Abstract
Human drug use involves repeated choices to take drugs or to engage in alternative behaviors. The purpose of this study was to examine how response cost for cocaine and the value of an alternative reinforcer (opportunity to play a game of chance) and how ‘free’ doses (with minimal response cost) affected cocaine choice. Two laboratory studies of cocaine self-administration were conducted in a group of humans who were habitual cocaine smokers and in a group of rhesus monkeys that intravenously self-administered cocaine. Nine human cocaine smokers who were not seeking treatment for their cocaine were repeatedly presented with the choice to smoke 25 mg cocaine base or play a game of chance for a monetary bonus paid at study completion. The response cost for choosing cocaine varied (up to 4000 responses/dose) and the number of game plays varied (up to 8). In this sample of humans, increasing either the response cost for cocaine or increasing the value of the alternative reinforcer did not significantly affect cocaine choice, while increasing both simultaneously slightly decreased cocaine choice and increased choice of the alternative. In monkeys, the dose–response function for cocaine self-administration (10 choices of 0.0125–0.1 mg/kg/infusion vs. candy coated chocolate) was steep and we failed to achieve a 50/50 cocaine/candy choice even after substantially manipulating cost and number of candies available. Providing a large ‘free’ self-administered cocaine dose to humans did not significantly affect cocaine choice, whereas in monkeys, a large free dose of cocaine decreased cocaine choice when higher doses of cocaine were available for self-administration. The present results demonstrate that in the laboratory, it is difficult to modify on-going cocaine self-administration behavior in both humans and non-human primates.
Keywords: Cocaine, Self-administration, Relapse model, Translational model, Human, Non-human primate
1. Introduction
In human cocaine users, smoked and intravenous cocaine is almost always taken in a “binge” pattern of repeated administration over a short time period. Therefore a primary goal of treatment research is to identify factors that can disrupt the choice to continue the binge once initial doses have been consumed, i.e., relapse. Laboratory models of self-administration often aim to test interventions that might decrease the number of times cocaine is chosen during a binge by providing a non-drug reinforcer or another alternative to cocaine-taking (Hart et al., 2000; Stoops et al., 2012). In theory, as the perceived value of the alternative gets larger the rate of cocaine choice should decrease, but the results are inconsistent across studies, and participant populations. In many studies using money as the non-drug reinforcer, increasing monetary values failed to disrupt choice of intravenous or smoked cocaine doses (Hart et al., 2000; Donny et al., 2003; Walsh et al., 2001), but did decrease choice of an intranasal or intravenous cocaine dose (Donny et al., 2004; Stoops et al., 2010) and various forms of contingency management can be effective in decreasing cocaine use in humans seeking treatment (e.g., DeFulio et al., 2009; Higgins et al., 1991). Similarly in non-human primates and rodents alternatives decrease cocaine taking but often with large differences in response cost or reinforcer numbers (e.g., Nader and Woolverton, 1991; Negus, 2003; Thomsen et al., 2013). Of note in human and non-human laboratory studies the effects are often all or none with little evidence of intermediate choice levels.
One potential reason cocaine choice is so difficult to disrupt in laboratory studies may be that the cocaine is available immediately while the money or other alternative is often not available until the conclusion of the study, days or weeks later. In a previous study, we attempted to address this problem of delayed reinforcement by creating a choice between cocaine and the opportunity to play a game of chance to earn money (Vosburg et al., 2010). In this paradigm, participants could draw balls from a bingo wheel each worth monetary amounts from $0 to $20 as an alternative to receiving a dose of smoked cocaine (25 mg). The value of the alternative reinforcer was varied by changing the number of bingo balls that could be drawn (2, 4 or 6). It was hypothesized that the “excitement” and immediacy of playing a game of chance would increase the perceived value of the alternative to cocaine choice, even if actual receipt of the winnings was still delayed. As hypothesized, cocaine choice decreased as the number of balls to be drawn increased, i.e., as the value of the alternative reinforcer increased. Notably, these data were consistent with the clinical efficacy of prize-based contingency management procedures for stimulant abusers (Petry et al., 2005). The current study sought to expand upon the method of Vosburg et al. (2010) by maintaining the game of chance as an alternative and adding a response cost to the cocaine choice (space bar presses on a keyboard) to attempt to model the real-life situation of varying monetary costs (or effort required) to procure cocaine.
In addition to developing a model with human cocaine abusers that approximates real life use conditions, an additional objective was to develop a model in non-human primates (Shively and Clarkson, 2009; Weerts et al., 2007) and assess the validity of translational observations between such studies. Non-human primates offer the advantage that experimental studies can be longer in duration so a greater range of variables can be parametrically manipulated. Further, compared to rats, non-human primates can have longer histories of cocaine self-administration which better model problematic human drug use. Thus, a second study was accomplished in rhesus monkeys and a range of response-independent and self-administered cocaine doses and alternatives was tested.
In addition to making choices to continue taking cocaine during a binge human cocaine users also make choices about resuming cocaine taking after a period of abstinence, i.e., relapse. This aspect of drug taking is commonly modeled in laboratory animals using reinstatement procedures to mimic the relapse to drug use that is a defining feature of substance use disorders (e.g., Bossert et al., 2013; Shelton et al., 2013; Waters et al., 2014). Reinstatement models have three stages: 1) acquisition or maintenance of baseline levels of drug self-administration; 2) extinction of drug-reinforced operant behavior, typically via response-contingent delivery of saline; and 3) evaluation of the ability of a test stimulus (e.g., drug, environmental cues, stress) to provoke/trigger drug responding that most often leads to the delivery of saline.
There are, however, a number of critical differences between the reinstatement paradigm used with laboratory animals and clinical relapse in human drug users. First, low rates of drug taking in laboratory animals are typically induced by substituting placebo for drug, i.e., extinction, while low rates of drug taking by human drug users generally associated with motivational changes from intrinsic or external sources. Second, laboratory animals are often given response-independent (non-contingent or priming) doses of test drugs during a test session, while humans self-administer drugs. Third, during reinstatement tests, laboratory animals respond for drug, but only receive placebo, while human drug taking during relapse is reinforced by active drug delivery. Thus, typical laboratory animal reinstatement models provide relatively pure measures of drug-seeking behavior, i.e., responding on a drug-associated lever that is not influenced by the direct effects of self-administered drug. In the animal and human models presented in the current study the goal was to better model human drug seeking, drug taking and relapse by 1) decreasing cocaine use by means of presenting alternatives and increasing response cost for drug taking; 2) presenting the “priming” dose response-dependently; and 3) having drug available during the relapse sessions. Thus, we tested in humans and non-human primates whether our two laboratory models could be used to study factors affecting the choice to continue using cocaine in the face of alternatives and the choice to start using cocaine at a greater level after a period of controlled lower-level use. The effect of providing response-independent amounts of an alternative reinforcer (candy) was also tested in the rhesus monkeys.
With respect to the choice to continue taking cocaine we hypothesized that cocaine choice would decrease as the response cost increased, and cocaine choice would be further decreased when combined with the opportunity to play the game of chance as an alternative reinforcer. With respect to the choice to relapse to cocaine after receiving a single dose of cocaine we hypothesized that providing the participant a dose of cocaine at no cost, i.e., a “priming” dose, prior to a session would increase cocaine choice. If the data obtained in rhesus monkeys complemented the data obtained in human cocaine users, then this cocaine choice procedure developed with laboratory animals would gain validity as a model for the context and experiences of human cocaine abusers during periods of active use, and importantly, during periods of attempted reductions in cocaine use.
2. Method for human participants
2.1. Participants
Sixteen research volunteers (14 Black, 2 Hispanic; 14 men and 2 non-pregnant women), 31 to 49 years of age (mean = 41.8 years) and with an average of 12.4 ± 1.8 (mean ± S.D.) years of education, participated in this study. Participants were solicited via word-of-mouth referral and newspaper advertisements in New York City, and signed a consent form approved by the Institutional Review Board of The New York State Psychiatric Institute, which described the study, outlined the possible risks, and indicated that cocaine would be administered. Repeated queries were made to ensure that no potential participant was seeking, or had recently been in, drug treatment. Before study enrollment, participants passed comprehensive medical and psychiatric evaluations, including a Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (DSM-IV SCID; First et al., 1995). Participants met a minimal cocaine use criterion set in advance based on our prior experience with this non-treatment seeking population: each had smoked crack cocaine at least 2 times a week for the past 6 months, and was currently spending at least $70 per week on cocaine. From our experience, this quantitative use threshold is more pertinent than the DSM-IV notion of cocaine dependence, as many of our participants did not endorse the DSM criterion of experiencing “significant impairment or distress” as a result of their use. No participant met criteria for any other Axis I disorder other than cocaine use disorders.
On average, participants reported using cocaine by the smoked route for the past 17.1 ± 8.3 years, using cocaine 4.4 ± 1.4 days per week, and spending $75 to $2000 per week on cocaine ($433 ± 488; the cost of cocaine was about $30/g in the New York City area when these data were collected). Fourteen of the participants smoked tobacco cigarettes, smoking an average of 7.6 ± 5.9 tobacco cigarettes per day. 16 participants completed the initial day of training sessions. Nine participants completed the choice sessions and 7 participants completed the entire study. One participant's relapse choice data was not included in the analysis as 1 or 2 choices were withheld for safety reasons each session. Six participants left for personal reasons and 3 participants were discontinued due to the occurrence of asymptomatic electrocardiogram abnormalities.
2.2. Design
The participants were admitted to the Irving Institute for Clinical and Translational Research in the Presbyterian Hospital for the 24-day study. Participants were not permitted to leave the unit unless accompanied by a staff member and visitors were prohibited. Urine samples were collected daily for drug monitoring, with no indication of drug consumption aside from study-related dosing. Participants' private rooms were equipped with a television, stereo, and DVD player to help alleviate boredom. Nicotine replacement was provided to tobacco smokers during their inpatient stays as nicotine polacrilex (Nicorette gum, 2 mg or 4 mg doses, one per hour on request; up to 5 times per day) in order to avert nicotine craving or withdrawal symptoms. No gum use was permitted during laboratory sessions.
Two laboratory sessions occurred each weekday; the first session began at 0900 h and the second session began at 1500 h and each session lasted approximately 2 h. Training sessions: There were two training sessions on the first experimental day prior to 31 choice sessions. The purpose of the two training sessions was to teach participants to associate 25 mg cocaine with the label of ‘Dose A.’ Participants were told that they needed to learn about Dose A because they would later be asked in choice sessions if they wanted to receive Dose A under a range of experimental conditions. There was no cost (operant requirement) for cocaine during training sessions, and there were no alternatives available. During training sessions, participants smoked six doses of cocaine (25 mg) at 14-min intervals. Choice sessions: During choice sessions, the first dose of cocaine was administered at no cost (similar to no-cost cocaine smoking during training sessions). The initial free dose of cocaine was followed by 6 choice trials, at 14-min intervals. Across sessions, the response cost for Dose A was systematically varied (0–4000 responses on the space bar) as was the response cost for the alternative reinforcer (opportunity to draw between 0 and 8 bingo balls in a game of chance, each ball with an assigned monetary value, see below). After the initial free dose, participants were asked to choose either to take cocaine and complete the response requirement (space bar presses) or to draw the number of bingo balls available that session, with a chance to accumulate money.
2.2.1. Response cost and alternative alone
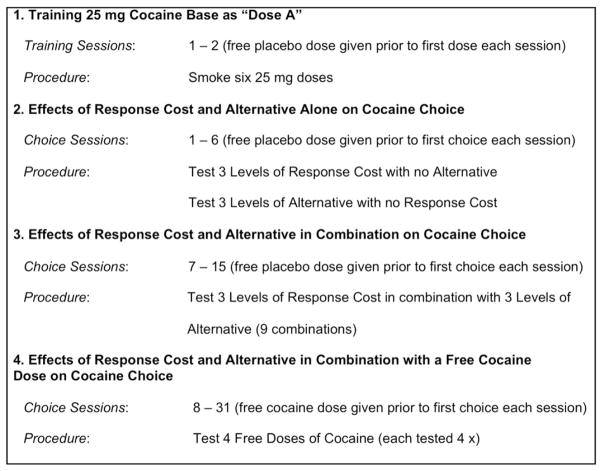
As shown in Fig. 1, for the first 15 choice sessions, the initial free “dose” was always placebo. For three of the 6 sessions, there was no alternative and the number of bar presses to earn cocaine was small (500–1000), medium (1000–2000), or large (2000–4000). All participants experienced the three levels, but the number of bar presses at each level varied among participants, e.g., 500, 1000, 2000 vs 1000, 2000, and 4000. The smaller number of bar presses was tested with the initial participants and the larger values tested with the later participants. For the other three sessions, there was no cost associated with the cocaine choice and the number of ball draws to win money at the game of chance was small (1–3), medium (3–5) or large (5–8). All participants experienced the three levels but, as with response cost, the number of game plays at each level varied among participants, e.g., 1, 3, 5 vs. 3, 5, 8 ball draws. The smaller number of game plays was tested with the initial participants and the larger number of plays tested with the later participants. For our initial participants, we found that the smaller response costs and fewer game plays did not alter drug taking in a manipulation-dependent manner, leading us to test larger response costs and number of game plays in participants run later in the study. The order of testing each cost and alternative condition during the first six choice sessions was determined randomly from all combinations of conditions. For the remaining choice sessions in this phase, each participant was tested with all 9 combinations of cost and alternatives; the order of testing each cost and alternative condition was determined randomly by sampling without replacement from all combinations of conditions.
Fig. 1.
Overview of experimental design used with human participants.
2.2.2. Response cost and alternative in combination
For the remaining 16 choice sessions, the cost of selecting cocaine and the alternative was set individually for each participant at amounts that in prior sessions resulted in 3 to 5 cocaine choices. We then examined the effects of providing a variable free dose [0, 12, 25 (“Dose A”), or 50 mg] of cocaine prior to each choice session; each “priming” dose was tested four times. Because the goal was to model the effects of a free drug dose on active cocaine smoking the effects of a free dose on placebo smoking were not assessed. Order of testing free doses was determined randomly by sampling without replacement from all dose conditions.
2.3. Experimental sessions
During the experimental sessions, the participants were seated in a reclining chair in front of a Macintosh computer and video monitor with a mouse manipulandum. As a safety measure, a 22-gauge catheter (Quik-Cath, Travenol Laboratories, Deerfield, IL) was inserted in a subcutaneous vein of one arm to permit ready intravenous access if needed in an emergency during the cocaine sessions. 12-lead electrocardiograms (ECGs) were monitored continuously with chest electrodes (MAC PC, Marquette Electronics, Milwaukee, WI), and heart rate (HR) and blood pressure (systolic, SP; diastolic, DP) were recorded every 2 min (Sentry II-Model 6100 automated vital signs monitor, NBS Medical, Costa Mesa, CA) beginning 20 min prior to cocaine administration. A Macintosh computer located in an adjacent control room was used for automated data collection. Participants were monitored via a one-way mirror by a physician and research nurses located in the control room, with communication via an intercom system.
During cocaine administration, participants were blindfolded and presented with cocaine base in a glass pipe (“stem”) fitted with smoke screens, and were instructed to take one large inhalation and to hold the vapor as long as they normally would outside of the laboratory. Vaporization of the cocaine base was accomplished by holding the flame from a pipe lighter beneath the cocaine base in the pipe. Cocaine or chances to play the game of chance were not given on any trial in which cardiovascular activity was above the criteria for safe drug administration (systolic pressure (SP) > 60 mm Hg; diastolic pressure (DP) > 100 mm Hg or a heart rate (HR) ≥ 220 – subject age × 0.85, sustained for more than 6 min prior to the next scheduled dose administration). Each session ended 30 min after the last possible cocaine delivery.
2.4. Game of chance
There were 20 balls in a bingo wheel (Vosburg et al., 2010), and each ball was labeled with a monetary value ranging from $0 to $20. Participants turned the wheel and a ball was delivered when they stopped; earnings for that trial were the sum of value of the balls. After participants chose the requisite number of balls, the balls were replaced in the bingo wheel for the next trial. When four bingo balls were drawn, the minimal value earned was $0 and the maximal value was $53. When 6 balls were drawn, the minimal value earned was $2 and the maximal value was $65. When 8 balls were drawn, the minimal value earned was $4 and the maximal value was $75. On choice sessions participants were told that part of their study pay was determined by how often they chose to play the game and how much they earned by chance.
2.5. Subjective effects questionnaire
A computerized subjective effects battery displayed on the participant's monitor was completed prior to the first cocaine dose (baseline), 4 min after each cocaine dose was delivered, and twice after the last cocaine dose of the session. The battery consisted of a series of 100 mm visual analog scales (VASs) anchored by “not at all” (0 mm) at one end and “extremely” (100 mm) at the other end. Participants registered their current state by setting a cursor appropriately along the VAS displayed on their monitor. To reduce the number of dependent variables, we used cluster analysis for the VAS, based on data used to measure the subjective effects of cocaine in our laboratory since 2002 (e.g., Evans et al., 2002; Foltin and Haney, 2004). A cluster analysis of previous subjective VAS effects of cocaine yielded five clusters of adjectives that are correlated, i.e., changes in one item being predictive of the changes in the other items in the same cluster, but do not predict changes in items in the other clusters (Evans et al., 2002). Each cluster was derived by taking the arithmetic average of the items in the cluster. Twenty of the VAS items resulted in the following five clusters: ‘bad drug effect’ consisted of seven items related to negative drug effects (e.g., ‘bad drug effect’, ‘anxious’), ‘self-esteem’ consisted of five items (e.g., ‘self-confident’, ‘social’), ‘calm’ consisted of two items (‘calm’ and ‘able to concentrate’), ‘good drug effect’ consisted of three items (‘high’, ‘good drug effect’, and ‘stimulated’), and ‘drug rating’ consisted of three items related to the cocaine dose the participant had just received (‘drug quality’, ‘drug potency’, and ‘drug liking’). Four VASs were used to operationalize drug craving, and were labeled ‘I want …’ ‘… cocaine’, ‘… heroin’, ‘… ethanol’, and ‘… nicotine’. A final question asked the participants ‘How much would you pay for the dose you just received?’ with a range of $0–25.
2.6. Cocaine
Cocaine base, derived from cocaine hydrochloride (Mallinckrodt Pharmaceuticals, St. Louis, MO), was prepared in pellets of 12, 25 and 50 mg by the New York State Psychiatric Institute Pharmacy (Foltin et al., 1990). The 0 mg dose consisted of inhaling warm air from the glass stem.
2.7. Data analysis
The primary outcome measure for choice sessions was the number of cocaine and alternative choices. Separate repeated-measures analyses of variance (ANOVAs) were conducted for 1) the effects of response cost on cocaine choice (3 levels); and 2) the effects of the alternative on cocaine choice (3 levels); the effect of response cost and alternative on cocaine choice (3 levels × 3 levels); and the effect of free cocaine doses on cocaine choice. When given a free dose of cocaine participants were consistent (±1 choice across the 4 assessments so the mean of 4 replications within each of 4 dose levels was used in the analysis). The outcome measures for training sessions were baseline and peak HR, DP, SP, VAS cluster scores, craving ratings and dose value: baseline cardiovascular measures were the mean of readings obtained (from t = −14 to −4 min) before the first dose. Training session data were analyzed using ANOVAs with completer status as a between groups variable (7 individuals who completed entire study vs. 9 individuals who completed training sessions, but not the entire study) and training session number (morning session vs. afternoon session) as repeated measures. In order to determine if the demographic and cocaine use characteristics of the sample predicted the response to cocaine during the training sessions, correlations between demographic information and dependent measures were calculated. Results for all analyses were considered statistically significant at P < 0.05 using Huynh–Feldt corrections for repeated-measures analyses.
3. Method for laboratory animals
3.1. Animals
Four adult, female rhesus monkeys (Macaca mulatta) weighing between 9 and 12 kg were fitted with a chronic indwelling catheter in the femoral vein (Access Technologies, Skokie, IL) that terminated in a subcutaneous vascular access port (VAP; Wojnicki et al., 1994; Cooper et al., 2013). Monkeys were housed in customized, squeeze-capable, rack-mounted, non-human primate cages (Hazleton Systems, Inc, Aberdeen, MD) in the AAALAC-approved animal care facility of The New York State Psychiatric Institute. Each monkey had access to 2 identically-sized chambers (61.5 cm wide × 66.5 cm deep × 88 cm high) connected by a 40 cm × 40 cm opening. Water was freely available from spouts located on the back wall of both chambers. Monkeys received a ration of approximately 7–9 chow each day (high protein monkey diet #5047, 3.37 kcal/g; LabDiets®, PMI Feeds, Inc., St. Louis, MO), designed to maintain consistent body weight. Fluorescent room lights were controlled by an automatic timer, and were illuminated from 0700 to 1900. All monkeys had experience responding for cocaine under a progressive ratio schedule of reinforcement (Cooper et al., 2013) and were experienced in getting in and out of primate restraint chairs. All aspects of animal maintenance and experimental procedures complied with the U.S. National Institutes of Health Guide for Care and Use of Laboratory Animals, and were approved by the New York State Psychiatric Institute Animal Care and Use Committee.
3.2. Self-administration set-up
For self-administration sessions, monkeys were taken from their home cage and placed into primate chairs. A custom-designed right angle Huber needle infusion set was used to connect the VAP to drug and saline infusion pumps (Multi-Phaser, Model NE-1000, New Era Pump Systems, Inc.). The response panel was mounted on the wall in front of each primate chair. Session lights were evenly spaced around the outside edges of each panel. Each panel had 2 Lindsley levers (Gerbrands, Arlington, MA), with stimulus lights above each lever, one for drug and one for candy mounted at the bottom. Each drug/saline delivery activated the drug pump for 10 s followed by the saline pump for 10 s. A pair of green lights, located at monkey eye level above the lever, flashed for 20 s (1 s on/1 s off) during the delivery of cocaine. For candy delivery, a food hopper, a pair of lights over the hopper, and a pellet-dispenser (BRS-LVE model PDC-005, Beltsville, MD) were mounted on the outside of the panel. Red lights above the food hopper were illuminated during candy delivery.
3.3. Cocaine and candy reinforcement
Intravenous cocaine reinforcement consisted of a 0.4 ml infusion of cocaine (0, 0.0125, 0.0250, 0.050, 0.100, and 0.300 mg/kg/infusion) followed by 1.25 ml infusion of saline, over a 20 s period. Cocaine hydrochloride (provided by The National Institute on Drug Abuse) was dissolved into USP sterile saline for injection and filtered using a 0.22 μm millipore filter.
Candy reinforcement consisted of 10 plain chocolate M&Ms® (Mars, Hackettstown, NJ; about 4.5 kcal per piece: 0.6-g carbohydrate, 0.2-g fat, 0.1-g protein); a highly preferred treat for rhesus monkeys (Foltin and Evans, 2001, 2002).
3.4. Schedule of reinforcement
Responding on the left manipulandum resulted in cocaine delivery, while responding on the right manipulandum resulted in candy delivery. Completion of a single fixed ratio [FR; 45 (n = 1) or 50 (n = 3) for cocaine, 10 for candy] resulted in the delivery of cocaine, or the alternative, and the presentation of the stimuli paired with reinforcement. During initial choice training none of the monkeys would chose candy when the response cost for cocaine was FR10. For this reason we increased the cocaine response requirement in order to decrease cocaine choice and increase candy choice. Monkeys had to fulfill the requirements of the FR schedule on the cocaine lever prior to beginning the choice part of the session. The first dose was always placebo, except on test days, when active doses of cocaine were used; in this way all doses were self-administered. After the first reinforcer delivery, the light over the lever extinguished, and a 6-min timeout occurred, and the choice trials began. Two-h choice sessions consisted of 10 discrete choice trials with a 6-min interval between choice opportunities. Choices were signaled by the illumination of the lights over the cocaine lever and over the candy lever. Making a single pull on either lever turned off the light over the non-selected lever and indicated the choice of that option. Once the choice was made, the ratio on that lever had to be completed for delivery of the reinforcer. Completion of the FR requirement resulted in the delivery of cocaine, or candy, and the presentation of the stimuli paired with reinforcement. There was a 5-min limited hold in effect for each choice trial. If an animal failed to complete the FR on the selected lever in 5 min, the choice opportunity terminated, and after a 6-min time out, both lever lights again illuminated signaling another choice trial. Occasionally a monkey would make a choice and not complete the response requirement. These uncompleted trials were recorded as no-choice trials. All schedule contingencies were programmed using Pascal on Macintosh (Cupertino, CA) computers located, along with the interface, in an adjacent room.
3.5. Procedure
Because all animals were accustomed to working for intravenous cocaine in the primate chairs, animals needed only to be trained in the choice procedure. This was accomplished by locking the lever for 1 commodity until responding stabilized. This was repeated over a 4–6 week interval until animals reliably switched between levers. The FRs for both commodities were systematically varied and a series of cocaine doses were examined. One of the 4 monkeys developed endometriosis and was sacrificed before completing the first phase. The 3 remaining monkeys completed the second phase which consisted of examining the effects of free candy (5, 10 or 20) or a free dose of cocaine (0, 0.05, 0.1, 0.3 mg/kg) administered prior to the choice trials; candy was dropped by the caretaker into the candy hopper prior to the first choice trial, and cocaine was substituted for the free initial placebo dose before the first choice trial. The 7 free conditions were each examined when monkeys self-administered 3 different doses of cocaine (0.025, 0.05, 0.1 mg/kg/inf).
4. Results with human participants
4.1. Training Sessions (n = 16)
The 25 mg cocaine doses smoked during the training sessions produced robust expectable physiological and subjective effects relative to baseline. Peak increases in heart rate were about 29 bpm, peak increases in diastolic pressure were about 30 mm Hg, peak increases in systolic pressure were about 40 mm Hg and peak ratings on the good drug effect cluster were about 55 mm. Cocaine craving (ratings on the “I want cocaine” VAS item) increased by about 25 mm from a baseline of about 40 mm. On average, participants indicated that they would pay $2.68 ± $1.75 to buy each 25 mg dose.
The only difference in demographics between the 7 participants who finished the entire study and the 9 who dropped out before study completion was that the completers reported greater daily tobacco cigarette use (11.7 vs 4.5, P < 0.02). There was only one difference between the completers and the non-completers in response to cocaine: peak diastolic pressure was greater (106 + 4 mm Hg) in completers than non-completers [94 ± 12.5; F (1,14) = 4.73, P < 0.0473]. There were, however, several demographic variables that were related to the response to cocaine during the morning training session. The older the participant the smaller the peak heart rate (r = −.049, P < 0.03), and the more years of cocaine use, the smaller the peak heart rate (r = −0.44, P < 0.01); both measures were also positively related (r = 0.58). It was also noted that the more years of cocaine use the smaller was the peak cocaine craving (r = −.042, P < 0.02).
4.2. Effects of response cost and alternative on cocaine choice (n = 9)
At minimal cost, the 9 participants who completed this phase of the study chose cocaine on 5.5 of the 6 trials (Fig. 2; left panel). Doubling or quadrupling the response cost had no effect on cocaine choice (P < 0.13). When the alternative to cocaine was 1 to 4 plays of the game of chance, participants chose cocaine on 4 of the 6 trials (Fig. 2; right panel). Doubling or quadrupling the number of game plays did not decrease cocaine choice; in fact, there was a trend for cocaine choice to increase from 4.0 to 4.9 (P < 0.09). Although mean earnings per trial increased from $3.80 to $28.00, this increase was not significant due to the large variability in earnings. Note that mean earnings per trial greatly exceeded participants' estimates of the street value of each dose.
Fig. 2.
Mean number of cocaine choices as a function of response cost in the absence of an alternative reinforcer (left panel) and value of the alternative reinforcer when cocaine choice did not have a response requirement (right panel) in 9 human cocaine smokers. Error bars represent 1 SEM.
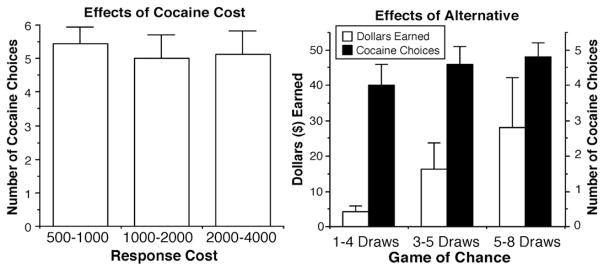
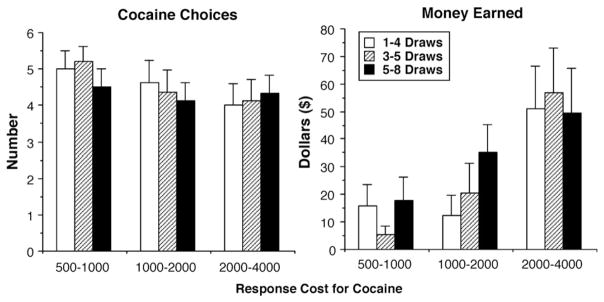
The combination of increased response cost and alternative reinforcers significantly influenced cocaine choice. Increasing the response cost for cocaine significantly decreased cocaine choice from 4.7 to 4.4 (Fig. 3; left panel) when playing the game of chance for money was available as an alternative to cocaine smoking [F (2,16) = 3.84, P < 0.04]. There was no effect of increasing the number of game plays (P < 0.17) and there was no significant interaction between response cost and alternative on cocaine choice. There was a significant increase in money earned playing the game of chance, from $23 to $36, as the number of plays increased [Fig. 3; right panel: F (2,16) = 7.31, P < 0.02], yet this increase in earnings was not associated with a shift in cocaine choice.
Fig. 3.
Mean number of cocaine choices as a function of response cost and value of the alternative together in 9 human cocaine smokers (left panel). Money earned as a function of response cost and value of the alternative together in 9 human cocaine smokers (right panel). Error bars represent 1 SEM.
4.3. Effects of free dose of cocaine (n = 6)
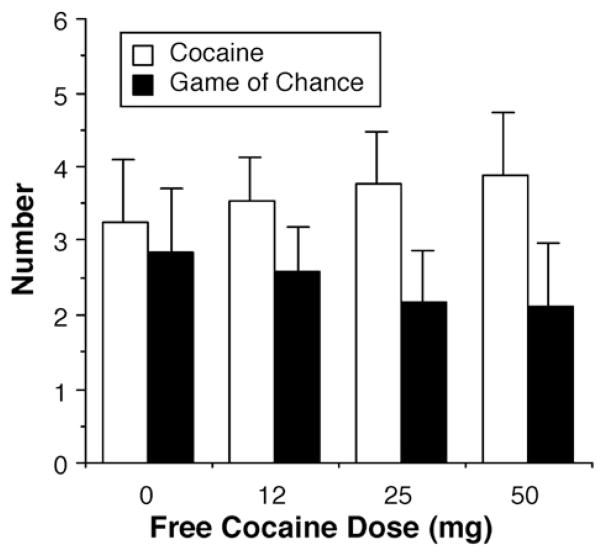
For the 6 participants whose data were included for this part of the study, the response cost and number of game plays that we estimated would result in 3 to 5 cocaine choices was used for the final phase of the study. Neither a free choice of cocaine prior to the session (at any of the doses tested: placebo, 12, 25 or 50 mg) nor the number of alternative reinforcers significantly influenced cocaine choice (Fig. 4).
Fig. 4.
Mean number of cocaine and game of chance choices as a function of dose of cocaine smoked at no cost before the choice session in 6 human cocaine smokers. Error bars represent 1 SEM.
5. Results with non-human primates
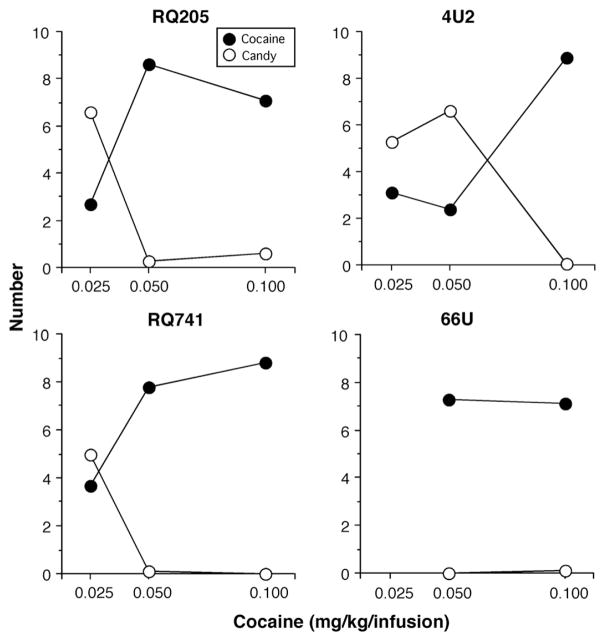
When the relatively low 0.025 mg/kg/infusion was available, the 3 monkeys tested with that dose all preferred to work for candy (Fig. 5). Increasing the cocaine dose shifted choice to 7–9 cocaine doses. Once choice shifted to cocaine, monkeys rarely chose candy such that monkeys would select cocaine or make no selection during each choice trial. Although the data are not presented, the FR value for candy and cocaine was varied in an attempt to get a more even distribution of candy and cocaine choices at the 0.05 mg/kg/infusion cocaine dose. After several months and several extinction re-training trials, all animals continued to choose nearly all cocaine doses at the 2 larger cocaine unit doses, i.e., we were unable to obtain a shift away from choosing predominantly cocaine to an intermediate shift in choice behavior.
Fig. 5.
Mean number of cocaine and candy choices as a function of intravenous cocaine dose available for self-administration in 4 rhesus monkeys.
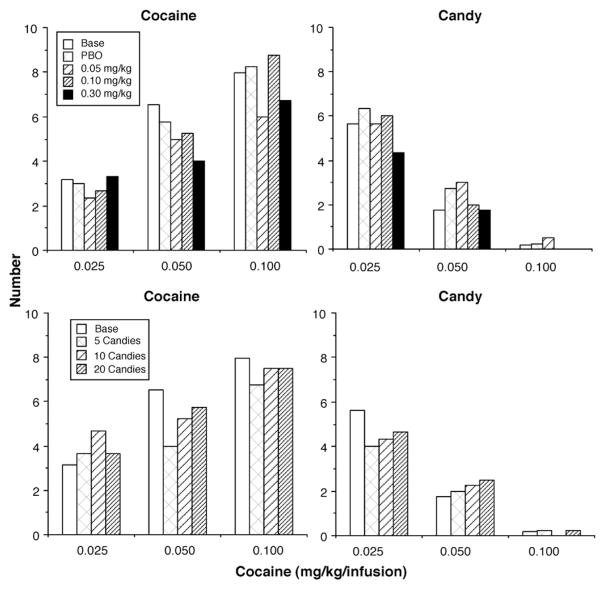
Each of the 3 monkeys who completed the second phase of the study experienced 3 free doses of cocaine (and placebo) and 3 free “doses” of candy while self-administering each of the 3 unit doses of cocaine. Again, increasing the dose of cocaine increased cocaine choice from about 2.5 to 8 (Fig. 6; top left panel) and decreased candy choice from about 4.5 to 0 (Fig. 6; top right panel). Placebo and the 3 free doses of cocaine did not alter choice behavior when monkeys had access to the 0.025 and 0.1 mg/kg/infusion cocaine doses. When monkeys had access to the middle dose (0.05 mg/kg/infusion), there was a decrease in cocaine choice and an increase in candy choice after free placebo and 0.05 mg/kg cocaine. The larger free cocaine doses decreased cocaine choice without affecting candy choice. Giving monkeys 5 to 20 free candies before the choice trials did not alter choice behavior under any of the 3 cocaine self-administration doses.
Fig. 6.
Mean number of cocaine and candy choices as a function of dose of intravenous cocaine self-administered at no cost before the choice session in 3 rhesus monkeys (top panels). Number of cocaine and candy choices as a function of number of candies eaten at no cost before the choice session in 3 rhesus monkeys (bottom panels).
6. Discussion
In the natural ecology, cocaine users regularly face choices to expend their resources on cocaine rather than on alternative non-drug reinforcers that might represent health or social benefits. As a result, decisions to use cocaine increase the risk of adverse health and social consequences, as users readily describe when sober. A goal of drug abuse treatment is to increase interest in such non-drug reinforcers and increase the salience of the adverse consequences of drug use. The purpose of this study was to develop a laboratory model for this clinical situation. Further, we aimed to develop models in human cocaine users smoking cocaine and rhesus monkeys taking cocaine intravenously in order to improve the translational utility of preclinical models to the human situation.
The results of the present study clearly show that ongoing cocaine self-administration is difficult to shift both in non-treatment seeking humans and in non-human primates. In our participants, increasing the motor response cost (up to 4000 bar presses over 12 min; a rapid and effortful rate of 5 presses a second) only reduced cocaine taking by 1/4 of a dose when there was no alternative reinforcer. Similarly, when cocaine was available without responding (“free”), increasing the value of the game alternative did not shift cocaine choice even though individuals earned as much as $36 (when 8 game plays was the alternative) each time they chose to play the game rather than smoke a dose of cocaine. Furthermore, there was a non-significant decrease in cocaine choice by 1 dose when both the cost for choosing cocaine was large and the value of the alternative was large. Rhesus monkeys showed a steep cocaine dose–response function such that cocaine choice was an all or none phenomenon for each animal.
As mentioned above increasing the value of the alternative reinforcer has been shown to decrease cocaine taking both preclinically in humans and laboratory animals and clinically in humans (e.g., DeFulio et al., 2009; Donny et al., 2004; Higgins et al., 1991; Negus, 2003; Nader and Woolverton, 1991; Thomsen et al., 2013; Vosburg et al., 2010). Yet the value of the alternative or its response cost must be quite a bit greater than that of cocaine, especially in the preclinical studies. Our shifts in cocaine choice were quite small, suggesting that a greater range of alternative costs and values needed to be tested. Donny et al. (2003, 2004) demonstrated that the order in which alternatives are presented within a session also affects their utility in decreasing cocaine choice. Beginning a choice session with a low value alternative and increasing the monetary value with each choice trial from $1 to $16 did not alter cocaine choice (Donny et al., 2003), but beginning a choice session with a high value alternative and decreasing monetary value with each choice from $19 to $1 did decrease cocaine choice (Donny et al., 2004). Our previous study (Vosburg et al., 2010) that showed a greater decrease in cocaine choice as a function of playing a game of chance for money used 3 different alternative values within each session with each value tested multiple times such that the largest alternative was experienced within the first 3 trials of each session. These results in combination with those of Donny et al. (2004) suggest that having larger value alternatives when cocaine is first available increases the utility of an alternative in decreasing cocaine choice.
Procedural details may have also played a role in the small shifts in cocaine choice. Humans experienced 2 sessions a day and each manipulation occurred during a single session. The large number of sessions may have blurred the manipulations for the participants, who we suspect approached each session with a plan, rather than responding carefully to the contingency in place for that single session. For example, some participants said that they planned to always take several does of cocaine each session regardless of alternative, but sometimes once the session started they were unable to follow the plan. In contrast the monkeys only had 1 session a day, but limiting the number of choice trials to 10 may have limited sensitivity to the manipulations.
We also examined whether our procedure that resulted in moderate levels of cocaine choice could be used as an alternative approach for testing cocaine-induced relapse to greater levels of cocaine use, i.e., would a large free dose of cocaine before the session increase subsequent cocaine choice. The most common model for studying human drug relapse in laboratory animals, mostly rodents, uses reinstatement procedures which have shown consistent patterns of drug-induced, stress-induced, and drug-paired stimuli-induced reinstatement, i.e., responding reinforced with placebo (for reviews see Bossert et al., 2013; Epstein et al., 2006; Shaham et al., 2003; Soria et al., 2008). In human cocaine users we examined the effect of a free dose of cocaine on active drug taking rather than placebo as is used in laboratory animal reinstatement models in order to see if free drug would increase drug choice. Of note, when no free cocaine doses were given these individuals chose cocaine on half of the trials in contrast to the nearly exclusive cocaine choice observed at the start of the study in the larger group. Perhaps the value of the alternative increased as the delay to spending the earned money after study completion decreased. Although cocaine choice slightly increased in humans as the size of the free dose increased (~0.75 doses) and alternative choice decreased, this effect of response-independent cocaine dosing was not significant. We examined the effects of a free cocaine dose on cocaine choice in 3 monkeys: in contrast to humans the largest size free cocaine doses decreased cocaine choice when the larger cocaine doses were available for self-administration, but not the smallest dose. As the largest free dose was 6 times the self-administered dose the decrease in cocaine choice may have been related to satiation. Providing free candy prior to the session did not alter cocaine nor candy choice.
Studies of reinstatement in monkeys have shown that drug given by the experimenter before the session (see review by Spealman et al., 1999) produces dose-dependent increases in responding for placebo (Gerber and Stretch, 1975; Spealman et al., 1999) and several studies report cocaine- and cue-induced reinstatement of responding previously reinforced with cocaine or other drugs of abuse in rhesus monkeys (Andersen et al., 2010; Banks et al., 2008; Gasior et al., 2004; Henry and Howell, 2009; Murnane et al., 2013; Sawyer et al, 2012). By contrast, in our small sample, in the context of ongoing cocaine choice we showed minimal effects of our manipulations both in the ability to decrease cocaine choice and the ability to elicit drug-induced relapse. The less robust response increases may have been due to the greater response requirement used, reinforcing choices with cocaine, or using a choice procedure. Perhaps the reinstatement effect in the present study was obfuscated by the larger current response requirement of thousands of responses in humans and 45 or 50 responses in monkeys compared to as few as a single response in rodents. The current results, rather than being a failure to model the clinical situation, may actually be a better model of the difficulty of decreasing drug use in long-term drug users.
Giving monkeys a small free cocaine dose produced a non-significant 0.7 increase in cocaine choice accompanied by a non-significant 0.7 decrease in candy choice in monkeys replicating Negus (2003) who also showed minimal effects of response-independent cocaine infusions in a cocaine vs. food choice procedure in rhesus monkeys. This subtle increase in cocaine intake replicates several studies in humans showing that response-independent alcohol increases measures of drinking behavior in both alcoholics (Bigelow et al., 1977; Hodgson et al., 1979) and social drinkers (de Wit and Chutuape, 1993) when they are consuming alcohol, not placebo during sessions. In contrast, the larger free cocaine doses decreased responding for the 2 larger self-administered cocaine doses in monkeys. This shows the value of obtaining complete dose–response functions in non-human primates that could be difficult to obtain in human cocaine users in an acceptable timeframe of study participation.
The 25 mg cocaine dose in humans produced robust increases in heart rate (29 bpm), blood pressure (40 mm Hg) and scores on the “good drug effect” cluster (50 on a 100 mm scale) relative to baseline. For safety reasons, cocaine is not given to human participants when vital signs are elevated above preset safety levels. It is important to test doses in a choice experiment that are robust reinforcers but rarely needed to be withheld because withholding a dose could alter later choice behavior: 25 mg is just such a dose. Our experience indicates that using a larger dose for self-administration would lead to choices being withheld for safety reasons in humans. One participant did have 1 or 2 doses withheld each session only during the free dose sessions and his data were not included in the analyses. In the monkeys we were able to test a larger dose range of both self-administered doses and free doses self-administered prior to the choice options. This highlights the difficulties of doing such studies in humans and the value of using non-human primates who will stop making choices when the cocaine interferes with motor behavior. The failure to see an increase in candy choice when cocaine choice decreased in the monkeys suggests that non-specific effects may have decreased responding. It is also possible that sex may play a role in the differences between the groups. Lynch et al. (2000) reported that female rats showed greater cocaine-induced reinstatement, i.e., responding for placebo, effects than male rats. The human cocaine users were nearly all males, while the rhesus monkeys were all females. The present findings fail to support this suggestion as the female monkeys did not show any cocaine-induced increases in responding, while the predominantly male group of humans showed modest increases.
This study was unique in its attempt to validate a non-human animal model concurrently with a human model for choice behavior. The similarity in results between the two species suggests it was successful in that regard, but the minimal effects of the procedures designed to alter choice in both species suggests that it was less successful at developing a non-human primate model for the human clinical situation. An alternative interpretation is that the procedures did model the human treatment condition for a nearly intractable problem in which effects are often quite small, inducing behavioral change is difficult and even small improvements merit encouragement.
A significant limitation to the study findings is the small sample size of both groups. While 16 humans began the study only 6 provided data for the relapse component and only 3 rhesus monkeys provided data for the entire study. The majority of the humans who did not complete the study left for personal reasons but others were excluded for medical reasons such that the final sample may represent a subgroup of cocaine users; a subgroup that may have been more sensitive to the cocaine response cost and value of the alternative such that their choices at testing were abut 50:50 cocaine and the alternative. Further selection bias may have arisen from confining our study to human participants who were not seeking treatment for their cocaine use. While there are concerns about giving cocaine to individuals seeking treatment for their cocaine use, such studies when conducted as part of a treatment plan may provide valuable information about predictors of treatment efficacy. The positive effects of contingency management in decreasing cocaine use (e.g., DeFulio et al., 2009; Higgins et al., 1991; Petry et al., 2005) argues that greater effects of alternatives would be observed in treatment seekers.
In summary, although we failed to develop a translational model using humans and non-human primates the present study demonstrates the possibility of attempting to do so. In the context of ongoing use, cocaine choice was difficult to disrupt in both groups and a self-administered free dose of cocaine had minimal effects on cocaine choice in both groups. Modeling variables relating to treatment in the laboratory setting is difficult. Our experimental findings confirm the sizeable challenge of the task (decreasing ongoing drug use) that drug abusers face when they decide to seek treatment.
Acknowledgments
This research was supported by DA-021319 from The National Institute on Drug Abuse, and approved by the New York State Psychiatric Institute Animal Care and Use Committee and the Internal Review Board. The research with humans was supported in part by Columbia University's CTSA grant no. UL1 RR024156 from NCATS-NCRR/NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The expert nursing assistance of Alicia Couraud R.N. and Audrey Perez R.N. and the assistance of Jean Willi, Angel Ramirez, Malgorzata Zawodna, Nicholas Urban, Ashley Soh and Drs. Gillinder Bedi, and Moshe Shalev is gratefully acknowledged.
References
- Andersen ML, Kessler E, Murnane KS, McClung JC, Tufik S, Howell LL. Dopamine transporter-related effects of modafinil in rhesus monkeys. Psychopharmacology (Berl) 2010;210:439–48. doi: 10.1007/s00213-010-1839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Sprague JE, Czoty PW, Nader MA. Effects of ambient temperature on the relative reinforcing strength of MDMA using a choice procedure in monkeys. Psycho-pharmacology (Berl) 2008;196:63–70. doi: 10.1007/s00213-007-0932-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow GE, Griffiths RR, Liebson IA. Pharmacological influences upon human ethanol self-administration. Adv Exp Med Biol. 1977;85B:523–38. doi: 10.1007/978-1-4615-9038-5_33. [DOI] [PubMed] [Google Scholar]
- Bossert J, Marchant N, Calu D, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 2013;229:453–76. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Foltin RW, Evans SM. Effects of menstrual cycle phase on cocaine self-administration in rhesus macaques. Horm Behav. 2013;63:105–13. doi: 10.1016/j.yhbeh.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Chutuape MA. Increased ethanol choice in social drinkers following ethanol preload. Behav Pharmacol. 1993;4:29–36. [PubMed] [Google Scholar]
- DeFulio A, Donlin WD, Wong CJ, Silverman K. Employment-based abstinence reinforcement as a maintenance intervention for the treatment of cocaine dependence: a randomized controlled trial. Addiction. 2009;104:1530–8. doi: 10.1111/j.1360-0443.2009.02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Bigelow GE, Walsh SL. Choosing to take cocaine in the human laboratory: effects of cocaine dose, inter-choice interval, and magnitude of alternative reinforcement. Drug Alcohol Depend. 2003;69:289–301. doi: 10.1016/s0376-8716(02)00327-7. [DOI] [PubMed] [Google Scholar]
- Donny EC, Bigelow GE, Walsh SL. Assessing the initiation of cocaine self-administration in humans during abstinence: effects of dose, alternative reinforcement and priming. Psychopharmacology (Berl) 2004;189:1–16. doi: 10.1007/s00213-003-1655-z. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;172:316–23. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (SCID-I/P, Version 2.0) Biometrics Research Department. New York State Psychiatric Institute; 1995. [Google Scholar]
- Foltin RW, Evans SM. The effects of D-amphetamine on responding for candy and fruit drink using a fixed ratio and a progressive ratio schedule of reinforcer delivery. Pharmacol Biochem Behav. 2001;69:125–31. doi: 10.1016/s0091-3057(01)00496-8. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Evans SM. Effect of response-independent candy on responding maintained by candy using a novel model of commodity acquisition and consumption in nonhuman primates. Pharmacol Biochem Behav. 2002;72:729–39. doi: 10.1016/s0091-3057(02)00746-3. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M. Intranasal cocaine in humans: acute tolerance, cardiovascular and subjective effects. Pharmacol Biochem Behav. 2004;78:93–101. doi: 10.1016/j.pbb.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Nestadt G, Stromberger H, Cornell EE, Pearlson GD. Demonstration of naturalistic methods for cocaine smoking by human volunteers. Drug Alcohol Depend. 1990;26:145–54. doi: 10.1016/0376-8716(90)90121-t. [DOI] [PubMed] [Google Scholar]
- Gasior M, Paronis CA, Bergman J. Modification by dopaminergic drugs of choice behavior under concurrent schedules of intravenous saline and food delivery in monkeys. J Pharmacol Exp Ther. 2004;308:249–59. doi: 10.1124/jpet.103.052795. [DOI] [PubMed] [Google Scholar]
- Gerber GJ, Stretch R. Drug-induced reinstatement of extinguished self-administration behavior in monkeys. Pharmacol Biochem Behav. 1975;3:1055–61. doi: 10.1016/0091-3057(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Hart C, Haney M, Foltin R, Fischman M. Alternative reinforcers differentially modify cocaine self-administration by humans. Behav Pharmacol. 2000;11:87–91. doi: 10.1097/00008877-200002000-00010. [DOI] [PubMed] [Google Scholar]
- Henry PK, Howell LL. Cocaine-induced reinstatement during limited and extended drug access conditions in rhesus monkeys. Psychopharmacology (Berl) 2009;204:523–9. doi: 10.1007/s00213-009-1485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, et al. A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatry. 1991;148:1218–24. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Hodgson R, Rankin H, Stockwell T. Alcohol dependence and the priming effect. Behav Res Ther. 1979;17:379–87. doi: 10.1016/0005-7967(79)90009-3. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology (Berl) 2000;152:132–9. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Murnane KS, Winschel J, Schmidt KT, Stewart LM, Rose SJ, Cheng K, et al. Serotonin 2A receptors differentially contribute to abuse-related effects of cocaine and cocaine-induced nigrostriatal and mesolimbic dopamine overflow in nonhuman primates. J Neurosci. 2013;33:13367–74. doi: 10.1523/JNEUROSCI.1437-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Effects of increasing the magnitude of an alternative reinforcer on drug choice in a discrete-trials choice procedure. Psychopharmacology (Berl) 1991;105:169–74. doi: 10.1007/BF02244304. [DOI] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with D-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–31. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Simcic F., Jr Prize reinforcement contingency management for cocaine dependence: integration with group therapy in a methadone clinic. J Consult Clin Psychol. 2005;73:354–9. doi: 10.1037/0022-006X.73.2.354. [DOI] [PubMed] [Google Scholar]
- Sawyer EK, Mun J, Nye JA, Kimmel HL, Voll RJ, Stehouwer JS, et al. Neurobiological changes mediating the effects of chronic fluoxetine on cocaine use. Neuropsychopharmacology. 2012;37:1816–24. doi: 10.1038/npp.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shelton KL, Hendrick ES, Beardsley PM. Efficacy of buspirone for attenuating cocaine and methamphetamine reinstatement in rats. Drug Alcohol Depend. 2013;129:210–6. doi: 10.1016/j.drugalcdep.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Clarkson TB. The unique value of primate models in translational research. Am J Primatol. 2009;71:715–21. doi: 10.1002/ajp.20720. [DOI] [PubMed] [Google Scholar]
- Soria G, Barbano MF, Maldonado R, Valverde O. A reliable method to study cue-, priming-, and stress-induced reinstatement of cocaine self-administration in mice. Psychopharmacology (Berl) 2008;199:593–603. doi: 10.1007/s00213-008-1184-x. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Barrett-Larimore RL, Rowlett JK, Platt DM, Khroyan TV. Pharmacological and environmental determinants of relapse to cocaine-seeking behavior. Pharmacol Biochem Behav. 1999;64:327–36. doi: 10.1016/s0091-3057(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Rush CR. Monetary alternative reinforcers more effectively decrease intranasal cocaine choice than food alternative reinforcers. Pharmacol Biochem Behav. 2010;95:187–91. doi: 10.1016/j.pbb.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PEA, Hays LR, Rush CR. Alternative reinforcer response cost impacts cocaine choice in humans. Prog Neuro-Psychopharmacol Biol Psychiatry. 2012;36:189–93. doi: 10.1016/j.pnpbp.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Barrett AC, Negus SS, Caine SB. Cocaine versus food choice procedure in rats: environmental manipulations and effects of amphetamine. J Exp Anal Behav. 2013;99:211–33. doi: 10.1002/jeab.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosburg SK, Haney M, Rubin E, Foltin RW. Using a novel alternative to drug choice in a human laboratory model of a cocaine binge: a game of chance. Drug Alcohol Depend. 2010;110:144–50. doi: 10.1016/j.drugalcdep.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Geter-Douglas B, Strain EC, Bigelow GE. Enadoline and butorphanol: evaluation of kappa-agonists on cocaine pharmacodynamics and cocaine self-administration in humans. J Pharmacol Exp Ther. 2001;299:147–58. [PubMed] [Google Scholar]
- Waters RP, Moorman D, Young A, Feltenstein M, See R. Assessment of a proposed “three-criteria” cocaine addiction model for use in reinstatement studies with rats. Psycho-pharmacology (Berl) 2014;231:3197–205. doi: 10.1007/s00213-014-3497-2. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Fantegrossi WE, Goodwin AK. The value of nonhuman primates in drug abuse research. Exp Clin Psychopharmacol (Berl) 2007;15:309–27. doi: 10.1037/1064-1297.15.4.309. [DOI] [PubMed] [Google Scholar]
- Wojnicki FH, Bacher JD, Glowa JR. Use of subcutaneous vascular access ports in rhesus monkeys. Lab Anim Sci. 1994;44:491–4. [PubMed] [Google Scholar]