Abstract
Considerable advances in our understanding of the mechanisms and functions of rapid-eye-movement (REM) sleep have occurred over the past decade. Much of this progress can be attributed to the development of new neuroscience tools that have enabled high-precision interrogation of brain circuitry linked with REM sleep control, in turn revealing how REM sleep mechanisms themselves impact processes such as sensorimotor function. This review is intended to update the general scientific community about the recent mechanistic, functional and conceptual developments in our current understanding of REM sleep biology and pathobiology. Specifically, this review outlines the historical origins of the discovery of REM sleep, the diversity of REM sleep expression across and within species, the potential functions of REM sleep (e.g., memory consolidation), the neural circuits that control REM sleep, and how dysfunction of REM sleep mechanisms underlie debilitating sleep disorders such as REM sleep behaviour disorder and narcolepsy.
Introduction
Although the first description of rapid-eye-movement (REM) sleep was met with skepticism and indifference, the initial report is now widely viewed as a true watershed moment in science, having forever changed the way scientists and the medical community view the sleeping brain. While historical accounts vary as to where true credit for this discovery might reside, the discovery of REM sleep links most strongly to Eugene Aserinsky, Nathaniel Kleitman, William Dement and Michel Jouvet. In 1953, Kleitman and Aserinsky showed in human infants that periods of ‘active’ sleep were marked by rapid eye movements and that these active sleep periods alternated with quiescent sleep periods [1]. Several years later Kleitman and Dement showed that these rapid eye movements in human adults were correlated with specific brain-wave patterns and that periods of dreaming occurred during periods of sleep with rapid-eye-movements [2,3]. Shortly after this, Jouvet identified REM sleep in cats, showing that, like humans, cats experience periods of rapid-eye-movements that occur in tandem with loss of muscle tone, muscle twitches and wake-like cortical activity [4,5].
While the behavioural state of REM sleep was initially distinguished on the basis of the rapid-eye-movements produced by bursting of oculomotor muscles, we now know that REM sleep is also characterized (and hence differentiated from wake and non-REM sleep) by other defining physiologic and behavioral features, including a reduced amplitude and faster frequency cortical electroencephalogram (EEG) that is reminiscent of waking, high-amplitude theta waves in the hippocampal EEG, active suppression of skeletal muscle activity, intermittent muscle twitches, autonomic and respiratory activation, fluctuations in brain/body temperature, and an elevated arousal threshold [6]. Because REM sleep is marked by a waking-like EEG pattern coupled with skeletal motor atonia, some scientists prefer the terms ‘active sleep’ or ‘paradoxical sleep’ when referring to this behavioral state [7–9]. In healthy humans, REM sleep alternates with, is preceded by, and occupies a significantly smaller fraction of our nightly sleep time than non-REM sleep (also termed slow-wave sleep) [6]. While long viewed as an enigmatic behavioural state, work over the past several decades has begun to inform our understanding of the biological functions of REM sleep [10,11] and has provided us with considerable insights into the anatomic, circuit and synaptic bases by which it is regulated [12–16]. For example, we now know that hippocampal neural activity during REM sleep is critically involved in memory consolidation [17], and that a distributed network of brain circuits and a myriad of neuromodulators regulates REM sleep timing, duration and its hallmark features [12,13,18–20].
REM Sleep Across Species
REM sleep — as operationally defined by the foregoing electro-physiological and behavioral signs (see introduction) — has been identified in terrestrial mammals [21,22], birds [23–25], reptiles (e.g., bearded dragons) [26] and some aquatic invertebrates (e.g., cuttlefish) [27], suggesting that this behavioral state is conserved across the animal kingdom. Possible exceptions include insects and nematodes, although there is some anecdotal evidence that even these animals may exhibit some of the classical features of mammalian REM sleep such as REM sleep-like muscle twitches [28].
Daily amounts of REM sleep vary considerably across species. For example, horses, giraffes and elephants spend very little time (if any) in REM sleep each day (e.g., <1 hour) [29–31], whereas ferrets, platypuses and house cats can spend 3 to 8 hours per day in REM sleep [32–37]. REM sleep expression patterns also differ across animal species. For example, in mice, periods of REM sleep occur roughly every 10–15 minutes [38], whereas, in humans, REM sleep periods occur every 90–120 minutes [39,40]. Why REM sleep amounts and expression differ between animals remains unclear. But, there is evidence that the length of the REM sleep cycle (i.e., the time between the start of an REM sleep period to the start of the next) scales with brain or body mass [41], which might suggest that small animals need more frequent periods of REM sleep than larger ones. However, the marked variation in REM sleep amounts across species has also been used to argue that REM sleep does not function to facilitate learning and memory processing given that some intelligent animals (e.g., elephants) spend very little time (if any) engaged in REM sleep [21,42].
Daily amounts of REM sleep are also labile even within a single species. REM sleep amounts depend on an animal's developmental age and can expand and contract as a function of environmental and ecological pressures. For example, newborn mammals, especially developmentally immature (i.e., altricial) ones, spend the majority of their early lives in REM sleep, with the amount of REM sleep declining and eventually plateauing at developmental maturity [43–45]. Northern fur-seals exhibit REM sleep while sleeping on land, but rarely when at sea [46], and elephants can go for days without sleeping under certain circumstances [30]. In addition, birds can effectively suspend sleep for days and weeks while migrating or flying out to sea, and some birds will suppress sleep (including REM sleep) in order to mate and take care of their hatchlings [47–50].
The physiological and behavioral markers of REM sleep also differ between animals. For example, the platypus and ostrich do not exhibit the classic EEG signatures of REM sleep; rather, they combine features of both REM and non-REM sleep into a single sleep-like state [24,34]. The platypus and ostrich both exhibit muscle atonia and twitches as well as rapid-eye-movements, but the appearance of these classic REM sleep features is concurrent with a non-REM sleep-like EEG (i.e., moderate or high in voltage) [24,34]. In contrast, monotremes (e.g., echidna) do not exhibit most of the classic features of REM sleep (i.e., reduced EEG amplitude, rapid-eye-movements, muscle twitches and atonia), but they do exhibit a pattern of cellular brainstem activity similar to that associated with REM sleep in cats, rats and mice [51]. Such findings suggest that REM sleep may manifest in different ways in different animals.
While all terrestrial mammals appear to exhibit some form of REM sleep, this may not hold true for some sea mammals. For example, dolphins and whales show little to no signs of REM sleep [52,53], but there is evidence that whales exhibit muscle jerks during periods of immobility and this could represent a form of REM sleep [54]. It nevertheless remains possible that these sea mammals may be similar to the echidna in that they do not exhibit the classic behavioural features of REM sleep, but may still exhibit patterns of ‘REM-like’ cellular activity in the brainstem [51].
Because the majority of animals studied to date exhibit some form of REM sleep, it seems reasonable to hypothesize that this behavioural state subserves an important biological purpose(s). Yet it remains unclear what that purpose is or whether REM sleep serves the same function(s) in all animals. What, however, is much more clear is that the brain circuits that generate REM sleep are generally conserved across species. Below, we consider the potential functions of REM sleep and discuss the neural mechanisms that produce this behavioural state.
Rem Sleep Function
The functional role of REM sleep in normal biology remains an open question as well as one of the more intriguing mysteries in science. Indeed, Science magazine identified how and why we dream during REM sleep as one of the most important and conspicuous knowledge gaps in science [55]. While the biological and neuropsychiatric function of dreams and dream mentation remains unclear, considerable insights into the biological and physiological functions of REM sleep have nevertheless been gleaned.
The observation that most newborn mammals spend a majority of their early lives in an REM sleep-like state (termed active sleep in newborns because they lack the cortical EEG features of adult REM sleep), inspired the hypothesis that REM sleep (or a REM sleep-specific behaviour) is important for brain development [44,56]. This concept is supported by the fact that altricial mammals, which have relatively immature and underdeveloped brains, spend longer periods in REM sleep than precocial animals do [56–59].
In immature animals, REM sleep is dominated by flurries of muscle twitches, with some estimates that newborn mammals experience tens of thousands of REM sleep-specific muscle twitches each day [44,60]. This observation has led some researchers to suggest that REM sleep twitches may function to engage brain development, and in particular motor learning, which is relatively underdeveloped at birth, in particular for altricial animals [61–63].
Muscle twitches were long viewed as functionless features of REM sleep [62,64,65]. But, recent research has raised the interesting possibility that twitches are a distinct class of movement that may function to aid sensorimotor system development. Unlike waking movements, REM sleep twitches occur against a background of muscle atonia, thereby allowing the central nervous system to more effectively monitor the specific origins of each muscle twitch on the basis of a greater signal-to-noise ratio [62,64,65]. Indeed, multiple brain regions are activated by muscle twitches during REM sleep; notably, these same regions are not activated by motor activity during wakefulness. For example, the hippocampus, cerebellar cortex and red nucleus are all activated by REM sleep twitches [61,63,66–69], providing evidence that REM sleep twitches contribute to activity-dependent development of the sensorimotor system.
One of the most widely supported ideas in sleep science is that REM sleep functions to facilitate the formation and consolidation of certain types of memory [10,70–72], although this notion is not universally upheld [21,42,73,74]. Numerous studies in rodents and humans have shown, for example, that REM sleep deprivation impairs formation (or expression) of spatial and emotional memories. A major and persisting critique of these types of studies is the fact that REM sleep deprivation typically impacts non-REM sleep [75], and that some of the methodologies employed to restrict REM sleep are inherently stressful [76], making it difficult to firmly establish a causal role for REM sleep per se in memory formation.
In an effort to resolve this controversy, Boyce and colleagues [17] took advantage of new optogenetic tools to temporally silence GABA cells in the medial septum that drive hippocampal theta activity, in turn permitting specific attenuation of the memory-associated theta rhythm during REM sleep without disturbing sleeping behaviour (Figure 1). They found that selectively silencing GABA neurons during REM sleep erased subsequent novel object place recognition and impaired fear-conditioned contextual memory. Silencing these same neurons outside of REM sleep episodes was without any detectable effect on memory processing, providing evidence that activity of GABA neurons in the medial septum during REM sleep is crucial for theta activity in the hippocampus, and subsequent memory consolidation.
Figure 1. Theta activity during REM sleep is associated with learning and memory consolidation.
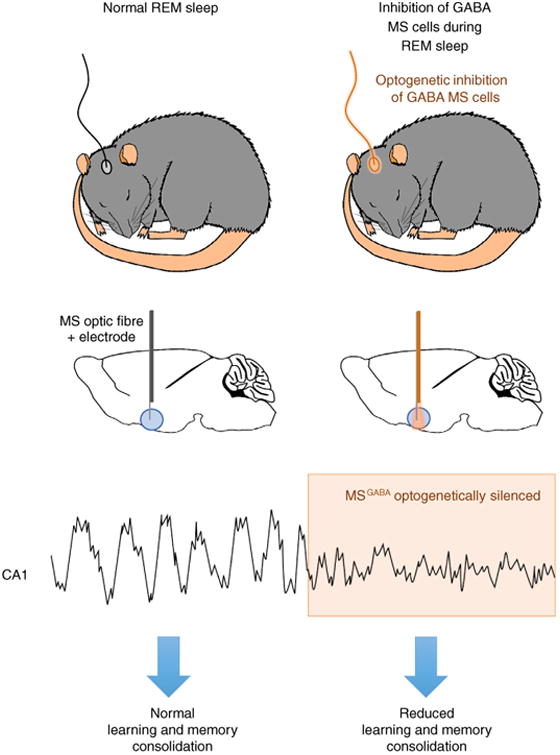
A schematic diagram demonstrating how optogenetic inhibition of GABA cells in the medial septum (MS) of mice affects acquisition of new memory associations. Top two figures show that naturally sleeping mice were fitted with optic fibers targeted above the MS. Delivery of orange laser light to the MS during REM sleep periods allowed for high precision optical inhibition of GABA cells in the MS since these cells expressed the inhibitory opsin archaerhodopsin (ArchT). The panel below the top two shows a sample recording of how optical inhibition of GABA inhibition during REM sleep impacts theta activity. Upon light-induced optogenetic inhibition (orange shading), a rapid reduction in amplitude of hippocampal theta rhythm is observed. REM sleep-specific inhibition of theta activity results in a reduction in learning and memory consolidation.
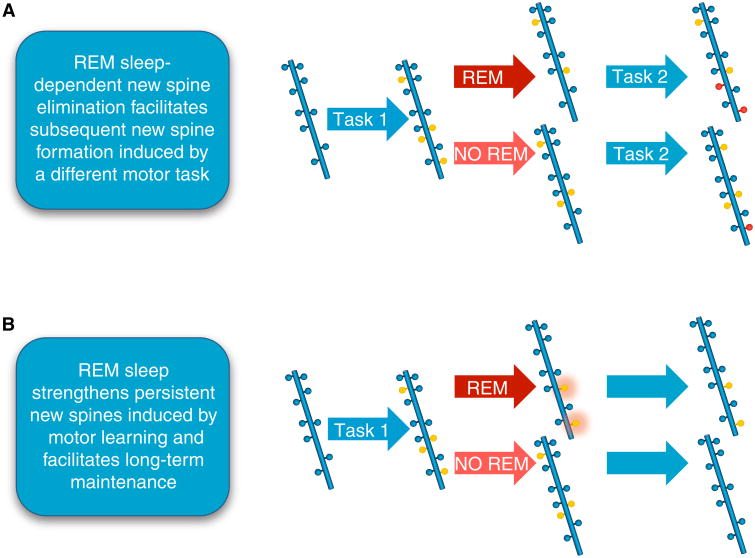
Intriguing new data also suggest that REM sleep may function to facilitate learning and memory by regulating neuronal synapses. Li and colleagues [77] showed that REM sleep appears to selectively prune and maintain new synapses associated with particular types of motor learning (Figure 2). Specifically, they found that REM sleep prunes newly formed postsynaptic dendritic spines on pyramidal neurons in the motor cortex after a new motor task is learned, and that REM sleep also strengthens and maintains these newly formed spines. They also showed that REM sleep-related calcium spikes within dendrites are important for pruning and strengthening new spines, suggesting that REM sleep facilitates learning and memory consolidation by selectively eliminating and maintaining new synapses by calcium spike-dependent mechanisms.
Figure 2. REM sleep facilitates learning and memory by regulating neuronal synapses.
A schematic diagram demonstrating that REM sleep strengthens persistent new spines induced by motor learning and facilitates long-term maintenance of synapses. (A) REM sleep prunes newly formed postsynaptic dendritic spines (yellow circles) of pyramidal neurons in the mouse motor cortex after motor learning (forward walking: Task 1). This REM sleep-dependent elimination of new spines facilitates subsequent spine formation (red circles) when a new motor task is learned (backward walking: Task 2), indicating a role for REM sleep in pruning to balance the number of new spines formed. (B) REM sleep also strengthens persistent new spines (orange halo around yellow circles) induced by motor learning and facilitates long-term maintenance, which are important for behavioral improvement after learning.
Several additional functions have also been ascribed to REM sleep, ranging from facilitating cortical plasticity [10,78] to restoration of aminergic cell/receptor function [79] and heightening general creativity [80,81]. It is worth noting that all of these proposals assume that REM sleep functions to restore some deficit incurred during prior wakefulness. Emerging evidence suggests, however, that REM sleep may also function to prepare for ensuing wakefulness by stimulating the central nervous system [64]. This idea derives from the facts that virtually all REM sleep periods are immediately followed by wakefulness [75], that the amount of time spent in REM sleep increases toward the end of the sleep period, and that humans and animals are more alert when woken from REM sleep than they are from non-REM sleep [82].
Recently, Brooks and Peever [64] found that the level of motor activity during individual REM periods reliably predicts the termination of REM sleep and the switch to wakefulness in rodents. Specifically, they found that levels of motor activity increase during REM sleep episodes and peak immediately before the transition from REM sleep to wakefulness, suggesting that motor activity during REM sleep may function to trigger and engage arousal from REM sleep into wakefulness. This observation aligns nicely with data showing that sensorimotor function is more intact following arousal from REM sleep as compared to arousal from non-REM sleep [82], and that animals are generally more alert when aroused from REM sleep than they are from non-REM sleep.
Brain Circuitry Controlling REM Sleep
The location of the brain circuitry governing REM sleep genesis was first investigated by Michel Jouvet, who during the course of his pioneering work identified a region in the dorsal pontine brainstem that appeared critical for the generation of muscle atonia during REM sleep [83]. Jouvet specifically found that lesions of this region in cats caused a perculiar state dissociation in which the animals appeared to ‘act out’ their dreams. Subsequent experimental work spanning several decades further refined the location of this ‘atonia generator’ to a region of the pons called the sublaterodorsal nucleus (SLD) in rodents (also termed the subcoeruleus region) [8,14,84–86] (Figure 3).
Figure 3. The circuit basis of REM sleep.
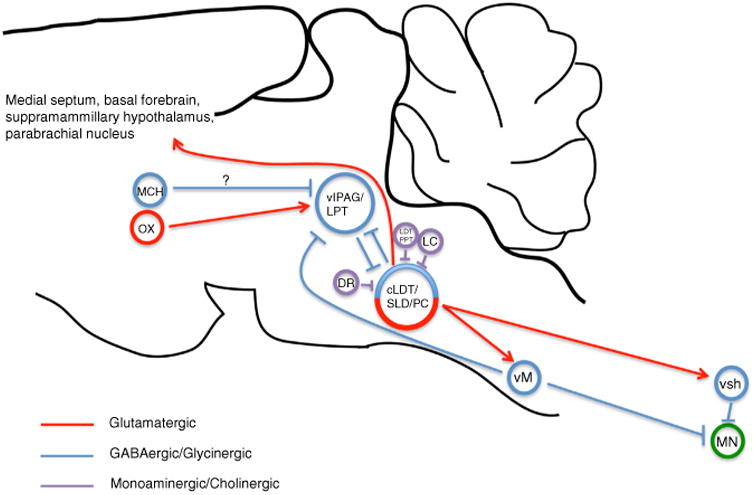
Together the caudal laterodorsal tegmental nucleus (cLDT), sublaterodorsal nucleus (SLD) and precoeruleus region (PC) comprise an executive pontine circuit element for REM sleep. REM-on glutamatergic neurons of the ventral SLD mediate REM motor atonia through direct synaptic activation of glycinergic interneurons of the spinal ventral horn (svh) as well as via GABAergic/glycinergic neurons of the ventral medial medulla (vM). vM neurons in turn project and inhibit motor neurons (MN) of the spinal ventral horn. REM-on GABAergic neurons of the SLD are reciprocally connected with REM-off GABAergic neurons of the ventrolateral periaqueductal gray and lateral pontine tegmentum (vlPAG/LPT), forming the basis of a pontine REM ‘flip-flop’ switch. GABAergic neurons of the vM can also drive REM sleep and do so by inhibiting REM-off vlPAG/LPT neurons. Lateral hypothalamic neurons containing orexin (OX) provide excitatory and stabilizing synaptic control over REM-off vlPAG/LPT neurons. REM-off noradrenergic locus coeruleus (LC) and serotoninergic dorsal raphe (DR) neurons provide additional synaptic control over SLD activity. Cholinergic laterodorsal tegmental and pedunculopontine tegmental (LDT/PPT) neurons may produce REM sleep through activation of REM-on SLD neurons. Lateral hypothalamic neurons containing melanin-concentrating hormone (MCH) also regulate REM sleep, possibly through direct inhibition of REM-off vlPAG/LPT neurons. Glutamatergic neurons of the SLD/PC regulate the corticohippocampal features of REM sleep, likely via projections to the medial septum, parabrachial nucleus, basal forebrain and supramamillary hypothalamus.
It is now well established that the ventral portion of the SLD contains a substantial population of spinally projecting neurons that function to produce the motor atonia of REM sleep. For example, work over the past two decades has shown that SLD neurons increase firing in anticipation of, and are maximally active in, REM sleep (i.e., REM-on cells), are glutamatergic, and produce motor atonia by activating inhibitory inter-neurons in both the ventral medulla (vM) and the spinal cord [14,60,87–89]. Selective disruption of glutamatergic transmission of SLD and dorsally adjacent caudal laterodorsal tegmental (cLDT) neurons produces REM sleep without atonia. Interestingly, inactivation of these same neurons also reduced total time spent in REM sleep in mice [86,90]. Hence in addition to regulating REM sleep motor atonia, glutamatergic cLDT-SLD neurons also play a role in generating the forebrain features of REM sleep. Recent experimental work has shown that selective and acute opto- or chemogenetic activation of cLDT-SLD glutamatergic neurons can potently drive REM sleep [91,92]. In fact, it now appears that the cLDT-SLC contains two functionally segregated sets of glutamatergic neurons, the first of which includes reticulospinal REM sleep atonia-generating neurons and the second of which, likely via the parabrachial nucleus (PB) and medially adjacent precoeruleus (PC) region, promote corticohippocampal activation during REM sleep as well as regulate REM sleep amounts [8,14,90,93] (Figure 3). Little more, however, is known about the ‘downstream’ circuits by which these excitatory SLD/cLDT/PC projections contribute to the forebrain features, e.g., reduction in cortical EEG amplitude and hippocampal EEG theta waves associated with REM sleep, suggesting a priority focus for future investigations. From a clinical perspective, the existence of two functionally segregated sets of glutamatergic SLD REM-on neurons may provide a framework for understanding the occasional dissociation of cortical activation and muscle atonia observed during various REM sleep pathologies, including cataplexy, sleep paralysis and REM sleep behavior disorder [94–96].
It is also unclear how SLD cells are regulated, although many different sources of synaptic inputs to the SLD have been identified, including those containing acetylcholine, noradrenaline, serotonin, GABA, glutamate and various peptides [14,97–101]. Of these inputs, the most important source arises from GABAergic neurons in the nearby ventrolateral periaqueductal gray matter (vlPAG) and adjacent lateral pontine tegmentum (LPT; also called the deep mesencephalic nuclei, DpMe) [14,15,97]. GABAergic vlPAG/LPT neurons appear to provide inhibitory control over SLD cells since lesions or pharmacological inactivation of these neurons produces excessive REM sleep as well as cataplexy-like events [14,15,102]. In addition, optogenetic inhibition of vlPAG GABA neurons promotes REM sleep [13]. Finally, application of a GABAA antagonist into the SLD of rats rapidly induces an REM sleep-like state, suggesting that disinhibition from GABAergic inputs is an important synaptic mechanism for ‘activating’ SLD REM-ON neurons [97,103,104]. On the collective basis of these findings it has been proposed that GABAergic vlPAG/LPT neurons tonically inhibit REM-ON SLD neurons. The vlPAG/LDT-SLD/PC circuit thus functions to prevent inappropriate activation of REM sleep atonia-generating neurons during wakefulness and at the same time ensures rapid reconstitution of muscle tone on awakening [14,19,96]. A dramatic example of failure of this circuitry (or at least the synaptic balance of this circuitry) is seen in animals and humans lacking orexin signalling [105,106]. The absence of excitatory orexin ‘tone’ is thought to disfacilitate vlPAG/LPT/ DpMe REM-off neurons (i.e., cells that are minimally active in REM sleep), in turn inappropriately biasing activation of SLD neurons during wake and leading to the loss of postural muscle tone, i.e., cataplexy.
Given the accumulating data on SLD function, it is perhaps not surprising that many investigators view the SLD as a critical region for REM sleep control. And while there is ample evidence to support this view, it is important to bear in mind that both old and recent studies have inferred the existence of at least one additional region that is important for REM sleep control. Specifically, outcomes of lesion, unit recording and chemogenetic studies have suggested an important role for the vM, including the medially located ventral gigantocellular reticular nucleus (GiV) and α-gigantocellular reticular nuclei (GiA) and laterally adjacent paragigantocellular (LPGi) cell groups, in REM sleep control and REM sleep atonia [8,107–109]. In general support of this concept, a recent optogenetic study found that activation of vM GABA neurons could potently trigger REM sleep and produce REM sleep atonia, and that this effect was likely mediated through the vlPAG-SLD circuit and direct, i.e., SLD-independent, projections to spinal motor neurons [13].
Thus, while glutamatergic neurons of the SLD and GABAergic neurons of the vM are widely viewed as key circuit elements that work jointly in REM sleep control, our interpretation of the literature yields the more nuanced view that REM sleep control is supported by a distributed network of circuits, with this distributed circuit network spanning the brainstem, midbrain and hypothalamus. We now know, for example, that lateral hypothalamic cells that contain melanin concentrating hormone (MCH) play a role in promoting REM sleep, possibly through inhibition of REM-off neurons in the vlPAG/LPT [16,110,111]. The synaptic mechanism by which MCH cells regulate the vlPAG/LPT remains unclear, but could be directly through MCH or GABA release or indirectly through glutamate-mediated feedforward inhibition [16,112]. Microinjections of MCH into the SLD also increase REM sleep, suggesting that MCH, acting at the level of the SLD, may contribute to both the descending (atonia) and ascending (EEG desynchronization and theta activity) aspects of REM sleep [113]. Also, cholinergic neurons of the pedunculopontine and laterodorsal tegmental nuclei (PPT/LDT) are active during REM sleep and project to the SLD and optogenetic activation of these neurons during non-REM sleep promotes REM sleep [114,115]. It should, however, be noted that more recent studies have cast doubt on the claim of sufficiency and necessity of the cholinergic PPT/LDT or cholinergic inputs to the SLD in REM sleep control [116,117]. Finally, a role for the supramammillary hypothalamus, in particular resident glutamate-releasing/Nos1-expressing neurons, in the regulation of REM sleep and REM hippocampal theta has been recently reported [118,119].
Because REM sleep propensity is greatest during the latter part of the rest phase and REM sleep propensity coincides with the nadir of the body temperature circadian rhythm, an active role for the circadian clock in REM sleep regulation is indicated [75,120,121]. Consistent with this, lesion studies in rodents have shown that the circadian clock actively promotes REM sleep during the rest, but not active, period but has almost no role in determining the total amount of REM sleep, which instead is determined by homeostatic mechanisms [122]. Other studies suggest that the circadian clock actively suppresses REM sleep during the active period, and that this suppression is mediated by orexin neurons [123].
Pathobiology of REM Sleep
Identifying the circuit mechanisms underlying REM sleep has also yielded a far deeper understanding of disorders associated with abnormal REM sleep control. For example, REM sleep behaviour disorder and narcolepsy are two common sleep disorders that link, at least in part, to pathogenic changes in the circuit control mechanisms underlying healthy REM sleep. Here, we discuss how recent advances in dissecting REM sleep biology have led to a deeper understanding of these two disorders.
REM Sleep Behaviour Disorder
REM sleep behaviour disorder (RBD) is a neurological condition characterized by loss of the biological mechanisms that cause REM sleep paralysis, resulting in excessive motor behaviors during REM sleep [124,125]. Movements in RBD range from shouting and sporadic limb jerks to more complex movements such as punching or kicking [126–128]. These movements in RBD are not innocuous as they often result in physical injury of the patient and/or their bed-partner [129]. But, the most alarming aspect of RBD is that the majority of patients develop a neurodegenerative disease within 6–15 years of initial RBD diagnosis [129]. RBD is currently the strongest known predictor of a class of neurodegenerative disorders known as synucleinopathies with 80–90% of patients developing Parkinson's disease, dementia with Lewy bodies, or multiple system atrophy [130–132].
The link between RBD and synucleinopathies suggests that RBD itself stems from a neurodegenerative process [133]. Emerging new data indicate that RBD patients exhibit degeneration within the brainstem circuits that control REM sleep (e.g., the SLD and vM, Figure 4). For example, neuro-imaging studies show that RBD patients exhibit signs of degeneration within the dorsal pons where REM sleep circuits are located [134]. In addition, post-mortem analysis of brains from RBD patients with comorbid Parkinson's disease demonstrate neural loss, gliosis and Lewy body and neurite deposition (indicators of synucleinopathies) in the brainstem areas that control REM sleep [124,135]. These data suggest that degeneration within the REM sleep circuits could underlie RBD.
Figure 4. Involvement of REM sleep circuitry in REM sleep behavior disorder (RBD).
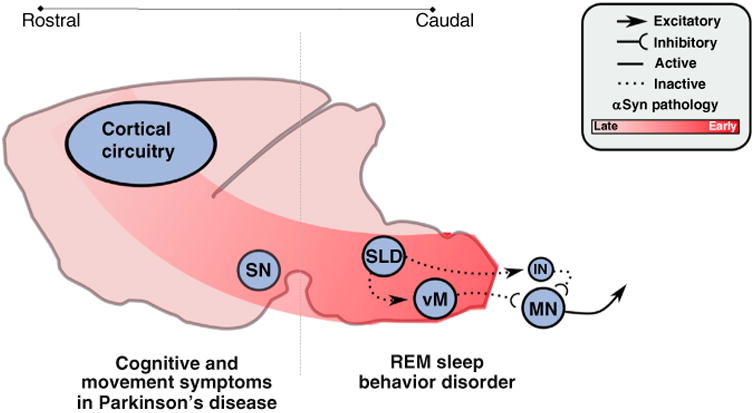
The hypothesized mechanisms and pathways by which synucleinopathic degeneration causes RBD. Synucleinopathic-mediated degeneration begins in the caudal brainstem and spreads inexorably rostrally, in a cell-to-cell fashion. Degeneration initially targets the sublaterodorsal nucleus (SLD) and ventral medulla (vM), impairing their functions and thereby causing loss of REM sleep atonia and the motor behaviors associated with RBD. But, as degenerative processes advance, they spread rostrally into the forebrain circuits that control normal cognition and waking motor behaviors (i.e., the substantia nigra, SN), thus leading to the classic cognitive and motor symptoms associated with synucleinopathies (e.g., Parkinson's disease) that follow initial RBD symptoms.
Classic lesion studies also suggest that damage to REM sleep circuitry contributes to RBD. For example, chemical, electrolytic and neuron-specific lesions of the REM sleep circuit, including the SLD and vM, result in loss of normal REM sleep paralysis and RBD-like motor behaviours in cats and rodents [8,14,84–86] (Figure 3). In addition, tumours or strokes that cause lesions of the dorsal pons where REM sleep circuits are located also cause RBD-like behaviours in humans [136,137]. Therefore, both clinical and basic science data indicate that RBD is caused by damage of the brainstem circuits that produce normal REM sleep.
Because 80% of RBD patients ultimately develop a synucleinopathy, it seems reasonable to assume that RBD is the earliest symptom in these disorders, that is, RBD is itself a synucleinopathy [132]. But, why does neurodegeneration affect REM sleep circuitry years before the onset of synpucleinopathies such as Parkinson's disease? New research concerning the root mechanisms of how synucleinopathies spread throughout the central nervous system is providing new clues about the potential link between RBD, neurodegeneration and synucleinopathies.
Emerging new research suggests that cell-to-cell transmission of mis-folded alpha-synuclein is the disease-causing mechanism in synucleinopathies, with mis-folded alpha-synuclein causing neuronal dysfunction and death [138,139]. Recent data show that synucleinopathies actually begin in the periphery and then spread into the central nervous system. Pathogenic alpha-synuclein appears to travel along vagus nerve afferents [140–142], which terminate in the nucleus of the solitary tract and dorsal motor nucleus of the vagus, which sits in close apposition to REM sleep circuitry (i.e., the SLD and vM). Therefore, synucleinopathies may initially impact the cells that control REM sleep atonia before spreading rostrally into the brain structures (e.g., substantia nigra and cortex) that cause the classic cognitive and motor impairments associated with synucleinopathies. Although this idea remains speculative it nonetheless fits with a classic model of Parkinson's disease pathogenesis, which proposes that neurodegeneration starts in the brainstem before ascending rostrally [143]. Several lines of clinical data also support the idea that RBD could result from synucleinopathic degeneration that starts in the periphery and then spreads into the brainstem. For example, RBD patients exhibit not only alpha-synuclein deposition in peripheral tissues [144] but also cell loss and alpha-synuclein deposition in the brainstem regions that control REM sleep motor atonia (i.e., the SLD and vM) [124].
While these new data suggest that synucleinopathic-mediated degeneration affects the circuits that control REM sleep, it remains unclear why REM sleep circuits themselves are the initial target of, and are vulnerable to, neurodegeneration. This is an important concept because the REM sleep circuits are located in a region of the brainstem that also houses circuits that control other vital behaviors (e.g., breathing, chewing and swallowing) that are largely unaffected in RBD. Therefore, understanding how and why cells in the REM sleep circuit are targeted by degeneration will not only shed light on the pathophysiology of RBD, but may also help identify the origins and mechanisms of synucleinopathies themselves.
Narcolepsy/Cataplexy
Narcolepsy is another common sleep disorder that is associated with changes in REM sleep function. Narcolepsy is linked to the loss of hypothalamic orexin cells and is characterized by excessive sleepiness, disturbed REM sleep, sleep paralysis, and dream-like hallucinations before falling asleep (called hypnagogic hallucinations) [145]. Another common symptom of narcolepsy is cataplexy, which is the involuntary onset of skeletal muscle paralysis or weakness during wakefulness (a link to video demonstrating cataplexy can be found here: https://www.youtube.com/watch?v=3MBCeKn0Oeo) [96]. The neural mechanisms that trigger cataplexy are hypothesized to result from intrusion of normal REM sleep paralysis into wakefulness.
Understanding mechanisms of REM sleep are helping to identify some of the potential causes of cataplexy. Converging lines of evidence suggest that cataplexy and REM sleep share a common neural mechanism. Tricyclic antidepressants are used to mitigate cataplexy, but they also suppress REM sleep, and withdrawal of these medications causes rebounds in cataplexy and REM sleep [146,147]. Neuroimaging studies in people with narcolepsy and electrophysiological recordings from neurons in narcoleptic dogs show that the brainstem circuitry involved in REM sleep has similar activity during both REM sleep and cataplexy [148–150]. In narcoleptic dogs, cells in the locus coeruleus (LC) are highly active during periods of wakefulness, but abruptly stop firing during cataplexy when muscle tone is lost [148], which mirrors LC activity during REM sleep. Because LC cells project to and activate motoneurons, their inactivity during cataplexy (and REM sleep) could be a neural mechanism by which motor paralysis occurs during these states [151]. Motor paralysis during cataplexy and REM sleep may also be mediated by GABA/ glycine cells in the vM (a region critical for promoting REM sleep paralysis) since their activity increases during both REM sleep and cataplexy [152].
Some patients with narcolepsy report hypnagogic hallucinations during cataplectic attacks, and some enter into REM sleep during cataplexy, suggesting that such attacks result from inappropriate recruitment of REM sleep circuits [96,145]. Recent data show that cataplexy-like attacks can be triggered in both wild-type and orexin knockout mice by directly activating the brainstem circuits that control REM sleep atonia (i.e., the SLD) [92]. This observation suggests that cataplexy may result from pathological recruitment of the circuits that cause REM sleep paralysis, and that muscle paralysis in REM sleep and cataplexy stem from a common neural mechanism.
Cataplexy is generally triggered by strong emotions such as hardy laughter, surprise or fear, and recent data suggest that there is a link between the brain structures that underlie emotional processing and those that trigger muscle atonia during REM sleep [96,153]. The amygdala not only processes emotions, but is also associated with REM sleep regulation and cataplexy [149]. Imaging studies showing that the amygdala is activated during cataplexy and REM sleep further support the link between the amygdala and cataplexy [149]. In narcoleptic dogs, neurons of the amygdala increase firing during cataplectic attacks [154]. Several recent studies indicate that the amygdala is associated with controlling cataplexy. Chemogenetic activation of GABA cells in the central nucleus of the amygdala (CeA) were found to promote cataplexy in orexin knockout mice, whereas, chemogenetic inhibition of these same cells reduced cataplexy [153,155,156]. It is hypothesized that GABA neurons in the CeA send descending projections to critical elements of the circuits that control REM sleep atonia, including the LC, the LPT and vlPAG, as well as the SLD. In narcolepsy, positive emotions activate GABA neurons in the CeA that inhibit cells in the LC, LPT, and vlPAG, which in turn disinhibit the SLD, which in turn activates the vM to produce motoneuron inhibition and muscle atonia during cataplexy [153,155,157] (Figure 5). But, in non-narcoleptics, positive emotions are unable to trigger muscle atonia because CeA-mediated inhibition of the LC/LDT/vlPAG is offset by excitatory or exin inputs, which prevent positive emotions from manipulating the SLD (Figure 5).
Figure 5. Circuits that produce REM sleep atonia underlie cataplexy.
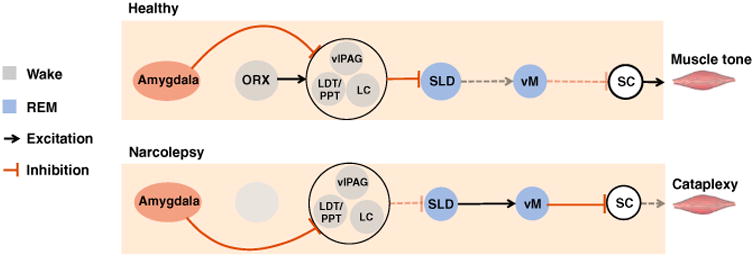
The sublaterodorsal nucleus (SLD) and ventral medulla (vM) represent the core circuits that produce REM sleep atonia. Glutamate cells in the SLD activate GABA and glycine neurons in the vM to trigger REM sleep atonia by inhibiting motoneurons in the spinal cord (SC). In narcolepsy, positive emotions activate GABA neurons in the central nucleus (CeA) that inhibit cells in the locus coeruleus (LC), laterodorsal tegmentum (LDT), and ventrolateral periaqueductal grey (vlPAG), which in turn disinhibit the SLD, which in turn activates the vM to produce motoneuron inhibition and muscle atonia during cataplexy. In non-narcoleptics, positive emotions are unable to trigger muscle atonia because CeA-mediated inhibition of the LC/LDT/vlPAG is offset by excitatory orexin inputs, which prevent positive emotions from manipulating the SLD.
If both RBD and narcolepsy arise from disturbances in REM sleep control, then there should be a link between these disorders. In fact, approximately 50% of patients with narcolepsy/ cataplexy experience RBD [158]. Also, RBD can be triggered or aggravated by antidepressant treatment in narcoleptic patients, and many narcoleptic patients exhibit marked increases in overall levels of motor activity during REM sleep, even if they do not experience frank RBD [158]. Therefore, one major commonality between RBD and narcolepsy is abnormal motor activity, with RBD resulting from loss of normal REM sleep paralysis, and cataplexy resulting from intrusion of REM sleep paralysis into wakefulness. These clinical observations suggest that RBD and narcolepsy may result from abnormal control of the circuits that underlie REM sleep, and particularly those that regulate REM sleep atonia (Figures 3–5).
Conclusions and Future Directions
Even though REM sleep was only identified as a distinct behavioural state in the early 1950s, considerable progress has been made in understanding its mechanisms and functions, with most of this headway being made within the past decade. And while it is undeniable that many of these advances in our understanding of REM sleep biology link to the development of new technologies (e.g., chemo- and opto-genetics), we would argue that it is the combinatorial application of these newer techniques with more classical approaches (e.g., functional anatomy, unit recording, and cellular imaging) that has truly enabled the effective probing and elucidation of the delimited circuits that drive REM sleep and, in turn, revealed how these circuits influence the function and activity of brain structures associated with learning, memory and motor control.
Although scientists have made important progress in identifying REM sleep mechanisms and elucidating some of its biological functions, many important questions remain. For example, is REM sleep actually present in all animals? While many biologists believe that sleep is a universal behaviour, we have only studied and identified sleep in a small fraction of all existing animal species. Understanding sleep biology across species, including differences, is important because such insight could provide new clues about the potential functions of REM sleep. For example, does REM sleep serve the same function in all animals?
Although REM sleep appears important for certain types of learning and memory, it remains virtually unknown how REM sleep actually facilitates plasticity and learning. While recent evidence indicates that theta activity during REM sleep (Figure 1) is critical for facilitating motor learning, it remains unknown how or why theta activity promotes memory-related plasticity. It also remains unclear if REM sleep serves the same biological function(s) across the lifespan. For example, why are REM sleep amounts typically higher in young and developing animals than in older ones? These age-related differences in REM sleep amounts suggest that REM sleep function may vary across an animal's lifespan, but what those potential functions are remains unclear.
While some of the circuits underlying REM sleep generation have been identified, the full extent of REM sleep circuits remains unidentified. For example, it was only within the last several years that neuroscientists identified that structures beyond the brain-stem (e.g., MCH cells in the hypothalamus) also play a critical role in modulating REM sleep. It also remains unclear how circuits that control REM sleep communicate with those that promote wakefulness and non-REM sleep. And this is a critical question in science and medicine because changes in REM sleep control are associated with some of the symptoms in narcolepsy (e.g., cataplexy).
Arguably, the most pressing issues in REM sleep biology relate to human disease. As discussed above, multiple lines of evidence indicate that degeneration of REM sleep circuits underlies RBD (Figure 4). A major challenge therefore for sleep biologists and clinicians is to gain a better understanding of how and why cells in the REM sleep circuit are targeted by degeneration. Answering this question will not only shed light on the pathophysiology of RBD, but may also help identify the origins and mechanisms of synucleinopathies themselves.
References
- 1.Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–274. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- 2.Dement W, Kleitman N. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalogr Clin Neurophysiol. 1957;9:673–690. doi: 10.1016/0013-4694(57)90088-3. [DOI] [PubMed] [Google Scholar]
- 3.Dement W, Kleitman N. The relation of eye movements during sleep to dream activity: an objective method for the study of dreaming. J Exp Psychol. 1957;53:339–346. doi: 10.1037/h0048189. [DOI] [PubMed] [Google Scholar]
- 4.Jouvet M, Michel F. Electromyographic correlations of sleep in the chronic decorticate & mesencephalic cat. Comptes rendus des seances de la Societe de biologie et de ses filiales. 1959;153:422–425. [PubMed] [Google Scholar]
- 5.Jouvet M. The states of sleep. Sci Am. 1967;216:62–68. doi: 10.1038/scientificamerican0267-62. passim. [DOI] [PubMed] [Google Scholar]
- 6.Siegel JM. In: REM sleep In Principles and Practice of Sleep Medicine. 4th. Kreiger MH, Roth T, Dement WC, editors. Philadelphia: W.B. Saunders Company; 2005. pp. 120–135. [Google Scholar]
- 7.Jones BE. Paradoxical REM sleep promoting and permitting neuronal networks. Arch Ital Biol. 2004;142:379–396. [PubMed] [Google Scholar]
- 8.Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci. 2002;16:1959–1973. doi: 10.1046/j.1460-9568.2002.02257.x. [DOI] [PubMed] [Google Scholar]
- 9.Xi MC, Morales FR, Chase MH. The motor inhibitory system operating during active sleep is tonically suppressed by GABAergic mechanisms during other states. J Neurophysiol. 2001;86:1908–1915. doi: 10.1152/jn.2001.86.4.1908. [DOI] [PubMed] [Google Scholar]
- 10.Dumoulin Bridi MC, Aton SJ, Seibt J, Renouard L, Coleman T, Frank MG. Rapid eye movement sleep promotes cortical plasticity in the developing brain. Sci Adv. 2015;1:e1500105. doi: 10.1126/sciadv.1500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh CM, Booth V, Poe GR. Spatial and reversal learning in the Morris water maze are largely resistant to six hours of REM sleep deprivation following training. Learn Mem. 2011;18:422–434. doi: 10.1101/lm.2099011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber F, Dan Y. Circuit-based interrogation of sleep control. Nature. 2016;538:51–59. doi: 10.1038/nature19773. [DOI] [PubMed] [Google Scholar]
- 13.Weber F, Chung S, Beier KT, Xu M, Luo L, Dan Y. Control of REM sleep by ventral medulla GABAergic neurons. Nature. 2015;526:435–438. doi: 10.1038/nature14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 15.Sapin E, Lapray D, Berod A, Goutagny R, Leger L, Ravassard P, Clement O, Hanriot L, Fort P, Luppi PH. Localization of the brainstem GABAergic neurons controlling paradoxical (REM) sleep. PLoS One. 2009;4:e4272. doi: 10.1371/journal.pone.0004272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jego S, Glasgow SD, Herrera CG, Ekstrand M, Reed SJ, Boyce R, Friedman J, Burdakov D, Adamantidis AR. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci. 2013;16:1637–1643. doi: 10.1038/nn.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyce R, Glasgow SD, Williams S, Adamantidis A. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science. 2016;352:812–816. doi: 10.1126/science.aad5252. [DOI] [PubMed] [Google Scholar]
- 18.Brooks PL, Peever JH. Identification of the transmitter and receptor mechanisms responsible for REM sleep paralysis. J Neurosci. 2012;32:9785–9795. doi: 10.1523/JNEUROSCI.0482-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scammell TE, Arrigoni E, Lipton JO. Neural circuitry of wakefulness and sleep. Neuron. 2017;93:747–765. doi: 10.1016/j.neuron.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiromani PJ, Peever JH. New neuroscience tools that are identifying the sleep-wake circuit. Sleep. 2017;40 doi: 10.1093/sleep/zsx032. https://doi.org/10.1093/sleep/zsx032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegel JM. The REM sleep-memory consolidation hypothesis. Science. 2001;294:1058–1063. doi: 10.1126/science.1063049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lesku JA, Roth TC, 2nd, Rattenborg NC, Amlaner CJ, Lima SL. History and future of comparative analyses in sleep research. Neurosci Biobehav Rev. 2009;33:1024–1036. doi: 10.1016/j.neubiorev.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Lesku JA, Rattenborg NC. Avian sleep. Curr Biol. 2014;24:R12–R14. doi: 10.1016/j.cub.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Lesku JA, Meyer LC, Fuller A, Maloney SK, Dell'Omo G, Vyssotski AL, Rattenborg NC. Ostriches sleep like platypuses. PLoS One. 2011;6:e23203. doi: 10.1371/journal.pone.0023203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beckers GJ, Rattenborg NC. An in depth view of avian sleep. Neurosci Biobehav Rev. 2015;50:120–127. doi: 10.1016/j.neubiorev.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Shein-Idelson M, Ondracek JM, Liaw HP, Reiter S, Laurent G. Slow waves, sharp waves, ripples, and REM in sleeping dragons. Science. 2016;352:590–595. doi: 10.1126/science.aaf3621. [DOI] [PubMed] [Google Scholar]
- 27.Frank MG, Waldrop RH, Dumoulin M, Aton S, Boal JG. A preliminary analysis of sleep-like states in the cuttlefish Sepia officinalis. PLoS One. 2012;7:e38125. doi: 10.1371/journal.pone.0038125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corner MA. Call it sleep – what animals without backbones can tell us about the phylogeny of intrinsically generated neuromotor rhythms during early development. Neurosci Bull. 2013;29:373–380. doi: 10.1007/s12264-013-1313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tobler I. Behavioral sleep in the Asian elephant in captivity. Sleep. 1992;15:1–12. [PubMed] [Google Scholar]
- 30.Gravett N, Bhagwandin A, Sutcliffe R, Landen K, Chase MJ, Lyamin OI, Siegel JM, Manger PR. Inactivity/sleep in two wild free-roaming African elephant matriarchs - does large body size make elephants the shortest mammalian sleepers? PLoS One. 2017;12:e0171903. doi: 10.1371/journal.pone.0171903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tobler I, Schwierin B. Behavioural sleep in the giraffe (Giraffa camelopardalis) in a zoological garden. J Sleep Res. 1996;5:21–32. doi: 10.1046/j.1365-2869.1996.00010.x. [DOI] [PubMed] [Google Scholar]
- 32.Marks GA, Shaffery JP. A preliminary study of sleep in the ferret, Mustela putorius furo: a carnivore with an extremely high proportion of REM sleep. Sleep. 1996;19:83–93. doi: 10.1093/sleep/19.2.83. [DOI] [PubMed] [Google Scholar]
- 33.Jha SK, Coleman T, Frank MG. Sleep and sleep regulation in the ferret (Mustela putorius furo) Behav Brain Res. 2006;172:106–113. doi: 10.1016/j.bbr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Siegel JM, Manger PR, Nienhuis R, Fahringer HM, Shalita T, Pettigrew JD. Sleep in the platypus. Neuroscience. 1999;91:391–400. doi: 10.1016/s0306-4522(98)00588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ursin R, Moses J, Naitoh P, Johnson LC. REM-NREM cycle in the cat may be sleep-dependent. Sleep. 1983;6:1–9. doi: 10.1093/sleep/6.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Ursin R. Sleep in the cat: a parallel increase of deep slow wave sleep and REM sleep following total sleep deprivation. Acta Physiol Scand. 1971;82:1A. doi: 10.1111/j.1748-1716.1971.tb05006.x. [DOI] [PubMed] [Google Scholar]
- 37.Ursin R. The two stages of slow wave sleep in the cat and their relation to REM sleep. Brain Res. 1968;11:347–356. doi: 10.1016/0006-8993(68)90030-9. [DOI] [PubMed] [Google Scholar]
- 38.Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral state instability in orexin knockout mice. J Neurosci. 2004;24:6291–6300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carskadon MA, Dement WC. Distribution of REM sleep on a 90 minute sleep-wake schedule. Sleep. 1980;2:309–317. [PubMed] [Google Scholar]
- 40.Weitzman ED, Czeisler CA, Zimmerman JC, Ronda JM. Timing of REM and stages 3 + 4 sleep during temporal isolation in man. Sleep. 1980;2:391–407. [PubMed] [Google Scholar]
- 41.Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horne J. Why REM sleep? Clues beyond the laboratory in a more challenging world. Biol Psychol. 2013;92:152–168. doi: 10.1016/j.biopsycho.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Blumberg MS, Seelke AM, Lowen SB, Karlsson KA. Dynamics of sleep-wake cyclicity in developing rats. Proc Natl Acad Sci USA. 2005;102:14860–14864. doi: 10.1073/pnas.0506340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blumberg MS, Seelke AM. The form and function of infant sleep: from muscle to neocortex. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. Oxford University Press; 2010. pp. 391–423. [Google Scholar]
- 45.Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- 46.Lyamin OI, Lapierre JL, Mukhametov LM. Sleep in aquatic species. In: Kushida C, editor. The Encyclopedia of Sleep. Vol. 1. Waltham, MA: Academic Press; 2013. pp. 57–62. [Google Scholar]
- 47.Rattenborg NC, Voirin B, Cruz SM, Tisdale R, Dell'Omo G, Lipp HP, Wikelski M, Vyssotski AL. Evidence that birds sleep in mid-flight. Nat Commun. 2016;7:12468. doi: 10.1038/ncomms12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lesku JA, Rattenborg NC, Valcu M, Vyssotski AL, Kuhn S, Kuemmeth F, Heidrich W, Kempenaers B. Adaptive sleep loss in polygynous pectoral sandpipers. Science. 2012;337:1654–1658. doi: 10.1126/science.1220939. [DOI] [PubMed] [Google Scholar]
- 49.Jones SG, Vyazovskiy VV, Cirelli C, Tononi G, Benca RM. Homeostatic regulation of sleep in the white-crowned sparrow (Zonotrichia leucophrys gambelii) BMC Neurosci. 2008;9:47. doi: 10.1186/1471-2202-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones SG, Paletz EM, Obermeyer WH, Hannan CT, Benca RM. Seasonal influences on sleep and executive function in the migratory White-crowned Sparrow (Zonotrichia leucophrys gambelii) BMC Neurosci. 2010;11:87. doi: 10.1186/1471-2202-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siegel JM, Manger PR, Nienhuis R, Fahringer HM, Pettigrew JD. The echidna Tachyglossus aculeatus combines REM and non-REM aspects in a single sleep state: implications for the evolution of sleep. J Neurosci. 1996;16:3500–3506. doi: 10.1523/JNEUROSCI.16-10-03500.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lyamin OI, Manger PR, Ridgway SH, Mukhametov LM, Siegel JM. Cetacean sleep: an unusual form of mammalian sleep. Neurosci Biobehav Rev. 2008;32:1451–1484. doi: 10.1016/j.neubiorev.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mukhametov LM. Unihemispheric slow-wave sleep in the Amazonian dolphin, Inia geoffrensis. Neurosci Lett. 1987;79:128–132. doi: 10.1016/0304-3940(87)90684-7. [DOI] [PubMed] [Google Scholar]
- 54.Lyamin OI, Shpak OV, Nazarenko EA, Mukhametov LM. Muscle jerks during behavioral sleep in a beluga whale (Delphinapterus leucas L.) Physiol Behav. 2002;76:265–270. doi: 10.1016/s0031-9384(02)00722-9. [DOI] [PubMed] [Google Scholar]
- 55.Kennedy D, Norman C. What don't we know? Science. 2005;309:75. doi: 10.1126/science.309.5731.75. [DOI] [PubMed] [Google Scholar]
- 56.Marks GA, Shaffery JP, Oksenberg A, Speciale SG, Roffwarg HP. A functional role for REM sleep in brain maturation. Behav Brain Res. 1995;69:1–11. doi: 10.1016/0166-4328(95)00018-o. [DOI] [PubMed] [Google Scholar]
- 57.Corner MA. From neural plate to cortical arousal-a neuronal network theory of sleep derived from in vitro “model” systems for primordial patterns of spontaneous bioelectric activity in the vertebrate central nervous system. Brain Sci. 2013;3:800–820. doi: 10.3390/brainsci3020800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frank MG. Sleep and developmental plasticity not just for kids. Prog Brain Res. 2011;193:221–232. doi: 10.1016/B978-0-444-53839-0.00014-4. [DOI] [PubMed] [Google Scholar]
- 59.Frank MG, Heller HC. Development of REM and slow wave sleep in the rat. Am J Physiol. 1997;272:R1792–R1799. doi: 10.1152/ajpregu.1997.272.6.R1792. [DOI] [PubMed] [Google Scholar]
- 60.Karlsson KA, Gall AJ, Mohns EJ, Seelke AM, Blumberg MS. The neural substrates of infant sleep in rats. PLoS Biol. 2005;3:e143. doi: 10.1371/journal.pbio.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tiriac A, Uitermarkt BD, Fanning AS, Sokoloff G, Blumberg MS. Rapid whisker movements in sleeping newborn rats. Curr Biol. 2012;22:2075–2080. doi: 10.1016/j.cub.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blumberg MS, Marques HG, Iida F. Twitching in sensorimotor development from sleeping rats to robots. Curr Biol. 2013;23:R532–R537. doi: 10.1016/j.cub.2013.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blumberg MS, Coleman CM, Gerth AI, McMurray B. Spatiotemporal structure of REM sleep twitching reveals developmental origins of motor synergies. Curr Biol. 2013;23:2100–2109. doi: 10.1016/j.cub.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brooks PL, Peever J. A temporally controlled inhibitory drive coordinates twitch movements during REM sleep. Curr Biol. 2016;26:1177–1182. doi: 10.1016/j.cub.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 65.Tiriac A, Del Rio-Bermudez C, Blumberg MS. Self-generated movements with “unexpected” sensory consequences. Curr Biol. 2014;24:2136–2141. doi: 10.1016/j.cub.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karlsson KA, Blumberg MS. Hippocampal theta in the newborn rat is revealed under conditions that promote REM sleep. J Neurosci. 2003;23:1114–1118. doi: 10.1523/JNEUROSCI.23-04-01114.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tiriac A, Blumberg MS. Gating of reafference in the external cuneate nucleus during self-generated movements in wake but not sleep. Elife. 2016;5:e18749. doi: 10.7554/eLife.18749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sokoloff G, Uitermarkt BD, Blumberg MS. REM sleep twitches rouse nascent cerebellar circuits: Implications for sensorimotor development. Dev Neurobiol. 2015;75:1140–1153. doi: 10.1002/dneu.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sokoloff G, Plumeau AM, Mukherjee D, Blumberg MS. Twitch-related and rhythmic activation of the developing cerebellar cortex. J Neurophysiol. 2015;114:1746–1756. doi: 10.1152/jn.00284.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frank MG. Sleep and plasticity in the visual cortex: more than meets the eye. Curr Opin Neurobiol. 2017;44:8–12. doi: 10.1016/j.conb.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Poe GR, Walsh CM, Bjorness TE. Cognitive neuroscience of sleep. Prog Brain Res. 2010;185:1–19. doi: 10.1016/B978-0-444-53702-7.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 73.Vertes RP. Memory consolidation in sleep; dream or reality. Neuron. 2004;44:135–148. doi: 10.1016/j.neuron.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 74.Rasch B, Pommer J, Diekelmann S, Born J. Pharmacological REM sleep suppression paradoxically improves rather than impairs skill memory. Nat Neurosci. 2009;12:396–397. doi: 10.1038/nn.2206. [DOI] [PubMed] [Google Scholar]
- 75.Benington JH, Heller HC. REM-sleep timing is controlled homeostatically by accumulation of REM-sleep propensity in non-REM sleep. Am J Physiol. 1994;266:R1992–R2000. doi: 10.1152/ajpregu.1994.266.6.R1992. [DOI] [PubMed] [Google Scholar]
- 76.Zhang J, Zhu Y, Zhan G, Fenik P, Panossian L, Wang MM, Reid S, Lai D, Davis JG, Baur JA, et al. Extended wakefulness: compromised metabolics in and degeneration of locus ceruleus neurons. J Neurosci. 2014;34:4418–4431. doi: 10.1523/JNEUROSCI.5025-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li W, Ma L, Yang G, Gan WB. REM sleep selectively prunes and maintains new synapses in development and learning. Nat Neurosci. 2017;20:427–437. doi: 10.1038/nn.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sterpenich V, Schmidt C, Albouy G, Matarazzo L, Vanhaudenhuyse A, Boveroux P, Degueldre C, Leclercq Y, Balteau E, Collette F, et al. Memory reactivation during rapid eye movement sleep promotes its generalization and integration in cortical stores. Sleep. 2014;37:1061–1075. 1075A–1075B. doi: 10.5665/sleep.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Siegel JM, Rogawski MA. A function for REM sleep: regulation of noradrenergic receptor sensitivity. Brain Res. 1988;472:213–233. doi: 10.1016/0165-0173(88)90007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cai DJ, Mednick SA, Harrison EM, Kanady JC, Mednick SC. REM, not incubation, improves creativity by priming associative networks. Proc Natl Acad Sci USA. 2009;106:10130–10134. doi: 10.1073/pnas.0900271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8:112–119. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Horner RL, Sanford LD, Pack AI, Morrison AR. Activation of a distinct arousal state immediately after spontaneous awakening from sleep. Brain Res. 1997;778:127–134. doi: 10.1016/s0006-8993(97)01045-7. [DOI] [PubMed] [Google Scholar]
- 83.Jouvet M, Michel F. Release of the “paradoxal phase” of sleep by stimulation of the brain stem in the intact and chronic mesencephalic cat. Comptes rendus des seances de la Societe de biologie et de ses filiales. 1960;154:636–641. [PubMed] [Google Scholar]
- 84.Henley K, Morrison AR. A re-evaluation of the effects of lesions of the pontine tegmentum and locus coeruleus on phenomena of paradoxical sleep in the cat. Acta neurobiologiae experimentalis. 1974;34:215–232. [PubMed] [Google Scholar]
- 85.Mouret J, Delorme F, Jouvet M. Lesions of the pontine tegmentum and sleep in rats. Comptes rendus des seances de la Societe de biologie et de ses filiales. 1967;161:1603–1606. [PubMed] [Google Scholar]
- 86.Valencia Garcia S, Libourel PA, Lazarus M, Grassi D, Luppi PH, Fort P. Genetic inactivation of glutamate neurons in the rat sublaterodorsal tegmental nucleus recapitulates REM sleep behaviour disorder. Brain. 2017;140:414–428. doi: 10.1093/brain/aww310. [DOI] [PubMed] [Google Scholar]
- 87.Lai YY, Siegel JM. Pontomedullary glutamate receptors mediating locomotion and muscle tone suppression. J Neurosci. 1991;11:2931–2937. doi: 10.1523/JNEUROSCI.11-09-02931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Onoe H, Sakai K. Kainate receptors: a novel mechanism in paradoxical (REM) sleep generation. Neuroreport. 1995;6:353–356. [PubMed] [Google Scholar]
- 89.Sanford LD, Cheng CS, Silvestri AJ, Tang X, Mann GL, Ross RJ, Morrison AR. Sleep and behavior in rats with pontine lesions producing REM without atonia. Sleep Res Online. 2001;4:1–5. [Google Scholar]
- 90.Krenzer M, Anaclet C, Vetrivelan R, Wang N, Vong L, Lowell BB, Fuller PM, Lu J. Brainstem and spinal cord circuitry regulating REM sleep and muscle atonia. PLoS One. 2011;6:e24998. doi: 10.1371/journal.pone.0024998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anaclet C, Fuller PM. Differential regulation of wake, sws and rem sleep by pontomedullary gabaergic, glutamatergic and phox2b-expressing neurons. Soc Neurosci Abstract. 2016;816:15. [Google Scholar]
- 92.Fraigne JJ, Torontali T, Thomasian A, Li DW, Peever JH. A dedicted brainstem circuit controls REM sleep. Sleep. 2017;40:A44. [Google Scholar]
- 93.Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519:933–956. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fuller PM, Saper CB, Lu J. The pontine REM switch: past and present. J Physiol. 2007;584:735–741. doi: 10.1113/jphysiol.2007.140160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peever J, Luppi PH, Montplaisir J. Breakdown in REM sleep circuitry underlies REM sleep behavior disorder. Trends Neurosci. 2014;37:279–288. doi: 10.1016/j.tins.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 96.Dauvilliers Y, Siegel JM, Lopez R, Torontali ZA, Peever JH. Cataplexy–clinical aspects, pathophysiology and management strategy. Nat Rev Neurol. 2014;10:386–395. doi: 10.1038/nrneurol.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boissard R, Fort P, Gervasoni D, Barbagli B, Luppi PH. Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially gating paradoxical sleep onset. Eur J Neurosci. 2003;18:1627–1639. doi: 10.1046/j.1460-9568.2003.02861.x. [DOI] [PubMed] [Google Scholar]
- 98.Arrigoni E, Chen MC, Fuller PM. The anatomical, cellular and synaptic basis of motor atonia during rapid eye movement sleep. J Physiol. 2016;594:5391–5414. doi: 10.1113/JP271324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Clement O, Sapin E, Libourel PA, Arthaud S, Brischoux F, Fort P, Luppi PH. The lateral hypothalamic area controls paradoxical (REM) sleep by means of descending projections to brainstem GABAergic neurons. J Neurosci. 2012;32:16763–16774. doi: 10.1523/JNEUROSCI.1885-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Verret L, Fort P, Gervasoni D, Leger L, Luppi PH. Localization of the neurons active during paradoxical (REM) sleep and projecting to the locus coeruleus noradrenergic neurons in the rat. J Comp Neurol. 2006;495:573–586. doi: 10.1002/cne.20891. [DOI] [PubMed] [Google Scholar]
- 101.Weng FJ, Williams RH, Hawryluk JM, Lu J, Scammell TE, Saper CB, Arrigoni E. Carbachol excites sublaterodorsal nucleus neurons projecting to the spinal cord. J Physiol. 2014;592:1601–1617. doi: 10.1113/jphysiol.2013.261800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kaur S, Thankachan S, Begum S, Liu M, Blanco-Centurion C, Shiromani PJ. Hypocretin-2 saporin lesions of the ventrolateral periaquaductal gray (vlPAG) increase REM sleep in hypocretin knockout mice. PLoS One. 2009;4:e6346. doi: 10.1371/journal.pone.0006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pollock MS, Mistlberger RE. Rapid eye movement sleep induction by microinjection of the GABA-A antagonist bicuculline into the dorsal subcoeruleus area of the rat. Brain Res. 2003;962:68–77. doi: 10.1016/s0006-8993(02)03956-2. [DOI] [PubMed] [Google Scholar]
- 104.Fenik VB, Kubin L. Differential localization of carbacholand bicuculline-sensitive pontine sites for eliciting REM sleep-like effects in anesthetized rats. J Sleep Res. 2009;18:99–112. doi: 10.1111/j.1365-2869.2008.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sasaki K, Suzuki M, Mieda M, Tsujino N, Roth B, Sakurai T. Pharmacogenetic modulation of orexin neurons alters sleep/ wakefulness states in mice. PLoS One. 2011;6:e20360. doi: 10.1371/journal.pone.0020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mochizuki T, Arrigoni E, Marcus JN, Clark EL, Yamamoto M, Honer M, Borroni E, Lowell BB, Elmquist JK, Scammell TE. Orexin receptor 2 expression in the posterior hypothalamus rescues sleepiness in narcoleptic mice. Proc Natl Acad Sci USA. 2011;108:4471–4476. doi: 10.1073/pnas.1012456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Siegel JM, Wheeler RL, McGinty DJ. Activity of medullary reticular formation neurons in the unrestrained cat during waking and sleep. Brain Res. 1979;179:49–60. doi: 10.1016/0006-8993(79)90488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kanamori N, Sakai K, Jouvet M. Neuronal activity specific to paradoxical sleep in the ventromedial medullary reticular formation of unrestrained cats. Brain Res. 1980;189:251–255. doi: 10.1016/0006-8993(80)90024-4. [DOI] [PubMed] [Google Scholar]
- 109.Chen MC, Vetrivelan R, Guo CN, Chang C, Fuller PM, Lu J. Ventral medullary control of rapid eye movement sleep and atonia. Exp Neurol. 2017;290:53–62. doi: 10.1016/j.expneurol.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Konadhode RR, Pelluru D, Blanco-Centurion C, Zayachkivsky A, Liu M, Uhde T, Glen WB, Jr, van den Pol AN, Mulholland PJ, Shiromani PJ. Optogenetic stimulation of MCH neurons increases sleep. J Neurosci. 2013;33:10257–10263. doi: 10.1523/JNEUROSCI.1225-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vetrivelan R, Kong D, Ferrari LL, Arrigoni E, Madara JC, Bandaru SS, Lowell BB, Lu J, Saper CB. Melanin-concentrating hormone neurons specifically promote rapid eye movement sleep in mice. Neuroscience. 2016;336:102–113. doi: 10.1016/j.neuroscience.2016.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chee MJ, Arrigoni E, Maratos-Flier E. Melanin-concentrating hormone neurons release glutamate for feedforward inhibition of the lateral septum. J Neurosci. 2015;35:3644–3651. doi: 10.1523/JNEUROSCI.4187-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Torterolo P, Sampogna S, Chase MH. MCHergic projections to the nucleus pontis oralis participate in the control of active (REM) sleep. Brain Res. 2009;1268:76–87. doi: 10.1016/j.brainres.2009.02.055. [DOI] [PubMed] [Google Scholar]
- 114.Boucetta S, Cisse Y, Mainville L, Morales M, Jones BE. Discharge profiles across the sleep-waking cycle of identified cholinergic, GABAergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J Neurosci. 2014;34:4708–4727. doi: 10.1523/JNEUROSCI.2617-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Van Dort CJ, Zachs DP, Kenny JD, Zheng S, Goldblum RR, Gelwan NA, Ramos DM, Nolan MA, Wang K, Weng FJ, et al. Optogenetic activation of cholinergic neurons in the PPT or LDT induces REM sleep. Proc Natl Acad Sci USA. 2015;112:584–589. doi: 10.1073/pnas.1423136112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kroeger D, Ferrari LL, Petit G, Mahoney CE, Fuller PM, Arrigoni E, Scammell TE. Cholinergic, glutamatergic, and GABAergic neurons of the pedunculopontine tegmental nucleus have distinct effects on sleep/wake behavior in mice. J Neurosci. 2017;37:1352–1366. doi: 10.1523/JNEUROSCI.1405-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Grace KP, Vanstone LE, Horner RL. Endogenous cholinergic input to the pontine REM sleep generator is not required for REM sleep to occur. J Neurosci. 2014;34:14198–14209. doi: 10.1523/JNEUROSCI.0274-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Renouard L, Billwiller F, Ogawa K, Clement O, Camargo N, Abdel-karim M, Gay N, Scote-Blachon C, Toure R, Libourel PA, et al. The supramammillary nucleus and the claustrum activate the cortex during REM sleep. Sci Adv. 2015;1:e1400177. doi: 10.1126/sciadv.1400177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pedersen NP, Ferrari L, Venner A, Wang JL, Abbott SBG, Vujovic N, Arrigoni E, Saper CB, Fuller PM. Supramammillary glutamate neurons are a key node of the arousal system. Nat Commun. 2017 doi: 10.1038/s41467-017-01004-6. In Press. https://doi.org/10.1038/s41467-017-01004-6. [DOI] [PMC free article] [PubMed]
- 120.Czeisler CA, Zimmerman JC, Ronda JM, Moore-Ede MC, Weitzman ED. Timing of REM sleep is coupled to the circadian rhythm of body temperature in man. Sleep. 1980;2:329–346. [PubMed] [Google Scholar]
- 121.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wurts SW, Edgar DM. Circadian and homeostatic control of rapid eye movement (REM) sleep: promotion of REM tendency by the suprachiasmatic nucleus. J Neurosci. 2000;20:4300–4310. doi: 10.1523/JNEUROSCI.20-11-04300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kantor S, Mochizuki T, Janisiewicz AM, Clark E, Nishino S, Scammell TE. Orexin neurons are necessary for the circadian control of REM sleep. Sleep. 2009;32:1127–1134. doi: 10.1093/sleep/32.9.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Iranzo A, Tolosa E, Gelpi E, Molinuevo JL, Valldeoriola F, Serra-dell M, Sanchez-Valle R, Vilaseca I, Lomena F, Vilas D, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 2013;12:443–453. doi: 10.1016/S1474-4422(13)70056-5. [DOI] [PubMed] [Google Scholar]
- 125.Schenck CH, Mahowald MW. REM sleep parasomnias. Neurol Clin. 1996;14:697–720. doi: 10.1016/s0733-8619(05)70281-4. [DOI] [PubMed] [Google Scholar]
- 126.Oudiette D, Leu-Semenescu S, Roze E, Vidailhet M, De Cock VC, Golmard JL, Arnulf I. A motor signature of REM sleep behavior disorder. Mov Disord. 2012;27:428–431. doi: 10.1002/mds.24044. [DOI] [PubMed] [Google Scholar]
- 127.Oudiette D, De Cock VC, Lavault S, Leu S, Vidailhet M, Arnulf I. Nonviolent elaborate behaviors may also occur in REM sleep behavior disorder. Neurology. 2009;72:551–557. doi: 10.1212/01.wnl.0000341936.78678.3a. [DOI] [PubMed] [Google Scholar]
- 128.Cygan F, Oudiette D, Leclair-Visonneau L, Leu-Semenescu S, Arnulf I. Night-to-night variability of muscle tone, movements, and vocalizations in patients with REM sleep behavior disorder. J Clin Sleep Med. 2010;6:551–555. [PMC free article] [PubMed] [Google Scholar]
- 129.Schenck CH, Mahowald MW. REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep. 2002;25:120–138. doi: 10.1093/sleep/25.2.120. [DOI] [PubMed] [Google Scholar]
- 130.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology. 1996;46:388–393. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 131.Iranzo A, Molinuevo JL, Santamaria J, Serradell M, Marti MJ, Valldeoriola F, Tolosa E. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 2006;5:572–577. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 132.St Louis EK, Boeve AR, Boeve BF. REM sleep behavior disorder in Parkinson's disease and other synucleinopathies. Mov Disord. 2017;32:645–658. doi: 10.1002/mds.27018. [DOI] [PubMed] [Google Scholar]
- 133.McKenna D, Peever J. Degeneration of rapid eye movement sleep circuitry underlies rapid eye movement sleep behavior disorder. Mov Disord. 2017;32:636–644. doi: 10.1002/mds.27003. [DOI] [PubMed] [Google Scholar]
- 134.Garcia-Lorenzo D, Longo-Dos Santos C, Ewenczyk C, Leu-Semenescu S, Gallea C, Quattrocchi G, Pita Lobo P, Poupon C, Benali H, Arnulf I, et al. The coeruleus/subcoeruleus complex in rapid eye movement sleep behaviour disorders in Parkinson's disease. Brain. 2013;136:2120–2129. doi: 10.1093/brain/awt152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Uchiyama M, Isse K, Tanaka K, Yokota N, Hamamoto M, Aida S, Ito Y, Yoshimura M, Okawa M. Incidental Lewy body disease in a patient with REM sleep behavior disorder. Neurology. 1995;45:709–712. doi: 10.1212/wnl.45.4.709. [DOI] [PubMed] [Google Scholar]
- 136.Zambelis T, Paparrigopoulos T, Soldatos CR. REM sleep behaviour disorder associated with a neurinoma of the left pontocerebellar angle. J Neurol Neurosurg Psychiatry. 2002;72:821–822. doi: 10.1136/jnnp.72.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Provini F, Vetrugno R, Pastorelli F, Lombardi C, Plazzi G, Marliani AF, Lugaresi E, Montagna P. Status dissociatus after surgery for tegmental ponto-mesencephalic cavernoma: a state-dependent disorder of motor control during sleep. Mov Disord. 2004;19:719–723. doi: 10.1002/mds.20027. [DOI] [PubMed] [Google Scholar]
- 138.Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, Trojanowski JQ, Lee VM. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Recasens A, Dehay B, Bove J, Carballo-Carbajal I, Dovero S, Perez-Villalba A, Fernagut PO, Blesa J, Parent A, Perier C, et al. Lewy body extracts from Parkinson disease brains trigger alpha-synuclein pathology and neurodegenerationin mice and monkeys. Ann Neurol. 2014;75:351–362. doi: 10.1002/ana.24066. [DOI] [PubMed] [Google Scholar]
- 140.VanderHorst VG, Samardzic T, Saper CB, Anderson MP, Nag S, Schneider JA, Bennett DA, Buchman AS. alpha-Synuclein pathology accumulates in sacral spinal visceral sensory pathways. Ann Neurol. 2015;78:142–149. doi: 10.1002/ana.24430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ulusoy A, Phillips RJ, Helwig M, Klinkenberg M, Powley TL, Di Monte DA. Brain-to-stomach transfer of alpha-synuclein via vagal preganglionic projections. Acta Neuropathol. 2017;133:381–393. doi: 10.1007/s00401-016-1661-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chandra R, Hiniker A, Kuo YM, Nussbaum RL, Liddle RA. alpha-Synuclein in gut endocrine cells and its implications for Parkinson's disease. JCI Insight. 2017;2 doi: 10.1172/jci.insight.92295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Braak H, Del Tredici K, Rub U, de Vos RAI, Steur E, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 144.Vilas D, Iranzo A, Tolosa E, Aldecoa I, Berenguer J, Vilaseca I, Marti C, Serradell M, Lomena F, Alos L, et al. Assessment of alpha-synuclein in submandibular glands of patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case-control study. Lancet Neurol. 2016;15:708–718. doi: 10.1016/S1474-4422(16)00080-6. [DOI] [PubMed] [Google Scholar]
- 145.Scammell TE. Narcolepsy. N Engl J Med. 2015;373:2654–2662. doi: 10.1056/NEJMra1500587. [DOI] [PubMed] [Google Scholar]
- 146.Guilleminault C, Raynal D, Takahashi S, Carskadon M, Dement W. Evaluation of short-termand long-term treatment of the narcolepsy syndrome with clomipramine hydrochloride. Acta Neurol Scand. 1976;54:71–87. doi: 10.1111/j.1600-0404.1976.tb07621.x. [DOI] [PubMed] [Google Scholar]
- 147.Ristanovic RK, Liang H, Hornfeldt CS, Lai C. Exacerbation of cataplexy following gradual withdrawal of antidepressants: manifestation of probable protracted rebound cataplexy. Sleep Med. 2009;10:416–421. doi: 10.1016/j.sleep.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 148.Wu MF, Gulyani SA, Yau E, Mignot E, Phan B, Siegel JM. Locus coeruleus neurons: cessation of activity during cataplexy. Neuroscience. 1999;91:1389–1399. doi: 10.1016/s0306-4522(98)00600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hong SB, Tae WS, Joo EY. Cerebral perfusion changes during cataplexy in narcolepsy patients. Neurology. 2006;66:1747–1749. doi: 10.1212/01.wnl.0000218205.72668.ab. [DOI] [PubMed] [Google Scholar]
- 150.Maquet P, Peters J, Aerts J, Delfiore G, Degueldre C, Luxen A, Franck G. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383:163–166. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- 151.Burgess CR, Peever JH. A noradrenergic mechanism functions to couple motor behavior with arousal state. Curr Biol. 2013;23:1719–1725. doi: 10.1016/j.cub.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 152.Siegel JM, Nienhuis R, Fahringer HM, Paul R, Shiromani P, Dement WC, Mignot E, Chiu C. Neuronal activity in narcolepsy: identification of cataplexy-related cells in the medial medulla. Science. 1991;252:1315–1318. doi: 10.1126/science.1925546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Snow MB, Fraigne JJ, Thibault-Messier G, Chuen VL, Thomasian A, Horner RL, Peever J. GABA cells in the central nucleus of the amygdala promote cataplexy. J Neurosci. 2017;37:4007–4022. doi: 10.1523/JNEUROSCI.4070-15.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gulyani S, Wu MF, Nienhuis R, John J, Siegel JM. Cataplexy-related neurons in the amygdala of the narcoleptic dog. Neuroscience. 2002;112:355–365. doi: 10.1016/s0306-4522(02)00089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mahoney CE, Agostinelli LJ, Brooks JN, Lowell BB, Scammell TE. GABAergic neurons of the central amygdala promote cataplexy. J Neurosci. 2017;37:3995–4006. doi: 10.1523/JNEUROSCI.4065-15.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hasegawa E, Maejima T, Yoshida T, Masseck OA, Herlitze S, Yoshioka M, Sakurai T, Mieda M. Serotonin neurons in the dorsal raphe mediate the anticataplectic action of orexin neurons by reducing amygdala activity. Proc Natl Acad Sci USA. 2017;114:E3526–E3535. doi: 10.1073/pnas.1614552114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pintwala S, Peever J. Circuit mechanisms of sleepiness and cataplexy in narcolepsy. Curr Opin Neurobiol. 2017;44:50–58. doi: 10.1016/j.conb.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 158.Nightingale S, Orgill JC, Ebrahim IO, de Lacy SF, Agrawal S, Williams AJ. The association between narcolepsy and REM behavior disorder (RBD) Sleep Med. 2005;6:253–258. doi: 10.1016/j.sleep.2004.11.007. [DOI] [PubMed] [Google Scholar]