Abstract
Background:
The poor hygiene of peri-implant tissues causes inflammation at tissue-implant interface, which may impair the rehabilitation success. The aim of this study was to evaluate the influence of external hexagon and Morse taper implants on peri-implant health in patients wearing mandibular overdentures for 1 year.
Materials and Methods:
A total of 46 implants were evaluated, 28 external hexagon and 18 Morse taper. Plaque index in the mini-abutment, bleeding index, peri-implant inflammation, keratinized mucosa zone, probing depth, and marginal mucosa level were evaluated after 3 months and 1 year of prostheses insertion.
Results:
Deeper probing was found in the external hexagon group compared with Morse taper (P = 0.024) after 1 year of rehabilitation. Although the Morse taper group exhibited worse scenario of peri-implant inflammation than the external hexagon group (P = 0.001), both groups showed reduced inflammation after 1 year. A larger keratinized mucosa zone was observed with external hexagon implants (P = 0.020). No significant difference was found between the groups for plaque index in the mini-abutment, bleeding index, and marginal mucosa level.
Conclusion:
In a follow-up period of 1 year, it was concluded that the external hexagon group had a larger probing depth than the Morse taper group. However, better periodontal conditions about inflammation and keratinized mucosa zone were found in external hexagon implants. It was found no influence of implant platform on plaque index in the mini-abutment, bleeding index, and marginal mucosa level.
Keywords: Dental implants, overdentures, peri-implantitis
INTRODUCTION
Mandibular implant-supported overdenture has been suggested as an efficient treatment for complete edentulous patients that had experienced problems with conventional dentures.[1] However, the poor hygiene of peri-implant tissues causes inflammation at tissue-implant interface, which may impair the rehabilitation success.[2]
The design of implant platform can influence the biomechanical and esthetic success of implant treatment.[3,4] In this sense, external connections present a microgap between implant and abutment while internal taper platforms (i.e., Morse taper) provide a tight contact at the interface.[3,5] This fit pattern avoids bacterial microleakage between the components and thus preserves peri-implant tissues.[3,6]
As periodontal health is crucial to maintain teeth in oral cavity, maintenance of peri-implant health is essential for longevity of dental implants. Continuous follow-up and supportive treatments reduce the risk factors of mucositis and peri-implantitis, which can lead to failure of implant-supported oral rehabilitation.[7] In general, studies comparing the influence of different implant connections on peri-implant health evaluate only the marginal bone level but the peri-implant soft tissues are not assessed.[8,9,10,11,12]
Assuming that peri-implant scenario is important for long-term implant success, the aim of this study was to evaluate the influence of external hexagon and Morse taper implants on peri-implant health of patients wearing mandibular overdentures during 1 year. The evaluation was based on data of plaque index, bleeding index, peri-implant inflammation, probing depth, keratinized mucosa zone, and marginal mucosa level.
MATERIALS AND METHODS
Experimental design and location
A nonrandomized controlled clinical trial was conducted in the Federal University of Rio Grande do Norte (UFRN) from January 2013 to February 2015. The experiment was approved by the Ethics Committee of the Institution (protocol 662.147).
Participants
Bimaxillary complete edentulous patients treated with maxillary conventional complete dentures and mandibular 2-implant overdentures with bar-clip attachment system were included in this study. The patients requesting implant-retained dentures were treated at the Department of Dentistry of UFRN. The individuals were divided into 2 groups, and those presenting bone availability ≥13 mm received Morse taper implants. The patients with bone height from 11 to 13 mm received external hexagon implants. Individuals with systemic dysfunctions and risk factors for implants insertion were excluded from the study.
Treatment
Maxillary conventional dentures and mandibular 2-implant overdentures with immediate loading were fabricated at the Department of Dentistry of UFRN. To reach the maximum extension of the prostheses, functional impression was taken and the casts were mounted in bilateral balanced occlusion. Two implants Neodent® Titamax (Neodent, Curitiba, Brazil; 3.75 mm in diameter; 11 mm in length) were inserted in the mandible in both groups. The external hexagon implants were placed at alveolar ridge level while the Morse taper implants were inserted 2 mm below the alveolar ridge. The protocols of implants insertion were established by the implant manufacturer (Neodent, Curitiba, Brazil).
Evaluation of peri-implant tissues
The peri-implant tissues were evaluated according to the clinical parameters described by Mombelli et al.[13,14] The analysis included plaque index in the mini-abutment, bleeding index, peri-implant inflammation, keratinized mucosa zone, marginal mucosa level, and probing depth in the mini-abutment.
Plaque index in the mini-abutment
Visual analysis was performed at mesial, distal, vestibular, and lingual surfaces of the mini-abutment after 3 months and 1 year of implant-supported rehabilitation. The score ranged from 0 to 3 (0 – no biofilm, 1 – detection of biofilm by probe sliding, 2 – visible biofilm at the marginal surface surrounding the implant, and 3 – excessive biofilm on implant surface).[13,14]
Bleeding index
The bleeding index was also evaluated at the four surfaces, and the score ranged from 0 to 3 (0 – no bleeding at probing, 1 – punctual bleeding, 2 – continuous bleeding line, and 3 – intense and deep bleeding). Score 0 represented the absence of peri-implant inflammation, while the scores 1, 2, and 3 were associated with mild, moderate, or intense peri-implant mucositis, respectively.[13,14]
Peri-implant inflammation
The clinical analysis was classified into scores ranging from 0 to 3 (0 – normal peri-implant mucosa, 1 – mild inflammation with minor color alteration, 2 – moderate inflammation with redness and swelling, and 3 – intense inflammation with redness and ulceration).[13,14]
Keratinized mucosa zone
The keratinized mucosa zone was measured (in mm) using a Williams periodontal probe. The measurement accomplished the extension from the mucogingival junction to peri-implant marginal mucosa at vestibular and lingual surfaces [Figure 1].[13,14]
Figure 1.
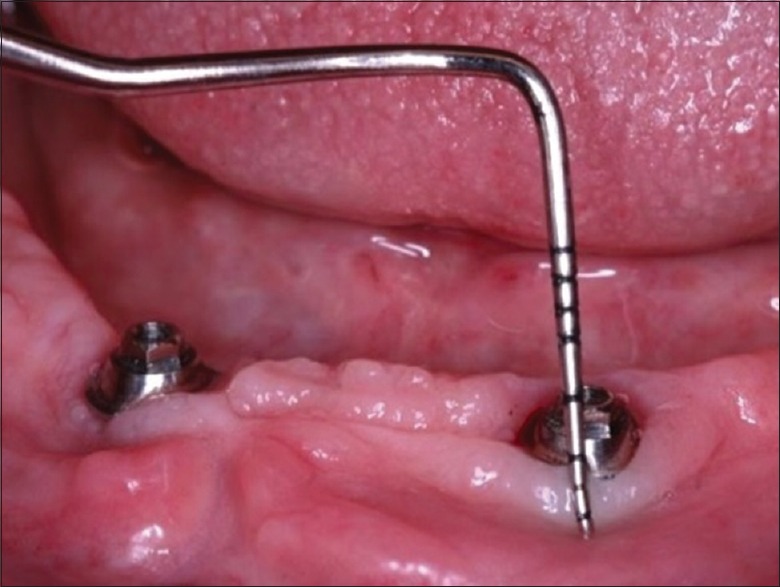
Clinical characteristics of the peri-implant tissues, abutment, and periodontal probe used in the examinations
Marginal mucosa level
The distance between the peri-implant mucosa and mini-abutment shoulder was measured (in mm) using a Williams periodontal probe. Positive values were recorded when the mucosa margin was coronal to the mini-abutment, while negative values were registered in the opposite scenario [Figure 1].[13,14]
Probing depth in the mini-abutment
The peri-implant probing depth was measured (in mm) with a Williams periodontal probe positioned at vestibular, lingual, mesial, and distal surfaces surrounding the implant [Figure 1].[13,14]
Statistical analysis
Statistical software (SPSS version 18.0, SPSS Inc., Chicago, IL, USA for Windows) was used for data analysis. Nonparametric tests were applied since the Kolmogorov–Smirnov test revealed abnormal data distribution. The Wilcoxon test was used to assess differences in peri-implant health between the 3-month and 1-year evaluations within each group. Mann-Whitney test was selected to reveal any correlation between the groups and peri-implant health. All tests were conducted at significance level of 5%.
RESULTS
A total of 23 patients (20 women and 3 men) wearing maxillary conventional dentures and mandibular 2-implant overdentures participated in the study. The age ranged from 42 to 75 years, with mean age of 58.4 years. A total of 46 implants were placed (28 external hexagon and 18 Morse taper).
The Morse taper group showed significant increase in plaque index between the 3-month and 1-year evaluations (P = 0.009). However, there was no statistically significant difference in plaque index comparing both groups of implant platform [Table 1].
Table 1.
Peri-implant indexes evaluated after 3 months (T1) and 1 year (T2) of rehabilitation with mandibular over dentures with external hexagon and Morse taper implants
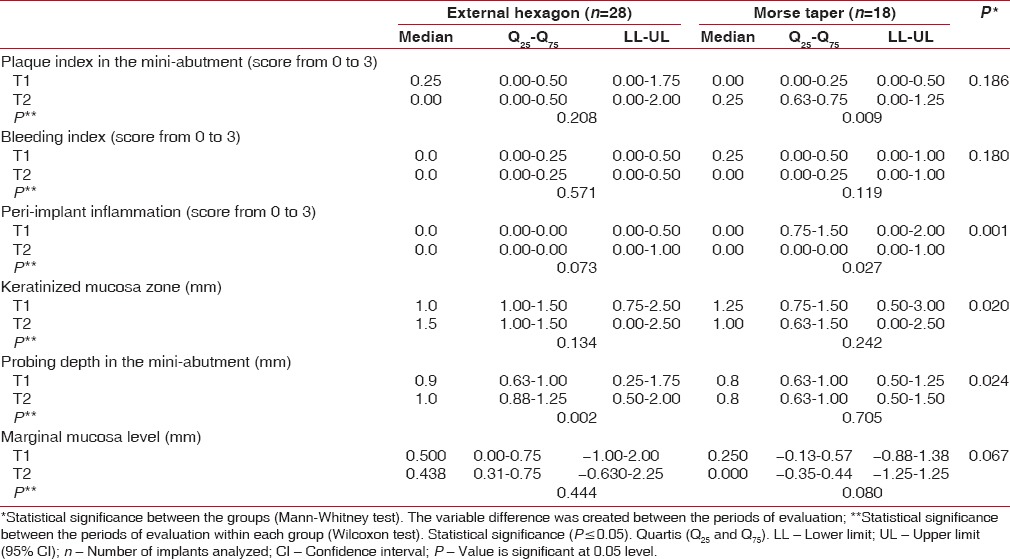
No significant difference in bleeding index and marginal mucosa level was found between the 3-month and 1-year evaluations in both groups. Similarly, no influence of implant platform was observed for those characteristics [Table 1].
Statistically significant difference in peri-implant inflammation (P = 0.001), keratinized mucosa zone (P = 0.020), and probing depth (P = 0.024) was showed between the implant platforms. Comparing the results obtained after 3 months and 1 year of evaluation within each group, only the external hexagon implants showed significant increase in probing depth, while the Morse taper implants maintained its value after 1 year of overdenture insertion [Table 1].
DISCUSSION
This controlled longitudinal trial evaluated the influence of external hexagon and Morse taper implants on peri-implant health during 3 months and 1 year. All patients were rehabilitated with mandibular 2-implant overdenture and maxillary conventional denture.
Some previous studies reported that a microgap existing at the implant-abutment interface allows microorganisms leakage into the implant.[3,4,5,6,15] As a consequence, the presence of microorganisms in the peri-implant tissues may cause chronic inflammation and implant failure.[15,16] In this sense, the tight connection obtained between the Morse taper implant and abutment has reduced microbial accumulation in comparison to the external connection that exhibits a microgap between the implant and abutment.[3,5,15]
In the present study, significant increase in biofilm accumulation in the mini-abutment between the 3-month and 1-year evaluations was found only in the Morse taper group. This result may be explained by the placement of Morse taper implants 2 mm below the alveolar ridge, which may difficult hygiene and increase biofilm accumulation. In the contrary, the external hexagon implants were fixed at the alveolar ridge level.
The Morse taper implants showed significant decrease in peri-implant inflammation between the 3-month and 1-year evaluations, which was not observed in the external hexagon group. This reduction may be associated with the location of the implant-abutment interface.[16] In Morse taper implants, this interface is located at the implant platform, reducing inflammatory cell infiltration.[17,18]
Comparing both platform designs, the external hexagon implants showed significant reduced inflammation than the Morse taper implants. This finding is probably associated with positioning of the implants in the alveolar ridge. In addition, the significant increase in plaque index between the 3-month and 1-year evaluations in the Morse taper group and the larger keratinized mucosa zone observed in the external hexagon group may also have influenced the result.[19]
A significant larger keratinized mucosa zone was found in the external hexagon group in comparison with the Morse taper. Similar findings were previously reported about the association between larger keratinized mucosa zones and minor inflammation.[19,20] In this study, the individuals rehabilitated with external hexagon also exhibited larger keratinized mucosa zone and reduced inflammation.
The occurrence of microgap at implant-abutment interface is a crucial factor for microbial leakage that leads to bone loss and deeper probing.[4,21,22] Significant deeper probing was measured between the 3-month and 1-year evaluations in the external hexagon group. Furthermore, there was statistically significant difference comparing this index between the groups, and the external hexagon implants showed worst probing depth than the Morse taper implants. This result is in accordance with the literature since external connections are usually associated with higher bone loss and deeper probing.[8,12]
It was found no influence of implant platform on plaque index in the mini-abutment, bleeding index, and marginal mucosa level after a 1-year follow-up. It is noteworthy that the marginal mucosa level remained within the significance limit, suggesting that a significant difference would probably occur after long-term evaluations. Although the protocols of implants placement and the location of implant-abutment interface were different between the groups, there was no influence of platform design on plaque and bleeding indexes probably because the overdenture is a removable prosthesis that favors oral hygiene.
Further clinical studies including radiographic evaluations of peri-implant health after a longer follow-up are required to evaluate rehabilitation with overdentures with different implant connections.
CONCLUSION
It was concluded that the external hexagon group presented deeper probing than the Morse taper after a 1-year follow-up. However, the external hexagon implants exhibited better periodontal indexes about inflammation and keratinized mucosa zone. The platform designs did not influence the results about plaque index in the mini-abutment, bleeding index, and marginal mucosa level.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to thank the Neodent company for donating the implants, which certainly contributed to this study.
REFERENCES
- 1.Meijer HJ, Raghoebar GM, Batenburg RH, Visser A, Vissink A. Mandibular overdentures supported by two or four endosseous implants: A 10-year clinical trial. Clin Oral Implants Res. 2009;20:722–8. doi: 10.1111/j.1600-0501.2009.01710.x. [DOI] [PubMed] [Google Scholar]
- 2.Real-Osuna J, Almendros-Marqués N, Gay-Escoda C. Prevalence of complications after the oral rehabilitation with implant-supported hybrid prostheses. Med Oral Patol Oral Cir Bucal. 2012;17:e116–21. doi: 10.4317/medoral.17099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machado LS, Bonfante EA, Anchieta RB, Yamaguchi S, Coelho PG. Implant-abutment connection designs for anterior crowns: Reliability and failure modes. Implant Dent. 2013;22:540–5. doi: 10.1097/ID.0b013e31829f1f2d. [DOI] [PubMed] [Google Scholar]
- 4.Ricomini Filho AP, Fernandes FS, Straioto FG, da Silva WJ, Del Bel Cury AA. Preload loss and bacterial penetration on different implant-abutment connection systems. Braz Dent J. 2010;21:123–9. doi: 10.1590/s0103-64402010000200006. [DOI] [PubMed] [Google Scholar]
- 5.Almeida EO, Freitas AC, Jr, Bonfante EA, Marotta L, Silva NR, Coelho PG, et al. Mechanical testing of implant-supported anterior crowns with different implant/abutment connections. Int J Oral Maxillofac Implants. 2013;28:103–8. doi: 10.11607/jomi.2443. [DOI] [PubMed] [Google Scholar]
- 6.Freitas-Júnior AC, Almeida EO, Bonfante EA, Silva NR, Coelho PG. Reliability and failure modes of internal conical dental implant connections. Clin Oral Implants Res. 2013;24:197–202. doi: 10.1111/j.1600-0501.2012.02443.x. [DOI] [PubMed] [Google Scholar]
- 7.Algraffee H, Borumandi F, Cascarini L. Peri-implantitis. Br J Oral Maxillofac Surg. 2012;50:689–94. doi: 10.1016/j.bjoms.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Peñarrocha-Diago MA, Flichy-Fernández AJ, Alonso-González R, Peñarrocha-Oltra D, Balaguer-Martínez J, Peñarrocha-Diago M, et al. Influence of implant neck design and implant-abutment connection type on peri-implant health. Radiological study. Clin Oral Implants Res. 2013;24:1192–200. doi: 10.1111/j.1600-0501.2012.02562.x. [DOI] [PubMed] [Google Scholar]
- 9.Bilhan H, Mumcu E, Erol S, Kutay O. Influence of platform-switching on marginal bone levels for implants with mandibular overdentures: A retrospective clinical study. Implant Dent. 2010;19:250–8. doi: 10.1097/ID.0b013e3181dc9d1a. [DOI] [PubMed] [Google Scholar]
- 10.Canullo L, Goglia G, Iurlaro G, Iannello G. Short-term bone level observations associated with platform switching in immediately placed and restored single maxillary implants: A preliminary report. Int J Prosthodont. 2009;22:277–82. [PubMed] [Google Scholar]
- 11.Canullo L, Fedele GR, Iannello G, Jepsen S. Platform switching and marginal bone-level alterations: The results of a randomized-controlled trial. Clin Oral Implants Res. 2010;21:115–21. doi: 10.1111/j.1600-0501.2009.01867.x. [DOI] [PubMed] [Google Scholar]
- 12.Koo KT, Lee EJ, Kim JY, Seol YJ, Han JS, Kim TI, et al. The effect of internal versus external abutment connection modes on crestal bone changes around dental implants: A radiographic analysis. J Periodontol. 2012;83:1104–9. doi: 10.1902/jop.2011.110456. [DOI] [PubMed] [Google Scholar]
- 13.Mombelli A, van Oosten MA, Schurch E, Jr, Land NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987;2:145–51. doi: 10.1111/j.1399-302x.1987.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 14.Macedo JP, Pereira J, Vahey BR, Henriques B, Benfatti CA, Magini RS, et al. Morse taper dental implants and platform switching: The new paradigm in oral implantology. Eur J Dent. 2016;10:148–54. doi: 10.4103/1305-7456.175677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mangano C, Mangano F, Piattelli A, Iezzi G, Mangano A, La Colla L, et al. Prospective clinical evaluation of 1920 Morse taper connection implants: Results after 4 years of functional loading. Clin Oral Implants Res. 2009;20:254–61. doi: 10.1111/j.1600-0501.2008.01649.x. [DOI] [PubMed] [Google Scholar]
- 16.Oh TJ, Yoon J, Misch CE, Wang HL. The causes of early implant bone loss: Myth or science? J Periodontol. 2002;73:322–33. doi: 10.1902/jop.2002.73.3.322. [DOI] [PubMed] [Google Scholar]
- 17.Broggini N, McManus LM, Hermann JS, Medina R, Schenk RK, Buser D, et al. Peri-implant inflammation defined by the implant-abutment interface. J Dent Res. 2006;85:473–8. doi: 10.1177/154405910608500515. [DOI] [PubMed] [Google Scholar]
- 18.Weng D, Nagata MJ, Bell M, Bosco AF, de Melo LG, Richter EJ, et al. Influence of microgap location and configuration on the periimplant bone morphology in submerged implants. An experimental study in dogs. Clin Oral Implants Res. 2008;19:1141–7. doi: 10.1111/j.1600-0501.2008.01564.x. [DOI] [PubMed] [Google Scholar]
- 19.Boynueǧri D, Nemli SK, Kasko YA. Significance of keratinized mucosa around dental implants: A prospective comparative study. Clin Oral Implants Res. 2013;24:928–33. doi: 10.1111/j.1600-0501.2012.02475.x. [DOI] [PubMed] [Google Scholar]
- 20.Schrott AR, Jimenez M, Hwang JW, Fiorellini J, Weber HP. Five-year evaluation of the influence of keratinized mucosa on peri-implant soft-tissue health and stability around implants supporting full-arch mandibular fixed prostheses. Clin Oral Implants Res. 2009;20:1170–7. doi: 10.1111/j.1600-0501.2009.01795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.IA. Analysis of the bacterial seal at the implant-abutment interface in external-hexagon and Morse taper-connection implants: An in vitro study using a new methodology. Int J Oral Maxillofac Implants. 2012;27:1091–5. [PubMed] [Google Scholar]
- 22.do Nascimento C, Miani PK, Pedrazzi V, Gonçalves RB, Ribeiro RF, Faria AC, et al. Leakage of saliva through the implant-abutment interface: In vitro evaluation of three different implant connections under unloaded and loaded conditions. Int J Oral Maxillofac Implants. 2012;27:551–60. [PubMed] [Google Scholar]


