Abstract
Mycobacterium tuberculosis is the causative agent of tuberculosis (TB). It acquires phenotypic drug resistance inside macrophages, and this resistance mainly arises from host-induced stress. However, whether cellular drug-efflux mechanisms in macrophages contribute to nonresponsiveness of M. tuberculosis to anti-TB drugs is unclear. Here, we report that xenobiotic nuclear receptors mediate TB drug nonresponsiveness by modulating drug-efflux transporters in macrophages. This was evident from expression analysis of drug-efflux transporters in macrophages isolated from TB patients. Among patients harboring rifampicin-susceptible M. tuberculosis, we observed increased intracellular survival of M. tuberculosis upon rifampicin treatment of macrophages isolated from patients not responding to anti-TB drugs compared with macrophages from patients who did respond. Of note, M. tuberculosis infection and rifampicin exposure synergistically modulated macrophage drug-efflux transporters in vitro. We also found that the xenobiotic nuclear receptor pregnane X receptor (PXR) modulates macrophage drug-efflux transporter expression and activity, which compromised the anti-TB efficacy of rifampicin. We further validated this finding in a TB mouse model in which use of the PXR antagonist ketoconazole rescued rifampicin anti-TB activity. We conclude that PXR activation in macrophages compromises the efficacy of the anti-TB drug rifampicin. Alternative therapeutic strategies, such as use of the rifampicin derivatives rifapentine and rifabutin, which do not activate PXR, or of a PXR antagonist, may be effective for tackling drug nonresponsiveness of M. tuberculosis that arises from drug-efflux systems of the host.
Keywords: drug resistance, drug transport, Mycobacterium tuberculosis, nuclear receptor, tuberculosis, Drug nonresponsiveness, PXR
Introduction
Mycobacterium tuberculosis is the primary causative agent of human tuberculosis (TB)2 and is responsible for maximum deaths than any other single bacterial pathogen today. The current choice for TB treatment is the use of chemotherapeutic drugs against various molecular targets of the pathogen. The greatest challenge in the treatment of TB is the rapid emergence of drug-resistant M. tuberculosis. Therefore, there is an urgent need to study the dynamics of drug resistance and to develop therapeutic strategies that can reduce the chances of development of drug-resistant pathogens (1). The present treatment for TB comprises of combination of chemotherapeutic drugs like isoniazid, rifampicin, pyrazinamide, and ethambutol for at least six months to achieve acceptable cure and relapse rates (2). However, a substantial variability in response to TB therapy is observed, even in patients harboring drug-susceptible strains (3). In some patients, M. tuberculosis is rapidly eradicated and patients are cured within 2–3 months of chemotherapy. Yet, in others, viable bacilli persist in sputum for considerably more time, despite being drug-susceptible in vitro. The observed differential response to anti-TB drugs among individuals is poorly understood and could be emanating from host factors such as xenobiotic sensing nuclear receptors, drug-metabolizing enzymes, and drug-efflux transporters (4–7). The majority of drug-metabolic processes take place in enterohepatic tissues (liver and intestine), as the drug-metabolizing enzymes that facilitate these processes are highly expressed in these tissues. The expression and activity of such enzymes in non-enterohepatic tissues is very little and here the drug response is majorly regulated by drug-efflux transporters (8–11). In addition to this, microRNAs have been implicated in drug response and chemotherapeutic resistance by modulating the expression of drug-metabolizing enzymes and transporters through post-transcriptional regulation (12). Previously, it has been reported that M. tuberculosis acquires drug nonresponsiveness inside macrophages through induction of its intrinsic drug-efflux transporters (13). However, macrophage innate drug-efflux mechanisms have not been addressed so far. In this study, we explored the host–pathogen and drug interactions in macrophages to identify the host cell factors contributing to drug nonresponsiveness. Our results highlight the role of host cell xenobiotic nuclear receptor pregnane X receptor (PXR) and macrophage drug-efflux transporters in the differential drug responsiveness observed in patients infected with drug-susceptible M. tuberculosis. In addition to this, we have also demonstrated that infection of macrophages with M. tuberculosis and treatment with anti-TB drug rifampicin modulates the expression of macrophage drug-efflux transporters through PXR. Our in vitro observations have been further validated in an in vivo mouse model of TB infection.
Results
Anti-tuberculosis efficacy of rifampicin is compromised in drug nonresponders harboring the drug-susceptible M. tuberculosis
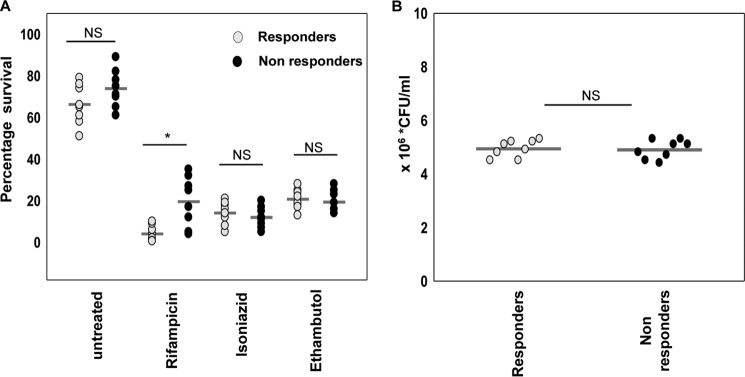
To understand the contribution of host cell determinants in the therapeutic outcome of TB, patients were divided into two categories, responders and nonresponders based on the sputum conversion from Acid-Fast Bacilli positive (AFB+) to Acid-Fast Bacilli negative (AFB−) after two months (intensive phase) of directly observed treatment, short course (DOTS) therapy. AFB+ and AFB− patients were considered as nonresponders and responders, respectively. The patients harboring drug-resistant bacteria were excluded from the study. The drug susceptibility of the M. tuberculosis was evaluated by culturing as well as by a multidrug-resistant TB rapid genotypic test of sputum as per Revised National Tuberculosis Control Programme (RNTCP) guidelines. We evaluated the intracellular survival of M. tuberculosis in human monocyte-derived macrophages (hMDMs) isolated from responders and nonresponders in the presence or absence of rifampicin, isoniazid, or ethambutol by a colony-forming unit (cfu) assay. M. tuberculosis survival was significantly higher in macrophages treated with rifampicin, isolated from nonresponders as compared with responders (Fig. 1A). However, we did not find any difference in M. tuberculosis survival in macrophages treated with the other two frontline drugs isoniazid and ethambutol. The uptake of M. tuberculosis was assessed after 4 h of infection in the absence of drug and was found similar in both study groups (Fig. 1B). The above data suggests that host cell mechanisms influence the anti-mycobacterial efficacy of rifampicin and the intracellular M. tuberculosis survival, despite the intrinsic susceptibility of the bacteria to rifampicin.
Figure 1.
Anti-tuberculosis efficacy of rifampicin, isoniazid, and ethambutol against the intracellular survival of M. tuberculosis in macrophages isolated from drug responders and nonresponders. A, intracellular survival of drug-susceptible M. tuberculosis in hMDMs of TB drug responding (n = 8) or nonresponding (n = 8) patients in the absence or presence of frontline anti-TB drugs (rifampicin, isoniazid, or ethambutol) for 48 h. B, intracellular bacterial load in hMDMs of responders and nonresponders following 4 h of infection in the absence of drug. Bacterial survival was measured by a cfu assay. Horizontal lines represent the mean. *, p < 0.05 by the Mann-Whitney test; NS, non significant.
Expression and activity of macrophage drug-efflux transporters are modulated by M. tuberculosis infection and exposure to rifampicin
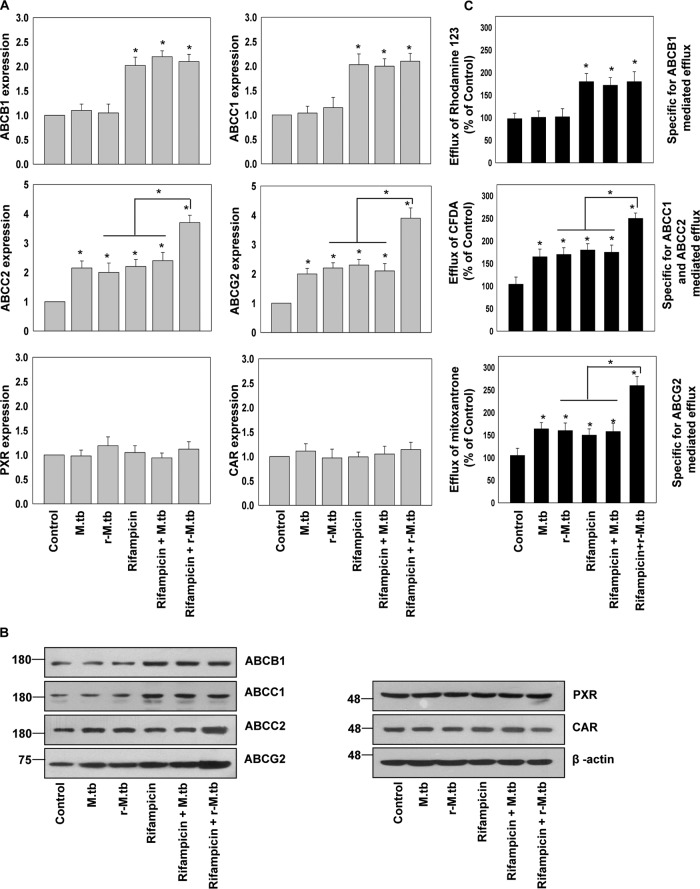
It has been reported that macrophage drug-efflux transporters are likely to play a crucial role in drug responsiveness in intracellular infections (14–16). We monitored the expression of P-glycoproteins/ATP-binding cassette transporter subfamily B member 1 (P-gp/ABCB1); multidrug-resistance associated proteins (MRP1/ABCC1 and MRP2/ABCC2), and breast cancer-resistant protein (BCRP/ABCG2), the prototype drug-efflux transporters by quantitative real-time PCR (qRT-PCR) and immunoblot analysis in hMDMs (isolated from healthy donors) infected with rifampicin-sensitive or -resistant M. tuberculosis and treated with or without rifampicin (Fig. 2, A and B). A dynamic host–pathogen and host–drug interaction was discovered.
Figure 2.
Expression and activity of macrophage drug-efflux transporters are modulated by M. tuberculosis infection and exposure to rifampicin. A, qRT-PCR. B, immunoblot analysis of ABC transporters (ABCB1, ABCC1, ABCC2, and ABCG2) and xenobiotic nuclear receptors (PXR and CAR) in hMDMs isolated from healthy volunteers infected with rifampicin sensitive or resistant M. tuberculosis (r-M.tb) and treated with or without rifampicin. C, rhodamine 123, CFDA, and mitoxantrone efflux potential of hMDMs isolated from healthy volunteers infected with rifampicin-sensitive or -resistant M. tuberculosis (r-M.tb) and treated with or without rifampicin. Data are mean ± S.D. from three independent experiments each performed in triplicate. *, p < 0.05 by two-tailed Student's t test.
We observed an increased expression of ABCC2 and ABCG2 but not of ABCB1 and ABCC1 in macrophages infected with rifampicin-sensitive or -resistant M. tuberculosis (Fig. 2, A and B). However, rifampicin treatment up-regulates the expression of all four transporters. Furthermore, the expression of ABCC2 and ABCG2 was significantly increased in macrophages infected with a rifampicin-resistant strain and cotreated with rifampicin as compared with either of them (Fig. 2, A and B). This synergistic effect was not observed in macrophages infected with the rifampicin-sensitive strain. This could be due to the anti-microbial activity of rifampicin in macrophages infected with a rifampicin-sensitive strain. Furthermore, we also looked for the expression of primary xenobiotic sensing nuclear receptors PXR and constitutive androstane receptor (CAR), which are reported to transcriptionally regulate drug-efflux transporters in various tissues (17, 18). However, we did not observe any significant change in the expression of PXR and CAR in M. tuberculosis (rifampicin-sensitive or -resistant) infection or rifampicin treatment (Fig. 2, A and B). We further monitored the functional activity of these transporters by their ability to efflux drug-like fluorescent molecules: rhodamine 123 (substrate for P-gp), 5,6-carboxyfluorescein diacetate (CFDA; specific for MRP-mediated efflux), and mitoxantrone (substrate for ABCG2) in hMDMs infected with rifampicin-sensitive or -resistant M. tuberculosis and treated with or without rifampicin (Fig. 2C). We observed that M. tuberculosis (rifampicin-sensitive or -resistant) infected hMDMs were more efficient in the efflux of mitoxantrone and CFDA but not rhodamine 123 when compared with uninfected control. Moreover, efflux of mitoxantrone and CFDA was further increased in rifampicin-resistant M. tuberculosis-infected hMDMs treated with rifampicin (Fig. 2C). This clearly suggests that M. tuberculosis infection and rifampicin treatment synergistically modulates the expression and activity of some of the macrophage prototype drug-efflux transporters.
M. tuberculosis infection and rifampicin treatment modulates the macrophage drug-efflux potential by modulating the ABC transporters expression through xenobiotic nuclear receptor
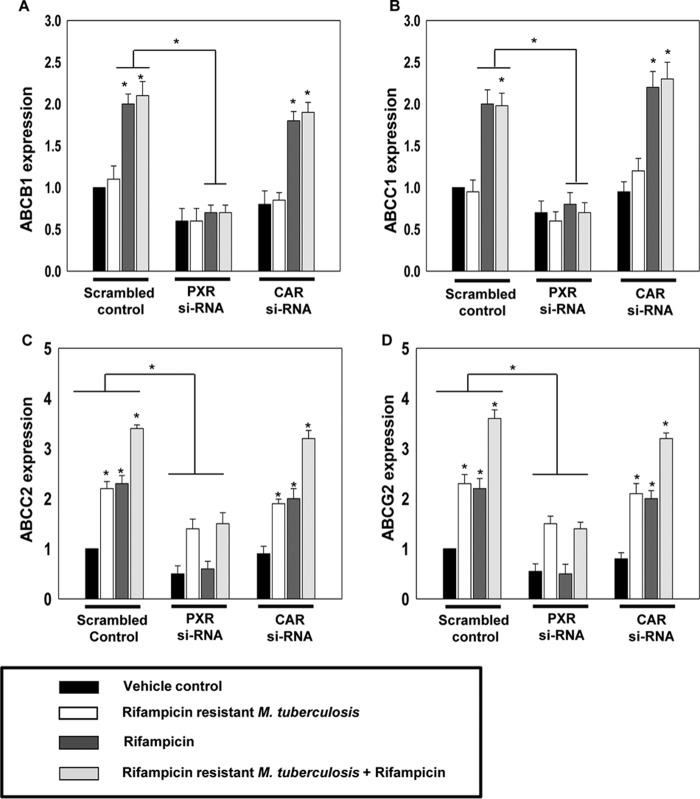
As stated above, PXR and CAR are known to regulate the expression of drug-efflux transporters and rifampicin is known to activate both PXR and CAR (19). Also, activated PXR and CAR leads to induction of a set of overlapping target genes (20). Therefore, we investigated the role of PXR and CAR in modulating the macrophage-efflux transporter expression induced by M. tuberculosis infection and rifampicin treatment. We monitored the expression of ABCB1, ABCC1, ABCC2, and ABCG2 in hMDMs with control, PXR, or CAR knockdown background, which were infected with rifampicin-resistant M. tuberculosis, in the presence or absence of rifampicin (Fig. 3).
Figure 3.
M. tuberculosis infection and rifampicin treatment modulates the macrophage drug-efflux potential by modulating the ABC transporters expression through xenobiotic nuclear receptor. A–D, qRT-PCR analysis of ABC transporters (ABCB1, ABCC1, ABCC2, and ABCG2) in control (Scrambled RNA), PXR knockdown (PXR si-RNA), and CAR (CAR si-RNA) knockdown hMDMs isolated from healthy volunteers infected with rifampicin-resistant M. tuberculosis (r-M.tb) and treated with or without rifampicin. Data are mean ± S.D. from three independent experiments each performed in triplicate. *, p < 0.05 compared with control or as indicated.
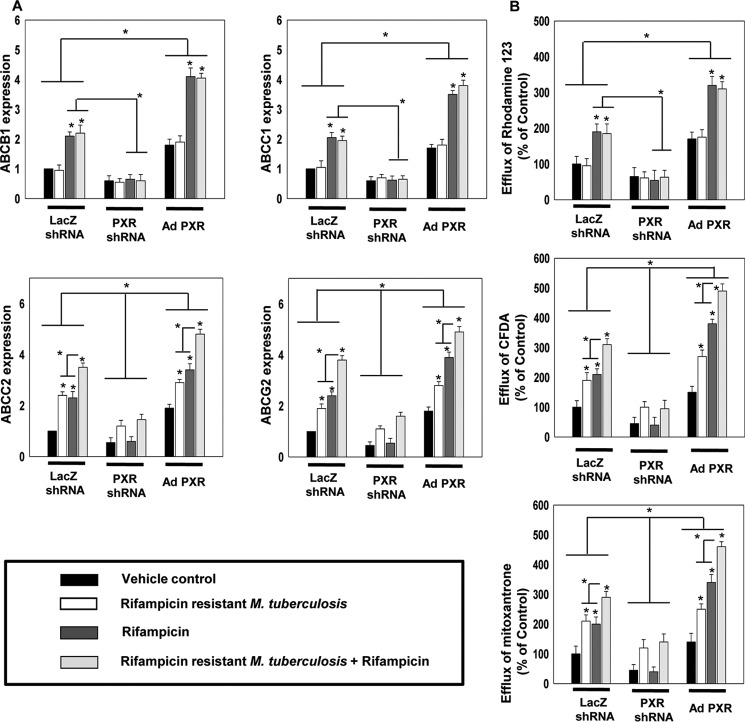
As observed earlier (Fig. 2), the expression of ABCB1 and ABCC1 was not modulated by M. tuberculosis infection but increased only upon rifampicin treatment. Interestingly, this increase was abrogated in PXR but not in CAR knockdown background (Fig. 3, A and B). On the other hand the expression of ABCC2 and ABCG2 was increased in both infected or rifampicin-treated control cells, with a synergistic increase in control infected macrophages cotreated with rifampicin. This increase was abrogated in PXR but not in CAR knockdown background (Fig. 3, C and D). This suggests that PXR but not CAR plays a crucial role in altered expression of the macrophage-efflux transporters induced by M. tuberculosis infection as well as rifampicin. Furthermore, to validate the role of PXR in the modulation of expression and activity of drug-efflux transporters in hMDMs, PXR gain and loss of function studies were done. In PXR knockdown cells, the expression and activity of drug-efflux transporters was very less in comparison to control and PXR overexpressed cells (Fig. 4, A and B). Upon comparing uninfected and infected hMDMs treated with or without rifampicin, we found that the expression of ABCC2 and ABCG2 as well as efflux of CFDA and mitoxantrone was comparatively high in infected cells than in uninfected cells that were further increased upon rifampicin treatment both in control and PXR overexpressed hMDMs but not in PXR knockdown hMDMs. This clearly suggests that the expression of PXR plays a key role in the modulation of macrophage drug-efflux transporters mediated by M. tuberculosis infection as well as rifampicin.
Figure 4.
PXR expression and activation are crucial for the macrophage drug-efflux potential modulated by M. tuberculosis infection and rifampicin treatment. A, qRT-PCR analysis of ABC transporters (ABCB1, ABCC1, ABCC2, and ABCG2) in control, PXR knockdown, and PXR overexpressing hMDMs isolated from healthy volunteers infected with rifampicin-resistant M. tuberculosis (r-M.tb) and treated with or without rifampicin. B, rhodamine 123, CFDA, and mitoxantrone efflux potential of hMDMs isolated from healthy volunteers infected with rifampicin-resistant M. tuberculosis and treated with or without rifampicin. Data are mean ± S.D. from three independent experiments each performed in triplicate. *, p < 0.05 compared with control or as indicated.
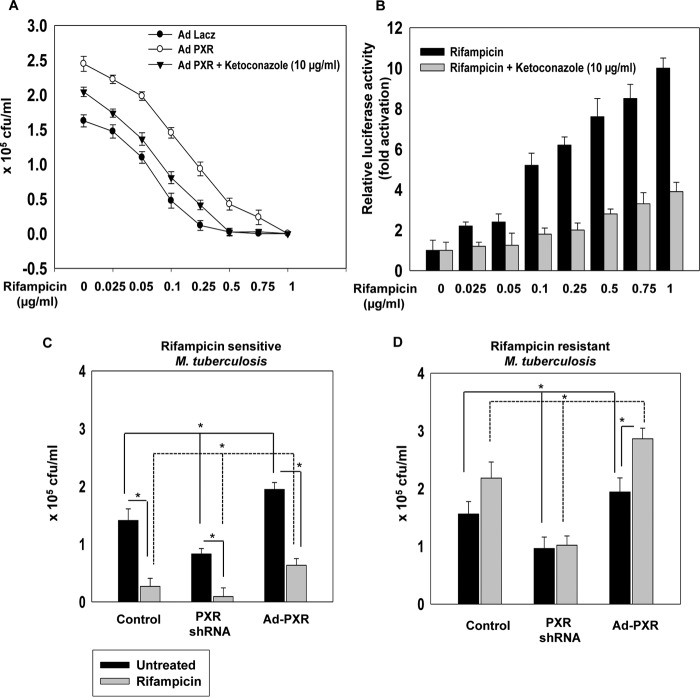
PXR contributes to nonresponsiveness to rifampicin
Given that PXR activation modulates macrophage drug-efflux potential, we investigated the function of PXR in drug-mediated intracellular M. tuberculosis clearance. We assessed the survival of rifampicin-sensitive M. tuberculosis in PXR overexpressing macrophages exposed to an increasing concentration of rifampicin in the presence or absence of ketoconazole (an antagonist of PXR) (Fig. 5A). After 48 h of infection, the intracellular survival of M. tuberculosis was evaluated by using the cfu assay. We observed an increase in the intracellular survival of M. tuberculosis as well as an increase in the minimal inhibitory concentration value of rifampicin in PXR overexpressed hMDMs as compared with LacZ control hMDMs. This increase was abrogated with ketoconazole treatment. We also verified the activation of PXR with a CYP3A4-XREM gene promoter assay and found that PXR was activated at all the used concentrations of rifampicin and this activation of PXR was abrogated when we used ketoconazole (Fig. 5B). We then selectively investigated the ability of rifampicin to cross-talk with PXR and modulate M. tuberculosis survival by employing a rifampicin-resistant M. tuberculosis strain in an intracellular bacterial survival cfu assay (Fig. 5, C and D). We compared the survival of rifampicin-sensitive and -resistant strains of M. tuberculosis in control, PXR knockdown, or PXR overexpressing hMDMs in the presence or absence of rifampicin. A significant increase in the intracellular survival of both rifampicin-resistant and rifampicin-sensitive M. tuberculosis strains was observed in PXR overexpressed hMDMs as compared with LacZ control or PXR knockdown hMDMs. Interestingly, survival of rifampicin-resistant M. tuberculosis was significantly increased upon rifampicin treatment of PXR overexpressing hMDMs. However, rifampicin-sensitive M. tuberculosis was effectively cleared in control and PXR knockdown hMDMs and to a lesser extent in PXR-overexpressing hMDMs upon treatment with rifampicin. This clearly suggests that PXR overexpression leads to nonresponsiveness to rifampicin treatment in human macrophages.
Figure 5.
Human PXR activation in hMDMs leads to drug nonresponsiveness. A, intracellular survival assay of M. tuberculosis H37Rv, exposed to an incremental concentration of rifampicin (0.025 to 1 μg/ml) in the presence and absence of ketoconazole in control and PXR overexpressing hMDMs. B, CYP3A4-XREM promoter reporter assay in COS1 cells overexpressing hPXR treated with different concentrations of rifampicin (0.025 to 1 μg/ml) in the presence or absence of ketoconazole. C and D, intracellular survival of rifampicin-sensitive (C) and rifampicin-resistant (D) M. tuberculosis as monitored by CFU assay in control, PXR knockdown, and PXR overexpressing hMDMs in the presence or absence of 0.25 μg/ml of rifampicin for 48 h. Data are mean ± S.D. from three independent experiments each performed in triplicate. *, p < 0.05 compared with control or as indicated.
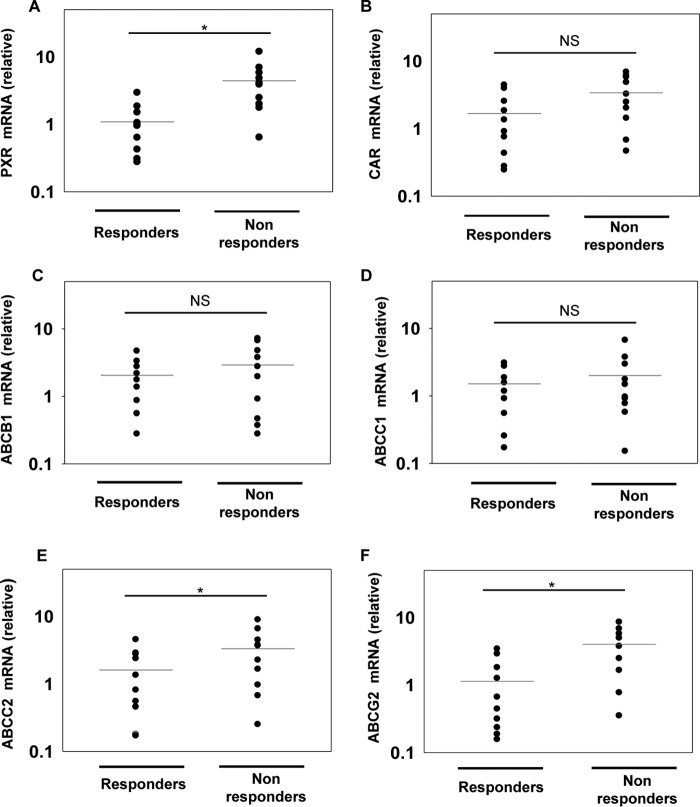
Relative expression of xenobiotic nuclear receptors and drug-efflux transporters in macrophages derived from patients responding differentially to anti-TB drugs
To expand our findings that xenobiotic nuclear receptors are responsible for drug nonresponsiveness to frontline anti-TB drug rifampicin, we extended our study to clinical samples. We compared the relative expression of PXR and CAR in macrophages isolated from patients who were responding differentially to anti-TB drugs by using qRT-PCR (Fig. 6, A–F). Interestingly, we observed a significantly higher expression of PXR in drug-nonresponsive patients when compared with drug responders (Fig. 6, A and B). In addition to PXR and CAR, we also monitored the expression of drug-efflux transporters (ABCB1, ABCC1, ABCC2, and ABCG2). Expression of ABCC2 and ABCG2 was significantly higher in drug-nonresponsive patients when compared with drug responders (Fig. 6, E and F).
Figure 6.
Relative mRNA abundance of xenobiotic nuclear receptors and drug transporters in hMDMs derived from TB patients. A–F, relative mRNA abundance of PXR, CAR, and drug transporters (ABCB1, ABCC1, ABCC2, and ABCG2) in hMDMs derived from drug responding and nonresponding TB patients. Relative mRNA abundance was calculated as 2−(ΔΔCt) relative to healthy individuals. Horizontal lines represent the mean. *, p < 0.05 by the Mann-Whitney test; NS, non significant.
Human PXR modulates drug nonresponsiveness in mice model of TB
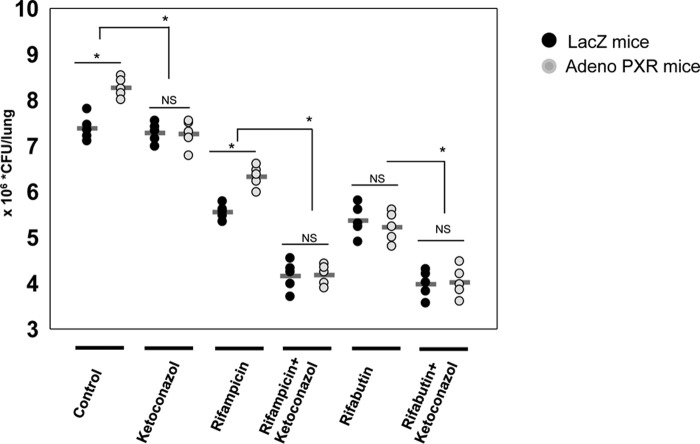
To validate our in vitro findings in an in vivo setting we used the C57BL/6 mice model of TB. We overexpressed hPXR in C57BL/6 mouse lungs using an adenoviral overexpression system. Control LacZ and hPXR-overexpressing mice were infected with rifampicin-sensitive M. tuberculosis. After 15 days of infection, mice were divided into six groups as shown in Fig. 7 and treated with the respective single drug or a combination of drugs. After 1 month of treatment the total number of bacilli in lungs was determined by cfu assay (Fig. 7). We observed a significant increase in number of bacilli in the lungs of hPXR overexpressing mice as compared with LacZ control mice and this increase was abrogated upon ketoconazole treatment, which is an antagonist of activated hPXR. Furthermore, on treatment with the frontline TB drug rifampicin or its derivative rifabutin, there was a significant decrease in the number of bacilli in the lungs as compared with untreated mice both in control LacZ and hPXR overexpressed mice. However, when we compared the anti-mycobacterial efficacy of rifampicin and rifabutin in LacZ and hPXR overexpressed mice, we observed that the efficacy of rifampicin and not rifabutin is compromised in PXR overexpressed mice as compared with LacZ mice and this effect was abrogated when mice were cotreated with ketoconazole. This could be due to the fact that rifabutin activates PXR to a lesser extent as compared with rifampicin (21). The above described observation suggests that increased expression and activity of hPXR plays a crucial role in drug nonresponsiveness to rifampicin.
Figure 7.
Expression and activity of hPXR plays a crucial role in drug nonresponsiveness to rifampicin in the mice model of TB. Humanized PXR mice model was generated by overexpression of hPXR in C57BL/6 mouse lungs using an adenoviral overexpression system and LacZ was used as a control. These control LacZ and hPXR overexpressing mice were infected with M. tuberculosis H37Rv via an aerosol challenge. After 15 days of infection the mice were divided into six treatment groups as shown and treated with the respective single or a combination of drugs. After 30 days of treatment the total number of bacilli in lungs was determined by cfu assays. Each group contained five mice. Horizontal lines represent the mean.*, p < 0.05 by the Mann-Whitney test; NS, non significant.
Discussion
The prevalence of drug-resistant TB poses a major risk to public health (22). The mechanisms by which M. tuberculosis is able to withstand the action of antibiotic therapy are highly diverse (23) and are largely defined by the genetic and epigenetic determinants of M. tuberculosis (24). Our study expands the drug-resistance mechanisms exploited by M. tuberculosis and reveals a novel interaction at the interface of infection and consequent anti-mycobacterial drug exposure. Here, we have explored the role of macrophage xenobiotic nuclear receptors in drug nonresponsiveness exhibited by individuals infected with M. tuberculosis. We found that the intracellular survival of drug-susceptible M. tuberculosis was higher in macrophages treated with rifampicin, isolated from drug nonresponders as compared with the responders (Fig. 1A). This result validates the contribution of host cell factors in anti-TB efficacy of rifampicin in TB patients who are not responding to anti-TB treatment despite of harboring drug-susceptible M. tuberculosis.
Drug-efflux transporters play a crucial role in modulating the efficacy of antimicrobial compounds that target intracellular pathogens (14–16). We found that rifampicin treatment modulates the expression and activity of macrophage drug-efflux transporters ABCB1, ABCC1, ABCC2, and ABCG2 and M. tuberculosis infection modulates ABCC2 and ABCG2 only. Furthermore, infected macrophages cotreated with rifampicin showed a synergistic increase in the expression and activity of ABCC2 and ABCG2 (Fig. 2). PXR and CAR are known to play a key role in modulating the host cell drug-efflux transporters and it has also been reported that rifampicin modulates efflux transporters by modulating the activity of PXR (17, 18, 25). Moreover, our previous study demonstrated that M. tuberculosis modulates PXR activity through its cell wall lipids (26). These reports instigated us to monitor the expression and activity of drug-efflux transporters in M. tuberculosis-infected macrophages with PXR and CAR knockdown background in the presence or absence of rifampicin. We observed a decrease in the expression of ABCB1, ABCC1, ABCC2, and ABCG2 in rifampicin-treated hMDMs with PXR knockdown as compared with control (Fig. 3). However, we observed a partial decrease in ABCC2 and ABCG2 (Fig. 3, C and D) in M. tuberculosis-infected hMDMs with PXR knockdown as compared with control. This effect could be due to some other host pathways being modulated by M. tuberculosis in addition to PXR. Furthermore, no change was observed in CAR knockdown hMDMs.
A proof of concept experiment using PXR gain of function revealed that hMDMs overexpressing PXR were more efficient in the efflux of drug-like fluorescent molecules CFDA, rhodamine 123, and mitoxantrone, as compared with the hMDMs with control and PXR knockdown background (Fig. 4B). This enhanced efflux was observed due to the increased expression of ABCB1, ABCC1, ABCC2, and ABCG2 (Fig. 4). In addition to this, a significant increase in the expression of ABCC2 and ABCG2 was observed in M. tuberculosis-infected macrophages as compared with the uninfected control, which resulted in efflux of CFDA and mitoxantrone but not rhodamine 123. Our observations also support the previous finding that bone marrow mesenchymal stem cells expressing ABCG2 provide an ideal niche for non-replicating M. tuberculosis (27). Similarly, PXR overexpressing macrophages seem to be an ideal protective niche for M. tuberculosis to evade from drug therapy.
Rifampicin, a frontline antibiotic for TB treatment also activates hPXR, therefore we investigated how hPXR expression and activity modulates the effective dosage of rifampicin treatment and intracellular survival of M. tuberculosis. Interestingly, an increase in the minimum inhibitory concentration value of rifampicin was observed in hPXR overexpressing hMDMs, which was abrogated in the presence of ketoconazole (Fig. 5A). This suggests that expression and activity of hPXR modulates the efficacy of rifampicin. This was further confirmed by using rifampicin-sensitive and -resistant strains of M. tuberculosis (Fig. 5, C and D). To the best of our knowledge, we are first to report the role of PXR in conferring TB drug nonresponsiveness, and to show that the expression of PXR correlates with TB drug nonresponsiveness. Furthermore, we profiled the expression levels of human PXR, CAR, and drug-efflux transporters in drug-responding and nonresponding TB patients (Fig. 6). We discovered that expression of PXR and ABCC2 and ABCG2 were relatively high in drug nonresponders in comparison to the drug responders harboring the drug-susceptible strain of M. tuberculosis (Fig. 6, A, E, and F). Our data suggests that the expression of PXR and its target genes may affect the tissue-specific pharmacokinetics of anti-TB drugs at the site of infection. Previously it has been reported that intracellular drug tolerance in M. tuberculosis infection is acquired through induction of its own drug-efflux transporter inside macrophages (13). Here in this study, we have shown that M. tuberculosis and host transcription factor PXR together modulate the expression of macrophage drug-efflux transporters, which in turn leads to drug nonresponsiveness. It seems that intracellular drug tolerance or drug nonresponsiveness exhibited by M. tuberculosis to frontline drugs such as rifampicin could be due to this mutual cross-regulation of drug-efflux transporters of macrophage and M. tuberculosis.
To validate our in vitro observations, we generated a humanized PXR mouse model by transducing hPXR in mouse. Comparing the efficacy of rifampicin and rifabutin in control and hPXR overexpressed mice, we observed that the efficacy of rifampicin but not rifabutin is compromised, which was restored when mice were cotreated with ketoconazole (Fig. 7). These results suggested that PXR expression and activity may affect the outcome of TB infection and TB therapy. Consequently, inclusion of a PXR antagonist in combinatorial drug treatment is likely to be a promising strategy to treat TB.
Experimental procedures
Human ethics statement
The project was approved by the Ethics Committee of the Government Medical College and Hospital, Sector 32 (Chandigarh, India), and the Ethics and Biosafety Committee of IMTECH, Sector 39A (Chandigarh, India). The study was conducted strictly in accordance with the Ethical Guidelines for Biomedical Research on Human Subjects by the Central Ethics Committee on Human Research, Indian Council of Medical Research-2000 and those as contained in the Declaration of Helsinki. Each subject was provided with written information about the study, and written consent on the consent form was obtained from each healthy volunteer before his or her induction in the study in the language (English, Hindi, and Punjabi) familiar to them (26).
Animal study and ethics statement
C57BL/6 mice (male, 6–8 weeks old) were procured from the animal facility at IMTECH and were housed at the Biosafety Level 3 (BSL3) facility of the Institute. All experiments were approved by the Institutional Animal Ethics Committee of IMTECH as stated earlier (26).
Study plan and study subjects
To assess the role of host cell determinants in the differential drug responsiveness in TB, adult patients (18–55 years) diagnosed with active TB were enrolled into the study based on certain decisive factors: (i) therapeutic response to anti-TB drugs after an initial 2 months (Intensive phase) of DOTS therapy, (ii) harboring drug-susceptible M. tuberculosis, and (iii) HIV negative. These approaches reduce the possibility of potential confounding effects of treatment failure as a result of bacterial drug resistance and disparity in therapeutic response due to altered immune functions. Patients were divided into two categories, responders and nonresponders based on the sputum conversion from AFB+ to AFB− after the initial 2 months of DOTS therapy. The drug susceptibility of the M. tuberculosis in these patients was evaluated as per the Revised National Tuberculosis Control Program guidelines. Gene expression profiling of xenobiotic nuclear receptors and drug transporters was performed to identify host cell genes with putative roles in differential drug responsiveness in TB patients. Macrophages derived from peripheral blood-derived monocytes were used for functional validation assays.
Aerosol infection and determination of M. tuberculosis burden
Experimental mice were exposed to aerosol inhalation of M. tuberculosis H37Rv (∼100 cfu/ lung, as per the standardized dose, observed after day 1 of infection) using the inhalation exposure system (Glas-Col; Terre Haute, IN) in the BSL3 facility, at IMTECH as described previously (28). Following infection, mice were divided into different treatment groups (five mice per group) and were allowed to establish the infection for 15 days. After 15 days of infection mice were treated with drugs 5 days/week for 1 month and after 1 month of treatment mice were sacrificed and lungs were homogenized in 2 ml of sterile PBS. Serial dilutions of lung homogenate were plated on Middlebrook 7H11 agar plates. Colonies were counted after 20 days of incubation at 37 °C, and cfu/lung were determined. Drugs were used in the experiments at the following concentrations: ketoconazole, 75 mg/kg; rifampicin, 10 mg/kg; and rifabutin, 150 mg/kg. Control group mice were treated with vehicle.
Bacterial strains and culture conditions
M. tuberculosis (H37Rv) was obtained from IMTECH-Microbial Type Culture Collection Chandigarh or the National Institute for Research in Tuberculosis, Chennai, India. Rifampicin-resistant M. tuberculosis (Zopf) Lehmann and Neumann (ATCC-35838) was obtained from the American Type Culture Collection (ATCC). The two strains were cultured in 7H9 broth medium (Becton Dickerson Difco Laboratories, 271310) containing 0.2% glycerol and 0.05% Tween 80. 10% Middlebrook oleic acid albumin dextrose catalase (Becton Dickerson Difco Laboratories, 211886) was added as a supplement, and the culture was incubated with shaking at 37 °C. Frozen stocks were made by resuspending the log phase cultures in sterile 7H9 broth containing 15% glycerol and stored at −80 °C until use.
Bacterial viability assays
Macrophages were washed with incomplete RPMI medium after 4 h of infection with M. tuberculosis, and incubated in complete RPMI media for 48 h. After 48 h the cells were then solubilized in 100 μl of 0.06% SDS in PBS, and the bacterial suspension was serially diluted and 100 μl of each diluted sample was plated on 7H11 agar plates. The plates were then incubated at 37 °C for 15 to 20 days and then the cfu were counted.
Luciferase reporter assays
hMDMs were transiently transfected with CYP3A4-XREM, pSG5-hPXR, and pBind plasmids for promoter reporter assay using TurboFect transfection reagent (Thermo Scientific, R0533). pBind vector having Renilla luciferase was used as transfection control. Dual luciferase activity was monitored as described previously (29). Firefly luciferase activity was normalized against Renilla luciferase and the normalized luciferase activities (relative light units) were plotted as an average of triplicate samples.
Gene silencing and overexpression
For gene silencing, we used siRNA and shRNA approachs. For overexpression studies, we used recombinant adenovirus expressing LacZ (Ad-LacZ) and hPXR (Ad-PXR), which were produced by using Adeno-X Expression System 1 (Clontech, catalogue number 631513), according to the manufacturer's instructions. For transduction in vitro, hMDMs were incubated on day 7 of culture with adenovirus in RPMI 1640 medium supplemented with 10% fetal bovine serum and penicillin/streptomycin (100 units/ml). The cells were incubated in a humidified CO2 (5%) incubator at 37 °C for 24 h, followed by aspiration of supernatant and replacement of media. Cells were further incubated for an additional 24 h before experimental assays. For in vivo studies, mice were injected in the tail vein and aerosol challenged with Ad-LacZ or Ad-PXR by using the nebulization system as described previously (26).
Efflux assay
hMDMs were seeded on a 12-well plate at a density of 5 × 105 cells/well and allowed to adhere overnight. Cells were then transduced with adenovirus either for knockdown or overexpression of hPXR. After 48 h of transduction, cells were infected with M. tuberculosis H37Rv as described under “Infection” and incubated for 48 h in the presence or absence of rifampicin. Cells were then washed with PBS and incubated in 2 μm rhodamine 123, 5 μm CFDA, or 3 μm mitoxantrone for 15 min at 37 °C. Cells were then washed with cold PBS and incubated for 30 min in 500 μl of Hanks' balanced salt solution at room temperature to allow the efflux. This conditioned Hanks' balanced salt solution was then filtered and fluorescence was measured by using Biotech synergy fluorimeter (30).
Cell culture and differentiation
Peripheral blood-derived monocytes were isolated from fresh blood using a Ficoll-Paque gradient centrifugation. Cells were incubated for 2–3 h at 37 °C and then washed with phosphate-buffered saline (PBS) to remove non-adherent cells, followed by 7 days of incubation in RPMI supplemented with 10% fetal bovine serum and macrophage colony-stimulating factor (50 ng/ml) at 37 °C and 5% CO2 (26).
Immunoblot analysis
Whole cell lysates were prepared and standard procedures were followed for immunoblotting. Briefly, equal amounts of total proteins were separated by SDS-PAGE on a 10% acrylamide gel and then transferred onto polyvinylidene difluoride membranes. The membranes were then blocked with 5% BSA for 1 h at room temperature, followed by overnight incubation at 4 °C with primary antibodies against ABCB1, ABCC1, ABCC2, ABCG2, PXR, CAR, and β-actin. Membranes were then washed three times with 1× PBST, pH 7.4, and incubated with appropriate horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature and developed with chemiluminescent horseradish peroxidase substrate.
Infection
hMDMs were infected either with M. tuberculosis H37Rv or rifampicin-resistant strains at a multiplicity of infection 1:5 (5 bacteria/cell). After 4 h, infected cells were washed three times with RPMI to remove unphagocytosed bacteria and incubated with repletion medium.
RNA isolation and qRT-PCR
Total RNA from hMDMs was isolated by the TRIzol reagent. 1 μg of RNA was reverse transcribed for cDNA synthesis and subsequently subjected to qRT-PCR using gene-specific primers. 18s rRNA was used as control. The relative abundance of the gene was calculated by using the formula 2−(ΔΔCt) as described previously (31).
Statistical analysis
Results are expressed as the mean ± S.D. unless otherwise mentioned. Sigma Plot was used for statistical analysis. Two-tailed Student's t tests and Mann-Whitney test were performed to obtain p values. Statistical significance was established at p < 0.05 (*).
Author contributions
E. B. and P. G. designed the experiments, E. B., D. T., N. A., R. N., A. S., R. K., and S. K. performed the experiments, E. B., P. G., and A. K. J. analyzed the data, E. B. and P. G. wrote the manuscript, P. G. supervised the project.
Acknowledgments
We thank the patients who participated in this study and the Government Medical College and Hospital pulmonary division staff for support in collecting blood from patients. We thank IMTECH, a constituent laboratory of the CSIR, for the facilities and financial support.
This work was supported by Department of Biotechnology-India Project BT/01/IYBA/2009, BT/HRD/NBA/37/01/2015, and Council of Scientific and Industrial research (CSIR) 12th Plan Network project Bugs to Drugs, Infectious Disease Grants BSC0211 and BSC0210 (to P. G.). The authors declare that they have no conflicts of interest with the contents of this article.
- TB
- tuberculosis
- AFB
- Acid-Fast Bacilli
- BCRP/ABCG2
- breast cancer-resistant protein
- CAR
- constitutive androstane receptor
- CFDA
- 5,6-carboxyfluorescein diacetate
- hMDM
- human monocyte-derived macrophages
- MRP1/ABCC1 and MRP2/ABCC2
- multidrug-resistance associated proteins
- P-gp/ABCB1
- P-glycoproteins/ATP-binding cassette transporter subfamily B member 1
- PXR
- pregnane X receptor
- DOTS
- directly observed treatment, short course
- qRT-PCR
- quantitative real-time PCR.
References
- 1. Mitchison D., and Davies G. (2012) The chemotherapy of tuberculosis: past, present and future. Int. J. Tuberc. Lung Dis. 16, 724–732 10.5588/ijtld.12.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Joshi J. M. (2011) Tuberculosis chemotherapy in the 21 century: back to the basics. Lung India 28, 193–200 10.4103/0970-2113.83977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Srivastava S., Pasipanodya J. G., Meek C., Leff R., and Gumbo T. (2011) Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J. Infect. Dis. 204, 1951–1959 10.1093/infdis/jir658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahmed S., Zhou Z., Zhou J., and Chen S. Q. (2016) Pharmacogenomics of drug metabolizing enzymes and transporters: relevance to precision medicine. Genomics Proteomics Bioinformatics 14, 298–313 10.1016/j.gpb.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DeGorter M. K., Xia C. Q., Yang J. J., and Kim R. B. (2012) Drug transporters in drug efficacy and toxicity. Annu. Rev. Pharmacol. Toxicol. 52, 249–273 10.1146/annurev-pharmtox-010611-134529 [DOI] [PubMed] [Google Scholar]
- 6. Prakash C., Zuniga B., Song C. S., Jiang S., Cropper J., Park S., and Chatterjee B. (2015) Nuclear receptors in drug metabolism, drug response and drug interactions. Nucl. Receptor Res. 2, 101178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sloan D. J., McCallum A. D., Schipani A., Egan D., Mwandumba H. C., Ward S. A., Waterhouse D., Banda G., Allain T. J., Owen A., Khoo S. H., and Davies G. R. (2017) Genetic determinants of the pharmacokinetic variability of rifampin in Malawian adults with pulmonary tuberculosis. Antimicrob. Agents Chemother. 61, e00210–17 10.1128/AAC.00210-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murray G. I., Barnes T. S., Sewell H. F., Ewen S. W., Melvin W. T., and Burke M. D. (1988) The immunocytochemical localisation and distribution of cytochrome P-450 in normal human hepatic and extrahepatic tissues with a monoclonal antibody to human cytochrome P-450. Br. J. Clin. Pharmacol. 25, 465–475 10.1111/j.1365-2125.1988.tb03331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Waziers I., Cugnenc P. H., Yang C. S., Leroux J. P., and Beaune P. H. (1990) Cytochrome P450 isoenzymes, epoxide hydrolase and glutathione transferases in rat and human hepatic and extrahepatic tissues. J. Pharmacol. Exp. Ther. 253, 387–394 [PubMed] [Google Scholar]
- 10. Schuetz J. D., Swaan P. W., and Tweedie D. J. (2014) The role of transporters in toxicity and disease. Drug Metab. Dispos. 42, 541–545 10.1124/dmd.114.057539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sodani K., Patel A., Kathawala R. J., and Chen Z. S. (2012) Multidrug resistance associated proteins in multidrug resistance. Chin. J. Cancer 31, 58–72 10.5732/cjc.011.10329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang W., and Dolan M. E. (2010) The emerging role of microRNAs in drug responses. Curr. Opin. Mol. Therap. 12, 695–702 [PMC free article] [PubMed] [Google Scholar]
- 13. Adams K. N., Takaki K., Connolly L. E., Wiedenhoft H., Winglee K., Humbert O., Edelstein P. H., Cosma C. L., and Ramakrishnan L. (2011) Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell 145, 39–53 10.1016/j.cell.2011.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seral C., Michot J. M., Chanteux H., Mingeot-Leclercq M. P., Tulkens P. M., and Van Bambeke F. (2003) Influence of P-glycoprotein inhibitors on accumulation of macrolides in J774 murine macrophages. Antimicrob. Agents Chemother. 47, 1047–1051 10.1128/AAC.47.3.1047-1051.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roy U., Barber P., Tse-Dinh Y. C., Batrakova E. V., Mondal D., and Nair M. (2015) Role of MRP transporters in regulating antimicrobial drug inefficacy and oxidative stress-induced pathogenesis during HIV-1 and TB infections. Front. Microbiol. 6, 948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gómez M. A., Navas A., Márquez R., Rojas L. J., Vargas D. A., Blanco V. M., Koren R., Zilberstein D., and Saravia N. G. (2014) Leishmania panamensis infection and antimonial drugs modulate expression of macrophage drug transporters and metabolizing enzymes: impact on intracellular parasite survival. J. Antimicrob. Chemother. 69, 139–149 10.1093/jac/dkt334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whyte-Allman S. K., Hoque M. T., Jenabian M. A., Routy J. P., and Bendayan R. (2017) Xenobiotic nuclear receptors Pregnane X receptor and constitutive androstane receptor regulate antiretroviral drug efflux transporters at the blood-testis barrier. J. Pharmacol. Exp. Therap. 363, 324–335 10.1124/jpet.117.243584 [DOI] [PubMed] [Google Scholar]
- 18. Amacher D. E. (2016) The regulation of human hepatic drug transporter expression by activation of xenobiotic-sensing nuclear receptors. Expert Opin. Drug Metab. Toxicol. 12, 1463–1477 10.1080/17425255.2016.1223626 [DOI] [PubMed] [Google Scholar]
- 19. Chen J., and Raymond K. (2006) Roles of rifampicin in drug-drug interactions: underlying molecular mechanisms involving the nuclear pregnane X receptor. Ann. Clin. Microbiol. Antimicrob. 5, 3 10.1186/1476-0711-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Banerjee M., Robbins D., and Chen T. (2015) Targeting xenobiotic receptors PXR and CAR in human diseases. Drug Discov. Today 20, 618–628 10.1016/j.drudis.2014.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shehu A. I., Li G., Xie W., and Ma X. (2016) The pregnane X receptor in tuberculosis therapeutics. Expert Opin. Drug Metab. Toxicol. 12, 21–30 10.1517/17425255.2016.1121381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loddenkemper R., and Hauer B. (2010) Drug-resistant tuberculosis: a worldwide epidemic poses a new challenge. Deutsches Arzteblatt Int. 107, 10–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Palomino J. C., and Martin A. (2014) Drug resistance mechanisms in Mycobacterium tuberculosis. Antibiotics 3, 317–340 10.3390/antibiotics3030317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith T., Wolff K. A., and Nguyen L. (2013) Molecular biology of drug resistance in Mycobacterium tuberculosis. Curr. Top. Microbiol. Immunol. 374, 53–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Benson E. A., Eadon M. T., Desta Z., Liu Y., Lin H., Burgess K. S., Segar M. W., Gaedigk A., and Skaar T. C. (2016) Rifampin regulation of drug transporters gene expression and the association of microRNAs in human hepatocytes. Front. Pharmacol. 7, 111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhagyaraj E., Nanduri R., Saini A., Dkhar H. K., Ahuja N., Chandra V., Mahajan S., Kalra R., Tiwari D., Sharma C., Janmeja A. K., and Gupta P. (2016) Human xenobiotic nuclear receptor PXR augments Mycobacterium tuberculosis survival. J. Immunol. 197, 244–255 10.4049/jimmunol.1600203 [DOI] [PubMed] [Google Scholar]
- 27. Beamer G., Major S., Das B., and Campos-Neto A. (2014) Bone marrow mesenchymal stem cells provide an antibiotic-protective niche for persistent viable Mycobacterium tuberculosis that survive antibiotic treatment. Am. J. Pathol. 184, 3170–3175 10.1016/j.ajpath.2014.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dkhar H. K., Nanduri R., Mahajan S., Dave S., Saini A., Somavarapu A. K., Arora A., Parkesh R., Thakur K. G., Mayilraj S., and Gupta P. (2014) Mycobacterium tuberculosis keto-mycolic acid and macrophage nuclear receptor TR4 modulate foamy biogenesis in granulomas: a case of a heterologous and noncanonical ligand-receptor pair. J. Immunol. 193, 295–305 10.4049/jimmunol.1400092 [DOI] [PubMed] [Google Scholar]
- 29. Mahajan S., Dkhar H. K., Chandra V., Dave S., Nanduri R., Janmeja A. K., Agrewala J. N., and Gupta P. (2012) Mycobacterium tuberculosis modulates macrophage lipid-sensing nuclear receptors PPARγ and TR4 for survival. J. Immunol. 188, 5593–5603 10.4049/jimmunol.1103038 [DOI] [PubMed] [Google Scholar]
- 30. Swales K. E., Moore R., Truss N. J., Tucker A., Warner T. D., Negishi M., and Bishop-Bailey D. (2012) Pregnane X receptor regulates drug metabolism and transport in the vasculature and protects from oxidative stress. Cardiovasc. Res. 93, 674–681 10.1093/cvr/cvr330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mahajan S., Saini A., Chandra V., Nanduri R., Kalra R., Bhagyaraj E., Khatri N., and Gupta P. (2015) Nuclear receptor Nr4a2 promotes alternative polarization of macrophages and confers protection in sepsis. J. Biol. Chem. 290, 18304–18314 10.1074/jbc.M115.638064 1 [DOI] [PMC free article] [PubMed] [Google Scholar]