Abstract
Context:
Sickle cell disease is a group of hemoglobin (Hb) disorders resulting from the inheritance of the sickle β-globin gene. It is the most common pathological Hb mutation worldwide with 75% being born in Sub-Saharan Africa.
Aims:
This study aims to determine if dried blood spots (DBSs) can be used for diagnosis of sickle cell in newborns. In Zambia, there is no neonatal screening program for sickle cell anemia (SCA), yet it has been proved that early diagnosis by newborn screening (NBS) using DBSs and access to comprehensive care results in survival to adulthood of over 96% of sickle cell patients.
Settings and Design:
A cross-sectional study was carried out at the University Teaching Hospital to determine whether DBSs can be used to diagnose sickle cell using Hb electrophoresis.
Subjects and Methods:
Results from DBSs stored for 2 weeks were then compared to those obtained using freshly collected whole blood.
Statistical Analysis Used:
To evaluate performance characteristics, the following values were used: true positive, false positive, true negative, and false negative.
Results:
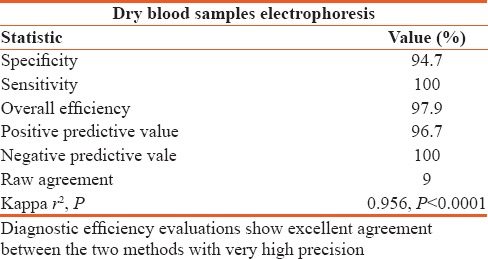
Ninety-seven participants were included in this study. DBSs had a sensitivity of 100%, a specificity of 94.7%, positive predictive value of 96.7%, negative predictive value of 100%, overall efficiency of 97.9%, and a Kappa r2, P < 0.0001 in comparison to fresh whole blood which we used as the gold standard.
Conclusions:
The use of DBSs can be recommended for NBS of SCA in Zambia due to its high sensitivity, specificity, and stability of hemoglobin.
Keywords: Dried blood spot, newborn, sickle cell
Introduction
Sickle cell disease is a group of hemoglobin (Hb) disorders resulting from the inheritance of the sickle β-globin gene. Sickle Hbs is the most common pathological Hb mutation worldwide.[1] It is estimated that the global number of neonates affected by sickle cell anemia (SCA) in 2010 was 312 000[2] with 75% being born in Sub-Saharan Africa.[3] Mortality in SCA historically has been extremely high, with death often occurring before a diagnosis is made.[4,5] In high-income countries, early diagnosis by newborn screening (NBS) and access to comprehensive care have resulted in survival to adulthood of over 95%.[6] Implementation of NBS and early preventive care also has led to improved SCA survival in resource-limited settings, as demonstrated in Jamaica.[7] Neonatal screening of SCA enables early diagnosis thereby improving overall mortality rates [8] and reducing complications by undertaking prompt management including administration of prophylactic antibiotic treatment and immunization with pneumococcal vaccines.[9] It also underlines the importance of parental education and comprehensive sickle cell disease care in improving clinical outcome.[9] It has been revealed that majority of parents have low levels of knowledge on SCA and this influences their understanding of the disease and negatively affects the way they care for the affected child and increases the burden of care.[10] NBS and treatment program have also been found to be highly cost-effective across all scenarios for Angola by the WHO criteria.[11] Zambia and Angola are neighbors, so implementing the program in a resource-limited setting like Zambia can be of great benefit to both the patients and the government.
Zambia is one of the countries in Sub-Saharan Africa, and despite having a high burden of sickle cell, there is no neonatal screening program for SCA. It is also a resource-limited country with most health centres in rural areas not equipped with instruments to screen for SCA. It is for this reason that we decided to test the efficacy of dry blood spots (DBSs) in the diagnosis of SCA using Hb electrophoresis as this can facilitate the easy transportation of the DBS cards to far-off hospitals in the cities where the screening can be carried out. This will improve the level of care and awareness of the disease by healthcare providers and parents and ultimately improve the levels of care and reduce mortality in Zambians living with SCA.
In comparison to conventional blood testing, DBS offers practical, clinical, and financial advantages.[12] It has been shown that drying the blood spot on blotting paper damages the capsid of viruses (HIV, cytomegalovirus, hepatitis C virus, human T-lymphotropic virus).[13] This makes DBS specimens less infectious and can be shipped by mail or other courier with no reasonable expectations of occupational exposure to blood or other potentially infectious materials.
Subjects and Methods
This cross-sectional study was carried out between November 2014 and April 2015 at the University Teaching Hospital in Lusaka, the capital city of Zambia. The study included infants from the sickle cell clinic and those without sickle cell from other wards at the University Teaching Hospital (n = 97). After obtaining informed consent from the parents, whole blood samples of at least 2 ml were collected in ethylenediaminetetraacetic acid (EDTA) containers, and at the same time, DBSs were made. Blood from EDTA containers was immediately analyzed for sickle cell using Hb electrophoresis, and DBSs were allowed to dry for a minimum of 3 h before storage at 18°C–25°C. The DBSs were stored in paper bags with desiccant to prevent moisture which can facilitate deterioration of Hb. Both EDTA blood and DBSs were analyzed for sickle cell using gel Hb electrophoresis on the Helena SAS-1 plus and Helena SAS-2 Auto-stainer.
Commercial AFSC/A2 controls were run together with patients' samples and used as a standard for analyzing the gels. Samples containing Hbs A, F, and S were also used as controls.
The University of Zambia Biomedical Research Ethics Committee (UNZABREC) IRB00001131 of IORG0000774 approved the study protocol; assurance No. FWA00000338. Written informed consent was obtained from all study participants.
Statistical analysis
To evaluate performance characteristics, the following values were used: true positive (TP), false positive (FP), true negative (TN), and false negative (FN). A TP is when the test is positive by the gold standard (whole blood electrophoresis) but negative for DBS. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and efficiency (E) were calculated. In addition, agreement of EDTA blood samples and DBS sample results for detecting Hb A and S was assessed using Cohen's Kappa statistic for agreement. All statistical tests were performed at the 5% significance level, and differences were considered significant if two tailed P < 0.05 for all tests applied.
Results
The study included a total of 97 participants. Of the 97 samples analyzed from EDTA containers and DBS, only 2 false results were observed for which positive sample in EDTA turned out negative in DBS analysis [Table 1]. DBS results after 2 weeks storage had a sensitivity and NPV of 100%, a Kappa r2 0f 0.956, P < 0.0001 [Table 2].
Table 1.
Cross tabulation comparison of dried blood spots samples and ethylenediaminetetraacetic acid blood samples
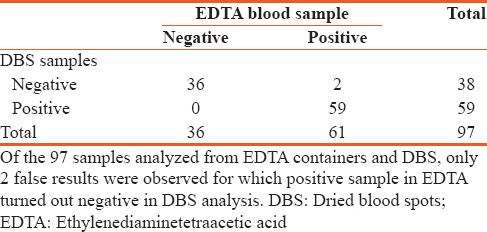
Table 2.
Sensitivity, specificity, positive predictive value, negative predictive value, efficiency, raw agreement, and Kappa on 97 dried blood samples against gold standard of ethylenediaminetetraacetic acid anticoagulated blood
Discussion
Venous blood is the gold standard method for blood collection for the screening of SCA in Zambia using Hb electrophoresis. However, this method is not ideal for the collection of blood in neonates, especially for the screening of disorders such as sickle cell hence the need to employ DBSs. The concept that capillary whole blood, obtained by heel or finger prick and blotted onto a filter paper, could be used to screen for metabolic disease in large populations of neonates was introduced in Scotland by Guthrie and Susie in 1963.[14] Since then, DBS samples from neonates have been collected routinely in many countries to screen for many disorders including sickle cell.[15]
In this study as demonstrated in Table 2, DBS results after two weeks storage had a sensitivity of 100%, a specificity of 94.7%, PPV of 96.7%, NPV of 100%, overall efficiency of 97.9% and a Kappa r2 of 0.956, P < 0.0001. This study agrees with that by Kerti et al. who determined Hb levels in sheep. They found that after 10 days storage of DBS, the elution required more time to get the same Hb results as whole blood.[16] This probably meant that Hb oxidation had begun; thus, greater quantities of Hb were required for detection of similar values to that of whole blood. The sensitivity of a clinical test refers to the ability of the test to correctly identify those patients with the disease.[17] Our results revealed a sensitivity of 100% demonstrating that the use of DBS for neonatal screening of sickle cell correctly identified all patients with sickle cell disease (TPs). On the other hand, the specificity of a clinical test refers to the ability of the test to correctly identify those patients without the disease.[17] The specificity for our study was 94.7%, which demonstrated that it correctly reported 94.7% of patients without sickle cell as TNs, but 5.3% patients without the disease were incorrectly reported as positive for sickle cell. It is an ideal but unrealistic situation to have a test whose accuracy is absolute.[17] Therefore, the use of DBS according to our study may lead to 5.3% of patients who do not have sickle cell being told of the possibility that they have it. This may imply that all those who are detected as positive for sickle cell may have to undergo further investigations to confirm that they actually have it. The 100% sensitivity makes the implementation of DBS for neonatal screening of sickle cell using gel electrophoresis ideal because no neonate with sickle cell can hypothetically be missed and none without sickle cell can be wrongly diagnosed to have it.
The PPV and the NPV of our study support the sensitivity and specificity discussed above. According to our study, it would be 96.7% likely that one has SCA when the test is positive and 100% likely that one does not have SCA when the test result is negative. This supports the theory that all those who are detected as positive may have to undergo additional investigations to confirm that they have SCA before further medical considerations.
Conclusions
Our findings support the recommendation of DBSs for NBS of SCA in Zambia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Weatherall D, Akinyanju O, Fucharoen S, Olivieri N, Musgrove P. Inherited Disorders of Hemoglobin: Disease Control Priorities in Developing Countries. 2nd ed. New York: Oxford University Press; 2006. [Google Scholar]
- 2.Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, et al. Global epidemiology of sickle haemoglobin in neonates: A contemporary geostatistical model-based map and population estimates. Lancet. 2013;381:142–51. doi: 10.1016/S0140-6736(12)61229-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Management of Birth Defects and Haemoglobin Disorders: Report of a Joint WHO-March of Dimes Meeting. Geneva: World Health Organization; 2006. [Google Scholar]
- 4.Grosse SD, Odame I, Atrash HK, Amendah DD, Piel FB, Williams TN, et al. Sickle cell disease in Africa: A neglected cause of early childhood mortality. Am J Prev Med. 2011;41:S398–405. doi: 10.1016/j.amepre.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global burden of sickle cell anaemia in children under five, 2010-2050: Modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013;10:e1001484. doi: 10.1371/journal.pmed.1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115:3447–52. doi: 10.1182/blood-2009-07-233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King L, Fraser R, Forbes M, Grindley M, Ali S, Reid M, et al. Newborn sickle cell disease screening: The Jamaican experience (1995-2006) J Med Screen. 2007;14:117–22. doi: 10.1258/096914107782066185. [DOI] [PubMed] [Google Scholar]
- 8.Vichinsky E, Hurst D, Earles A, Kleman K, Lubin B. Newborn screening for sickle cell disease: Effect on mortality. Pediatrics. 1988;81:749–55. [PubMed] [Google Scholar]
- 9.Lê PQ, Ferster A, Cotton F, Vertongen F, Vermylen C, Vanderfaeillie A, et al. Sickle cell disease from africa to belgium, from neonatal screening to clinical management. Med Trop (Mars) 2010;70:467–70. [PubMed] [Google Scholar]
- 10.Wasomwe MM, Ngoma C. A study to assess problems encountered by immediate family members in caring for children affected with sickle cell disease at university teaching hospital, Lusaka, Zambia. Med J Zambia. 2011;38:2–7. [Google Scholar]
- 11.McGann PT, Grosse SD, Santos B, de Oliveira V, Bernardino L, Kassebaum NJ, et al. A cost-effectiveness analysis of a pilot neonatal screening program for sickle cell anemia in the republic of angola. J Pediatr. 2015;167:1314–9. doi: 10.1016/j.jpeds.2015.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehmann S, Delaby C, Vialaret J, Ducos J, Hirtz C. Current and future use of “dried blood spot” analyses in clinical chemistry. Clin Chem Lab Med. 2013;51:1897–909. doi: 10.1515/cclm-2013-0228. [DOI] [PubMed] [Google Scholar]
- 13.Resnick L, Veren K, Salahuddin SZ, Tondreau S, Markham PD. Stability and inactivation of HTLV-III/LAV under clinical and laboratory environments. JAMA. 1986;255:1887–91. [PubMed] [Google Scholar]
- 14.Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. 1963;32:338–43. [PubMed] [Google Scholar]
- 15.Henderson SJ, Fishlock K, Horn ME, Oni L, Bellingham AJ. Neonatal screening for haemoglobin variants using filter paper-dried blood specimens. Clin Lab Haematol. 1991;13:327–34. doi: 10.1111/j.1365-2257.1991.tb00295.x. [DOI] [PubMed] [Google Scholar]
- 16.Kerti A, Morlin MZ, Pajor F, Bárdos L. XIII Risk Factors og Food Chain. Gödöllő, Hungary: Research Gate; 2013. Determination of hemoglobin content in whole blood and in dried blood spots in lambs. [Google Scholar]
- 17.Lalkhen AG, McCluskey A. Clinical tests: Sensitivity and specificity. Contin Educ Anaesth Crit Care Pain. 2008;8:221–3. [Google Scholar]


