographic and baseline clinical characteristics of the participants randomized to placebo and lofexidine + dronabinol and completed week 12 QLES-Q-SF (N = 62)
| Characteristic | Mean or n | SD or % |
|---|---|---|
| Demographic characteristics | ||
| Age (years) | 36.74 | 10.98 |
| Male | 41 | 66.13 |
| Race/Ethnicity | ||
| Hispanic | 16 | 25.81 |
| Black | 14 | 22.58 |
| White | 29 | 46.77 |
| Other | 3 | 4.84 |
| Education | ||
| High school | 14 | 22.58 |
| Some college | 13 | 20.97 |
| College | 23 | 36.10 |
| Graduate school | 12 | 19.35 |
| Employment status | ||
| Full-time | 24 | 38.71 |
| Part-time | 8 | 12.90 |
| Student | 9 | 14.52 |
| Unemployed/others | 21 | 32.87 |
| Currently married | 14 | 22.58 |
| Randomized to Lofexidine + Dronabinol | 31 | 50.00 |
| Clinical characteristics | ||
| Baseline QLES-Q-SF raw score (n = 52)a | 49.83 | 9.28 |
| Drug use in the 28 days prior to study entry | Median | IQR |
| Total dollars spent | 368.03 | 188.00–660.00 |
| Total grams used (n = 61)b | 37.12 | 17.29–63.24 |
| Average grams used per week | 9.28 | 4.32–15.81 |
| Proportion of using days during the past 28 days | 1.00 | .964–1.00 |
| Predictors | ||
| Last 2 weeks Abstinence | 29 | 46.77 |
| Last 2 weeks proportion of using days | .071 | 0.00–0.50 |
| Average grams used per week during the last 4 weeks | 1.71 | 0.53–4.45 |
| Average reduction in grams of cannabis use per week (n = 40)b,c | 5.49 | 3.19–12.16 |
| Outcome | ||
| QLES-Q-SF raw score at Week 12 | 56.11 | 9.38 |
IQR denotes “Interquartile Range.”
10 subjects were missing baseline QLES-Q-SF scores;
1 subject missing total grams used at baseline;
21 subjects were abstinent during the last 4 weeks and were not included
Baseline Cannabis Use and Quality of Life
At baseline, QLES-Q-SF was collected on 52 participants (10 participants’ baseline scores were missing due to: initiation of the study prior to IRB approval of use of the QLES-Q-SF as a measure (n = 6) and failure to complete the measure (n = 4)). The mean raw score was 49.83 (standard deviation (SD) = 9.28), which is equivalent to 64.0% of the maximum possible score on the QLES-S-SF (Table 1). The median total dollar amount and median total grams of cannabis used in the 28 days prior to treatment were $368.03 (interquartile range (IQR) = $188.00–$660.00) and 37.12 grams (IQR = 17.29g–63.24 g), respectively. At baseline, no significant correlations were discovered between QLES-Q-SF raw scores and log transformed cannabis use in dollar amount (r(50) = −.091, p = .520) or grams used (r(50) = −.158, p = .264).
Cannabis Use and Quality of Life at End of Study
All results of the main effect models predicting QLES-Q-SF raw scores at week 12 are described in detail below and summarized in Table 2.
TABLE 2.
Main effect models predicting QLES-Q-SF raw score at week 12
| Predictors | Model 1 (N = 52)a | Model 2 (N = 52)b | Model 3 (N = 32)c | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| b | SE | t | p | b | SE | t | p | b | SE | t | p | |
| Lofex-Dro (vs. Placebo) | 2.01 | 2.44 | 0.83 | .413 | 1.15 | 2.40 | 0.48 | .635 | 3.72 | 3.16 | 1.18 | .249 |
| Female (vs. Male) | 0.54 | 2.55 | 0.21 | .832 | 1.88 | 2.54 | 0.74 | .462 | −0.36 | 3.27 | −0.11 | .913 |
| Baseline QLESQ-SF raw score | 0.45 | 0.13 | 3.55 | .001 | 0.35 | 0.13 | 2.72 | .009 | 0.50 | 0.15 | 3.31 | .003 |
| Last 2 weeks Abstinence | 6.78 | 2.35 | 2.89 | .006 | ||||||||
| Last 2 weeks Proportion of using days | −11.14 | 3.62 | −3.08 | .004 | ||||||||
| Reduction in Cannabis use | 0.08 | 0.16 | 0.50 | .620 | ||||||||
b, beta coefficient; SE, standard error; t, t-statistic; p, p-value.
Gender by Last 2 weeks abstinence interaction was not significant (F1,46 = 0.67, p = .418) and was omitted from the final model;
Gender by Last 2 weeks Proportion of using days interaction was not significant (F1,46 = 0.42, p = .521) and was omitted from the final model;
Gender by Reduction in cannabis use interaction was not significant (F1,26 = 0.08, p = .786) and was omitted from the final model
Model 1: Effect of Abstinence During Last 2 Weeks
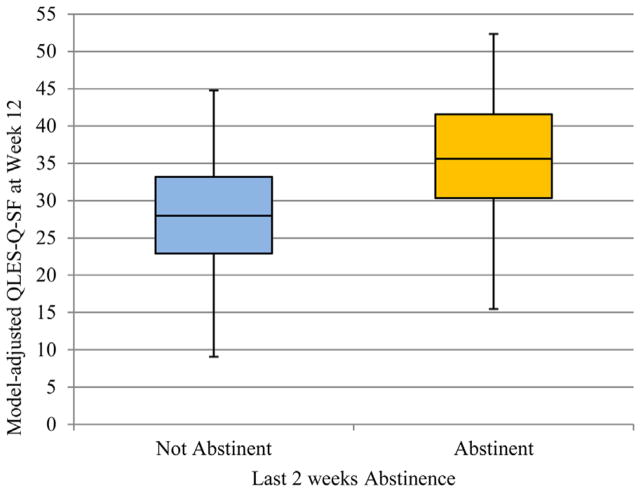
Of the subjects who completed QLES-Q-SF at week 12, the observed proportion of subjects achieving abstinence during the last 2 weeks of the trial was 29/62 (46.8%). There was no significant moderation effect of gender (F1,46 = 0.70, p = .408) on last 2 weeks abstinence, so the interaction term was omitted from the final model. Last 2 weeks abstinence was significantly associated (F1,47 = 8.34, p = .006) with higher QLES-Q-SF raw scores at week 12, while adjusting for study arm, gender, and baseline QLES-Q-SF score (see Table 2, Model 1). The model adjusted box plot for QLES-Q-SF raw scores and last 2 weeks abstinence is shown in Figure 1. Participants who were abstinent during the last 2 weeks of the trial reported higher QLES-Q-SF raw score on average by 6.78 points (or 12% of the maximum possible change in QLES-Q-SF score) compared to those who continued to use cannabis.
FIGURE 1.
Plot of model adjusted QLES-Q-SF at week 12 and last 2 weeks abstinence (N = 52).
Model 2: Effect of Proportion of Using Days
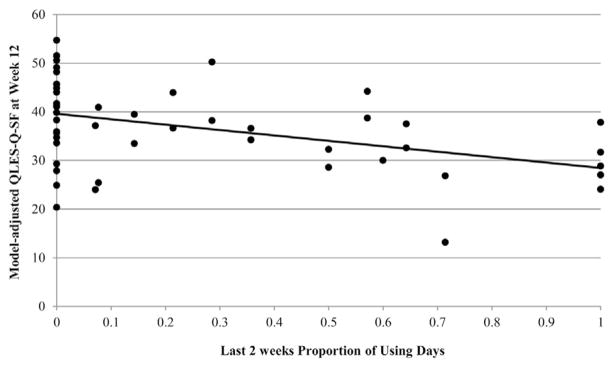
For subjects who completed the QLES-Q-SF at week 12, the median of the within-person average proportion of using days per week during the last 2 weeks was 7.14% (IQR = 0%–50%). There was no significant moderation effect of gender (F1,46 = 0.42, p = .521) on the proportion of using days during the last 2 weeks of the trial, so the interaction term was omitted from the final model. The proportion of using days during the last 2 weeks was significantly associated (F1,47 = 9.48, p = .004) with QLES-Q-SF raw scores at week 12, while adjusting for study arm, gender, and baseline QLES-Q-SF score (see Table 2, Model 2). The model adjusted scatter plot for QLES-Q-SF raw scores and proportion of using days is shown in Figure 2. With one more cannabis using day during the last 2 weeks, QLES-Q-SF raw scores decreased on average, by 0.80 points (or 1.4% maximum possible score).
FIGURE 2.
Plot of model adjusted QLES-Q-SF at week 12 and proportion of using days during the last 2 weeks (N = 52).
3.3.3 Model 3: Effect of Reduction in Cannabis Use
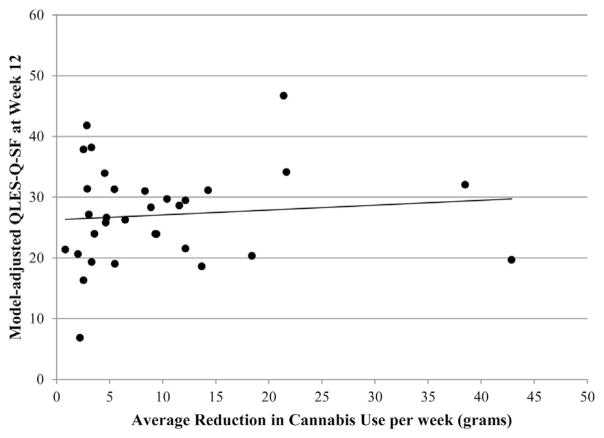
Of subjects who were not abstinent (n = 40) during the last 4 weeks of the trial, the median reduction in grams of cannabis used from the weekly mean at baseline to the weekly mean during the last 4 weeks of the trial (weeks 9–12) was 5.49 g (IQR = 3.19–12.16). There was no significant moderation effect of gender (F1,26 = 0.08, p = .786) on the reduction in grams of cannabis use, so the interaction term was removed from the model. Reduction in cannabis use was not significantly associated with QLES-Q-SF raw scores (F1,27 = 0.25, p = .620; see Table 2, Model 3), while adjusting for study arm, gender, and baseline QLES-Q-SF score. The model adjusted scatter plot for QLES-Q-SF raw scores and reduction in grams of cannabis used is shown in Figure 3.
FIGURE 3.
Plot of model adjusted QLES-Q-SF at week 12 and reduction in grams of cannabis used from average weekly use at baseline to average weekly use during weeks 9–12 (N = 32).
DISCUSSION
Contrary to our first hypothesis, no baseline associations were found between severity of cannabis use and quality of life, which differs from previous findings. This is likely attributable to the limited range of baseline variability in cannabis use in our sample. Our study’s inclusion criteria required that all participants had a DSM-IV diagnosis of cannabis dependence and as per results from the primary outcomes paper, all were daily users.3 Our study sample’s severe and heavy cannabis use likely corresponded to a lower and limited range in quality of life at baseline. Previous studies that demonstrated dose-related associations of cannabis use and self-reports of QoL included individuals with CUD and occasional, recreational cannabis users, providing a broader range of both cannabis use and associated effects on quality of life.4 However, similar to our findings, Lev-Ran et al.4 found that among individuals with CUD, no association in QoL ratings were found with increasing doses of cannabis used, suggesting that once a certain severity of cannabis use is met clinically, quality of life ratings are no longer affected. Compared to past studies of normative community samples,18 the participants entered our study with moderately severe levels of impairment in QoL (mean QLES-Q-SF score of 49.83 or 64.0%) at baseline corresponding to their heavy cannabis use.
Consistent with our hypothesis, abstinence, and lower proportion of cannabis using days (reduced frequency of use) are associated with higher QoL. Reduction in grams of cannabis used (amount), however, was not associated with improvements in QoL. This suggests that the overall burden on QoL as a result of cannabis use is driven by the frequency of use (proportion of using days) and not the total amount of cannabis consumed (grams of cannabis used). This is consistent with previous studies demonstrating that greater frequency of cannabis use is associated with lower measures of QoL,19,20 and that spontaneous remission or decreases in frequency of use (reducing days of use) resulted in improvements in QoL.21,22 In our study it is notable that for each additional cannabis using day, QLES-Q-SF scores were lower by 0.8 on the raw score or 1.4%. These findings suggest that treatments targeting reduced frequency of use may be most effective. For individuals who were abstinent, their QLES-Q-SF scores were higher by 6.78 points (12%), correlating with greater than one standard deviation of improvement as seen in community samples (SD = 11.3%). This is a clinically meaningful improvement as supported by previous studies examining changes in QLES-Q-SF during treatment for major depressive disorder.23–25 Individuals with CUD, by definition, are having impairment across many areas of their life as a result of their cannabis use. It is not surprising that likely meaningful reductions in cannabis use (being able to go full day(s) without any use) and abstinence as a result of treatment lead to significant, positive associations with participants’ multi-dimensional self-assessments of their degree of enjoyment and satisfaction experienced across different areas of daily functioning as measured with quality of life assessments.
Our study adds to the small but growing literature on quality of life in the treatment of CUD. Our findings are consistent with other treatment studies for non-cannabis substance use disorders. Studies examining medication or psychosocial treatments for alcohol,26,27 opioid,28 cocaine,29 nicotine,30 and multiple substance use disorders31 showed improvements in measures of QoL over the course of treatment with reductions in frequency of use or abstinence. Our findings are supported by the range in natural variability in cannabis use severity seen in large, cross-sectional, epidemiological studies without interventions, showing this negative association of cannabis use with QoL—non-users and occasional users have higher QoL indices as compared to heavy cannabis users or those who meet criteria for a CUD.4,7 A recent secondary analysis looking at quality of life changes in a treatment study for CUD did not find QoL changes associated with reductions in cannabis use.32 This study notably did not use a validated measure for QoL but a non-validated proxy measure of number of past days with mental or physical problems. The study did find that measures of mood, anxiety, and sleep quality, all factors that can influence QoL, did change over time as a result of changes in cannabis use. The differences in outcome measure may explain the overall differences in findings.
Unlike previous studies looking at QoL and cannabis use, our study did not find gender differences. Previous studies that found an effect of cannabis use on negative self-report ratings on QoL in women compared to men had substantial sample, methods, and study design differences, making comparisons challenging. The most recent studies finding gender differences all utilized data from the National Epidemiological Survey of Alcohol and Related Conditions (NESARC),4,9,12 a large, general population-based, cross-sectional survey. Further, they used different measures for both cannabis use and QoL than our study, and notably had significant psychiatric comorbidity that was greater in women, including depressive and anxiety disorders, which may contribute to lower QoL scores as compared to men.9,12 Our study analyzed data from a randomized controlled trial of outpatient medication treatment for individuals meeting criteria for CUD, resulting in a more homogenous and substantially smaller sample. Because of exclusionary criteria in our study for significant or unstable co-occurring psychiatric disorders, our sample did not include participants with symptomatic or notable mental health comorbidity. The differential effects of cannabis use and QoL observed in women may be more relevant in a dual-diagnosis population. Further, of the participants who completed end of study QoL ratings, two-thirds were men. The small number of women in our study likely diminished our power to detect gender differences.
While our study has a number of important strengths (eg, first study to assess a validated measure of QoL and find abstinence and reduced frequency of cannabis use during treatment to be associated with higher QoL), there are some limitations that should be noted. Because one of the medications (dronabinol) used in the trial results in positive urine drug screens for cannabis, our measure for cannabis use is based on self-report and could not be confirmed with quantitative urine THC levels. However, as previously noted in Levin et al.,3 given that there were no negative consequences for reporting use, the self-reports are likely consistent with urine results as previous studies have demonstrated. Another limitation is our assessment of QoL. While the QLES-Q-SF is a validated measure to assess QoL in participants engaged in treatment, we could have looked at additional factors, such as subjective and objective measures of sleep, exercise, stress, and mood that may impact QoL, particularly in individuals with CUD undergoing treatment in order to have a more comprehensive picture of the relationship between changes in cannabis use and QoL. As previously shown by Hser et al.,32 differential effects may be seen during treatment across measures. Finally, although our hypotheses suggest changes in cannabis use related to frequency and abstinence are a predictor of QoL at end of study, directionality between changes in QoL and changes in cannabis use need to be further explored in reverse, where improved QoL may precede reduction in cannabis use. Our study focused on QoL at the end of study, but future studies should assess QoL more frequently in order to capture the temporal relationships between QoL and changes in cannabis use.
Finally, we only analyzed data for study completers, and not missing QoL data at end of study. Those subjects who did complete the study as a group are probably different than all randomized participants. Without knowing the actual values of missing data, we are unable to identify these differences. Our results are likely not generalizable due to the attrition to the larger population of treatment-seeking individuals with CUD. Future studies should assess QoL more frequently during trials.
In summary, our results highlight the importance of QoL as an outcome measure in CUD treatment trials. As seen in other chronic disease models from cancer to heart failure, QoL indices are meaningful subjective measures to track over the trajectory of illness and treatment. As healthcare shifts from a system focused on volume of services delivered (as a result of fee-for-service reimbursement) to value created for patients, identifying and measuring outcomes that are patient-centered is critical. There has been recent interest in the substance abuse treatment and research communities in determining whether changes in illicit drug use are associated with positive changes in health-related and other functional outcomes in individuals with substance use disorders which cannot be measured or understood with urine toxicology and TLFB alone.33 Demonstrating, as we have in this study, that abstinence and decreased frequency in cannabis use are associated with positive changes in one’s QoL helps to support QoL as a useful outcome measure in clinical treatment trials for CUD. Future substance abuse treatment studies should target these gaps in our knowledge and examine measures, both objective and subjective, that are meaningful to patients, linked to reductions in cannabis use, and reflective of functionality.
Acknowledgments
Funding for this research was provided by NIDA grants K24DA029647 and 5P50DA009236. Dr. Brezing is supported by T32DA007294. We want to thank the staff of the Substance Treatment and Research Service (STARS) of the New York State Psychiatric Institute for their support.
Footnotes
Declaration of Interest
Drs. Brezing, Pavlicova, Mariani, Ms. Choi, Mr. Brooks, and Ms. Mahony report no competing interests and no financial relationships with commercial interests. Dr. Levin received medication from the US WorldMed for this trial and served as a consultant to GW Pharmaceuticals and Eli Lily. The authors alone are responsible for the content and writing of this paper.
References
- 1.Sherman BJ, McRae-Clark AL. Treatment of cannabis use disorder: Current science and future outlook. Pharmacotherapy. 2016;36:511–535. doi: 10.1002/phar.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balter RE, Cooper ZD, Haney M. Novel pharmacologic approaches to treating cannabis use disorder. Curr Addict Rep. 2014;1:137–143. doi: 10.1007/s40429-014-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin FR, Mariani JJ, Pavlicova M, et al. Dronabinol and lofexidine for cannabis use disorder: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2016;159:53–60. doi: 10.1016/j.drugalcdep.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall W, Kozlowski LT. The diverging trajectories of cannabis and tobacco policies in the United States: Reasons and possible implications. Addiction. 2017:22. doi: 10.1111/add.13845. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Saiz F, Rojas OL, Castillo II. Measuring the impact of psychoactive substance on health-related quality of life: An update. Curr Drug Abuse Rev. 2009;2:5–10. doi: 10.2174/1874473710902010005. [DOI] [PubMed] [Google Scholar]
- 6.Gruber AJ, Pope HG, Hudson JI, et al. Attributes of long-term heavy cannabis users: A case-control study. Psychol Med. 2003;33:1415–1422. doi: 10.1017/s0033291703008560. [DOI] [PubMed] [Google Scholar]
- 7.Cougle JR, Hakes JK, Macatee RJ, et al. Quality of life and risk of psychiatric disorders among regular users of alcohol, nicotine, and cannabis: An analysis of the National Epidemiological Survey on Alcohol and Related Conditions (NESARC) J Psychiatr Res. 2015;66–67:135–141. doi: 10.1016/j.jpsychires.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Goldenberg M, IsHak WW, Danovitch I. Quality of life and recreational cannabis use. Am J Addict. 2017;26:8–25. doi: 10.1111/ajad.12486. [DOI] [PubMed] [Google Scholar]
- 9.Lev-Ran S, Le Foll B, McKenzie K, et al. Cannabis use and mental health-related quality of life among individuals with anxiety disorders. J Anxiety Disord. 2012;26:799–810. doi: 10.1016/j.janxdis.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Gates PJ, Sabioni P, Copeland J, et al. Psychosocial interventions for cannabis use disorder. Cochrane Database Syst Rev. 2016;5:CD005336. doi: 10.1002/14651858.CD005336.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gates P, Albertella L, Copeland J. Cannabis withdrawal and sleep: A systematic review of human studies. Subst Abus. 2016;37:255–269. doi: 10.1080/08897077.2015.1023484. [DOI] [PubMed] [Google Scholar]
- 12.Notzon DP, Pavlicova M, Glass A, et al. ADHD is highly prevalent in patients seeking treatment for cannabis use disorders. J Atten Disord. 2016:31. doi: 10.1177/1087054716640109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levin FR, Mariani JJ, Brooks DJ, et al. Dronabinol for the treatment of cannabis dependence: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116:142–150. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mainthia R, Reppart L, Reppart J, et al. A model for improving the health and quality of life of single mothers in the developing world. Afr J Reprod Health. 2013;17:14–25. [PubMed] [Google Scholar]
- 15.Mariani JJ, Brooks D, Haney M, et al. Quantification and comparison of marijuana smoking practices: Blunts, joints, and pipes. Drug Alcohol Depend. 2011;113:249–251. doi: 10.1016/j.drugalcdep.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Endicott J, Nee J, Harrison W, et al. Quality of life enjoyment and satisfaction questionnaire: A new measure. Psychopharmacol Bull. 1993;29:321–326. [PubMed] [Google Scholar]
- 17.Cohen RM, Greenberg JM, IsHak WW. Incorporating multidimensional patient-reported outcomes of symptom severity, functioning, and quality of life in the Individual Burden of Illness Index for Depression to measure treatment impact and recovery in MDD. JAMA Psychiatry. 2013;70:343–350. doi: 10.1001/jamapsychiatry.2013.286. [DOI] [PubMed] [Google Scholar]
- 18.Schechter D, Endicott J, Nee J. Quality of life of “normal” controls: Association with lifetime history of mental illness. Psychiatry Res. 2007;152:45–54. doi: 10.1016/j.psychres.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dey M, Gmel G, Mohler-Kuo M. Body mass index and health-related quality of life among young Swiss men. BMC Public Health. 2013;13:1028. doi: 10.1186/1471-2458-13-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dey M, Gmel G, Studer J, et al. Health-risk behaviors and quality of life among young men. Qual Life Res. 2014;23:1009–1017. doi: 10.1007/s11136-013-0524-4. [DOI] [PubMed] [Google Scholar]
- 21.Rubio JM, Olfson M, Villegas L, et al. Quality of life following remission of mental disorders: Findings from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2013;74:e445–e450. doi: 10.4088/JCP.12m08269. [DOI] [PubMed] [Google Scholar]
- 22.Caldeira KM, O’Grady KE, Vincent KB, et al. Marijuana use trajectories during the post-college transition: Health outcomes in young adulthood. Drug Alcohol Depend. 2012;125:267–275. doi: 10.1016/j.drugalcdep.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.IsHak WW, Mirocha J, Christensen S, et al. Patient-reported outcomes of quality of life, functioning, and depressive symptom severity in major depressive disorder comorbid with panic disorder before and after SSRI treatment in the star*d trial. Depress Anxiety. 2014;31:707–716. doi: 10.1002/da.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vilhauer JS, Cortes J, Moali N, et al. Improving quality of life for patients with major depressive disorder by increasing hope and positive expectations with future directed therapy (FDT) Innov Clin Neurosci. 2013;10:12–22. [PMC free article] [PubMed] [Google Scholar]
- 25.Ishak WW, Greenberg JM, Cohen RM. Predicting relapse in major depressive disorder using patient-reported outcomes of depressive symptom severity, functioning, and quality of life in the Individual Burden of Illness Index for Depression (IBI-D) J Affect Disord. 2013;151:59–65. doi: 10.1016/j.jad.2013.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan MY, Landron F, Lehert P New European Alcoholism Treatment Study G. Improvement in quality of life after treatment for alcohol dependence with acamprosate and psychosocial support. Alcohol Clin Exp Res. 2004;28:64–77. doi: 10.1097/01.ALC.0000108652.73143.4B. [DOI] [PubMed] [Google Scholar]
- 27.Francois C, Rahhali N, Chalem Y, et al. The effects of as-needed nalmefene on patient-reported outcomes and quality of life in relation to a reduction in alcohol consumption in alcohol-dependent patients. PLoS ONE. 2015;10:e0129289. doi: 10.1371/journal.pone.0129289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell SG, Gryczynski J, Schwartz RP, et al. Changes in quality of life following buprenorphine treatment: relationship with treatment retention and illicit opioid use. J Psychoactive Drugs. 2015;47:149–157. doi: 10.1080/02791072.2015.1014948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petry NM, Alessi SM, Hanson T. Contingency management improves abstinence and quality of life in cocaine abusers. J Consult Clin Psychol. 2007;75:307–315. doi: 10.1037/0022-006X.75.2.307. [DOI] [PubMed] [Google Scholar]
- 30.Hays JT, Croghan IT, Baker CL, et al. Changes in health-related quality of life with smoking cessation treatment. Eur J Public Health. 2012;22:224–229. doi: 10.1093/eurpub/ckq137. [DOI] [PubMed] [Google Scholar]
- 31.Andrade LF, Alessi SM, Petry NM. The impact of contingency management on quality of life among cocaine abusers with and without alcohol dependence. Am J Addict. 2012;21:47–54. doi: 10.1111/j.1521-0391.2011.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hser YI, Mooney LJ, Huang D, et al. Reductions in cannabis use are associated with improvements in anxiety, depression, and sleep quality, but not quality of life. J Subst Abuse Treat. 2017;81:53–58. doi: 10.1016/j.jsat.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tiffany ST, Friedman L, Greenfield SF, et al. Beyond drug use: A systematic consideration of other outcomes in evaluations of treatments for substance use disorders. Addiction. 2012;107:709–718. doi: 10.1111/j.1360-0443.2011.03581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]