Abstract
Rationale
Recent evidence indicates that histone deacetylase enzymes (HDACs) contribute to ischemia re-perfusion (I/R) injury, and pan-HDAC inhibitors have been shown to be cardioprotective when administered either before an ischemic insult or during reperfusion. We have shown previously that selective inhibition of class I HDACs provides superior cardioprotection when compared to pan-HDAC inhibition in a pretreatment model, but selective class I HDAC inhibition has not been tested during reperfusion, and specific targets of class I HDACs in I/R injury have not been identified.
Objective
We hypothesized that selective inhibition of class I HDACs with the drug MS-275 (entinostat) during reperfusion would improve recovery from I/R injury in the first hour of reperfusion.
Methods and results
Hearts from male Sprague-Dawley rats were subjected to ex vivo I/R injury ± MS-275 class I HDAC inhibition during reperfusion alone. MS-275 significantly attenuated I/R injury, as indicated by improved LV function and tissue viability at the end of reperfusion. Unexpectedly, we observed that HDAC1 is present in the mitochondria of cardiac myocytes, but not fibroblasts or endothelial cells. We then designed mitochondria-restricted and mitochondria-excluded HDAC inhibitors, and tested both in our ex vivo I/R model. The selective inhibition of mitochondrial HDAC1 attenuated I/R injury to the same extent as MS-275, whereas the mitochondrial-excluded inhibitor did not. Further assays demonstrated that these effects are attributable to a decrease in SDHA activity and subsequent metabolic ROS production in reperfusion.
Conclusions
We demonstrate for the first time that HDAC1 is present within the mitochondria of cardiac myocytes, and mitochondrial HDAC1 contributes significantly to I/R injury within the first hour of reperfusion.
Keywords: Ischemia reperfusion injury, Histone deacetylases, Mitochondria, Basic science research, Ischemia, Epigenetics, Metabolism, Oxidant stress
1. Introduction
Lysine acetylation is a reversible post-translational protein modification that occurs on a multitude of proteins and regulates vital cellular processes including metabolism [1–3], cell cycle regulation [4,5], chromatin remodeling [6,7], nuclear transport [4,8–10], and autophagy [11–14], among others. It was initially proposed [15], and later confirmed that acetylation of histone tails regulates gene expression by loosening chromatin compaction and allowing transcription factors to bind DNA [16]. Eventually, non-histone protein acetylation was also discovered, starting with p53 [17] and expanding to non-histone proteins throughout the cell [18]. As the field has expanded, it has come to be appreciated that the acetylation state of non-histone proteins and enzymes can dramatically affect characteristics of the acetylated proteins, including protein stability [19,20], enzymatic activity [21,22], DNA binding [23], and intracellular localization [24]. These changes in protein characteristics can have a profound effect on cellular processes, including those determining the fate of cells subjected to injury.
Lysine acetylation occurs via the addition of an acetyl group to the ε-amino moiety of lysine residues in a reaction catalyzed by histone acetyltransferases (HATs). The removal of acetyl groups, termed deacetylation, is achieved by histone deacetylases (HDACs). HDACs consist of 4 classes, delineated based on their similarity to histone deacetylase enzymes in yeast. HDACs 1, 2, 3, and 8 comprise the class I HDACs. Class II HDACs are subgrouped into class IIa (HDACs 4, 5, 7, and 9), and class IIb HDACs (HDACs 6 and 10). Class III HDACs are the sirtuin family, differentiated from the other classes because they use NAD+ as a cofactor. HDAC11 is the sole known class IV HDAC. In the last two decades, the function of HDACs and their promise as a treatment target in cardiovascular disease has become a topic of great interest in cardiovascular research [25,26]. Accordingly, the pharmacological inhibition of HDAC activity has been shown to be beneficial in animal models of multiple cardiac pathologies, though these discoveries have yet to be translated to the clinic.
Ischemic heart disease is the leading cause of death worldwide and the majority of these deaths are due to acute myocardial infarction [27]. Treatment for AMI is reperfusion via primary percutaneous coronary intervention (PCI), or coronary angioplasty [28]. Although PCI and angioplasty limit ischemic injury, reperfusion itself injures the heart by increasing ROS. We and others have shown that HDAC inhibition preceding ischemia reperfusion (I/R) injury in the heart can preserve left ventricular function and myocardial survival [29–31]. Importantly, we demonstrated that the selective inhibition of class I HDACs was more effective than pan-HDAC inhibition in preserving myocardial viability and function in the setting of I/R injury. Recently, pan-HDAC inhibition solely during the reperfusion phase of I/R injury was shown to preserve myocardial function in multiple animal models, although the exact mechanisms behind this cardioprotection have yet to be fully elucidated [32,33].
Here, we utilized a Langendorffisolated heart model to test the hypothesis that selective pharmacological class I HDAC inhibition during the reperfusion phase alone would confer cardioprotection from I/R injury. In doing so, we discovered that HDAC1 localizes to the mitochondria of cardiac myocytes and modifies oxidative metabolism, contributing to ROS production and mitochondrial injury in the re-perfusion phase of I/R injury.
2. Methods
2.1. Langendorffheart isolation
Rats weighing 300–400 g (Harlan, Frederick MD) were cared for in accordance with the National Institutes of Health (NIH) guidelines and those of the Institutional Animal Care and Use Committee (IACUC) of the Medical University of South Carolina. Rats were anesthetized with ketamine/xylazine (85/15 mg/kg) via intraperitoneal injection. Following confirmation of anesthesia, rats were tracheotomized with a 16 g catheter and ventilated with 8 mL/kg/min room air at a rate of 70 strokes/min with a rodent ventilator. 1000 U/kg heparin was administered into the jugular vein and allowed to circulate for 30 s prior to thoracotomy. Midsternal thoracotomy was performed to expose the heart, followed by in situ cannulation of the aorta. The hearts were removed and immediately attached to a non-recirculating Langendorff constant pressure perfusion apparatus. Hearts were perfused with oxygenated (95% O2 + 5% CO2) modified Krebs Henseleit buffer (in mM: 112 NaCl, 5 KCl, 1.2 MgSO4, 1 K2HPO4, 1.25 CaCl2, 25 NaHCO3, 11 D-glucose, 0.2 octanoic acid, pH = 7.4) maintained at 37.4 °C through the use of custom crafted water-jacketed glassware.
2.2. Left ventricular functional assessment
A saline filled balloon attached to a pressure transducer was inserted into the left ventricle and set to a minimum diastolic pressure of 10 mm Hg to allow for real time monitoring of left ventricular function. The pressure transducer was attached to a PowerLab 8/30 analog to digital converter and to a computer running LabChart Pro software (ADInstruments, Colorado Springs CO). This allowed the direct measurement of heart rate, systolic pressure, diastolic pressure, dP/dtmax and −dP/dtmax. Developed pressure was calculated as the difference between maximum systolic and minimum diastolic pressures. Rate pressure product was calculated as the product of the heart rate and the developed pressure. Coronary flow was continuously measured via an inline flow meter (Transonic Systems, Ithica NY).
2.3. Ischemia reperfusion injury and HDAC inhibition
Global ischemia was achieved by complete cessation of buffer flow for 30 min, followed by 60 min of reperfusion. Administration of 10 nM MS-275, 10 nM LL-66, or 10 nM LL-224 at reperfusion was achieved by dissolving MS-275, LL-66, or LL-224 in the perfusion buffer during the ischemic period. At the termination of reperfusion, hearts were briefly placed at −80 °C until firm but not frozen, then sliced into 2 mm sections. Slices were then either used for infarct staining or flash frozen in liquid nitrogen and stored at −80 °C.
2.4. Infarct staining
Triphenyl Tetrazolium Chloride (TTC) was used to stain for area of infarction at the termination of the I/R experiments, using the method of Ferrera et al. [34]. One slice from each heart was incubated in 1% TTC at 37.4 °C for 20 min, then fixed in 10% formalin solution overnight. Slices were photographed and analyzed for infarct area using imageJ software. Infarct size was reported as a percent of total left ventricular area.
2.5. Mitochondrial isolation and proteinase K digestion
Mitochondria were isolated from rat ventricular cardiac tissue by differential centrifugation, as described previously [35]. Briefly, hearts were excised from rats, the atria were removed, and the ventricles were minced in ice-cold mitochondrial isolation medium (in mM: 220 mannitol, 75 sucrose, 5 MOPS, 0.5 EGTA, 2 taurine, pH 7.25). The minced tissue was then homogenized by polytron. Trypsin was added to the homogenate (1 mg/100 mg wet tissue) for 5 min to digest contractile proteins, and 0.2% BSA was added to stop the digestion. Homogenates were then centrifuged at 600g for 10 min at 4 °C. The supernatant was removed and re-centrifuged at 600g for 10 min at 4 °C. The supernatant was again removed, and centrifuged at 5500g for 15 min. The pellet was then re-suspended in buffer B (in mM: 137 KCl, 2 KH2PO4, 2.5 MgCl2, 20 HEPES, 0.5 EGTA). This procedure was also used for isolation of skeletal muscle mitochondria. For isolation of liver mitochondria, the trypsin digestion was omitted. For western blotting, antibodies against HDAC1 (Santa Cruz sc-6298, Abcam 53091), HDAC2 (Santa Cruz sc-7899), HDAC3 (Santa Cruz sc-11417), HDAC8 (Santa Cruz sc-11,405), α/β-tubulin (Cell Signaling 2148) and histone H3 (Cell Signaling 9715) were used. Proteinase K digestion was performed according to standard protocols [36]. Isolated mitochondria (100 μg per sample) were re-suspended in buffer B or buffer B containing 2% triton-X100. Mitochondria were then incubated in the presence of 50 μg/mL proteinase K (Qiagen, Germantown, MD) for 0, 20, 40 or 60 min at 37 °C. Following digestion, samples were immediately boiled in SDS and processed for western blotting with HDAC1, HDAC2, and ACAA2 (Santa Cruz sc-100847).
2.6. Myocyte isolation and immunofluorescence staining
Rats were anesthetized using 5% isoflurane, subjected to midsternal thoracotomy and removal of the heart. The hearts were then cannulated and attached to a perfusion apparatus, flushed with 37 °C perfusate (in mM: 113 NaCl, 4.7 KCl, 0.6 KH2PO4, 0.6 Na2HPO4, 1.2 MgSO4, 12 NaHCO3, 10 KHCO3, 10 HEPES, 0.1 adenosine, 0.1 gadolinium chloride, 6.4 sodium pyruvate, 30 taurine, 10 2,3-butanedione monoxime (BDM)) and perfused with recirculating digestion medium (0.1 mg/mL Liberase TH, 0.1 mg/mL trypsin, 12.5 mM CaCl in perfusion medium) for 25 min. Following digestion, hearts were removed from perfusion apparatus and minced in perfusion buffer containing 30% BSA to halt the digestion. Samples were then pipetted up and down three times using a 25 mL serological pipette and allowed to settle 3 min. The supernatant was removed and transferred to a 15 cm culture dish, and the pellet was resuspended in 25 mL perfusion buffer containing 15% BSA, pipetted up and down three times and allowed to settle for 3 min. The supernatant was removed and added to the previous supernatant. This process was repeated 3 more times, while monitoring for myocyte quality. Calcium was then reintroduced to a final concentration of 1.8 mM in 6 additions, with a 5 min rest between each addition. Finally, cells were transferred to 50 mL conical and allowed to settle for 15 min. For fibroblast isolation, the supernatant was then removed, plated, and allowed to settle for 4 h before washing and switching to fibroblast specific medium. For myocyte isolation, the pellet was resuspended in MEM containing 6 mM NaHCO3, 5 mM BDM, penicillin, and 1× insulin-transferrin-selenium (ThermoFisher, Grand Island NY) and plated on laminin coated coverslips. The following day, cells were fixed in 3.7% formaldehyde for 15 min, permeabilized in 0.5% triton-x100 for 5 min, and blocked for 1 h in 10% normal donkey serum. HDAC1 and ACAA2 primary antibodies were used for immunostaining, followed by Alexafluor 488 and Alexafluor 555 secondary antibodies. ProlongGold antifade mounting medium with DAPI (ThermoFisher, Grand Island NY) was used for mounting, and the cells were imaged by confocal microscopy.
2.7. Synthesis of 2-(4-((2-(6-amino-3-iminio-3H-xanthen-9-yl)benzamido) methyl)benzamido) benzenaminium 2,2,2-trifluoroacetate (LL66)
4-(aminomethyl)benzoic acid (755 mg, 5 mmol) was dissolved in 15 mL 1 mol/L NaOH aqueous solution, to which was added 1308 mg Boc2O and 2 mL THF. The mixture was stirred overnight and THF condensed under vacuum. The residue was acidification by diluted hydrochloric acid and the resulting white solid was filtered to yield Boc-protected intermediate. The Boc-protected compound, 1925 mg TBTU and 1 mL TEA were dissolved in 50 mL DCM. 30 min later, 1,2-diaminobenzene (650 mg, 6 mM) was added and the mixture was allowed to stir at room temperature overnight. Volatiles were removed under vacuum, the residue was recrystallized by ethyl estate and hexane to yield a white solid. This was dissolved in 10 mL mixed solution of DCM and TFA [1,1], the solution was stirred at room temperature for 1 h. The volatiles were evaporated under vacuum to afford a color less oil. 110 mg oil was dissolved in 5 mL DMF then 193 mg N, N-Diisopropylethylamine and 114 mg Rhodamine 110 chloride was added. After the reaction finished, 30 mL water was added and the mixed solution was extracted by ethyl estate. The organic phase was dried by MgSO4 and evaporated under vacuum. The crude residue was purified on C18 reverse phase columns eluted with acetonitrile and water (containing 0.2% TFA) to yield the pure product.
2.8. Synthesis of 9-(2-((4-((2-aminophenyl)carbamoyl)benzyl)carbamoyl) phenyl)-9H–xanthene-3,6-diyl diacetate (LL224)
4-(aminomethyl)benzoic acid (755 mg, 5 mmol) was dissolved in 15 mL 1 mol/L NaOH aqueous solution, to which was added 1308 mg Boc2O and 2 mL THF. The mixture was stirred overnight and THF condensed under vacuum. The residue was acidification by diluted hydrochloric acid and the resulting white solid was filtered to yield Boc-protected intermediate. The Boc-protected compound, 1925 mg TBTU and 1 mL TEA were dissolved in 50 mL DCM. 30 min later, 1,2-diaminobenzene (650 mg, 6 mM) was added and the mixture was allowed to stir at room temperature overnight. Volatiles were removed under vacuum, the residue was recrystallized by ethyl estate and hexane to yield a white solid. This was dissolved in 10 mL mixed solution of DCM and TFA (1:1), the solution was stirred at room temperature for 1 h. The volatiles were evaporated under vacuum to afford a colorless oil. 209 mg dihydrofluorescein diacetate was dissolved in 2 mL DMF, followed by the addition of 175 mg TBTU and 0.1 mL TEA. 30 min later, 200 mg colorless oil obtained from last step and 0.1 mL TEA was added. After the reaction finished, 30 mL water was added and the mixed solution was extracted by ethyl estate. The organic phase was dried by MgSO4 and evaporated under vacuum. The crude residue was purified on C18 reverse phase columns eluted with acetonitrile and water to yield the pure product.
2.9. HDAC activity assays
HDAC activity was assayed in 5 μg of total rat heart lysate and 5 μg rat heart mitochondrial homogenates using the acetylated lysine aminomethyl-coumarin-Boc substrate, which is a substrate for class I and class IIb HDCACs but not class IIa or class IV HDACs [37,38]. HDAC6 activity was inhibited using 1μM Tubastatin A. Class I HDAC activity was assessed by comparing 1μM Tubastatin A inhibited activity with the 1μM Tubastatin A plus 5 μM MS-275 inhibited activity. The resulting inhibitor-enzyme solution was pre-incubated at 30 °C for 5 min. The activity assay was initiated with the addition of 100 μM (final concentration) acetylated lysine aminomethyl-coumarin-Boc substrate in HDAC buffer solution added and the resulting solution further incubated for 2 h at 30 °C. A developer solution containing 5 mg/mL trypsin protease and 1 mM Trichostain A was added to quench the deacetylation reaction. The resulting fluorescence which correlates to deacetylated substrates was read at 360(Ex)/460(Em) using a Tecan Infinity M200 Pro plate reader. Data were normalized to a standard curve of coumarin fluorescence.
2.10. Simulated hypoxia-reoxygenation and assay of metabolic activity in cardiac myocytes
Hypoxia-reoxygenation was simulated chemically using cyanide, as has been described by Nishimura et al. [39]. Cardiac myocytes were isolated and cultured overnight. The following morning cells were incubated in RS buffer (in mM: 1.8 CaCl2, 0.6 MgCl2, 0.5 KH2PO4, 5.33 KCl, 0.5 Na2HPO4, 130 NaCl, 4 glutamine, 1 pyruvate, 100 nM insulin, and 1% BSA; supplemented with 1× MEM Non-Essential Amino Acids, 1× MEM Amino Acids and 1× MEM Vitamins (Gibco)) containing 2.5 mM potassium cyanide (simulated ischemia) or RS buffer alone (normoxia) for 90 min. Following the simulated ischemic period, cells were washed three times in PBS, then incubated in RS buffer with either DMSO, 10 nM MS-275, or 10 nM LL-66 for 60 min to simulate re-perfusion. At the end of the simulated reperfusion period, plates were loaded into a Seahorse XF96 Extracellular Flux analyzer, and OCR measurements were taken over six cycles (2 min mixing, 3 min measuring per cycle). Prior to the seventh cycle, FCCP was added to a final concentration of 1 μM, and measurements were taken for four more cycles. After the 10th cycle, Antimycin D and rotenone were added, both to a final concentration of 1 μM, and the final four measurements were taken.
2.11. SDHA activity assay
The specific enzymatic activity (Unit/mg of protein) of succinate dehydrogenase (mitochondrial OXPHOX complexes II) was measured by spectrophotometry at 37 °C in rat ventricular homogenates from hearts subjected to I/R injury ± MS-275, LL-66, or LL-224 with a protocol adapted from [40,41]. Briefly, heart ventricles were minced in ice-cold mitochondrial resuspension buffer (280 mM sucrose and 10 mM Tris, pH 7.25). The minced tissue was then homogenized by polytron. Homogenates were centrifuged at 600g for 10 min at 4 °C. The supernatant was collected and samples stored in ice. Succinate dehydrogenase activity was measured by following the reduction of 2,6-dichlorophenolindophenol (DCPIP), at 600 nm in measurement buffer (in mM 100 KH2PO4, 10 KCN, 500 disodium succinate, 20 Phenazine methosulfate, 40 Thenoyltrifluoroacetone). the reaction was initiated by adding 500 mM of dichlorophenolindophenol. Malonate was used to test the effect of a competitive inhibitor of succinate on the activity of the enzyme. Therefore, the specificity of our measurements.
2.12. Co-immunoprecipitation
Heart ventricles were minced in 1× ice-cold lysis buffer (Cell Signaling 9803). The minced tissue was then homogenized by polytron. Homogenates were then centrifuged at 600g for 10 min at 4 °C. The supernatant was collected and kept in ice. 2.5 mg of protein was used for each IP. Samples were pre-cleared by rocking them with 30 μL of protein A agarose beads (Cell Signaling 9863) at 4 °C for 45 min. After overnight incubation with gentle rocking at 4 °C, with 2.5 μg of IgG (Cell Signaling 5415 for control) or 2.5 μg of primary antibody anti SDHA (Cell Signaling 11998), 25 μL of protein A agarose beads were added to each sample and gentle rocking at 4 °C for 2 h was performed. Beads were collected by centrifugation at 2000g for 1 min at 4 °C and washed 5 times with lysis buffer. Beads were then resuspended with 3× SDS sample buffer (Cell Signaling 7722), boiled at 95 °C for 5 min and supernatant collected after 1 min centrifugation at 14,000g. Western was blotted with an HDAC1antibody (Abcam 53091).
2.13. Oxyblot
An Oxyblot Oxidized Protein Detection Kit (EMD Millipore, Billerica MA) was used to assess the degree of oxidative damage in response to I/R injury ± HDAC inhibition. Frozen Langendorff heart samples were homogenized in lysis buffer with 50 mM DTT. From each sample, 20 μg of protein were then incubated for 15 min in 2,4-dinitrophenylhy-drazine (DNPH) reagent to derivitize the carbonyl groups of oxidized proteins. Samples were then neutralized, subjected to SDS-PAGE, and western blotting was performed using an anti-DNP primary antibody. Quantitation of results was performed using ImageJ.
2.14. Statistics
For all comparative experiments, either Students t-tests or ANOVA were performed, as indicated. For ANOVA analyses, Bonferroni or Dunnet’s post-tests were applied.
3. Results
3.1. Inhibition of class I HDAC activity at reperfusion preserves left ventricular function and viable myocardium post I/R-injury ex vivo
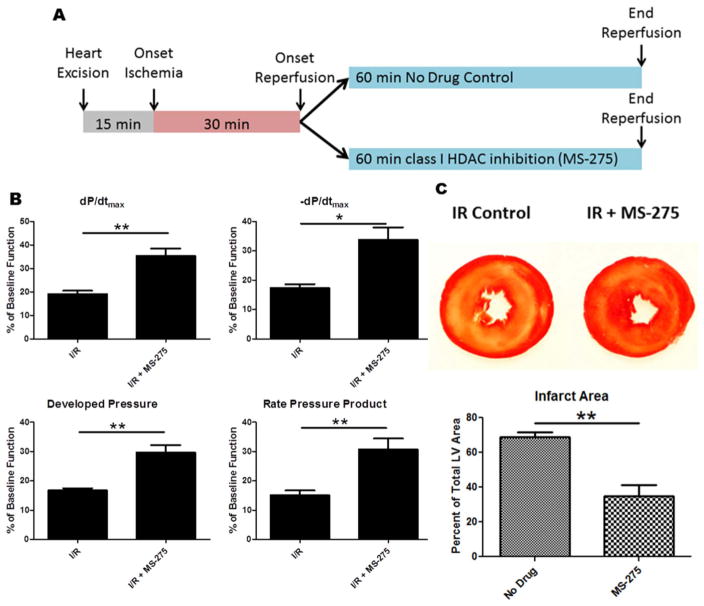
To test whether selective class I HDAC inhibition can postcondition the heart against I/R injury, we isolated hearts from Sprague-Dawley rats and perfused them using a constant pressure Langendorff apparatus. After 15 min of equilibration, we subjected the hearts to 30 min of ischemia, followed by 60 min of reperfusion. The selective class I HDAC inhibitor MS-275 was administered throughout the reperfusion phase and compared to drug-free control hearts (Fig. 1A). In order to measure left ventricular functional performance, a saline-filled balloon was inserted into the left ventricle and attached to a pressure transducer. We observed a significant preservation of multiple parameters of ventricular function with class I HDAC inhibition during reperfusion alone; including the rate of pressure generation (dP/dtmax), rate of pressure relaxation (−dP/dtmax), developed pressure and rate pressure product (Fig. 1B). At the conclusion of the 60 min reperfusion period, hearts were removed from the perfusion column and sliced into 2 mm transverse sections using a tissue slicing matrix. Hearts were then incubated in 2,3,5-triphenyltetrazolium chloride (TTC) in order to determine the amount of infarcted myocardium. MS-275 significantly reduced infarct area when compared to control hearts (Fig. 1C).
Fig. 1.
Experimental design, recovery of ventricular function and TTC staining following class I HDAC inhibition at reperfusion. (A) Experimental design for I/R procedures. (B) Graphs represent measurements of left ventricular function after 30 min of ischemia and 60 min of reperfusion. dP/dtmax indicates the rate of pressure development, −dP/dtmax indicates the rate of pressure relaxation, Developed Pressure was calculated as the difference between the systolic and diastolic pressures, Rate Pressure Product was calculated as the developed pressure multiplied by the heart rate. Measurements were taken at the end of reperfusion and represented as a percentage of baseline pre-ischemic function. (C) TTC staining for infarct area at the end of reperfusion; yellow/white region indicates area of infarction. Infarct areas were measured using imageJ software. All data are represented as mean ± S.D. N = 4, t-test *p, 0.05. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. HDAC1 is present in the mitochondria of normal rat ventricular cardiac tissue
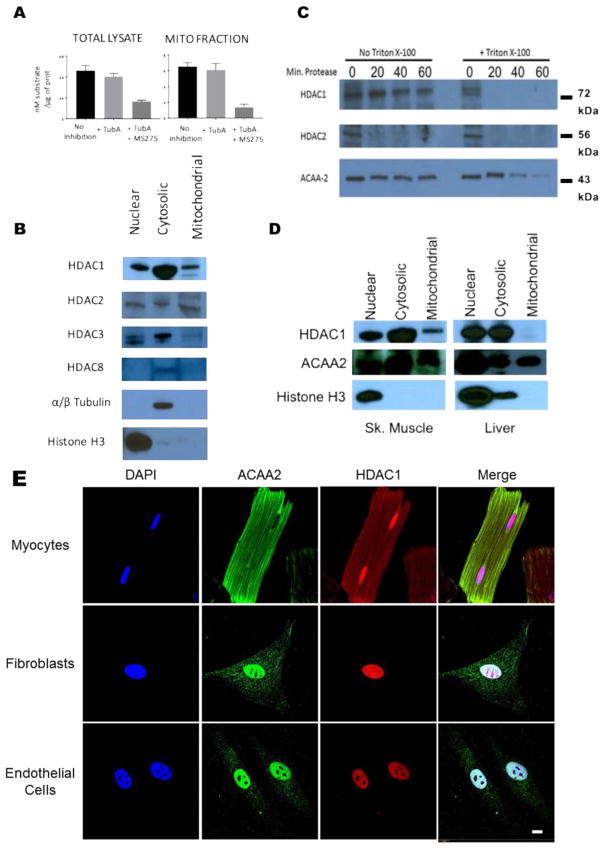
Given the very short timeframe (60 min) within which selective class I HDAC inhibition attenuated reperfusion injury, we decided to focus on examining the non-transcriptional effects of class I HDAC activity in I/R injury. Since mitochondria play a prominent role in early cell death signaling, and acetylation regulates metabolic pathways, we decided to investigate the possibility that class I HDACs either directly or indirectly modify the acetylation of mitochondrial proteins, thus altering the mitochondrial response to I/R injury. To do this, mitochondria were isolated from mouse hearts and subjected to a class I HDAC activity assay. As expected the total cardiac lysates have robust HDAC activity (Fig. 2A). In this assay the difference between no inhibitor and treatment with the class IIb HDAC inhibitor tubastatin A reveals HDAC6 activity. The difference between Tubastatin A treatment and Tubastatin A plus MS-275 shows class I HDAC activity. Surprisingly, about 15% of the total class I HDAC activity found in cardiac tissue lysates was detected in the mitochondrial samples. To determine which class I HDACs were present in rat mitochondrial isolates, Western blotting was performed for each class I HDAC. HDAC1 and HDAC2 were both detected in the mitochondrial isolates, while HDAC3 and HDAC8 were not (Fig. 2B). To further interrogate the localization of HDAC1 and HDAC2, rat mitochondrial isolates were subjected to proteinase K digestion. HDAC1 was still detected after 60 min of proteinase K digestion, supporting its presence within the mitochondria, while HDAC2 was rapidly digested, suggesting that it associates with the outer mitochondrial membrane. ACAA2, the final enzyme in the fatty acid β-oxidation pathway, was used as a marker for the mitochondrial matrix. Addition of triton-X100 to disrupt mitochondrial membranes demonstrated that HDAC1 is susceptible to proteinase K digestion. (Fig. 2C).
Fig. 2.
Characterization of HDAC localization in tissues and cells. (A) Class I HDAC activity in whole heart lysates and mitochondrial isolates. Acetylated substrate selective for only class I and class IIb HDACs. HDAC6 activity inhibited with 1 μM Tubastatin A (Tub A). Class I HDACs inhibited with 5 μM MS275. Activity is assessed from a 2 h. reaction. (B) Western blotting for class I HDACs in whole heart nuclear, cytoplasmic, and mitochondrial isolates. (C) Proteinase K digestion of whole heart mitochondrial isolates. (D) Western blotting for HDAC1 in skeletal muscle and liver mitochondrial isolates. (E) Staining for HDAC1 and ACAA2 in cardiac myocytes, fibroblasts and endothelial cells. Bar = 10 μm N = 3 per group for all experiments.
This is the first demonstration of a class I HDAC in mammalian mitochondria. Therefore, we decided to test other rat tissues to determine if mitochondrial localization of HDAC1 is widespread, restricted to muscle, or restricted to the heart. Mitochondria were isolated from rat quadriceps and liver and subjected to western blotting for HDAC1. Intriguingly, HDAC1 was detected in the mitochondrial isolate from skeletal muscle, while it was absent from the mitochondria of the liver (Fig. 2D).
3.3. HDAC1 localizes to the mitochondria of cardiac myocytes, but not fibroblasts or endothelial cells
In order to further characterize the distribution of HDAC1 within the heart, we subjected cardiac myocytes, fibroblasts, and endothelial cells to immunofluorescence staining for HDAC1 and the mitochondrial marker ACAA2. In the myocytes, HDAC1 was robustly detected within the nucleus, as expected. Confirming the results of our biochemical studies, HDAC1 was also detected throughout the myocyte in a pattern that closely overlaps with the mitochondrial marker ACAA2 (Fig. 2E). Fibroblasts and endothelial cells show canonical HDAC1 localization only in the nucleus, with no HDAC1 detected in the cytoplasm or mitochondria (Fig. 2E).
3.4. Mitochondria-targeted class I HDAC inhibition results in significant preservation of LV function and myocyte viability post-I/R injury
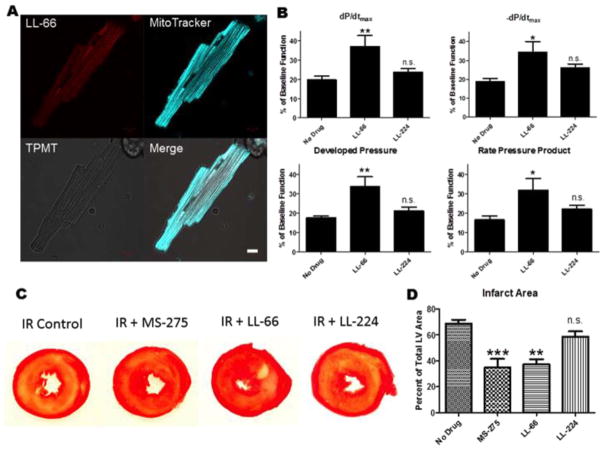
In order to differentiate the effects of mitochondrial HDAC activity from nuclear and cytosolic HDAC activity, we developed class I selective HDAC inhibitors that are targeted to, or excluded from, the mitochondria. To create the mitochondrial HDAC inhibitor, we conjugated a benzamide derivative (MS-275) to Rhodamine 123, a dye used to measure mitochondrial membrane potential [42]. This new inhibitor, LL-66, should concentrate many fold within respiring mitochondria and has the added advantage of direct visualization using confocal microscopy (Supplemental Fig. I). To create an HDAC inhibitor that does not concentrate in the mitochondria, we conjugated MS-275 to dihydrofluorescein diacetate, which accumulates in the cytosol. It can enter but is not concentrated within the mitochondria. Our data confirm that LL-66 co-localizes primarily with Mitotracker Deep Red FM, demonstrating that LL-66 is selectively targeted to the mitochondria and is absent from the nucleus in isolated adult rat ventricular myocytes (Fig. 3A) and HeLa cells and that LL-224 not concentrated in the mitochondria but is primarily in the cytosol and nucleus (supplemental Fig. I).
Fig. 3.
LL-66 localization and effect on ventricular recovery and myocardial viability post-I/R injury. (A) Confocal fluorescent images of myocytes loaded with LL-66 and Mitotracker Deep Red FM. Scale bar = 10 μm (B) LV functional measurements taken at the end of 30 min ischemia and 60 min of reperfusion, represented as a percentage of baseline pre-ischemia function. dP/dtmax indicates the rate of pressure development, −dP/dtmax indicates the rate of pressure relaxation, Developed Pressure was calculated as the difference between the systolic and diastolic pressures, Rate Pressure Product was calculated as the developed pressure multiplied by the heart rate. Measurements were taken at the end of reperfusion. (C) TTC staining for infarct at the end of reperfusion; yellow/white region indicates area of infarction. Infarct areas were measured using imageJ software. Slices from Fig. 1C are shown for the purpose of comparison. (D) Area of Infarction for each of the groups in (C). N = 3 for (A), N = 4–6 for B–D. All data are represented as mean ± S.D. t-test for panel B, one way ANOVA with Bonferroni post-test for panel D *p < 0.05. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
LL-66 and LL-224 allowed us to directly examine the role of mitochondrial HDAC1 activity in ischemia-reperfusion injury in the heart by again utilizing the Langendorff isolated heart model. Hearts were subjected to 30 min of global ischemia, followed by 60 min of reperfusion ± LL-66 or LL-224. LL-66 and LL-224 were administered at a sub-pharmacologic dose of 10 nM, far below their IC50 for class I HDACs (supplemental Fig. II). Therefore, HDAC activity would only be inhibited in the mitochondria where LL-66, but not LL-224, is concentrated. LL-66 treatment during reperfusion resulted in the same magnitude of LV functional recovery from I/R injury as the non-targeted class I HDAC inhibitor MS-275, while LL-224 showed a slight improvement in −dP/dtmax, but no improvement in the other LV functional parameters (Fig. 3B). Further, LL-66 treatment at reperfusion rescued viable myocardium to the same extent as MS-275, while LL-224 did not (Fig. 3C). These experiments indicate that the detrimental effects of increased HDAC activity in the first hour of reperfusion injury largely occur within the mitochondria of cardiac myocytes.
3.5. Mitochondrial class I HDAC inhibition alters the metabolic response of cardiac myocytes to I/R injury
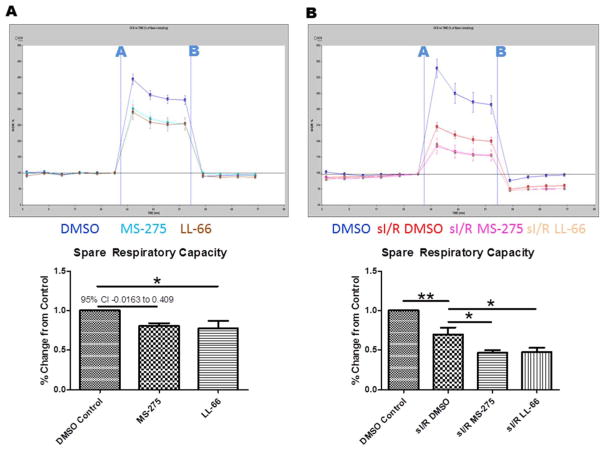
Since acetylation is known to regulate the activity of mitochondrial enzymes involved in multiple metabolic pathways, we decided to determine if class I HDAC inhibition affects metabolic activity in cardiac myocytes both at baseline and when subjected to simulated hypoxia-reoxygenation (sI/R). For these experiments, isolated adult rat cardiac myocytes were subjected to chemical hypoxia or normoxia control for 90 min, then reperfused in the presence of DMSO vehicle, MS-275, or LL-66 for 60 min. At the conclusion of the reperfusion period, myocytes were loaded into a Seahorse extracellular flux analyzer and the oxygen consumption rate (OCR) was analyzed at baseline and in response to FCCP (injection A) and antimycin D/rotenone (injection B). The presence of either HDAC inhibitor significantly decreased the maximal uncoupled OCR, both at baseline (Fig. 4A) and following sI/R (Fig. 4B).
Fig. 4.
Effect of class I HDAC inhibition on OCR and ROS. (A) Measurements of oxygen consumption rate (OCR) in isolated cardiac myocytes. FCCP was injected at point A and antimycin D/rotenone injected at point B. (B) Measurements of OCR in isolated myocytes subjected to normoxia (blue) or chemical hypoxia reoxygenation (red) and reperfused with DMSO (red), MS-275 (pink), or LL-66 (peach). For (A) and (B), top panels are representative, bottom panels are the uncoupled rate as a percentage of baeline rate over 4 runs. Representative seahorse data are represented mean ± SEM, Bar graphs are represented mean ± S.D. For all experiments, N = 4, one way ANOVA with Dunnet post test *p < 0.05, **p < 0.01. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.6. Mitochondrial HDAC1 modifies succinate dehydrogenase (SDHA) activity and alters ROS generation in reperfusion
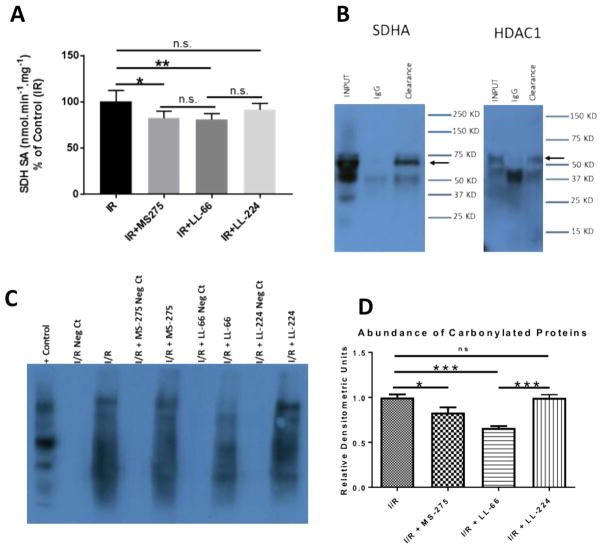
Chouchani et al. recently demonstrated that succinate accumulation during ischemia, and subsequent oxidation through SDHA in reperfusion, substantially contributes to ROS release in reperfusion [43], mediating early I/R injury. To determine if SDHA activity was affected by selective HDAC1 inhibition, we examined SDHA activity in ventricular homogenates from rat hearts subjected to I/R injury ± MS-275, LL-66 and LL-224. We found that whole cell (MS-275) and mitochondrial-selective (LL-66) HDAC inhibition resulted in a significant reduction of SDHA activity, while cytoplasmic-enriched HDAC inhibition (LL-224) did not (Fig. 5A). Co-immunoprecipitation experiments in rat ventricular homogenates confirmed the interaction between SDHA and HDAC1 (Fig. 5B). Further, these results correlate with a reduction of ROS burden in the presence of whole cell (MS-275) and mitochondrial-enriched (LL-66) HDAC inhibition, as demonstrated by oxyblotting of ventricular homogenates subjected to I/R ± MS-275, LL-66, and LL-224 (Fig. 5C).
Fig. 5.
Class I HDAC inhibition reduces SDHA activity and ROS burden in reperfusion. (A) Measurements of SDHA specific activity in rat ventricular homogenates from hearts subjected to I/R injury ± MS-275, LL-66, or LL-224. (B) Co-immunoprecipitation of SDHA and HDAC1. Immunoprecipitation of SDHA from rat cardiac ventricular homogenates followed by Western blot with anti-SDH or anti-HDAC1. (C) Oxyblotting of tissue homogenates from Langendorff hearts subjected to I/R ± LL-66, MS-275 or LL-224. All data are represented mean ± S.D. For all experiments, N = 4–5, one way ANOVA *p < 0.05, **p < 0.01.
4. Discussion
In this study, we demonstrate that class I HDAC inhibition during the reperfusion phase of I/R injury salvages viable myocardium and improves left ventricular function within the first hour of injury. This is the first report that HDAC1 is present in the adult cardiac myocyte mitochondria, and that inhibiting the activity of HDAC1 in the mitochondria alone is sufficient to provide a similar level of protection against reperfusion injury as global inhibition of HDAC1. We demonstrate that in both normal conditions and after simulated I/R injury, HDAC inhibition reduces the spare respiratory capacity of isolated cardiac myocytes, which correlates with a reduction in SDHA activity and oxidative damage during reperfusion, promoting myocyte survival.
Multiple groups, including ours, have observed that HDACs contribute to the deleterious effects of ischemia reperfusion injury in the heart [29–33]. However, previous studies examining the effect of HDAC inhibition as a postconditioning stimulus have focused mostly on pan-HDAC inhibitors, and on their effect late in reperfusion, 24 h or more after injury [32,33]. Here, we demonstrate that the adverse effects of increased HDAC activity begin within the first hour of reperfusion. The beneficial effects of inhibiting HDAC activity during this period may be synergistic with, or completely separate from, the established effects of inhibiting HDACs later in reperfusion [32,33]. More studies are needed to parse out the distinct pathways affected by HDAC inhibition at these different time points of reperfusion. Our study also demonstrates that a class-selective HDAC inhibitor is beneficial in salvaging viable myocardium when administered only during the reperfusion phase. Investigating the effects of inhibiting class I HDACs in reperfusion and comparing the results to the effects of pan-HDAC inhibition may help better define the exact pathways affected by HDAC activity during re-perfusion and aid in the design of better future therapies for reperfusion injury.
Many of the critical pathways determining the acute myocardial response to I/R injury either originate within the mitochondria or externally target the mitochondrial permeability transition pore (mPTP). Acetylation is a very important regulator of enzymatic function and protein-protein interaction in the mitochondria [1,4,44]. Other studies have demonstrated a potential role for mitochondrial protein acetylation in the response to I/R injury, with most studies focusing on the effects of SIRT3-mediated acetylation in I/R [45,46]. Interestingly, recent work utilizing a Cyclophilin D knockout, a model that is resistant to ischemia-reperfusion injury [47], demonstrated increased acetylation of mitochondrial proteins [48]. Importantly, only a small percentage of the peptides identified in this study were SIRT3 substrates, leaving open the possibility of another mitochondrial deacetylase.
The work presented here is the first to report that a class I HDAC is present within the mitochondria in an adult mammalian organ. The rapidly expanding field of acetylation proteomics has identified acetylation as the major regulatory PTM driving metabolic programs within the mitochondria [1,49,50]; control of which was previously thought to be solely the purview of Sirtuin 3 [51]. Here, we identify another mitochondrial deacetylase, HDAC1, which plays a critical role in regulating the metabolic response of the heart to injury. Given recent work demonstrating perturbations of the mitochondrial acetylome in heart failure [52] and other cardiac pathologies [53], the discovery of HDAC1 as a mitochondrial deacetylase in cardiac myocytes opens up exciting new opportunities in both dissecting the mitochondrial elements of the pathogenesis of these diseases and designing new cardiovascular therapeutics.
The inhibition of mitochondrial HDAC1 using whole cell (MS-275) or mitochondrial-targeted (LL-66) HDAC inhibitors resulted in improved functional recovery and greater tissue viability following I/R injury, compared to control hearts or hearts subjected to a cytoplasmic-targeted HDAC inhibitor (LL-224) (Fig. 3). These data indicate that mitochondrial HDAC1 activity has a deleterious effect on functional recovery of the heart during reperfusion injury. The IC50 of MS-275 for HDAC1 is ~200 nM [54], while the IC50 of LL-66 and LL-224 for HDAC1 are 3286 nM and 211.6 nM, respectively (Supplemental Fig. 1). Both LL-66 and LL-224 are administered to the hearts at 10 nM, well below their respective IC50. Although we did not recognize it at the beginning of these studies MS-275 is a hydrophobic cationic molecule, which would be accumulated in the mitochondrial matrix by the mitochondrial membrane potential Δψ. This allowed us show that it was also efficacious at a sub-pharmacologic dose of 10 nM. The fact that LL-66 has similar effects as MS-275, despite having a much higher IC50 than MS-275, supports the hypothesis that both are substantially accumulated in the mitochondria and inhibition of mitochondrial HDAC1 confers protection against I/R injury. LL-66 is concentrated in the mitochondria by Δψ so it will not be concentrated sufficiently to inhibit HDAC1 in mitochondria that are severely impaired or damaged following reperfusion. This may actually work in the favor of inhibiting HDAC1 activity only in those mitochondria which are capable of rescue. The role of mitochondrial HDAC1 inhibition in conferring protection from I/R is also supported by our data utilizing LL-224. LL-224 has a similar IC50 as MS-275, and much lower than LL-66. But LL-224 is not accumulated in the mitochondria and is largely restricted to the cytoplasm and nucleus (Supplemental Fig. 1). Therefore, our data demonstrating, that LL-224 does not confer protection whereas LL-66 and MS-275 do, suggests that only mitochondrial and not cytoplasmic and nuclear HDAC1 inhibition is sufficient to protect the heart from I/R. injury.
We further demonstrate that inhibiting the activity of mitochondrial HDAC1, using either MS-275 or the mitochondria-targeted LL-66, results in a reduction in the spare respiratory capacity of adult rat ventricular cardiac myocytes, both at baseline and after simulated I/R. This important finding demonstrates that changing the deacetylase activity of HDAC1 in the myocyte mitochondria affects the maximal rate at which myocyte mitochondria are able to consume oxygen, indicating that HDAC1 may regulate the activity of metabolic enzymes.
Given the demonstrated role of SDHA in exacerbating ROS production in reperfusion and exacerbating I/R injury [43], we examined whether SDHA is a specific target of HDAC1. Our data demonstrate that HDAC1 binds to SDHA, and that inhibition of HDAC1 with MS-275 or LL-66 in reperfusion reduce the activity of SDHA. Further, the observed decrease in maximal respiratory rate and SDHA activity in response to MS-275 or LL-66 treatment correlates with a reduction in oxidative damage during reperfusion ex vivo. This reduction in oxidative damage may promote myocyte survival by reducing the mitochondrial injury endured during early reperfusion.
Our data demonstrate a correlation between reduced oxygen consumption, decreased ROS stress, and preservation of myocyte viability post-I/R injury. This correlates with a reduction in SDHA activity, which is a known source of ROS during reperfusion. However, other undiscovered mitochondrial targets of HDAC1 may also play a role in I/R or other cardiac pathologies. Increasing evidence suggests that reduced metabolic flux through many different pathways is beneficial to the heart in reperfusion [55]. Recent work has shown that inhibiting fatty acid oxidation protects the heart from injury [56], as does inhibition of the malate aspartate shuttle [57], while metabolic succinate accumulation [43] sensitizes the heart to injury. Interestingly, emerging evidence demonstrates that acetylation of proteins involved in metabolic pathways acts as a powerful regulatory signal, providing a possible direct link between mitochondrial HDAC1 and the metabolic state of the cardiac myocyte. As such, further studies are needed to investigate the metabolic pathways affected by HDAC1 activity in the cardiac myocyte mitochondria in order to fully understand the pathological role of mitochondrial HDAC1 in I/R injury.
The data presented here demonstrate a previously unknown role for HDAC1 in directly modulating the mitochondrial acetylome, which results in an altered metabolic state in the reperfusion phase of I/R injury. This altered metabolism further correlates with reduced SDHA activity and reduced oxidative damage, increased viable myocardium and improved LV function post-I/R injury. Although reperfusion limits ischemic injury, the resulting oxidative stress accounts for up to 50% of the final damage from AMI [58]. The absence of any available clinical intervention to attenuate reperfusion injury with PCI or coronary angioplasty accentuates the exigency of a therapy that reduces ROS during reperfusion. This study identifies HDAC1 as a possible therapeutic target for I/R injury in the earliest hours of reperfusion.
Supplementary Material
Acknowledgments
We thank Gyda Beeson and Brett Hoover for excellent technical assistance.
Sources of funding
This project supported by VA Merit AwardBX002327-01 to DRM. Additional support by F30 HL129629, T32 GM008716, T32 HL007260, and the SCTR Institute, CTSA, NIH/NCATS grant numbers UL1TR001450 and TL1 TR000061.
Additional funding for Donald Menick : This publication was supported by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina CTSA, NIH/NCATS Grant Number UL1TR001450.
Dan Herr supported by F30 HL129629, T32 GM008716, T32 HL007260.
Abbreviations
- HAT
histone acetyltransferase enzyme
- HDAC
histone deacetylase enzyme
- I/R
ischemia reperfusion injury
- dP/dtmax
rate of LV pressure generation
- −dP/dtmax
rate of LV pressure relaxation
- TTC
2,3,5-triphenyltetrazolium chloride
- mPTP
mitochondrial permeability transition pore
- sI/R
simulated ischemia reperfusion injury in vitro
- OCR
oxygen consumption rate
- AMI
acute myocardial infarction
- PCI
primary percutaneous coronary intervention
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.yjmcc.2017.12.004.
Footnotes
Disclosures
None.
References
- 1.Choudhary C, Weinert BT, Nishida Y, Verdin E, Mann M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat Rev Mol Cell Biol. 2014;15:536–550. doi: 10.1038/nrm3841. [DOI] [PubMed] [Google Scholar]
- 2.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science (New York, NY) 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan KL. Regulation of cellular metabolism by protein lysine acetylation. Science (New York, NY) 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science (New York, NY) 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 5.Koprinarova M, Schnekenburger M, Diederich M. Role of histone acetylation in cell cycle regulation. Curr Top Med Chem. 2016;16(7):732–744. doi: 10.2174/1568026615666150825140822. [DOI] [PubMed] [Google Scholar]
- 6.Swygert SG, Peterson CL. Chromatin dynamics: interplay between remodeling enzymes and histone modifications. Biochim Biophys Acta. 2014;1839:728–736. doi: 10.1016/j.bbagrm.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corona DF, Clapier CR, Becker PB, Tamkun JW. Modulation of ISWI function by site-specific histone acetylation. EMBO Rep. 2002;3:242–247. doi: 10.1093/embo-reports/kvf056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ventura M, Mateo F, Serratosa J, Salaet I, Carujo S, Bachs O, Pujol MJ. Nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase is regulated by acetylation. Int J Biochem Cell Biol. 2010;42:1672–1680. doi: 10.1016/j.biocel.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Ishfaq M, Maeta K, Maeda S, Natsume T, Ito A, Yoshida M. Acetylation regulates subcellular localization of eukaryotic translation initiation factor 5A (eIF5A) FEBS Lett. 2012;586:3236–3241. doi: 10.1016/j.febslet.2012.06.042. [DOI] [PubMed] [Google Scholar]
- 10.Riolo MT, Cooper ZA, Holloway MP, Cheng Y, Bianchi C, Yakirevich E, Ma L, Chin YE, Altura RA. Histone deacetylase 6 (HDAC6) deacetylates survivin for its nuclear export in breast cancer. J Biol Chem. 2012;287:10885–10893. doi: 10.1074/jbc.M111.308791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yi C, Ma M, Ran L, Zheng J, Tong J, Zhu J, Ma C, Sun Y, Zhang S, Feng W, Zhu L, Le Y, Gong X, Yan X, Hong B, Jiang FJ, Xie Z, Miao D, Deng H, Yu L. Function and molecular mechanism of acetylation in autophagy regulation. Science (New York, NY) 2012;336:474–477. doi: 10.1126/science.1216990. [DOI] [PubMed] [Google Scholar]
- 12.Yi C, Yu L. How does acetylation regulate autophagy? Autophagy. 2012;8:1529–1530. doi: 10.4161/auto.21156. [DOI] [PubMed] [Google Scholar]
- 13.Webster BR, Scott I, Traba J, Han K, Sack MN. Regulation of autophagy and mitophagy by nutrient availability and acetylation. Biochim Biophys Acta. 2014;1841:525–534. doi: 10.1016/j.bbalip.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang R, Xu Y, Wan W, Shou X, Qian J, You Z, Liu B, Chang C, Zhou T, Lippincott-Schwartz J, Liu W. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol Cell. 2015;57:456–466. doi: 10.1016/j.molcel.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and T heir possible role in the regulation of Rna synthesis. Proc Natl Acad Sci U S A. 1964;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brownell JE, Zhou J, Ranalli T, Kobayashi R, Edmondson DG, Roth SY, Allis CD. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell. 1996;84:843–851. doi: 10.1016/s0092-8674(00)81063-6. [DOI] [PubMed] [Google Scholar]
- 17.Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 18.Spange S, Wagner T, Heinzel T, Kramer OH. Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int J Biochem Cell Biol. 2009;41:185–198. doi: 10.1016/j.biocel.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 19.Geng H, Liu Q, Xue C, David LL, Beer TM, Thomas GV, Dai MS, Qian DZ. HIF1alpha protein stability is increased by acetylation at lysine 709. J Biol Chem. 2012;287:35496–35505. doi: 10.1074/jbc.M112.400697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gronroos E, Hellman U, Heldin CH, Ericsson J. Control of Smad7 stability by competition between acetylation and ubiquitination. Mol Cell. 2002;10:483–493. doi: 10.1016/s1097-2765(02)00639-1. [DOI] [PubMed] [Google Scholar]
- 21.Samant SA, Pillai VB, Sundaresan NR, Shroff SG, Gupta MP. Histone deacetylase 3 (HDAC3)-dependent reversible lysine acetylation of cardiac myosin heavy chain isoforms modulates their enzymatic and motor activity. J Biol Chem. 2015;290:15559–15569. doi: 10.1074/jbc.M115.653048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Heide LP, Smidt MP. Regulation of FoxO activity by CBP/p300-mediated acetylation. Trends Biochem Sci. 2005;30:81–86. doi: 10.1016/j.tibs.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Arbely E, Natan E, Brandt T, Allen MD, Veprintsev DB, Robinson CV, Chin JW, Joerger AC, Fersht AR. Acetylation of lysine 120 of p53 endows DNA-binding specificity at effective physiological salt concentration. Proc Natl Acad Sci U S A. 2011;108:8251–8256. doi: 10.1073/pnas.1105028108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray S, Lee C, Hou T, Boldogh I, Brasier AR. Requirement of histone deacetylase1 (HDAC1) in signal transducer and activator of transcription 3 (STAT3) nucleocytoplasmic distribution. Nucleic Acids Res. 2008;36:4510–4520. doi: 10.1093/nar/gkn419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKinsey TA. Therapeutic potential for HDAC inhibitors in the heart. Annu Rev Pharmacol Toxicol. 2012;52:303–319. doi: 10.1146/annurev-pharmtox-010611-134712. [DOI] [PubMed] [Google Scholar]
- 26.McKinsey TA. Isoform-selective HDAC inhibitors: closing in on translational medicine for the heart. J Mol Cell Cardiol. 2011;51:491–496. doi: 10.1016/j.yjmcc.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, Alvis-Guzman N, Amrock S, Ansari H, Arnlov J, Asayesh H, Atey TM, Avila-Burgos L, Awasthi A, Banerjee A, Barac A, Barnighausen T, Barregard L, Bedi N, Belay Ketema E, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castaneda-Orjuela CA, Castillo-Rivas J, Catala-Lopez F, Choi JY, Christensen H, Cirillo M, Cooper L, Jr, Criqui M, Cundiff D, Damasceno A, Dandona L, Dandona R, Davletov K, Dharmaratne S, Dorairaj P, Dubey M, Ehrenkranz R, El Sayed Zaki M, Faraon EJA, Esteghamati A, Farid T, Farvid M, Feigin V, Ding EL, Fowkes G, Gebrehiwot T, Gillum R, Gold A, Gona P, Gupta R, Habtewold TD, Hafezi-Nejad N, Hailu T, Hailu GB, Hankey G, Hassen HY, Abate KH, Havmoeller R, Hay SI, Horino M, Hotez PJ, Jacobsen K, James S, Javanbakht M, Jeemon P, John D, Jonas J, Kalkonde Y, Karimkhani C, Kasaeian A, Khader Y, Khan A, Khang YH, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Kosen S, Krohn KJ, Kumar GA, Kwan GF, Lal DK, Larsson A, Linn S, Lopez A, Lotufo PA, El Razek HMA, Malekzadeh R, Mazidi M, Meier T, Meles KG, Mensah G, Meretoja A, Mezgebe H, Miller T, Mirrakhimov E, Mohammed S, Moran AE, Musa KI, Narula J, Neal B, Ngalesoni F, Nguyen G, Obermeyer CM, Owolabi M, Patton G, Pedro J, Qato D, Qorbani M, Rahimi K, Rai RK, Rawaf S, Ribeiro A, Safiri S, Salomon JA, Santos I, Santric Milicevic M, Sartorius B, Schutte A, Sepanlou S, Shaikh MA, Shin MJ, Shishehbor M, Shore H, Silva DAS, Sobngwi E, Stranges S, Swaminathan S, Tabares-Seisdedos R, Tadele Atnafu N, Tesfay F, Thakur JS, Thrift A, Topor-Madry R, Truelsen T, Tyrovolas S, Ukwaja KN, Uthman O, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Watkins D, Weintraub R, Werdecker A, Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Vos T, Naghavi M, Murray C. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hausenloy DJ, Yellon DM. Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol. 2016;13:193–209. doi: 10.1038/nrcardio.2016.5. [DOI] [PubMed] [Google Scholar]
- 29.Aune SE, Herr DJ, Mani SK, Menick DR. Selective inhibition of class I but not class IIb histone deacetylases exerts cardiac protection from ischemia reperfusion. J Mol Cell Cardiol. 2014;72:138–145. doi: 10.1016/j.yjmcc.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao TC, Cheng G, Zhang LX, Tseng YT, Padbury JF. Inhibition of histone deacetylases triggers pharmacologic preconditioning effects against myocardial is-chemic injury. Cardiovasc Res. 2007;76:473–481. doi: 10.1016/j.cardiores.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Qin X, Zhao Y, Fast L, Zhuang S, Liu P, Cheng G, Zhao TC. Inhibition of histone deacetylases preserves myocardial performance and prevents cardiac remodeling through stimulation of endogenous angiomyogenesis. J Pharmacol Exp Ther. 2012;341:285–293. doi: 10.1124/jpet.111.189910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Granger A, Abdullah I, Huebner F, Stout A, Wang T, Huebner T, Epstein JA, Gruber PJ. Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice. FASEB J. 2008;22:3549–3560. doi: 10.1096/fj.08-108548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie M, Kong Y, Tan W, May H, Battiprolu PK, Pedrozo Z, Wang ZV, Morales C, Luo X, Cho G, Jiang N, Jessen ME, Warner JJ, Lavandero S, Gillette TG, Turer AT, Hill JA. Histone deacetylase inhibition blunts ischemia/reperfusion injury by inducing cardiomyocyte autophagy. Circulation. 2014;129:1139–1151. doi: 10.1161/CIRCULATIONAHA.113.002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrera R, Benhabbouche S, Bopassa JC, Li B, Ovize M. One hour reperfusion is enough to assess function and infarct size with TTC staining in Langendorff rat model. Cardiovasc Drugs Ther. 2009;23:327–331. doi: 10.1007/s10557-009-6176-5. [DOI] [PubMed] [Google Scholar]
- 35.Chen M, Sato PY, Chuprun JK, Peroutka RJ, Otis NJ, Ibetti J, Pan S, Sheu SS, Gao E, Koch WJ. Prodeath signaling of G protein-coupled receptor kinase 2 in cardiac myocytes after ischemic stress occurs via extracellular signal-regulated kinase-dependent heat shock protein 90-mediated mitochondrial targeting. Circ Res. 2013;112:1121–1134. doi: 10.1161/CIRCRESAHA.112.300754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badugu R, Garcia M, Bondada V, Joshi A, Geddes JW. N terminus of calpain 1 is a mitochondrial targeting sequence. J Biol Chem. 2008;283:3409–3417. doi: 10.1074/jbc.M706851200. [DOI] [PubMed] [Google Scholar]
- 37.Bradner JE, West N, Grachan ML, Greenberg EF, Haggarty SJ, Warnow T, Mazitschek R. Chemical phylogenetics of histone deacetylases. Nat Chem Biol. 2010;6:238–243. doi: 10.1038/nchembio.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowers A, West N, Taunton J, Schreiber SL, Bradner JE, Williams RM. Total synthesis and biological mode of action of largazole: a potent class I histone deacetylase inhibitor. J Am Chem Soc. 2008;130:11219–11222. doi: 10.1021/ja8033763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishimura Y, Romer LH, Lemasters JJ. Mitochondrial dysfunction and cytoskeletal disruption during chemical hypoxia to cultured rat hepatic sinusoidal endothelial cells: the pH paradox and cytoprotection by glucose, acidotic pH, and glycine. Hepatology. 1998;27:1039–1049. doi: 10.1002/hep.510270420. [DOI] [PubMed] [Google Scholar]
- 40.Guillet V, Gueguen N, Verny C, Ferre M, Homedan C, Loiseau D, Procaccio V, Amati-Bonneau P, Bonneau D, Reynier P, Chevrollier A. Adenine nucleotide translocase is involved in a mitochondrial coupling defect in MFN2-related Charcot-Marie-Tooth type 2A disease. Neurogenetics. 2010;11:127–133. doi: 10.1007/s10048-009-0207-z. [DOI] [PubMed] [Google Scholar]
- 41.Baarine M, Beeson C, Singh A, Singh I. ABCD1 deletion-induced mitochondrial dysfunction is corrected by SAHA: implication for adrenoleukodystrophy. J Neurochem. 2015;133:380–396. doi: 10.1111/jnc.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ronot X, Benel L, Adolphe M, Mounolou JC. Mitochondrial analysis in living cells: the use of rhodamine 123 and flow cytometry. Biol Cell. 1986;57:1–7. doi: 10.1111/j.1768-322x.1986.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 43.Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord EN, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa AS, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, Murphy MP. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Still AJ, Floyd BJ, Hebert AS, Bingman CA, Carson JJ, Gunderson DR, Dolan BK, Grimsrud PA, Dittenhafer-Reed KE, Stapleton DS, Keller MP, Westphall MS, Denu JM, Attie AD, Coon JJ, Pagliarini DJ. Quantification of mitochondrial acetylation dynamics highlights prominent sites of metabolic regulation. J Biol Chem. 2013;288:26209–26219. doi: 10.1074/jbc.M113.483396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porter GA, Urciuoli WR, Brookes PS, Nadtochiy SM. SIRT3 deficiency exacerbates ischemia-reperfusion injury: implication for aged hearts. Am J Physiol Heart Circ Physiol. 2014;306:H1602–9. doi: 10.1152/ajpheart.00027.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bochaton T, Crola-Da-Silva C, Pillot B, Villedieu C, Ferreras L, Alam MR, Thibault H, Strina M, Gharib A, Ovize M, Baetz D. Inhibition of myocardial re-perfusion injury by ischemic postconditioning requires sirtuin 3-mediated deacetylation of cyclophilin D. J Mol Cell Cardiol. 2015;84:61–69. doi: 10.1016/j.yjmcc.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 47.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen TT, Wong R, Menazza S, Sun J, Chen Y, Wang G, Gucek M, Steenbergen C, Sack MN, Murphy E. Cyclophilin D modulates mitochondrial acetylome. Circ Res. 2013;113:1308–1319. doi: 10.1161/CIRCRESAHA.113.301867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson KA, Hirschey MD. Mitochondrial protein acetylation regulates metabolism. Essays Biochem. 2012;52:23–35. doi: 10.1042/bse0520023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Menzies KJ, Zhang H, Katsyuba E, Auwerx J. Protein acetylation in metabolism -metabolites and cofactors. Nat Rev Endocrinol. 2016;12:43–60. doi: 10.1038/nrendo.2015.181. [DOI] [PubMed] [Google Scholar]
- 51.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horton JL, Martin OJ, Lai L, Riley NM, Richards AL, Vega RB, Leone TC, Pagliarini DJ, Muoio DM, Bedi KC, Jr, Margulies KB, Coon JJ, Kelly DP. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight. 2016;2 doi: 10.1172/jci.insight.84897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith LE, White MY. The role of post-translational modifications in acute and chronic cardiovascular disease. Proteomics Clin Appl. 2014;8:506–521. doi: 10.1002/prca.201400052. [DOI] [PubMed] [Google Scholar]
- 54.Chou CJ, Herman D, Gottesfeld JM. Pimelic diphenylamide 106 is a slow, tight-binding inhibitor of class I histone deacetylases. J Biol Chem. 2008;283:35402–35409. doi: 10.1074/jbc.M807045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burwell LS, Nadtochiy SM, Brookes PS. Cardioprotection by metabolic shutdown and gradual wake-up. J Mol Cell Cardiol. 2009;46:804–810. doi: 10.1016/j.yjmcc.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lopaschuk GD, Barr R, Thomas PD, Dyck JR. Beneficial effects of trimetazidine in ex vivo working ischemic hearts are due to a stimulation of glucose oxidation secondary to inhibition of long-chain 3-ketoacyl coenzyme a thiolase. Circ Res. 2003;93:e33–7. doi: 10.1161/01.RES.0000086964.07404.A5. [DOI] [PubMed] [Google Scholar]
- 57.Nielsen TT, Stottrup NB, Lofgren B, Botker HE. Metabolic fingerprint of ischaemic cardioprotection: importance of the malate-aspartate shuttle. Cardiovasc Res. 2011;91:382–391. doi: 10.1093/cvr/cvr051. [DOI] [PubMed] [Google Scholar]
- 58.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.