Abstract
Objective
Corin is a serine protease that converts pro-atrial natriuretic peptide (pro-ANP) to atrial natriuretic peptide (ANP), a cardiac hormone that regulates salt-water balance and blood pressure. ANP is degraded by natriuretic peptide receptor (NPR). This study was to determine if aberrant pro-ANP/corin/NPR signaling is present in maternal vascular system in preeclampsia.
Study Design
Maternal venous blood was obtained from 197 pregnant women (84 normotensive, 16 complicated with chronic hypertension (CHT), 11 mild and 86 severe preeclampsia). Plasma corin and pro-ANP concentrations were measured by enzyme-linked immunosorbent assay. Maternal subcutaneous fat tissue was obtained from 12 pregnant women with cesarean section delivery (6 normotensive and 6 preeclampsia). Vascular ANP and its receptors NPR-A, NPR-B, and NPR-C expression were examined by immunostaining of paraffin embedded subcutaneous fat tissue sections.
Results
Corin concentrations were significantly higher in mild (2.78±0.67ng/ml, p<0.05) and severe (2.53±0.18ng/ml, p<0.01) preeclampsia than in normotensive (1.58±0.08ng/ml) and CHT (1.55±0.20ng/ml) groups. Pro-ANP concentrations were significantly higher in CHT (1.59±0.53ng/ml, p<0.05) and severe preeclampsia (1.42±0.24ng/ml, p<0.01) than in normotensive (0.48±0.06ng/ml) and mild preeclampsia (0.52±0.09ng/ml) groups. ANP and NPR-B expression was undetectable in maternal vessels from normotensive and preeclamptic pregnancies, but reduced NPR-A expression and increased NPR-C expression was found in maternal vessel endothelium in preeclampsia.
Conclusions
ANP is a vasodilator and NPR-C is a clearance receptor for ANP. The finding of upregulation of NPR-C expression suggests that circulating ANP clearance or degradation is increased in preeclampsia. These results also suggest that pro-ANP/corin/NPR signaling is dominant in the vascular system in preeclampsia.
Keywords: Corin, ANP, NPR, pregnancy, preeclampsia
Introduction
Corin is a serine protease of the trypsin superfamily. It is originally identified as a cardiac enzyme with abundant expression in atrial and ventricular myocytes in the heart [1]. Corin converts atrial natriuretic peptide (ANP) precursor (pro-ANP) to mature ANP, a circulating cardiac hormone that regulates salt balance, intravascular blood volume, and subsequently vascular tone. Therefore, corin and ANP signaling pathway molecules play an important compensative role in the cardiovascular system because of its diuretic, natriuretic, and vasodilating actions and also its inhibitory effects on renin and aldosterone secretion [2]. In humans, altered corin expression and production have been found in several cardiovascular and renal diseases. For example, plasma corin levels are reduced significantly in patients with heart failure [3]. Kidney corin expression is downregulated in proteinuric kidney diseases associated with increased sodium retention [4]. In mice, corin deficiency prevents pro-ANP processing and causes salt-sensitive hypertension and the hypertensive phenotype is exacerbated by high-salt diet when the mice become pregnant [5]. Therefore, altered corin and ANP production and activity have a significant impact on vascular and renal functional homeostasis.
Recent animal studies showed that corin is able to activate ANP in the pregnant uterus to promote spiral artery remodeling and to prevent pregnancy-induced hypertension [6–8]. In humans, up-regulation of corin was found in the late secretory phase in endometrium and in first trimester decidua tissue [7]. While, in corin- or ANP-deficient mice, trophoblast invasion was markedly impaired as compared to the wild type animals [6]. Study also showed that maternal ANP levels were significantly increased in pregnant women, and further increased in women with preeclampsia, a hypertensive disorder in human pregnancy [9]. It is known that the action of ANP is regulated via binding to its receptor on cell membrane. However, it is not known if altered ANP receptor expression occurs in preeclampsia. In the present study, we measured maternal plasma corin and pro-ANP levels and examined maternal vascular ANP and natriuretic peptide receptor A (NPR-A), NPR-B, and NPR-C expression in normotensive and preeclamptic pregnant women to test our hypothesis that aberrant pro-ANP/corin/NPR signaling is present in maternal vascular system in preeclampsia. We also measured corin and pro-ANP levels in pregnant women complicated with chronic hypertension to determine if differences exist for corin and/or pro-ANP levels in pregnant women complicated between preeclampsia and chronic hypertension.
Materials and Methods
Patient information and blood and subcutaneous fat tissue sample collection
Collections of maternal blood and subcutaneous fat tissue were approved by the Institutional Review Board for human research at Louisiana State University Health Sciences Center - Shreveport (LSUHSC-S), Louisiana and the study was conducted in the Department of Obstetrics and Gynecology, LSUHSC-S. Written consent was obtained from all study subjects. Maternal venous blood was obtained either at the Perinatal Clinics or admitted at Labor and Delivery Unit before delivery from 197 pregnant women: 84 from normotensive, 16 from pregnancy complicated with chronic hypertension, 11 from mild preeclampsia and 86 from severe preeclampsia. For blood collection, ethylenediamine tetraacetic acid (EDTA) was used as anticoagulant. Plasma sample was then extracted after centrifugation, aliquot and stored at −70C until analyzed. Maternal subcutaneous fat tissue was collected during cesarean section delivery from 12 pregnant women, 6 from normotensive and 6 from preeclampsia. Freshly obtained subcutaneous fat tissue was fixed with 10% formalin and then embedded with paraffin. Normotensive pregnancy is defined as pregnancy with maternal blood pressure <140/90mmHg, absence of proteinuria and medical complications. Diagnosis of chronic hypertension in pregnancy is defined as either a documented history of high blood pressure antedating pregnancy or persistent elevation of blood pressure (≥ 140/90 mmHg) on two occasions more than 24 hours apart before the 20th week of gestation. Mild preeclampsia is defined as follows: sustained systolic blood pressure ≥ 140 mmHg or a sustained diastolic blood pressure of ≥ 90mmHg on two separate readings; proteinuria measurement of 1+ or more on dipstick, or 24 hour urine protein collection with ≥ 300mg in the specimen (after 20 weeks of gestation). Severe preeclampsia is defined when one or more of the following criteria is present: maternal blood pressure ≥ 160mmHg or a sustained diastolic blood pressure ≥ 110mmHg on two separate readings at least six hours apart; proteinuria ≥ 3+ on dipstick or ≥ 2 gram/24 hours after 20 weeks of gestation; persistent headache or other cerebral or visual disturbances; persistent epigastric pain; or serum creatinine >1.2mg/dl. Smokers were excluded. None of the study subjects had signs of infection. To avoid clinical phenotypic differences in preeclamptic patients, patients complicated with HELLP syndrome (hemolysis, elevated liver enzyme and low platelet count), diabetes and/or renal disease were excluded. Clinical characteristics of study subjects whose plasma sample was tested are presented in Table 1 and whose subcutaneous fat tissue was used are present in Table 2.
Table 1.
Demographic data of study subjects in which plasma samples were used to determine maternal concentrations for corin and pro-ANP
| Group | Normal n=84 |
CHT n=16 |
mPE n=11 |
sPE n=86 |
|
|---|---|---|---|---|---|
| Maternal age | 23 ± 5 | 28 ± 7** | 26 ± 8 | 24 ± 6* | |
| Racial status n (%) | Black | 66 (79) | 13 (81) | 9 (82) | 60(70) |
| White | 15 (18) | 2 (13) | 2 (18) | 23(27) | |
| Other | 2 (2) | 1 (6) | 0 | 2(2) | |
| Unknown | 1 (1) | 0 | 0 | 1(1) | |
| Nulliparous: n (%) | 40 (48) | 10 (63) | 8 (73) | 48(56) | |
| BMI@ | 32 ± 6 | 41 ± 11** | 43 ± 11** | 35 ± 9§§ | |
| BP Systolic | 125 ± 14 | 159 ± 16** | 151 ± 16** | 170 ± 17**§§# | |
| Diastolic | 73 ± 10 | 90 ± 15** | 89 ± 12** | 102 ± 10**§§## | |
| Gestational age (weeks+days) | |||||
| (at sample collection) | 34+2 ± 5+0 | 30+1 ± 7+2**§ | 35+6 ± 3+4 | 32+6 ± 3+4 | |
| (at delivery) | 38+5 ± 2+3 | 35+5 ± 5+0## | 37+3 ± 2+1 | 32+6 ± 3+5**§§ | |
| Delivery mode: n (%) | |||||
| Vaginal delivery | 57 (68) | 6 (37.5) | 5 (45) | 32(37) | |
| C-section | 27 (32) | 10 (62.5) | 6 (55) | 54(63) | |
Data are expressed as Mean ± SD.
Maternal age: *p<0.05: sPE vs. CHT, **p<0.01: CHT vs. normal;
: BMI at enrollment; ** p<0.01: CHT and mPE, vs. Normal; §§ p<0.01: sPE vs. CHT and mPE;
BP systolic: ** p<0.01: CHT, mPE, and sPE vs. normal; §§ p<0.01: sPE vs. mPE; # p<0.05: sPE vs. CHT;
BP diastolic: ** p<0.01: CHT, mPE, and sPE vs. normal; §§ p<0.01: sPE vs. mPE; ## p<0.01: sPE vs. CHT;
Gestational age at sample collection: ** p<0.01: CHT vs. normal; § p<0.05: CHT vs. mPE and sPE;
Gestational age at delivery: ** p<0.01: sPE vs. normal and mPE; §§ p<0.01: sPE vs. CHT; ## p<0.01: CHT vs. normal.
Table 2.
Clinical information of study subjects from which maternal vessels were examined in the study
| Variables | Normotensive (n=6) | Preeclampsia (n=6) | p value |
|---|---|---|---|
| Maternal age: years | 24 ± 4 (19–28) | 30 ± 6 (23–38) | 0.055 |
| Racial Status | |||
| White | 1 | 3 | --- |
| Black | 4 | 3 | --- |
| Other | 1 | 0 | --- |
| BMI | 30 ± 4 (24–34) | 31 ± 6 (27–39) | 0.831 |
| Blood Pressure: mmHg | |||
| Systolic | 119 ± 6 (111–128) | 174 ± 14 (160–193) | 0.009 |
| Diastolic | 73 ± 6 (67–83) | 102 ± 8 (93–110) | 0.009 |
| Gestational Age at delivery (weeks) | 34 ± 2 (32–37) | 30 ± 2 (28–33) | 0.016 |
| Primigravida: n (%) | 3 (50) | 3 (50) | --- |
Data are expressed as Mean ± SD (range).
Measurement of plasma concentrations of corin and pro-ANP
Maternal plasma concentrations of corin and stable N-terminal prohormone forms of ANP (pro-ANP) were determined by enzyme-linked immunosorbent assay (ELISA). DuoSet ELISA development kits for human corin (DY2209) and human pro-ANP (DY8247) were purchased from R&D Systems, Inc. (Minneapolis, MN). All assays were carried out according to the manufacturer’s instruction. The range of the standard curve for corin was 15.6 to 4,000 pg/ml and the range of the standard curve for pro-ANP was 15.6 to 2,000 pg/ml. Plasma sample was 1:2 diluted for corin assay and assayed directly for pro-ANP. All samples were tested in duplicate and blinded to outcome. Within assay variations were <7% for both assays.
Immunohistochemistry
Expression of ANP and its receptors NPR-A, NPR-B and NPR-C were examined using paraffin embedded subcutaneous fat tissue sections and placental villous tissue sections by a standard immunohistochemistry staining procedure. Antibody for ANP (AF3366) was obtained from R&D system (Minneapolis, MN), antibodies for NPR-A (sc-137041) and NPR-B (sc-293451) were obtained from Santa Cruz Biotechnology (San Diego, CA), and antibody for NPR-C (ab37617) was purchased from Abcam (Cambridge, MA), respectively. The antibody concentration used for the experiment was recommended by the manufacturers: 2μg/ml for ANP, 4μg/ml for both NPR-A and NPR-B, and 20μg/ml NPR-C, respectively. Stained slides were counterstained with Gill’s formulation hematoxylin. Tissue sections stained with isotype IgG or secondary antibody only served as negative control. Slides stained with the same antibody were processed at the same time. Stained slides were reviewed under an Olympus microscope (Olympus IX71, Japan), and images were captured by a digital camera and recorded into a microscope-linked PC computer.
Data analysis
Clinical demographic data is presented as mean ± SD. Data for plasma corin and pro-ANP concentrations were presented as mean ± SD ng/ml. Statistical analysis was performed with ANOVA or un-paired test by computer software Prism 5 (GraphPad Software, Inc. La Jolla, CA). Tukey’s test was used as a post hoc test. A probability level less than 0.05 was considered statistically significant.
Results
Patient Clinical Data
Patient clinical information including maternal age, racial status, gravida, body mass index (BMI), blood pressure, and gestational age at blood draw and delivery and delivery mode was obtained by chart review. The clinical characteristics for subjects whose plasma sample was assayed for corin and pro-ANP are shown in Table 1. Maternal age was higher in chronic hypertensive group than in normotensive pregnant group, but not different between mild or severe preeclamptic group vs. normotensive group. BMI was significantly higher in the chronic hypertensive and mild preeclamptic groups vs. normotensive and severe preeclamptic groups, but not different between severe preeclamptic vs. normotensive groups. Blood pressure was significantly higher in chronic hypertension, mild and severe preeclamptic groups vs. normotensive pregnant group. The demographic data for subjects whose subcutaneous fat tissue was used to determine ANP, NPR-A, NPR-B, and NPR-C expression are shown in Table 2.
Elevated maternal plasma levels of corin and pro-ANP in severe preeclampsia
Maternal plasma concentrations for corin and pro-ANP in normotensive pregnant women, pregnant women complicated with chronic hypertension, mild preeclampsia and severe preeclampsia are shown in Table 3. Maternal corin concentrations were significantly higher in mild preeclampsia (* p<0.05) and severe preeclampsia (** p<0.01) vs. normotensive pregnant women and pregnancy complicated with chronic hypertension. Maternal pro-ANP concentrations were significantly higher in pregnancy complicated with chronic hypertension (* p<0.05) and severe preeclampsia (** p<0.01) than in normotensive pregnant women. Pro-ANP concentrations were also significantly higher in severe preeclampsia than in mild preeclampsia (# p<0.05).
Table 3.
Maternal plasma concentrations for corin and pro-ANP
| Normotensive n=84 |
CHT n=16 |
mPE n=11 |
sPE n=86 |
|
|---|---|---|---|---|
| Corin (ng/ml) | 1.58 ± 0.70 | 1.55 ± 0.79 | 2.78 ± 2.23* | 2.53 ± 1.75**^ |
| (range) | (0.66 – 4.42) | (0.69 – 3.27) | (1.08 – 6.95) | (0.85 – 10.67) |
| Pro-ANP (ng/ml) | 0.49 ± 0.53 | 1.59 ± 2.11* | 0.52 ± 0.29 | 1.42 ± 2.12**# |
| (range) | (0.09 – 2.73) | (0.13 – 8.53) | (0.10 – 1.07) | (0.15 – 15.59) |
Data are expressed as mean ± SD (range).
Corin: * p<0.05 and ** p<0.01: mPE vs. normotensive or sPE vs. normotensive; ^ p<0.05: sPE vs. CHT.
Pro-ANP: * p<0.05 and ** p<0.01: CHT vs. normotensive or sPE vs. normotensive; # p<0.05: sPE vs. mPE.
Expression of ANP and NPR-C in maternal vasculature
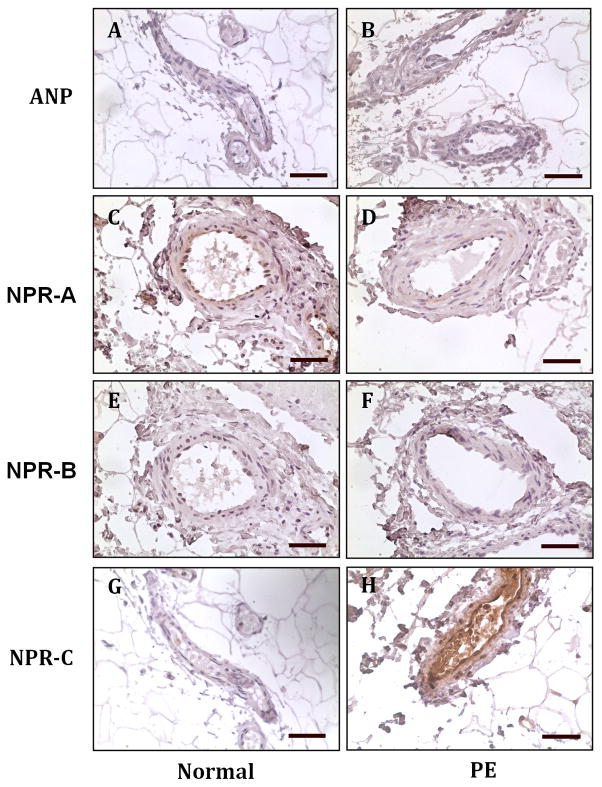
We examined maternal vascular expression of ANP and its receptors NPR-A, NPR-B, and NPR-C in subcutaneous fat tissue sections to determine if altered ANP and its receptor signaling were present in maternal vasculature in preeclampsia. Our results showed that ANP expression was not detectable in maternal vessels from normotensive and preeclamptic pregnant women (Figure 1, A and B). However, different patterns for its receptor expression were noticed in maternal vessels between normotensive and preeclamptic pregnant women. NPR-A expression was detected in endothelium of maternal vessels from normotensive (Figure 1, C), but not from preeclamptic (Figure 1, D), pregnant women. NPR-B expression was undetectable in maternal vessels from both normotensive and preeclamptic pregnancies (Figure 1, E and F). However, strong NPR-C expression was detected in endothelium of maternal vessels from preeclamptic (Figure 1, H), but not from normotensive (Figure 1, G), pregnancies.
Figure 1.
A: Representative image of maternal vessel endothelium expression for ANP and its receptors NPR-A, NPR-B and NPR-C in normotensive and preeclamptic pregnancies. A, C, E, and G: normotensive pregnancy; B, D, F, and H: preeclampsia. A and B: ANP; C and D: NPR-A; E and F: NPR-B, and G and H: NPR-C. ANP and NPR-B expression was undetectable in maternal vessels from both normotensive and preeclamptic pregnancies. NPR-A expression was detected in endothelium of maternal vessels from normotensive, but not preeclamptic, pregnancies. In contrast, NPR-C expression was detected vessel endothelium in preeclamptic, but not in normotensive, pregnancies. Bar = 50micron.
Discussion
Recent studies found that maternal corin levels are significantly higher in women with preeclampsia, therefore considered corin as a new biomarker for this hypertensive pregnancy disorder [10–12]. In our study, we found that not only maternal corin but also maternal pro-ANP levels were significantly elevated in women complicated with severe preeclampsia. In addition, our results also revealed that NPR-A expression was downregulated and NPR-C expression was upregulated in maternal vessel endothelium in preeclampsia compared to that in normotensive pregnant controls. These results suggest that pro-ANP/corin/NPR signaling pathway is altered in maternal systemic vasculature in women with preeclampsia.
Corin is a serine protease and it cleaves pro-ANP into N-terminal ANP and ANP, the latter one binds to its receptors and produces downstream biological effects on targeting cells/organs or is rapidly removed from the circulation [13] by binding to its receptor and cleared out through kidneys. The soluble corin proteolytic activity in vivo is still elusive, but an in vitro study suggests that both active and inactive fragments of corin are generated either by the action of metalloprotease or by corin autocleavage [14]. Corin has three soluble fragments with 180, 160, and 100kDa, respectively. Using protease inhibitors, ionomycin, phorbol myristate acetate stimulation, as well as small interfering RNA knockdown and site-directed mutagenesis, Jiang et al demonstrated that a disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) was primarily responsible for shedding corin at its juxtamembrane region to release the 180kDa fragment, corresponding to the near-entire extracellular region [14]. In contrast, the 160 and 100kDa fragments were from corin autocleavage at Arg-164 in frizzled 1 domain and Arg-427 in LDL receptor 5 domain of the extracellular region, respectively [14]. The source(s) of increased maternal corin levels in preeclampsia have not been defined. Other than the heart, which is the major source of corin in response to high blood pressure [15], placenta might contribute to the increased maternal corin levels, since strong corin expression was detected in syncytiotrophoblasts [11] and the key corin shedding enzyme ADAM10 expression was upregulated in placental syncytiotrophoblasts in preeclampsia [16].
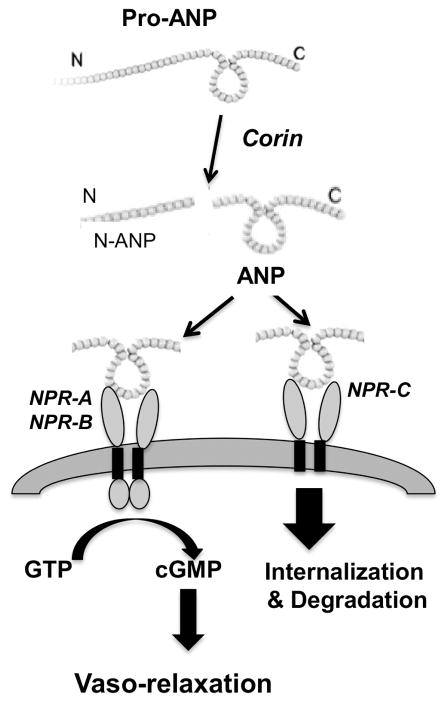
Natriuretic peptide family has three types of transmembrane receptors, NPR-A, NPR-B, and NPR-C, which regulate ANP activity. As shown in Figure 2, NPR-A and NPR-B are guanylyl cyclase receptors. Activation of NPR-A or -B would stimulate intracellular guanylate cyclase activity to convert GTP to cGMP. cGMP stimulates cGMP-dependent protein kinase (PKG), which could then induce smooth muscle relaxation. On vasculature, by binding to NPR-A or -B, ANP leads to an increase in cGMP production and produces relaxation effect. On kidney, by binding to NPR-A or -B, ANP possesses diuretic and natriuretic properties, resulting from the direct inhibition of sodium absorption in the renal collecting duct. Therefore, ANP decreases blood pressure, modulates endothelial permeability, and plays important roles in the regulation of volume and pressure homeostasis in the circulatory system. In contrast, NPR-C is a non-guanylyl cyclase receptor and it is coupled to adenylyl cyclase inhibition or phospholipase C activation through inhibition of guanine nucleotide regulatory protein (Gi) [17]. Circulating ANP is subjected to rapid clearance by NPR-C-mediated mechanism and proteolytic degradation by neutral endopeptidase. Moreover, NPR-C is the most abundantly expressed ANP receptor and is widely distributed in various cell types including platelets and vascular smooth muscle cells (VSMCs) [18]. In mice, knockout of Npr3 (NPR-C gene) leads to hypotension and skeletal deformities due to rapid bone turnover [19]. Therefore, NPR-C is not only considered a clearance receptor for ANP, but also involved in cellular proliferation, migration, and vascular remodeling. In humans, upregulation of NPR-C expression was reported in the intima of advanced carotid artery plaques in atherosclerosis patients [20]. In our study, we found that NPR-A expression was downregulated and NPR-C expression was upregulated in maternal vessel endothelium in women with preeclampsia. In contrast, NPR-B was not present in vessel endothelium. This notion is supported by our observation of positive expression of NPR-A and negative expression of NPR-B in placental villous core endothelium (data not shown). Although the exact mechanism of downregulation of NPR-A expression and upregulation of NPR-C expression is not known, since NPR-C is considered a clearance receptor for circulating ANP, increased NPR-C expression suggests that circulating ANP clearance/degradation process is increased in this pregnancy disorder.
Figure 2.
ANP and corin signaling pathway. Corin cleavages pro-ANP into N-ANP and ANP. ANP binds to NPR-A/-B leading to increase in production of cGMP. ANP binds to NPR-C with high affinity facilitating its clearance from circulation through receptor-mediated internalization and degradation.
Human corin gene is located on chromosome 4p12-p13, has 22 exons and spans 244 kb [21]. Reduce corin activity associated with corin gene variants and mutations was identified in patients with hypertension and heart diseases [22]. Whether increased maternal corin levels are associated with reduced corin activity in preeclampsia is elusive, but two CORIN gene mutations were reported in preeclamptic patients, one mutation changed amino acid Lys317 to Glu in LDLR2 domain and another changed amino acid Ser472 to Gly in frizzled 2 domain [23]. Since both LDLR2 and frizzled 2 domains are important for corin to process pro-ANP [24], mutation of Lys317Glu and Ser472Gly might reduce pro-ANP processing activity [6] and result in higher plasma levels of pro-ANP in preeclampsia. In addition, two corin polymorphisms (C for rs2271036 and G for rs2271037) were also reported in patients with preeclampsia [25]. Although we did not measure corin activity in the present study, increased both pro-ANP and corin levels combined with increased endothelial NPR-C expression clearly indicate that altered pro-ANP/corin/NPR signaling is present in the vascular system in preeclampsia.
In this study, we also noticed that pro-ANP, but not corin, levels were elevated in pregnant women complicated with chronic hypertension. Although the sample size is small for the chronic hypertension group and little is known about corin and ANP regulation in patients with chronic hypertension during pregnancy, the phenomenon of differences in maternal pro-ANP and corin levels between pregnancy complicated with chronic hypertension and preeclampsia may represent a diverse regulatory mechanism related to the pathophysiological process in the systemic vasculature in patients between chronic hypertension and preeclampsia.
Taken together, in the present study we found that maternal levels of corin and pro-ANP levels and maternal vascular endothelial expression of NPR-C were significantly increased in women with preeclampsia. ANP is a vasodilator. In contrast, endothelial NPR-A expression was reduced in preeclampsia. Although it is not clear if increased corin levels are a compensative mechanism to offset increased vasoconstriction in preeclampsia, upregulation of NPR-C expression in maternal vessel endothelium in preeclampsia implies that endothelial activity for clearance/degradation of ANP is altered in this pregnancy disorder. Nonetheless, our data of increased maternal plasma pro-ANP and corin levels together with downregulation of NPR-A expression and upregulation of NPR-C expression in maternal vascular endothelium in preeclampsia clearly support the notion that aberrant ANP/NPR pathway signaling contributes to vascular dysfunction in preeclampsia. Whether altered pro-ANP/corin/NPR signaling also occurs in the placental vasculature in preeclampsia warrant further investigation.
Highlights.
Maternal levels of pro-ANP and its converting enzyme corin are elevated in preeclampsia.
Upregulation of NPR-C expression suggests increased ANP clearance/degradation in preeclampsia.
Aberrant pro-ANP/corin/NPR signaling may contribute to vascular dysfunction in preeclampsia.
Acknowledgments
This study was supported in part by grant from the Eunice Kennedy Shriver National Institute Child Health and Human Development (NICHD) R21HD076289 to YW.
Abbreviations
- pro-ANP
pro-atrial natriuretic peptide
- ANP
atrial natriuretic peptide
- NPR
natriuretic peptide receptor
- CHT
chronic hypertension
- ELISA
enzyme-linked immunosorbent assay
- BMI
body mass index
- GTP
guanosine triphosphate
- cGMP
cyclic guanosine monophosphate
- VSMCs
vascular smooth muscle cells
Footnotes
Conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yan W, Sheng N, Seto M, Morser J, Wu Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J Biol Chem. 1999;274:14926–14935. doi: 10.1074/jbc.274.21.14926. [DOI] [PubMed] [Google Scholar]
- 2.Armaly Z, Assady S, Abassi Z. Corin: a new player in the regulation of salt-water balance and blood pressure. Curr Opin Nephrol Hypertens. 2013;22:713–722. doi: 10.1097/01.mnh.0000435609.35789.32. [DOI] [PubMed] [Google Scholar]
- 3.Dong N, Chen S, Yang J, et al. Plasma soluble corin in patients with heart failure. Circ Heart Fail. 2010;3:207–211. doi: 10.1161/CIRCHEARTFAILURE.109.903849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polzin D, Kaminski HJ, Kastner C, et al. Decreased renal corin expression contributes to sodium retention in proteinuric kidney diseases. Kidney Int. 2010;78:650–659. doi: 10.1038/ki.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan JC, Knudson O, Wu F, Morser J, Dole WP, Wu Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc Natl Acad Sci U S A. 2005;102:785–790. doi: 10.1073/pnas.0407234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui Y, Wang W, Dong N, et al. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature. 2012;484:246–250. doi: 10.1038/nature10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaitu’u-Lino TJ, Ye L, Tuohey L, et al. Corin, an enzyme with a putative role in spiral artery remodeling, is up-regulated in late secretory endometrium and first trimester decidua. Hum Reprod. 2013;28:1172–1180. doi: 10.1093/humrep/det028. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Y, Wu Q. Role of corin and atrial natriuretic peptide in preeclampsia. Placenta. 2013;34:89–94. doi: 10.1016/j.placenta.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adam B, Malatyalioğlu E, Alvur M, Kökçü A, Bedir A. Plasma Atrial Natriuretic Peptide Levels in Preeclampsia and Eclampsia. J Matern Fetal Investig. 1998;8:85–88. [PubMed] [Google Scholar]
- 10.Duvekot JJ, Roeters van Lennep JE. Searching for new biomarkers for preeclampsia: is there a role for corin? J Womens Health (Larchmt) 2015;24:546–547. doi: 10.1089/jwh.2015.28999.jjd. [DOI] [PubMed] [Google Scholar]
- 11.Miyazaki J, Nishizawa H, Kambayashi A, et al. Increased levels of soluble corin in pre-eclampsia and fetal growth restriction. Placenta. 2016;48:20–25. doi: 10.1016/j.placenta.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Jadli A, Sharma N, Damania K, Satoskar P, Bansal V, Ghosh K, Shetty S. Promising prognostic markers of preeclampsia: new avenues in waiting. Thromb Res. 2015;136:189–195. doi: 10.1016/j.thromres.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Cusson JR, Thibault G, Kuchel O, Hamet P, Cantin M, Larochelle P. Cardiovascular, renal and endocrine responses to low doses of atrial natriuretic factor in mild essential hypertension. J Hum Hypertens. 1989;3:89–96. [PubMed] [Google Scholar]
- 14.Jiang J, Wu S, Wang W, Chen S, Peng J, Zhang X, Wu Q. Ectodomain shedding and autocleavage of the cardiac membrane protease corin. J Biol Chem. 2011;286:10066–10072. doi: 10.1074/jbc.M110.185082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen S, Sen S, Young D, Wang W, Moravec CS, Wu Q. Protease corin expression and activity in failing hearts. Am J Physiol Heart Circ Physiol. 2010;299:H1687–H1692. doi: 10.1152/ajpheart.00399.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao S, Gu Y, Fan R, Groome LJ, Cooper D, Wang Y. Proteases and sFlt-1 release in the human placenta. Placenta. 2010;31:512–218. doi: 10.1016/j.placenta.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anand-Srivastava MB. Natriuretic peptide receptor-C signaling and regulation. Peptides. 2005;26:1044–1059. doi: 10.1016/j.peptides.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 18.Rubattu S, Sciarretta S, Morriello A, Calvieri C, Battistoni A, Volpe M. NPR-C: a component of the natriuretic peptide family with implications in human diseases. J Mol Med (Berl) 2010;88:889–897. doi: 10.1007/s00109-010-0641-2. [DOI] [PubMed] [Google Scholar]
- 19.Chusho H, Tamura N, Ogawa Y, et al. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci U S A. 2001;98:4016–4021. doi: 10.1073/pnas.071389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zayed MA, Harring SD, Abendschein DR, Vemuri C, Lu D, Detering L, Liu Y, Woodard PK. Natriuretic Peptide Receptor-C is Up-Regulated in the Intima of Advanced Carotid Artery Atherosclerosis. J Med Surg Pathol. 2016;1:131. doi: 10.4172/2472-4971.1000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan J, Hinzmann B, Yan W, Wu F, Morser J, Wu Q. Genomic structures of the human and murine corin genes and functional GATA elements in their promoters. J Biol Chem. 2002;277:38390–38398. doi: 10.1074/jbc.M205686200. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Li H, Zhou J, et al. A corin variant identified in hypertensive patients that alters cytoplasmic tail and reduces cell surface expression and activity. Sci Rep. 2014;4(7378) doi: 10.1038/srep07378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong N, Zhou T, Zhang Y, et al. Corin mutations K317E and S472G from preeclamptic patients alter zymogen activation and cell surface targeting. J Biol Chem. 2014;289:17909–17916. doi: 10.1074/jbc.M114.551424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knappe S, Wu F, Madlansacay MR, Wu Q. Identification of domain structures in the propeptide of corin essential for the processing of proatrial natriuretic peptide. J Biol Chem. 2004;279:34464–34471. doi: 10.1074/jbc.M405041200. [DOI] [PubMed] [Google Scholar]
- 25.Stepanian A, Alcaïs A, de Prost D, et al. Highly significant association between two common single nucleotide polymorphisms in CORIN gene and preeclampsia in Caucasian women. PLoS One. 2014;9:e113176. doi: 10.1371/journal.pone.0113176. [DOI] [PMC free article] [PubMed] [Google Scholar]