Abstract
Purpose of the Review
Evidence is rapidly accumulating implicating gut dysbiosis in hypertension (HTN). However, we are far from understanding whether this is a cause or consequence of HTN, and how to best translate this fundamental knowledge to advance the management of HTN. This review aims to summarize recent advances in the field, illustrate the connections between the gut and hypertension, and establish that the gut microbiota (GM)-gut interaction is centrally positioned for consideration as an innovative approach for HTN therapeutics.
Recent Findings
Animal models of HTN have shown that gut pathology occurs in HTN, and provides some clues to mechanisms linking the dysbiosis, gut pathology, and HTN.
Summary
Circumstantial evidence links gut dysbiosis and HTN. Gut pathology, apparent in animal HTN models, has not been fully investigated in hypertensive patients. Objective evidence and an understanding of mechanisms could have a major impact for new antihypertensive therapies and/or improved applications of current ones.
Keywords: Hypertension, Gut microbiota, Gut pathology, Renin angiotensin aldosterone system, Brain-gut-immune axis
Introduction
The latest American Heart Association statistics for 2009–2012 note approximately 80 million US adults or a third of Americans over 20 years old have hypertension (HTN) [1]. These hypertensive patients are at a greater risk for cardiovascular disease, kidney disease, stroke, and death than normotensive subjects. Indeed, 34% of deaths in 10 years between 2003 and 2013 were attributable to high blood pressure [1]. HTN creates an enormous health and economic burden, not just in the USA, but globally. Unfortunately, despite lifestyle modifications, new therapies, and intensive medical interventions, approximately a third of hypertensive patients do not achieve blood pressure control when prescribed ≥3 medications, and have “treatment-resistant” hypertension (RHTN) [1]. Black Americans have some of the highest rates of HTN in the world, especially black women where almost half have hypertension. This disparity is worsened by earlier onset, higher blood pressures, and higher rates of RHTN [1]. Effective treatment paradigms are urgently needed for HTN, especially for RHTN, and the causes of HTN are poorly understood in many, particularly those with RHTN. This review will highlight recent developments in our understanding of the pathogenesis of HTN and will focus on a role for the gut.
Potential for the Involvement of the Gut in Blood Pressure Control and HTN
Gastrointestinal Tract Anatomy and Ecology
The gastrointestinal tract has distinct structures, functions, motility and mucin layers along its length. It is also one of the few organs where the epithelial cell lining is capable of regeneration every 5 days. These properties provide a dynamic environment and varied ecological niches for bacteria to exploit along the length of the tract. This results in different types and communities of bacteria residing at different levels. There are not only bacteria inhabiting these ecological niches but also viruses and in some people fungi and parasites. The complexity of the mix is influenced by the gut itself which secretes antimicrobial proteins, such as defensins, to favor certain bacteria over others. A healthy gut maintains a balance of these, often competing, elements. Thus, the gut microbial community plays a key role in maintaining normal physiological homeostasis in the host.
Gut epithelium has a vast surface area, necessary for efficient absorption and secretion. This large surface area presents a challenge to maintain its epithelial barrier function and it is estimated that ∼70% of the body’s immune cells reside in the gut, constituting the “gut associated lymphatic tissue” (GALT). The GALT moderates the continuous interactions of the bacterial communities and their byproducts with the gut [2]. The gut microbiome (GM) interacts with the immune system of the host to “educate” it, as germ-free mice have a defective immune system [3]. This “education”, begins perinatally and continues until about 3 years of age in humans. The result is a symbiosis between the immune status and the GM that is beneficial and balanced to both sides, e.g., eubiosis. This eubiosis is very stable such that perturbations to the system are generally only short term, [4]. There are circadian and seasonal rhythms to the GM, but in general, the GM is considered imperturbable. The GM of an individual is stable, but there are differences between individuals, and even greater differences between individuals living in different communities. Occasionally, this eubiosis is thrown into disarray, resulting in dysbiosis with major pathophysiological consequences to the host.
The notion that the gut and its contents influence blood pressure has been in existence for more than half a century based on epidemiological studies. These studies identified salt [5], and alcohol [6] intake, the hyperglycemia related to increased carbohydrate consumption [7], and a lack of fiber in the diet [8] as risk factors for HTN. Their common element is that the gastrointestinal tract is the initial point of contact of these dietary elements with the body. Connections between the gut and HTN are further suggested by the important interactions of the gut and its contents with the immune system, the afferent and efferent portions of the autonomic nervous system, pre-autonomic brain areas and the enteric nervous system (ENS), and the renal axis [9]; all of which become dysregulated in HTN. Life-style choices and illnesses that affect the risk for developing HTN, impact the GM and gut motility. Finally, dysbiosis of the GM has recently been linked to metabolic HTN [10], HTN during obese pregnancies [11], lifetime risk of developing HTN [12••], and found in pre-hypertensive as well as hypertensive populations of Asian descent [13••]. These connections and their link to HTN is the focus of this review.
Gut Microbiota Metabolites and Their Effects on Blood Pressure
GM produce unique metabolites that are potentially important in BP control. Some of these metabolites with clear links to blood pressure regulation are discussed. The bacteria of the GM are the only source of short chain fatty acids (SCFA) for the body. They are predominantly acetate, proprionate and butyrate, and are derived from the digestion of dietary fiber by the GM. SCFA are an energy source for colonic epithelium, influencing cell growth, gut motility [14] and proliferation to maintain the gut barrier [15]. They epigenetically alter epithelial cells to effect transcription, for example acting on histone deacetylases [16]. They bind to the aryl hydrocarbon receptor (AHR) to increase transcription of IL10R via an AHR element in the IL10R promotor to reduce inflammation in ileal epithelial cells [17]. Furthermore, intestinal immunity is under regulation of AHR signaling [18]. Polymorphisms of AHR are associated, with other genes in its signaling pathway, with essential hypertension [19]. SCFA bind the olfactory receptors gpr41, gpr43, and olfO79 in the kidney, heart, sympathetic ganglia, and blood vessels to modulate blood pressure [20, 21]. SCFA modify gut motility by actions on the ENS [22]. And finally, the lack of butyrate due to depletion of butyrate producing bacteria in the GM was identified as a factor increasing blood pressure in obese pregnant women. SCFA maintain the epithelial barrier to reduce inflammation, directly affect immune cells and reduce sympathetic nerve activity. Thus, the GM directly mediates the effects of dietary fiber, a known modulator of risk for HTN, as well as blood pressure per se. Collectively, these findings support the hypothesis that SCFA, the products of GM digestion of dietary fiber, affect immune-, epithelial-, nervous system-, and blood vessel function to modulate blood pressure and mediate the decrease in the risk of HTN due to a fiber rich diet.
Formate and alanine serve as urinary markers of blood pressure and cardiovascular disease risk, especially as related to diet and the amount of animal protein consumed, [23]. Toxic byproducts of the microbiota produced by the metabolism of phosphatidylcholine to trimethylamine N-oxide also increase blood pressure [24]. Products of the GM, such as hydrogen sulfate, can directly act on blood vessels to modulate blood pressure [25]. Thus, by-products of gut bacterial metabolism have direct effects on blood pressure.
Tryptophan and its metabolites are important in host-GM communication. Firstly, they are precursors of neurotransmitters and directly affect the autonomic and ENS. Tryptophan metabolites can readily traverse the blood brain barrier (BBB) to influence the inflammatory status in the brain, as can SCFA and neuroactive steroids made by the GM. Neuroinflammation and its link to the GM have been demonstrated in many neurological diseases/disorders, for example multiple sclerosis [26], and hypertension, [27]. Therefore, GM dysbiosis may modulate centrally acting GM metabolites to promote neuroinflammation, a state strongly associated with HTN in animal models. The finding that a centrally penetrating anti-inflammatory antibiotic, minocycline, produced long-lasting blood pressure reduction in a hypertensive individual [28] suggests that GM metabolites may also promote neuroinflammation, and the related human pathology. Secondly, the tryptophan metabolite kynurenine acts on ileal epithelial cells to promote wound healing [17], while another metabolite, tryptophol, modulates release of IFN gamma after LPS stimulation [29]. GM metabolites can influence mitochondrial function. For example, pieces of mitochondrial DNA in the CSF cause HTN through activation of TGFß-mediated pathways and neuroinflammation [30]; propionate influences the TCA cycle and secondarily carnitine metabolism in mitochondria to cause dysfunction [31]. To summarize, GM-mediated tryptophan metabolism supports the epithelia of the ileum by increasing expression of receptors for anti-inflammatory cytokines and modulates immune responses to stimuli such as LPS. Microbiota-derived metabolites such as SCFA, neurosteroids and of tryptophan modulate central glial activation, a hallmark of HTN in animal models and possibly some humans.
Gut-Immune Interactions
Germ-free mice have an altered microglial population in their brains that is associated with defects in innate immune responses [3]. These deficits were recapitulated by conventional mice treated with antibiotics and mice raised with reduced gut microbial diversity, [3], suggesting that there must be a continuous conversation between the immune system and GM metabolites to maintain a normally responsive immune system. GM dysbiosis accompanies HTN in rodents [32•, 33, 34] and alterations in the human GM occur in the prehypertensive state [13••]. The microbiome of the human prehypertensive subject is very similar to that of the hypertensive patient. This suggests that changes in the GM precede the onset of HTN. Furthermore, FMT from hypertensive humans to germfree mice resulted in increased blood pressure [13••]. It is not clear whether GM metabolites increase blood pressure, whether the gut needs to be colonized with HTN-associated bacteria to effect HTN, or if the FMT included bacteria harboring lysogenic viruses that infected the host to increase gut inflammation and permeability. However, the implication is that GM would have a different interaction with the immune system in the prehypertensive and hypertensive states compared to the normotensive. Systemic immune system dysregulation is a hallmark of HTN in animal models and humans [35] and neuroinflammation in blood pressure-relevant brain areas has been demonstrated in animal models of HTN [27]. This results from both resident microglial activation and recruitment of precursors from bone marrow that differentiate once in place in the brain [27]. Whether this inflammation results from a prohypertensive signal from the brain, the gut or the bone marrow, or a combination of these is unknown. Humans known to have “leaky guts” with stimulated immune responses, gut fibrosis, etc. for example inflammatory bowel disease sufferers, are less likely than the general population to be hypertensive [36]. So, the simple explanation of HTN resulting from immune stimulation by bacteria leaking into the host is neither accurate nor does it arise directly from the signal of gut inflammation. Taken together, these studies indicate that chronic systemic inflammation resulting from immune activation is associated with HTN; dysbiosis of the gut microbiota and the consequent immune responses likely contribute to this inflammation.
The GM is remarkably stable in the adult, [4, 37], but is malleable in early childhood [38], so environmental or lifestyle changes in adulthood are unlikely to manipulate the GM to cause a long-term outcome like HTN. So how could this GM dysbiosis-linked inflammation arise? The DOHaD (developmental origins of health and disease) hypothesis, that posits that non-communicable diseases result from early life influences, was initially based on epidemiological studies showing that low birth weight infants were more likely to die from CAD and CVD in later life than normal birth weight infants [39, 40]. The intrauterine environment coupled with early postnatal exposures contribute to this susceptibility [41]. It is tempting to suggest that the GM-gut-immune-brain axis is incorrectly established in early life in these small-for-gestational age infants resulting in a predisposition towards HTN, but this hypothesis remains to be tested. A recent study found the lifetime risk of cardiovascular disease to be associated with six bacterial genera and overall microbial richness, [12••]. It would be very helpful to better understand when these GM conditions are achieved in the lifespan and their stability, to be able to propose new HTN treatment options. Could, for example, an undernourished mother pass epigenetic markers to her offspring that predispose to a gut-GM-immune-brain interaction favoring HTN development? Is the gut microbiome truly as stable over the lifespan as it appears from the limited studies reported or could HTN modify or be modified by changes over the lifespan? Therefore, perinatal and early life conditions influence the risk of developing HTN, and this may occur through modulation of the establishment of the gut-immune brain axis. In summary, gut-GM-immune interactions are altered during HTN.
Enteric Nervous System, GM, and Autonomic Nervous System Interactions
The ENS, the nervous system of the gut, is a plexus primarily utilizing the neurotransmitter, serotonin, produced from the precursor tryptophan. It controls gut motility in collaboration with the autonomic nervous system. The GM is an important component; depleting the GM of mice with antibiotics induced changes in host serotonin biosynthesis and intestinal motility [42]. Constipation, a result of changed gut motility, is linked to HTN in postmenopausal women [43] and in men with chronic kidney disease and end stage renal disease [44, 45]. The ENS communicates with the central nervous system, receiving sympathetic and parasympathetic input to the plexus, as well as neuro-hormonal input. In return, it sends neural and neuro-hormonally coded information to the central nervous system. The GM metabolizes tyrosine with potential to alter sympathetic transmitters (dopamine, norepinephrine, and epinephrine) and contribute to sympathetic dysregulation, another hallmark of HTN. These findings demonstrate that ENS activity is altered in HTN.
Autonomic system: Altered sympathetic nervous system activity is associated with HTN in humans and animal models of HTN. Rodent studies describe a change in microbiota secondary to dysfunctional autonomic nervous system activity (ANS) [32•] but it is not clear which occurs first in people. Metabolites of amino acids produced by actions of the GM, such as tryptophan metabolites, and the glutamate metabolite, GABA, can directly impact the central and peripheral nervous system, [46, 47, 48]. Many of these metabolites are freely accessible centrally as well as systemically and have potential to influence blood pressure at multiple sites in the cardiovascular control centers of the brain, as well as peripherally. SCFA also influence ENS activity that in turn signals to the CNS, alterations of this interaction can impact sympathetic activity. The autonomic nervous system integrates input from the ENS and GM metabolites at peripheral and central sites to change autonomic system activity and blood pressure. Dysregulated autonomic nervous system activity is coincident with, or precedes, HTN [33••], and the ENS under the influence of a dysbiotic GM contributes to this dysregulation.
Gut Pathology Related to HTN
There are few studies of gut pathology related to hypertension in the human, barring a potentially-related finding of decreased blood flow in pro-inflammatory states [49]. This is an area that deserves more study in the wake of recent findings in rodent models of HTN. The SHR, prior to increased blood pressure, reveals both increased sympathetic activity to the gut, and a decrease in tight junction proteins that are essential for the barrier function of gut epithelium. As HTN becomes established, gut pathology becomes more pronounced, with increased permeability, increased stiffness, fibrosis and muscle thickness, and decreased goblet cells and villi length in the small intestine. Similar changes occur in the colon. In the chronic angiotensin II (Ang II)-infusion model of HTN, the pathology is almost the same but with a smaller loss of goblet cells and no changes in the length of villi, perhaps related to the shorter time period of HTN. ACE inhibition with captopril in the SHR reversed the changes including those on sympathetic activity, but not those on goblet cells; however, captopril had no effect on the gut of the WKY, in which there was a relatively smaller decrease in blood pressure [33••]. The implications here are either that the active molecule is Ang II, since captopril prevents the conversion of Ang I to Ang II; or, that high blood pressure is directly related to the gut pathology. Hematopoietic stem cells treated with Ang II had altered differentiation potential and reduced homing capacity [50], suggesting that Ang II rather than high blood pressure is causative at least for the immune dysregulation in Ang II-induced HTN. Ang II has been shown to cause matrix accumulation, inflammation and apoptosis via TGF-ß and its downstream signaling molecules in the kidney [51], but whether this is true in the gut is unknown. If gut pathology precedes HTN in humans or even if it occurs with established HTN remains to be investigated. In summary, gut pathology precedes HTN in animal models, but has not been investigated in patients with hypertension.
Gut RAAS and HTN
The gut has a local renin angiotensin aldosterone system that is important for the uptake of sodium and water from the colon and for the control of gut contractility. Key members of both arms of the RAAS are present in the gut. The effector arm includes the angiotensin type I receptor (AT1R), angiotensin converting enzyme (ACE), angiotensin II (Ang II), aldosterone, and the mineralocorticoid receptor (MR), and its counter regulatory system consists of angiotensin converting enzyme 2 (ACE2), the Mas receptor (MAS1R), the angiotensin type 2 receptor (AT2R), and angiotensin 1–7 (Ang1–7). There are high levels of the AT1R and MR in colonic epithelium, but low or non-existent levels of AT2R or MAS1R (the receptor for Ang1–7) [52], although injury increases the expression of the MAS1R [53]. The AT1R is expressed solely in enteroendocrine L-cells that make Glucagon like Peptide 1 (Glp1) and PYY, and affects gut epithelial flux of anions and fluid [52], as does the MR. The AT1R also modulate gut contractility. This arm of the RAS is important for water and salt movement across the gut, but can be pro-hypertensive and pro-inflammatory if in imbalance with the RAAS counter-regulatory arm. Some beneficial actions of antihypertensive drugs that act on the RAAS are caused by altering these effects in the gut. For example, Losartan®, the AT1R blocker, decreases the number of AT1R in the gut and alters gut motility [54].
ACE2 is located primarily in the epithelium of the small intestine [55]; there is little to no RNA expression for ACE2 in the colon and the protein was not detectable there by immunohistochemistry. ACE2 acts as an anti-inflammatory agent by increasing Ang1–7 and decreasing Ang II content of the colon, and has important beneficial effects in some diseases of the gut such as colitis [53].
Interactions between the GM and the gut RAAS that Relate to HTN
Production of RAAS Activators and Inhibitors by the GM
Some symbiotic bacteria produce ACE inhibitors, renin inhibitors, and antioxidant molecules during the digestion of mucin, thus GM dysbiosis could trigger HTN, as reviewed in [56]. Aldosterone (and other steroid metabolites) are synthesized from bile salt-conjugated steroids in the enterohepatic circulation by the microbiota, and elicit HTN [57]. GM-mediated synthesis of steroids that reduce the inactivation of cortisol by acting as inhibitors of 11ß-hydroxysteroid dehydrogenase 2 (11HSD2) have also been described [58]. These two types of steroids produced by the GM have potential to be prohypertensive through local actions in the gut and, being freely diffusible, the brain [59, 60, 61] and kidney. Further, metabolic activity of the GM may explain the pro-hypertensive actions of stress in some of the population if we hypothesize that they have a GM dysbiosis that inhibits the breakdown of cortisol by 11HSD2. Cortisol binds two receptors to have pro-hypertensive actions, the mineralocorticoid receptor, and in the absence of 11HSD2, to the glucocorticoid receptor [62].
The HTN resulting from GM-mediated synthesis of aldosterone was prevented by antibiotic knockdown of the gut microbiota in rodents [57], illustrating the important role of the GM in HTN. This role has been extended to humans by a recent case report showing that HTN was reversed by a course of brain penetrating, anti-inflammatory antibiotics, and returned 6 months after antibiotic therapy ended [28]. This may have been the result of actions on both the GM and the brain, especially since steroid-activated MR can be pro-inflammatory by actions in macrophages and microglia, and the antibiotics have anti-inflammatory as well as antibiotic actions. From these data, we can conclude that anti-inflammatory antibiotics may be useful to treat HTN by actions in the gut and brain.
ACE2 Actions on the GM
ACE2 is the most studied component of the gut RAAS that has direct effects on the GM. ACE2 alters antimicrobial peptide secretion in the small intestine leading to altered GM composition in the colon [55]. Polymorphisms of the ACE2 gene have been linked to hypertension [63] and it is likely that this could be, in part, due to altered ACE2 activity in gut and resultant GM alterations. ACE2 is shed as an enzymatically active molecule, sACE2, from lung and kidney proximal tubule epithelia by the actions of ADAM17 [64, 65]. sACE2 interacts with viruses in various ways. sACE2 inhibits the binding of the SARS virus to the epithelium and prevents its uptake. ACE2 is also cleaved by the flu virus neuraminidase and subsequently degraded in the cell [66, 67]. It is unclear whether sACE2 has actions in the gut, or if ACE2 has any interactions with gut viruses, but it would be interesting to have this information and realize its potential in HTN, considering the huge viral load in the gut, estimated at 1011 per gram. Essentially, the gut RAAS and GM interact, and dysbiosis affects HTN by modulating the gut RAAS.
Some interesting questions arise when considering interactions of GM and RAAS. If antihypertensive drugs, such as ACE inhibitors were prescribed to an individual with GM already producing ACE inhibitors, would these be less effective to treat HTN than in an individual without these GM? Do inter-individual antihypertensive responses vary depending upon the background GM? Can the GM metabolize drugs differently depending upon the composition of the GM [68•]? Is resistance to development of HTN in some individuals due to a particularly beneficial combination of GM and RAAS? Could ACE2’s antimicrobial peptide activity be exploited to correct dysbiotic colonic GM populations? Answers to these questions could greatly potentiate the ability to prescribe effective antihypertensive agents.
Genetic Models of ACE2 Expression in Mice
Mice with global overexpression of ACE2 (ACE2 KI) are resistant to anxiety [69]. ACE2 stabilizes the neutral amino acid transporter B0AT1 in gut epithelia [55]. Overexpression would be expected to increase uptake of tryptophan, the precursor for serotonin, into the host; serotonin decreases anxiety. Central MAS1 receptor antagonism blocks the anxiolytic effect of ACE2 KI, suggesting a central mechanism of anxiolysis via Ang1–7 generation. Dysbiosis of the GM occurs in ACE2 knockout mice (ACE2 KO) where lack of tryptophan uptake in the small intestine changed the secretion of antimicrobial proteins and altered colonic bacterial populations [55]. These studies led to the hypothesis that ACE2 has a major impact on the GM. The ACE2 KI mice were used to test this hypothesis and to discover the contribution of the ACE2 genetically modified mice to our understanding of BP regulation. ACE2 KI mice have a small trend towards a decrease in BP at baseline compared to their littermates, but respond much less to hypertensive stimuli (Ang II infusion), unpublished data. Similarly, ACE2 KO mice have little change in baseline blood pressure. We performed analysis of the fecal microbiota (collected and analyzed as described in [32•]), from ACE2 KI mice and their controls, described in [69] with protocols approved by the Institutional Care and Use Committee at the University of Florida. ACE2 KI mice have increased OTU (or species) abundance, Fig. 1a, and increased alpha diversity of bacteria in the GM in two estimations of alpha diversity (Shannon diversity index, p < 0.01 and Fisher’s alpha test, p < 0.05) compared to their littermate controls, Fig. 1b, and their bacterial populations were distinctly separated in Bray-Curtis PcoA plots, Anosim p = 0.001, Fig. 1c. One of the pathways suggested to be significantly upregulated in the bacteria present in the GM of ACE2 KI mice compared to littermate controls by PICRUSt analysis [70] is the phenylalanine-tyrosine-tryptophan biosynthetic pathway, (LDA score of 2.8), § in Fig. 1f. This is consistent with the previously described actions of ACE2 on tryptophan uptake in the gut [55]. While tryptophan metabolism was altered in the GM following manipulation of ACE2 gene expression coincident with dysbiosis, the multiple gene pathways and the abundance and diversity of the bacteria modeled as significantly different following ACE2 overexpression suggested that this was not the only cause of the dysbiosis, Fig. 1d, e and f.
Fig. 1.
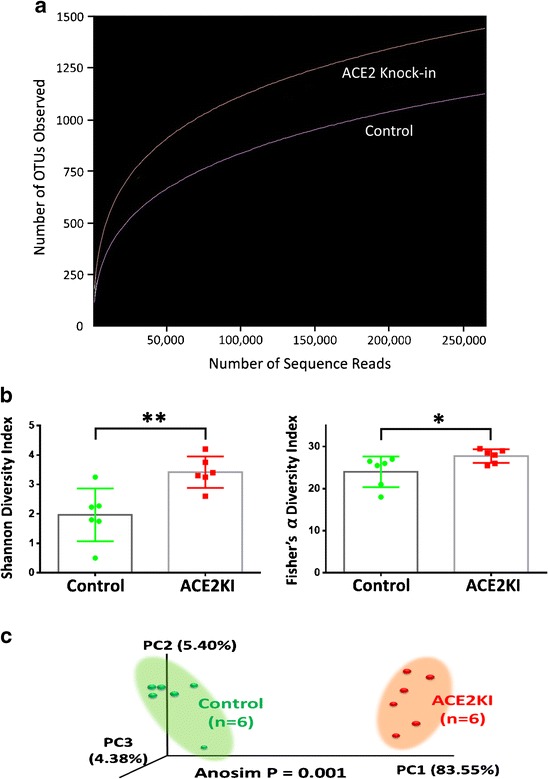
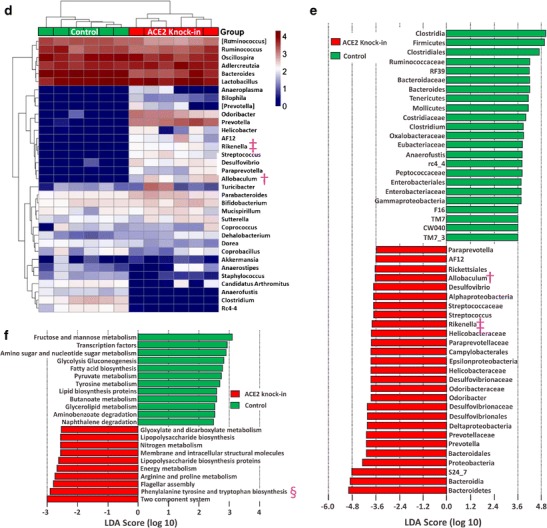
ACE2 knock-in mice have altered gut microbiota compared to littermate controls. 16S rRNA gene sequence-based identification of bacteria in ACE2 knock-in mice. a Number of OTUs (or species) found at multiple rarefaction depths; ACE2 knock-in red, littermate controls purple. b The richness (# of OTUs) and evenness (distribution across OTUs) between ACE2 knock-ins and their littermate controls were significantly different using two tests of alpha diversity, Shannon index (left) and Fisher’s alpha test (right), * = p ≤ 0.05, ** = p ≤ 0.001. c Principal coordinate analysis (PCoA) plot showing the separation between the bacterial communities found in the feces of ACE2 knock-in and their littermate control mice. The variance explained by each of the first three axes is shown in parentheses (83.55, 4.38, and 5.40%, respectively). d Heatmap illustrating the genus-level changes in bacterial abundance in the littermate controls and ACE2 knock-in mice. The relative abundance of a bacterial genus (row) in individual animals (column) is indicated by the color of the cell (blue, low abundance; red, high abundance). Bacterial genera altered in expression in both ACE2 knock-out [55] and ACE2 knock-in mice are illustrated by † (Allobaculum) and ‡ (Rikenella). e Bacterial taxa with significantly different abundances between littermate controls and ACE2 knock-in mice identified by linear discriminant analysis coupled with effect size (LEfSe). Bacterial taxa enriched in the ACE2 knock-in mice are shown in red, in littermate controls in green. Bacterial taxa altered in expression in both ACE2 knock-out [55] and ACE2 knock-in mice are illustrated by † (Allobaculum) and ‡ (Rikenella). f Phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt) analysis showing the significantly different functional capabilities predicted for the bacterial communites in ACE2 knock-in and littermate control mice. The phenylalanine, tyrosine, and tryptophan biosynthetic pathway expected to be altered in the knock-in mice is indicated by §
ACE2 KI mice, as well as the ACE2 KO mouse, show changes in the abundances of Allobacullum and Rikenella, Fig. 1d and e; † and ‡ respectively, suggesting that the level of expression of ACE2 can modulate these bacterial populations. The health consequences of changes in abundance of Rikenella in the GM are not well researched. Their abundance is decreased by a high-fat diet (HFD) and increased towards the levels seen on a normal diet by the administration of tea with a high-fat diet, coincident with an improvement in health [71]. Allobaculum is considered a beneficial bacterium. It is also decreased in mice on a HFD and increased in mice fed prebiotics, that improved health, in association with a HFD [72]; treatment with berberine prevents obesity and insulin resistance (both associated with metabolic HTN) in rats on a HFD, while also increasing Allobacullum abundance [73]. However, Allobaculum is a tryptophan auxotrophe, so its increase in ACE2 KI mice GM was unexpected. Further metabolic studies are needed to resolve this conundrum. Nonetheless, these data suggest that gut ACE2 is involved in regulation of the GM in a way that could interact with HTN, as obesity and insulin resistance are risk factors for metabolic HTN. The physiological significance of this regulation remains to be investigated. However, initial evidence suggests the GM-ACE2 interaction to be important; reduced blood pressure responses to hypertensive stimuli (unpublished data) and the anxiolysis in ACE2 KI mice supports this view.
Conclusions
Accumulating evidence suggests the gut and its microbiota are likely important players in control of blood pressure, joining the team that includes autonomic activity, immune activation, and neuroinflammation. The gut and its GM interface with these components in animal models of hypertension, and there are emerging suggestions that similar interfaces occur in human HTN. But what is missing for us to be able to translate this new knowledge into better therapy for HTN? To what extent is gut pathology involved in human hypertension? A better understanding of the state of the gut barrier in HTN would be very helpful, as recently suggested [74]. For example, could bacteria, viruses or fungi escape into the systemic circulation to trigger immune responses to precipitate HTN? Might there be a HTN signature of bacterial, fungal or viral DNA expressed in the serum of hypertensive patients that could be used to predict and treat hypertension? Could the dysbiosis of HTN create a unique profile of metabolites in the blood that could be similarly used? Can GM diversity between individuals result in different metabolism of antihypertensive drugs, such that effective or resistant drug responses are driven by differences in GM composition [68•]. If drug therapy could be tailored individually based on understanding of how the GM will metabolize various classes of antihypertensive agents, significant advances in treating HTN might be achieved. What is the interplay of miRNAs in the communication between the host and the microbiome in HTN [75]. MiRNAs play a role in HTN [76], have recently been shown to be key players in controlling glial activation in diseases such as multiple sclerosis and autoimmune encephalitis [77], and thus may have an important role in mediating HTN. Yet we have no information about miRNAs of the gut and HTN. And finally, would the disparities associated with HTN in African Americans be reflected in these measures, resulting in greater ability to treat the disease and mitigate the damage it does? There are many avenues and treatment opportunities to research in the gut-HTN interaction, it will take guts and intestinal fortitude to fully explore and exploit these potentials.
Compliance with Ethical Standards
Conflict of Interest
Drs. Richards, Pepine, Raizada, and Kim declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
The animal studies reported here were performed with protocols approved by the Institutional Animal Care and Use Committee at the University of Florida that conforms to nationally accepted standards for animal experimentation.
Footnotes
This article is part of the Topical Collection on Gut Microbiome, Sympathetic Nervous System, and Hypertension
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Writing Group Members. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, DK MG, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sortie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee. Stroke Statistics Subcommittee Executive summary: heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Vighi G, Marcucci F, Sensi L, Di Cara G, Frati F. Allergy and the gastrointestinal system. Clin Exp Immunol. 2008;153(Suppl 1):3–6. doi: 10.1111/j.1365-2249.2008.03713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erny D, Hrabě de Angelis AL, Jaitin D, Peter Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermöhlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meneely GR, Dahl LK. Electrolytes in hypertension: the effects of sodium chloride: the evidence from animal and human studies. Med Clin North Am. 1961;45:271–283. doi: 10.1016/S0025-7125(16)33891-3. [DOI] [PubMed] [Google Scholar]
- 6.Klatsky AL, Friedman GD, Siegelaub AB. Gérard MJ alcohol consumption and blood pressure Kaiser-Permanente multiphasic health examination data. N Engl J Med. 1977;296:1194–1200. doi: 10.1056/NEJM197705262962103. [DOI] [PubMed] [Google Scholar]
- 7.du Florey VC, Uppal S, Lowy C. Hyperglycaemia; a risk factor for hypertension. Rev Epidemiol Sante Publique. 1976;24:313–319. [PubMed] [Google Scholar]
- 8.Wright A, Burstyn PG, Gibney MJ. Dietary fibre and blood pressure. Br Med J. 1979;2:1541–1543. doi: 10.1136/bmj.2.6204.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jose PA, Raj D. Gut microbiota in hypertension. Curr Opin Nephrol Hypertens. 2015;24:403–409. doi: 10.1097/MNH.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Z, Xiong S, Liu D. The gastrointestinal tract: an initial organ of metabolic hypertension? Cell Physiol Biochem. 2016;38:1681–1694. doi: 10.1159/000443107. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M, SPRING Trial Group Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension. 2016;68:974–981. doi: 10.1161/HYPERTENSIONAHA.116.07910. [DOI] [PubMed] [Google Scholar]
- 12.Kelly TN, Bazzano LA, Ajami NJ, He H, Zhao J, Petrosino JF, Correa A, He J. Gut microbiome associates with lifetime cardiovascular disease risk profile among Bogalusa heart study participants. Circ Res. 2016;119:956–964. doi: 10.1161/CIRCRESAHA.116.309219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.•• Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, Zhang W, Weldon R, Auguste K, Yang L, Liu X, Chen L, Yang X, Zhu B, Cai J. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(14) doi:10.1186/s40168-016-0222-x. Paper describes gut dysbiosis before the onset of hypertension in an Asian population [DOI] [PMC free article] [PubMed]
- 14.Cherbut C, Aubé AC, Blottière HM, Galmiche JP. Effects of short-chain fatty acids on gastrointestinal motility. Scand J Gastroenterol Suppl. 1997;222:58–61. doi: 10.1080/00365521.1997.11720720. [DOI] [PubMed] [Google Scholar]
- 15.Blottière HM, Buecher B, Galmiche JP, Cherbut C. Molecular analysis of the effect of short-chain fatty acids on intestinal cell proliferation. Proc Nutr Soc. 2003;62:101–106. doi: 10.1079/PNS2002215. [DOI] [PubMed] [Google Scholar]
- 16.Krautkramer KA, Kreznar JH, Romano KA, Vivas EI, Barrett-Wilt GA, Rabaglia ME, Keller MP, Alan D, Attie AD, Federico E, Rey FE, Denu JM. Diet-microbiota interactions mediate global epigenetic programming in multiple host tissues. Mol Cell. 2016;64:982–992. doi: 10.1016/j.molcel.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanis JM, Alexeev EE, Curtis VF, Kitzenberg DA, Kao DJ, Battista KD, Gerich ME, Glover LE, Kominsky DJ, Colgan SP. Tryptophan metabolite activation of the aryl hydrocarbon receptor regulates IL-10 receptor expression on intestinal epithelia. Mucosal Immunol. 2017 doi: 10.1038/mi.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiering C, Wincent E, Metidji A, Iseppon A, Li Y, Potocnik AJ, Omenetti S, Henderson CJ, Wolf CR, Nebert DW, Stockinger B. Feedback control of AHR signalling regulates intestinal immunity. Nature. 2017;542:242–245. doi: 10.1038/nature21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polonikov AV, Bushueva OY, Bulgakova IV, Freidin MB, Churnosov MI, Solodilova MA, Shvetsov YD, Ivanov VP. A comprehensive contribution of genes for aryl hydrocarbon receptor signaling pathway to hypertension susceptibility. Pharmacogenet Genomics. 2017;27:57–69. doi: 10.1097/FPC.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 20.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110:4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyamoto J, Kasubuchi M, Nakajima A, Irie J, Itoh H, Kimura I. The role of short-chain fatty acid on blood pressure regulation. Curr Opin Nephrol Hypertens. 2016;25:379–383. doi: 10.1097/MNH.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 22.Ono S, Karaki S, Kuwahara A. Short-chain fatty acids decrease the frequency of spontaneous contractions of longitudinal muscle via enteric nerves in rat distal colon. Jpn J Physiol. 2004;54:483–493. doi: 10.2170/jjphysiol.54.483. [DOI] [PubMed] [Google Scholar]
- 23.Holmes E, Loo RL, Stamler J, Bictash M, Yap IK, Chan Q, Ebbels T, De Iorio M, Brown IJ, Veselkov KA, Daviglus ML, Kesteloot H, Ueshima H, Zhao L, Nicholson JK, Elliott P. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature. 2008;453:396–400. doi: 10.1038/nature06882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–455. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomasova L, Dobrowolski L, Jurkowska H, Wróbel M, Huc T, Ondrias K, Ostaszewski R, Ufnal M. Intracolonic hydrogen sulfide lowers blood pressure in rats. Nitric Oxide. 2016;60:50–58. doi: 10.1016/j.niox.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao CC, Patel B, Yan R, Blain M, Alvarez JI, Kébir H, Anandasabapathy N, Izquierdo G, Jung S, Obholzer N, Pochet N, Clish C, Prinz M, Prat A, Antel J, Quintana FJ. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22:586–597. doi: 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santisteban MM, Ahmari N, Carvajal JM, Zingler MB, Qi Y, Kim S, Joseph J, Garcia-Pereira F, Johnson RD, Shenoy V, Raizada MK, Zubcevic J. Involvement of bone marrow cells and neuroinflammation in hypertension. Circ Res. 2015;117:78–191. doi: 10.1161/CIRCRESAHA.117.305853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi Y, Aranda JM, Rodriguez V, Raizada MK, Pepine CJ. Impact of antibiotics on arterial blood pressure in a patient with resistant hypertension—a case report. Int J Cardiol. 2015;201:157–158. doi: 10.1016/j.ijcard.2015.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, ter Horst R, Jansen T, Jacobs L, Bonder MJ, Kurilshikov A, Fu J, Joosten LAB, Zhernakova A, Huttenhower C, Wijmenga C. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. 2016;167:1125–1136. doi: 10.1016/j.cell.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alé A, Zhang Y, Han C, Cai D. Obesity-associated extracellular mtDNA activates central TGFβ pathway to cause blood pressure increase. Am J Physiol Endocrinol Metab. 2017;312:E161–E174. doi: 10.1152/ajpendo.00337.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacFabe DF. Enteric short-chain fatty acids: microbial messengers of metabolism, mitochondria, and mind: implications in autism spectrum disorders. Microb Ecol Health Dis. 2015;26 doi:10.3402/mehd.v26.28177. [DOI] [PMC free article] [PubMed]
- 32.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, Cole-Jeffrey CT, Lobaton GO, Stewart DC, Rubiano A, Simmons CS, Garcia-Pereira F, Johnson RD, Pepine CJ, Raizada MK. Hypertension-linked pathophysiological alterations in the gut. Circ Res. 2017;120:312–323. doi: 10.1161/CIRCRESAHA.116.309006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durgan DJ, Ganesh BP, Cope JL, Ajami NJ, Phillips SC, Petrosino JF, Hollister EB, Bryan RMJ. Role of the gut microbiome in obstructive sleep apnea–induced hypertension. Hypertension. 2016;67:469–474. doi: 10.1161/HYPERTENSIONAHA.115.06672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santisteban MM, Kim S, Pepine CJ, Raizada MK. Brain-gut-bone marrow Axis: implications for hypertension and related therapeutics. Circ Res. 2016;118:1327–1336. doi: 10.1161/CIRCRESAHA.116.307709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yarur A, Deshpande AR, Pechman DM, Tamariz L, Abreu MT, Sussman DA. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am J Gastroenterol. 2011;106:741–747. doi: 10.1038/ajg.2011.63. [DOI] [PubMed] [Google Scholar]
- 37.Coyte KZ, Schluter J, Foster KR. The ecology of the microbiome: networks, competition, and stability. Science. 2015;350:663–666. doi: 10.1126/science.aad2602. [DOI] [PubMed] [Google Scholar]
- 38.Yassour M, Vatanen T, Siljander H, Hämäläinen A, Härkönen T, Ryhänen SJ, Franzosa EA, Vlamakis H, Huttenhower C, Gevers D, Lander ES, Knip M, on behalf of the DIABIMMUNE Study Group. Xavier RJ. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Science translational medicine. 2016;8:343ra81. doi: 10.1126/scitranslmed.aad0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/S0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 41.Okada T, Takahashi S, Nagano N, Yoshikawa K, Usukura Y, Hosono S. Early postnatal alteration of body composition in preterm and small-for-gestational-age infants: implications of catch-up fat. Pediatr Res. 2015;77:136–142. doi: 10.1038/pr.2014.164. [DOI] [PubMed] [Google Scholar]
- 42.Ge X, Ding C, Zhao W, Xu L, Tian H, Gong J, Zhu M, Li J, Li N. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J Transl Med. 2017;15 doi:10.1186/s12967-016-1105-4. [DOI] [PMC free article] [PubMed]
- 43.Salmoirago-Blotcher E, Crawford S, Jackson E, Ockene J, Ockene I. Constipation and risk of cardiovascular disease among postmenopausal women. Am J Med. 2011;124:714–723. doi: 10.1016/j.amjmed.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sumida K, Molnar MZ, Potukuchi PK, Thomas F, Lu JL, Matsushita K, Yamagata K, Kalantar-Zadeh K, Kovesdy CP. Constipation and incident CKD. J Am Soc Nephrol. 2016;28 doi:10.1681/ASN.2016060656. [DOI] [PMC free article] [PubMed]
- 45.Andersen K, Kesper JA, Konrad L, Ryu M, Kumar SVR, Kulkarni OP, Mulay SR, Romoli S, Demleitner J, Schiller P, Dietrich A, Muller S, Gross O, Ruscheweyh HJ, Huson D, Stecher B, Anders HJ. Intestinal dysbiosis, barrier dysfunction, and bacterial translocation account for CKD–related systemic inflammation. J Am Soc Nephrol. 2017;28:76–83. doi: 10.1681/ASN.2015111285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris G, Berk M, Carvalho A, Caso JR, Sanz Y, Walder K, Maes M. The role of the microbial metabolites including tryptophan catabolites and short chain fatty acids in the pathophysiology of immune-inflammatory and neuroimmune disease. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0004-2. [DOI] [PubMed] [Google Scholar]
- 47.Dinan TG, Cryan JF. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin N Am. 2017;46:77–89. doi: 10.1016/j.gtc.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 48.O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 49.Granger DN, Holm L, Kvietys P. The gastrointestinal circulation: physiology and pathophysiology. Compr Physiol. 2015;5:1541–1583. doi: 10.1002/cphy.c150007. [DOI] [PubMed] [Google Scholar]
- 50.Kim S, Zingler M, Harrison JK, Scott EW, Cogle CR, Luo D, Raizada MK. Angiotensin II regulation of proliferation, differentiation, and engraftment of hematopoietic stem cells. Hypertension. 2016;67:574–584. doi: 10.1161/HYPERTENSIONAHA.115.06474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rüster C, Wolf G. Angiotensin II as a morphogenic cytokine stimulating renal fibrogenesis. J Am Soc Nephrol. 2011;22:1189–1199. doi: 10.1681/ASN.2010040384. [DOI] [PubMed] [Google Scholar]
- 52.Pais R, Rievaj J, Larraufie P, Gribble F, Reimann F. Angiotensin II type 1 receptor-dependent GLP-1 and PYY secretion in mice and humans. Endocrinology. 2016;157:3821–3831. doi: 10.1210/en.2016-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khajah MA, Fateel MM, Ananthalakshmi KV, Luqmani YA. Anti-inflammatory action of angiotensin 1-7 in experimental colitis. PLoS One. 2016;11:e0150861. doi: 10.1371/journal.pone.0150861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patten GS, Abeywardena MY. Effects of antihypertensive agents on intestinal contractility in the spontaneously hypertensive rat: angiotensin receptor system downregulation by losartan. J Pharmacol Exp Ther. 2017;360:260–266. doi: 10.1124/jpet.116.237586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SM, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dave LA, Hayes M, Montoya CA, Rutherfurd SA, Moughan PJ. Human gut endogenous proteins as a potential source of angiotensin-I-converting enzyme (ACE-I)-, renin inhibitory and antioxidant peptides. Peptides. 2016;76:30–44. doi: 10.1016/j.peptides.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 57.Honour JW. Historical perspective: gut dysbiosis and hypertension. Physiol Genomics. 2015;46:443–446. doi: 10.1152/physiolgenomics.00063.2015. [DOI] [PubMed] [Google Scholar]
- 58.Morris DJ, Latif SA, Hardy MP, Brem AS. Endogenous inhibitors (GALFs) of 11beta-hydroxysteroid dehydrogenase isoforms 1 and 2: derivatives of adrenally produced corticosterone and cortisol. J Steroid Biochem Mol Biol. 2007;104:161–168. doi: 10.1016/j.jsbmb.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 59.de Kloet ER, Joels M. Brain mineralocorticoid receptor function in control of salt balance and stress-adaptation. Physiol Behav. 2017 doi: 10.1016/j.physbeh.2016.12.045. [DOI] [PubMed] [Google Scholar]
- 60.Fujita M, Fujita T. The role of CNS in the effects of salt on blood pressure. Curr Hypertens Rep. 2016;18:10. doi: 10.1007/s11906-015-0620-7. [DOI] [PubMed] [Google Scholar]
- 61.Ito K, Hirooka Y, Sunagawa K. Cardiac sympathetic afferent stimulation induces salt-sensitive sympathoexcitation through hypothalamic epithelial Na+ channel activation. Am J Physiol Heart Circ Physiol. 2015;308:H530–H539. doi: 10.1152/ajpheart.00586.2014. [DOI] [PubMed] [Google Scholar]
- 62.Busillo JM, Azzam KM, Cidlowski JA. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J Biol Chem. 2011;286:38703–38713. doi: 10.1074/jbc.M111.275370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang YL, Mo YP, He YS, Yang F, Xu Y, Li CC, Wang J, Reng HM, Long L. Correlation between renin-angiotensin system gene polymorphisms and essential hypertension in the Chinese Yi ethnic group. J Renin-Angiotensin-Aldosterone Syst. 2015;16:975–981. doi: 10.1177/1470320315598697. [DOI] [PubMed] [Google Scholar]
- 64.Xiao F, Zimpelmann J, Burger D, Kennedy C, Hébert RL, Burns KD. Protein kinase C-δ mediates shedding of angiotensin-converting enzyme 2 from proximal tubular cells. Front Pharmacol. 2016 doi: 10.3389/fphar.2016.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jia HP, Look DC, Tan P, Shi L, Hickey M, Gakhar L, Chappell MC, Wohlford-Lenane C, McCray PBJ. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2009;297:L84–L96. doi: 10.1152/ajplung.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu X, Yang N, Tang J, Liu S, Luo D, Duan Q, Wang X. Downregulation of angiotensin-converting enzyme 2 by the neuraminidase protein of influenza a (H1N1) virus. Virus Res. 2014;185:64–71. doi: 10.1016/j.virusres.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lai ZW, Lew RA, Yarski MA, Mu FT, Andrews RK, Smith A. The identification of a calmodulin-binding domain within the cytoplasmic tail of angiotensin-converting enzyme-2. Endocrinology. 2009;150:2376–2381. doi: 10.1210/en.2008-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ll J, Anzenbacher P, Anzenbacherova E. Human gut microbiota plays a role in the metabolism of drugs. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160:317–326. doi: 10.5507/bp.2016.039. [DOI] [PubMed] [Google Scholar]
- 69.Wang L, de Kloet AD, Pati D, Hiller H, Smith JA, Pioquinto DJ, Ludin JA, Oh SP, Katovich MJ, Frazier CJ, Raizada MK, Krause EG. Increasing brain angiotensin converting enzyme 2 activity decreases anxiety-like behavior in male mice by activating central Mas receptors. Neuropharmacology. 2016;105:23. doi: 10.1016/j.neuropharm.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Z, Chen Z, Guo H, He D, Zhao H, Wang Z, Zhang W, Liao L, Zhang C, Ni L. The modulatory effect of infusions of green tea, oolong tea, and black tea on gut microbiota in high-fat-induced obese mice. Food Funct. 2016;7:4869–4879. doi: 10.1039/C6FO01439A. [DOI] [PubMed] [Google Scholar]
- 72.Everard A, Lazarevic V, Gaıa N, Johansson M, Stahlman M, Backhed F, Delzenne NM, Schrenzel J, Francois P, Cani PD. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. The ISME Journal. 2014;8:2116–2130. doi: 10.1038/ismej.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang X, Zhao Y, Zhang M, Pang X, Xu J, Kang C, Li M, Zhang C, Zhang Y, Li X, Nin G, Zhao L. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS One. 2012;7:e42529. doi: 10.1371/journal.pone.0042529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ufnal M, Pham K. The gut-blood barrier permeability–a new marker in cardiovascular and metabolic diseases? Med Hypotheses. 2017;98:35–37. doi: 10.1016/j.mehy.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 75.Liu S, da Cunha AP, Rezende RM, Cialic R, Wei Z, Bry L, Comstock LE, Gandhi R, Weiner HL. The host shapes the gut microbiota via fecal MicroRNA. Cell Host Microbe. 2016;19:32–43. doi: 10.1016/j.chom.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klimczak D, Jazdzewski K, Kuch M. Regulatory mechanisms in arterial hypertension: role of microRNA in pathophysiology and therapy. Blood Press. 2017;26:2–8. doi: 10.3109/08037051.2016.1167355. [DOI] [PubMed] [Google Scholar]
- 77.Ponomarev ED, Veremeyko T, Weiner HL. MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia. 2013;61:91–103. doi: 10.1002/glia.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]


