Abstract
Purpose of review
The quantity of tissue removed during an oncologic surgical procedure is not standardized and there are numerous reports of local recurrence despite histologically adequate resection margins. The oral cavity is one of the sites in the head and neck with high chances of recurrence following negative margins. To address this need, this article reviews the recent applications of Dynamic Optical Contrast Imaging (DOCI) towards both oral screening and the intraoperative evaluation of tumor margins in head and neck surgery.
Recent findings
Human ex vivo and in vivo trials suggest DOCI is safe, low cost, and sensitive for differentiating cancerous from normal tissues throughout the head and neck, in addition to the oral cavity. Ex vivo imaging of OSCC specimens generated histologically-verified image contrast. Furthermore, in vivo intraoperative results demonstrate significant potential for image-guided detection and resection of oral cavity squamous cell carcinoma (OSCC).
Summary
DOCI augments tissue contrast and may enable surgeons to: clinically screen patients for oral cancer, make histologic evaluations in vivo with fewer unnecessary biopsies, delineate clinical margins for tumor resection, provide guidance in the choice of biopsy sites, and preserve healthy tissue to increase the postoperative functionality and quality of life of the patient.
Keywords: Optical imaging, dynamic optical contrast imaging, surgical margins, squamous cell carcinoma, intraoperative image-guided surgery
Introduction
The prognosis for patients with oral cavity squamous cell carcinoma (OSCC) is largely determined by the stage of disease at clinical presentation [1–3]. Patients diagnosed with advanced OSCC have shorter survival times (5-year mortality, 85%, 75%, 47%, and 35% among patients diagnosed as having distant, regional, local, and in situ cancer, respectively) and higher costs (average total hospital payments, $53,741, $58,387, $42,698, and $37,434, respectively) [4]. Despite medical advances in the management of OSCC, the global incidence, morbidity, and mortality associated with this disease remains relatively unchanged over the past three decades [4–7]. One of the major factors accounting for these dismal reports is that over 60% of OSCC cases are detected at a late stage (III or IV), when diagnostic evaluation, treatment, and management of complications and recurrences are often lengthy, complex, and burdened by poor outcomes [6,8,9].
The primary surgical management of OSCC (successful when diagnosed at earlier stages) may result in significant functional morbidity; impairments in speech, swallowing, taste, smell, and has the potential to greatly affect the quality of life in these patients [1,10]. Unfortunately, surgeons are only equipped with their natural senses of sight and physical touch to localize the contours of a tumor followed by limited frozen section biopsy to establish “clean margins”[11–13]. Consequently, the determination of tumor margins by palpation and visual inspection has led to recurrence rates of 25-50% [14,15]. These challenges substantiate both a clinical need for improved screening and a surgical need for intraoperative guidance to localize tumor margins.
In order to address these challenges, our lab has developed a novel optical image-guided technology termed dynamic optical contrast imaging (DOCI). This review focuses on the recent applications of DOCI towards both preventative oral screening and also tissue identification in the intraoperative setting for margin control in head and neck oncologic surgery.
DOCI: screening for oral cancer
The prognostic implications of diagnosis and treatment of early intra-epithelial stages of OSCC carcinogenesis have already shown to be highly significant due to the high survival rates (~80%) of patients diagnosed with early OSCC [16]. Early screening of pre-cancerous oral lesions has the potential to minimize the progression of these lesions to frank carcinoma. Thus, improvements in early detection would allow for frequent patient monitoring, dietary changes (i.e. tobacco and alcohol use), and timely lesion excision, thus maximizing patient survival.
In the current OSCC literature, screening (i.e. detection) and case-finding (i.e. diagnosis) have often been mistakenly used interchangeably in epidemiological studies designed to detect pre-cancerous oral lesions in the patient population [17]. Screening should always be evaluated with respect to sensitivity, specificity, and predictive values. Such analysis requires that the test outcome from a sample of subjects be compared to the results of an appropriate gold standard (i.e. diagnosis as confirmed by histology of the biopsy) on the same population [17]. Currently, the sensitivity of biopsy is uncharacterized as false negative rates (the probability of missing a lesion) cannot be determined unless the entire anatomic site (e.g. entire tongue) is submitted for histologic sectioning following tissue biopsy (Figure 1) informed by clinical indication [18,1,19,20]. Similarly, current specificity rates are unknown as true negative rates (probability of correct rejection) are not accurately tested and false positive rates (probability of false positive) are not always tabulated at the time of biopsy. For this reason, the reported sensitivity and specificity of the following OSCC screening techniques vary significantly and may not be easily compared.
Figure 1. Sampling error associated with biopsy.
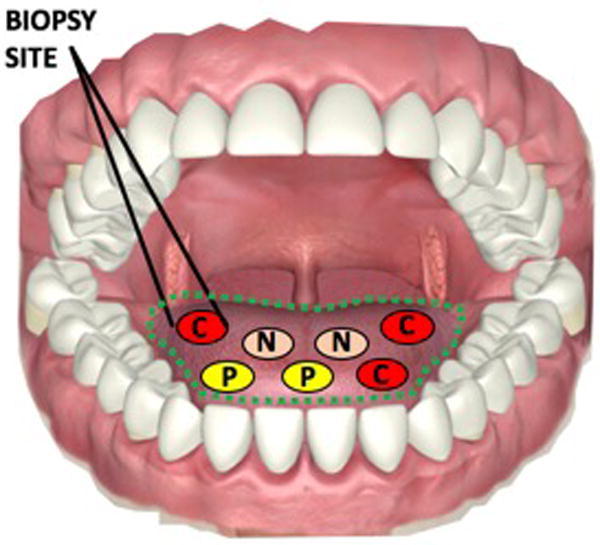
“C,” “P,” and “N” denote cancerous, pre-cancerous, and normal tissue in the oral cavity. The site biopsied may not be representative of neighboring tissue in the field of evaluation (dotted green region), and therefore measured sensitivity and sensitivity is often incomparable.
Original.
The existing standard of OSCC screening is conventional oral examination (COE) that can be followed by biopsy [1,21]. The clinician is limited in the visual identification of precancerous and early-stage OSCC lesions with COE due to the difficulty of differentiating harmful lesions from similar-looking benign lesions. Pre-cancerous oral lesions are often asymptomatic and vary greatly in their clinical appearance and often lack clinical characteristics and biomarkers associated with advanced OSCC, (e.g., ulceration, induration, and bleeding) [22–24]. Improvements in the real-time differentiation between different tissue types would represent a considerable advancement towards improving surgical decision making (i.e. biopsy selection), and thus, patient outcomes.
Dynamic Optical Contrast Imaging (DOCI), is a technique that enables macroscopic delineation of abnormal from normal tissue based on the temporal decay of endogenous fluorophores after excitation [25–27]. This fluorescence decay (i.e. lifetime) is a fluorophore-specific characteristic that is not influenced by the local concentration of fluorophores, the optical path, the local excitation intensity, or the local fluorescence detection efficiency (which is not possible in autofluorescence imaging) [28]. The intrinsic contrast mechanism accessed by DOCI parallels fluorescence lifetime imaging microscopy (FLIM), in which the endogenous fluorophore lifetime of tissue is probed by illumination with a pulsed, long-wave ultraviolet light source [26]. In comparison to FLIM, DOCI produces pixel values that are proportional to the aggregate fluorophore of the probed tissue without the requirement of fitting acquired data to complex mathematical models through a unique image frame normalization scheme [26]. The avoidance of mathematical complexity in calculations enables scalable mapping of fluorophore lifetimes over macroscopic (not microscopic) fields of view with high contrast to noise ratios in a relatively short time frame (<60 sec image acquisition time) with all pixels acquired simultaneously. This capability contrasts with autofluorescence imaging, where optical tissue properties (e.g., absorption and scattering) markedly influence image quality [17,28,29]. The benefit of this technology is a potential decrease in the sampling error of biopsy and a subsequent improvement in the discriminatory power between normal and abnormal tissue. This capability may allow the provider to biopsy lesions that appear higher risk and make appropriate referrals to specialists.
Recent investigations in both human ex vivo and in vivo specimens demonstrate the ability of this technique to generate statistically significant contrast between tumor tissue and surrounding normal tissue in biopsies taken from OSCC patients [26]. In addition, a recent in vivo pilot study suggests the capability of delineating differences between pre-cancerous and normal tissues (Figure 2). Collectively, the preliminary data indicates the potential utility of DOCI for early OSCC screening. Furthermore, the DOCI images reveal detailed tissue characteristics that are confirmed by histology but not visible to the naked eye alone [26].
Figure 2. DOCI permits differentiation of pre-cancer from inflammation.
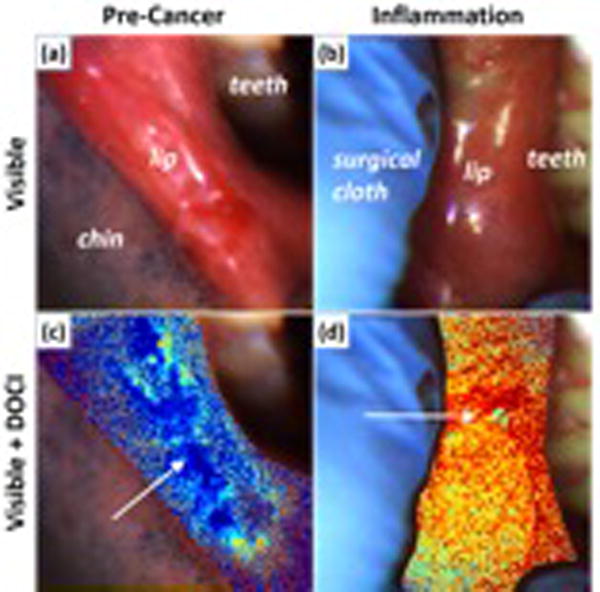
(a) visible image of precancerous lesion and (b) inflammation of the lip. (c) visible image and DOCI overlay of pre-cancerous lesion and (d) inflammation of the lip.
Original.
DOCI: Intraoperative assessment of tumor margins
A pilot in vivo study of 15 consecutive patients undergoing surgical resection for OSCC was performed to evaluate the diagnostic utility of DOCI in the intraoperative detection of OSCC [30]. Biopsy-proven squamous cell carcinoma neoplasms were obtained from the following head and neck sites and subsites: auricle, parotid, scalp, oral cavity, oropharynx, hypopharynx, and neck. All specimens were imaged with the DOCI system prior to resection. Following tumor ablation, specimens were immediately sectioned into multiple fresh samples containing tumor and contiguous normal tissue of suspect lesions and submitted for histological assessment. Areas of neoplasm were then confirmed by a pathologist (blinded to the DOCI image results) and relative lifetime values were computed independent of pathologic diagnosis.
In vivo DOCI images demonstrated contrast between OSCC tissue and the surrounding normal tissue (Figure 3). The DOCI image color map transforms blue to the global minimum relative decay lifetime and red to the maximum relative decay lifetime. A reduced DOCI pixel value indicates a more rapid decay of fluorescence signal, indicating an overall shorter lifetime.
Figure 3. In vivo imaging of tongue cancer.
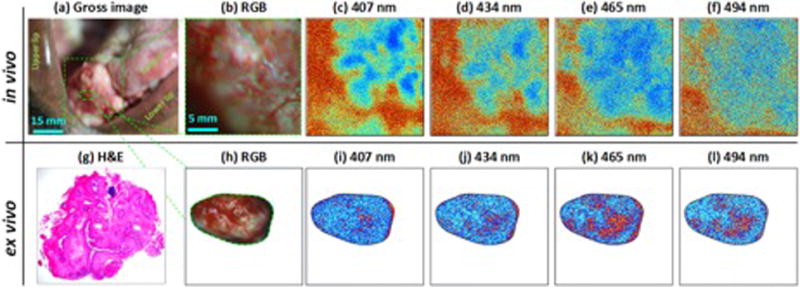
(a-b) visible image of cancer, (c-f) in vivo DOCI images at corresponding wavelengths (g) Histology (h) visible image of biopsy (i-l) DOCI images of ex vivo biopsy. DOCI contrast is displayed using an absolute color map scale where shorter relative lifetimes are mapped to blue and longer lifetimes are mapped to red. The in vivo DOCI image of the tumor tissue has a substantially shorter relative lifetime than the surrounding normal tissue and the tissues are thus displayed in bluer shades and redder shades, respectively. Similar contrast is observed in ex vivo DOCI images at all emission wavelengths.
Original.
Areas of OSCC were characterized by reduced relative lifetime compared to the lifetime of surrounding normal tissue. Comparable relative lifetime measurements were observed in the post-resection ex vivo images of excited tissue. Strong positive correlations between ex-vivo OSCC and in-vivo OSCC over the entire range of emission wavelengths suggest that DOCI and its associated image analysis methods are directly translatable to in vivo clinical use [30].
DOCI: histologic tissue characterization
The aggregate fluorophore decay time that describes pixel values in DOCI images may also potentially be used to accurately and rapidly distinguish histologically-verified tissue components. DOCI was performed in a large trial of fresh ex vivo OSCC biopsies (45 patients; 88 individual samples) to further investigate this hypothesis [30]. Since DOCI images are acquired from the epithelial surface in vivo, DOCI images of ex vivo specimens acquired along the same imaging plane are clinically relevant and revealed details on tissue structure/type that are not evident on gross examination (Figure 4). Ex vivo tissue and corresponding histology were sectioned parallel to the imaged plane and epithelial surface to capture both cancerous and adjacent stroma. Differences in relative lifetimes were reported between areas of tumor, fat, muscle, and collagen that were assessed with DOCI [25].
Figure 4. DOCI permits optical evaluation of OSCC histology.
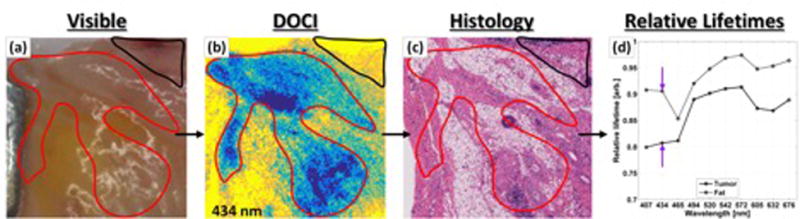
(a) Gross visible images. (b) DOCI data with (c) accompanying co-registered histologic section. Regions of interest have been drawn by a physician blinded to the DOCI images and then superimposed upon the DOCI images and histology. The absolute color map associated with DOCI imagery transforms blue to the global minimum relative decay lifetime and yellow to the maximum relative decay lifetime. Malignant OSCC (red contour) is associated with lower relative decay lifetimes than normal, fat tissue (black contour). (d) Statistical analysis is performed on the pooled DOCI data grouped by tissue type.
Original.
DOCI lifetime mapping produced statistically significant differences in contrast between all four tissue types under investigation (tumor, fat, muscle, and collagen) across most emission wavelengths. A decrease in fluorescence lifetime was observed in malignant tissue that is consistent with the short lifetimes reported for biochemical markers of tumors as well as previous ex vivo and in vivo published work [31,32,29,33]. Statistical significance by Wilcoxon rank-sum test (P < .05) between muscle and tumor was established for 10 of 10 emission wavelengths, between collagen and tumor for 8 of 10 emission wavelengths, and between fat and tumor for 2 of 10 wavelengths [30]. This study thus demonstrated the feasibility of DOCI to accurately distinguish OSCC and surrounding normal tissue and its potential to maximize the efficacy of surgical resection.
Future directions
In initial surgical use, DOCI images may be acquired immediately after tumor resection but before frozen-section biopsies as an image-guided margin-biopsy system. Local recurrences occur in up to 50% of head and neck cancer patients even with histologically negative margins by frozen section [34]. This may in part be a byproduct of the frozen-section technique; only representative areas of the tissues at risk are sampled, resulting in geographical misses of positive margins located in non-sampled tissue regions. Potentially, imaging the tumor bed after resection can minimize the sample bias. The process of DOCI imaging and the generation of decay maps requires less than a minute and is much faster than intraoperative frozen section analysis, a process that not only adds 20 to 30 minutes to anesthesia time but is also costly (averaging ~$500 per sample) [35]. DOCI could potentially increase tumor resection accuracy, decrease operation time, and decrease costs associated with random-sample frozen sections.
Following the validation of DOCI in clinical trials, the system can be further optimized by upgrading the imaging core, resulting in an inexpensive, compact, lightweight, and more efficient device. Interchangeable lens systems will be designed for endoscopic, transoral, and open head and neck dissection applications with each configuration providing a large FOV. These improvements are designed to integrate DOCI into the standard of care by helping guide the intraoperative assessment and staging of cancers in the head and neck.
Conclusion
The introduction of real-time DOCI imaging will advance patient screening and intraoperative tissue localization by providing surgeons with spatially resolved and pathology-specific maps of tissue. DOCI is a wide-field technique that images large surgical fields in real-time without disrupting the normal workflow in the operating room. In contrast to other imaging modalities, DOCI is safe, as it does not utilize ionizing radiation, and also avoids the use of dyes or injection of radioactive material. In addition, the visual output of the instrument does not demand special training or experience to interpret. In conclusion, our novel technology augments tissue contrast and enable surgeons to: clinically screen patients for oral cancer, make histologic evaluations in vivo with fewer unnecessary biopsies, delineate clinical margins for tumor resection, provide guidance in the choice of biopsy sites, and preserve healthy tissue to increase the postoperative functionality and quality of life of the patient.
KEY POINTS.
- DOCI is a wide-field imaging technique that produces spatially resolved and pathology-specific maps of tissue.
- The imaging modality enables differentiation of inflammation from cancerous mucosal change and may be used for patient screening.
- The system produces real-time images that may permit intraoperative guidance for tumor margin delineation in surgery.
- Image resolution of ex vivo specimens permitted optical characterization of tissue histology.
Acknowledgments
We would like to greatly thank Harrison Cheng and Dr. Zachary Taylor for their huge amount of work throughout the development and realization of this large project.
Financial support and sponsorship
This work was supported by the UCLA Medical Scientist Training Program (grant T32GM008042; P.P.), the American Academy of Otolaryngology–American Head & Neck Society Surgeon Scientist Career Development Award (M.A.S.J.), the Tobacco-Related Disease Research Program of the University of California (M.A.S.J.), the STOP Cancer Foundation (M.A.S.J.), and the UCLA Henry Samueli School of Engineering and Applied Sciences, Los Angeles, CA.
Footnotes
Conflicts of interest
None.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
**of outstanding interest
- 1.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho AL, K LP. Influence of time delay and clinical upstaging in the prognosis of head and neck cancer. Oral Oncol. 37:94–98. doi: 10.1016/s1368-8375(00)00066-x. [DOI] [PubMed] [Google Scholar]
- 3.McGurk M, Chan C, Jones J, O’regan E, Sherriff M. Delay in diagnosis and its effect on outcome in head and neck cancer. Br J Oral Maxillofac Surg. 43:281–284. doi: 10.1016/j.bjoms.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Lang K, Menzin J, Earle CC, Jacobson J, Hsu M-A. The economic cost of squamous cell cancer of the head and neck: findings from linked SEER-Medicare data. Arch Otolaryngol Neck Surg. 2004;130:1269–1275. doi: 10.1001/archotol.130.11.1269. [DOI] [PubMed] [Google Scholar]
- 5.Lee JM, Turini M, Botteman MF, Stephens JM, Pashos CL. Economic burden of head and neck cancer. Eur J Heal Econ Former HEPAC. 2004;5:70–80. doi: 10.1007/s10198-003-0204-3. [DOI] [PubMed] [Google Scholar]
- 6.Mignogna MD, Fedele S, Russo LL. The World Cancer Report and the burden of oral cancer. Eur J Cancer Prev. 13:139–142. doi: 10.1097/00008469-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Society AC. Cancer facts and figures 2013. American Cancer Society; Atlanta: 2013. [Google Scholar]
- 8.Allison P, Locker D, Feine JS. The role of diagnostic delays in the prognosis of oral cancer: a review of the literature. Oral Oncol. 1998;34:161–170. doi: 10.1016/s1368-8375(97)00071-7. [DOI] [PubMed] [Google Scholar]
- 9.Mignogna MD, Fedele S, Russo LL, Ruoppo E, Muzio LL. Oral and pharyngeal cancer: lack of prevention and early detection by health care providers. Eur J Cancer Prev. 10:381–383. doi: 10.1097/00008469-200108000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aliperti LA, Predina JD, Vachani A, Singhal S. Local and Systemic Recurrence is the Achilles Heel of Cancer Surgery. Ann Surg Oncol [Internet] 2011;18:603–7. doi: 10.1245/s10434-010-1442-0. Available from: http://download.springer.com/static/pdf/108/art%253A10.1245%252Fs10434-010-1442-0.pdf?originUrl=http%3A%2F%2Flink.springer.com%2Farticle%2F10.1245%2Fs10434-010-1442-0&token2=exp=1493709719~acl=%2Fstatic%2Fpdf%2F108%2Fart%25253A10.1245%25252Fs10434-010-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinni ML. Head and neck surgical reconstruction. Curr Opin Otolaryngol Head Neck Surg [Internet] 2013 doi: 10.1097/MOO.0b013e32836302a8. [cited 2017 Oct 29]; 21: 303–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23770829. [DOI] [PubMed]
- 13.Ho AS, Kraus DH, Ganly I, Lee NY, Shah JP, Morris LGT. Decision making in the management of recurrent head and neck cancer. Head Neck [Internet] 2014;36:144–51. doi: 10.1002/hed.23227. Available from: http://dx.doi.org/10.1002/hed.23227. [DOI] [PubMed] [Google Scholar]
- 14.Goodwin WJJ. Salvage surgery for patients with recurrent squamous cell carcinoma of the upper aerodigestive tract: when do the ends justify the means? Laryngoscope. 2000;110:1–18. doi: 10.1097/00005537-200003001-00001. [DOI] [PubMed] [Google Scholar]
- 15.Siegel R, Ward E, Brawley O, Jemal A. Cancer Statistics, 2011 The Impact of Eliminating Socioeconomic and Racial Disparities on Premature Cancer Deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 16.Epstein JB, Zhang L, Rosin M. Advances in the diagnosis of oral premalignant and malignant lesions. Journal-Canadian Dent Assoc. 2002;68:617–621. [PubMed] [Google Scholar]
- 17.Lingen MW, Kalmar JR, Karrison T, Speight PM. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008;44:10–22. doi: 10.1016/j.oraloncology.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greven KM, Keyes JW, Williams DW, McGuirt WF, Joyce WT. Occult primary tumors of the head and neck. Cancer. 1999;86:114–118. doi: 10.1002/(sici)1097-0142(19990701)86:1<114::aid-cncr16>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 19.Larson RA, Thomas H, Cleveland JC. Errors in management of head and neck tumors. CA Cancer J Clin. 1968;18:92–95. doi: 10.3322/canjclin.18.2.92. [DOI] [PubMed] [Google Scholar]
- 20.Harréus U. Surgical errors and risks–the head and neck cancer patient. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2013;12 doi: 10.3205/cto000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehanna H, Paleri V, West CM, Nutting C. Head and neck cancer–Part 1: Epidemiology, presentation, and prevention. Bmj. 2010;341:663–666. doi: 10.1136/bmj.c4684. [DOI] [PubMed] [Google Scholar]
- 22.Mashberg A, Feldman LJ. Clinical criteria for identifying early oral and oropharyngeal carcinoma: erythroplasia revisited. Am J Surg. 1988;156:273–275. doi: 10.1016/s0002-9610(88)80290-3. [DOI] [PubMed] [Google Scholar]
- 23.Sidransky D. Emerging molecular markers of cancer. Nat Rev Cancer. 2002;2:210–219. doi: 10.1038/nrc755. [DOI] [PubMed] [Google Scholar]
- 24.Silverman S. Early diagnosis of oral cancer. Cancer. 1988;62:1796–1799. doi: 10.1002/1097-0142(19881015)62:1+<1796::aid-cncr2820621319>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 25 **.Kim IA, Taylor ZD, Cheng H, Sebastian C, Maccabi A, Garritano J, et al. Dynamic Optical Contrast Imaging: A Technique to Differentiate Parathyroid Tissue from Surrounding Tissues. Otolaryngol Neck Surg [Internet] 2017;156:480–3. doi: 10.1177/0194599816686294. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28116982%0Ahttp://journals.sagepub.com/doi/10.1177/0194599816686294 This article demonstrates that DOCI is capable of efficiently distinguishing parathyroid tissue from adjacent tissues. [DOI] [PubMed] [Google Scholar]
- 26 **.Tajudeen BA, Taylor ZD, Garritano J, Cheng H, Pearigen A, Sherman AJ, et al. Dynamic optical contrast imaging as a novel modality for rapidly distinguishing head and neck squamous cell carcinoma from surrounding normal tissue. Cancer. 2016 doi: 10.1002/cncr.30338. This article demonstrates the feasibility of using DOCI to rapidly and accurately distinguish HNSCC from surrounding normal tissue. [DOI] [PubMed] [Google Scholar]
- 27 *.Taylor Z, Kim I, Pesce J, Grundfest W, St John MS. Dynamic Optical Contrast Imaging as a Novel Modality to Rapidly Distinguish Oral Squamous Cell Carcinoma From Surrounding Normal Tissue. Int J Radiat Oncol [Internet] 2016;94:921. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0360301615270058 This poster indicates that DOCI images reveal microscopic characterization sufficient for tissue type identification comparable to histology. [Google Scholar]
- 28.Keereweer S, Van Driel PBAA, Snoeks TJA, Kerrebijn JDF, Baatenburg de Jong RJ, Vahrmeijer AL, et al. Optical image-guided cancer surgery: challenges and limitations. Clin Cancer Res [Internet] 2013;19:3745–54. doi: 10.1158/1078-0432.CCR-12-3598. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23674494. [DOI] [PubMed] [Google Scholar]
- 29.De Veld DCG, Witjes MJH, Sterenborg HJCM, Roodenburg JLN. The status of in vivo autofluorescence spectroscopy and imaging for oral oncology. Oral Oncol. 2005;41:117–31. doi: 10.1016/j.oraloncology.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 30 **.Kim IA, Taylor ZD, Cheng H, Sebastian C, Maccabi A, Garritano J, et al. Dynamic Optical Contrast Imaging: A Technique to Differentiate Parathyroid Tissue from Surrounding Tissues. Otolaryngol Neck Surg [Internet] 2017;156:480–3. doi: 10.1177/0194599816686294. Available from: http://journals.sagepub.com/doi/10.1177/0194599816686294 This article demonstrates that DOCI is capable of efficiently distinguishing parathyroid tissue from adjacent tissues. [DOI] [PubMed] [Google Scholar]
- 31.Schwarz RA, Gao W, Redden Weber C, Kurachi C, Lee JJ, El-Naggar AK, et al. Noninvasive evaluation of oral lesions using depth-sensitive optical spectroscopy. Cancer [Internet] 2009;115:1669–79. doi: 10.1002/cncr.24177. Available from: http://doi.wiley.com/10.1002/cncr.24177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skala MC, Riching KM, Gendron-Fitzpatrick A, Eickhoff J, Eliceiri KW, White JG, et al. In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia. Proc Natl Acad Sci [Internet] 2007;104:19494–9. doi: 10.1073/pnas.0708425104. Available from: http://www.pnas.org/cgi/doi/10.1073/pnas.0708425104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Y, Phipps JE, Meier J, Hatami N, Poirier B, Elson DS, et al. Endoscopic Fluorescence Lifetime Imaging for In Vivo Intraoperative Diagnosis of Oral Carcinoma. Microsc Microanal [Internet] 2013;19:791–8. doi: 10.1017/S1431927613001530. Available from: http://www.journals.cambridge.org/abstract_S1431927613001530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nathan CAO, Liu L, Li BD, Abreo FW, Nandy I, DeBenedetti A. Detection of the proto-oncogene eIF4E in surgical margins may predict recurrence in head and neck cancer. Oncogene. 1997;15:579–84. doi: 10.1038/sj.onc.1201216. [DOI] [PubMed] [Google Scholar]
- 35.DiNardo LJ, Lin J, Karageorge LS, Powers CN. Accuracy, utility, and cost of frozen section margins in head and neck cancer surgery. Laryngoscope [Internet] 2000;110:1773–6. doi: 10.1097/00005537-200010000-00039. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11037842. [DOI] [PubMed] [Google Scholar]


