Abstract
Background:
To evaluate and compare the effect of curcumin (CMN) mouth rinse with chlorhexidine (CHX) mouth rinse on clinical parameters and reactive oxygen metabolites (ROM) levels in participants with chronic gingivitis.
Materials and Methods:
Thirty plaque-induced generalized chronic gingivitis participants were randomly assigned to three groups – Group I (control/saline), Group II (CHX), and Group III (CMN), respectively. Baseline plaque index (PI), gingival index (GI), and salivary ROM were estimated, and oral prophylaxis was done. The parameters were recorded and evaluated again at the end of 4 weeks.
Results:
Overall, the three groups have shown a significant reduction in PI, GI, and ROM levels at the end of 4 weeks. However, on intragroup comparison, only CMN group have shown significant reduction in ROM levels at the end of 4 weeks (P < 0.05).
Conclusion:
CMN mouth rinse can be considered as an alternative antigingivitis agent to CHX because of its anti-inflammatory and antioxidant properties.
Keywords: Chlorhexidine, curcumin, dental plaque, free radicals, gingivitis, mouthwash
INTRODUCTION
Inflammation of the gingiva induced by bacteria is the most common form of gingivitis. The universal features of gingivitis are the clinical signs and symptoms of inflammation, which are confined to the gingiva. Gingivitis is reversible, once the etiology, that is, bacterial-laden plaque is eradicated by mechanical therapy. The initial changes from health to plaque-induced gingivitis may be subclinical, but as gingivitis progresses to more advanced forms of the disease, the clinical features become more apparent.
Classical studies have demonstrated that accumulation of bacteria on teeth reproducibly induces gingival inflammatory response. Removal of plaque leads to the disappearance of the clinical signs of this inflammation.[1,2] However, regular maintenance to prevent further buildup of plaque is a primary requisite to prevent the recurrence of disease. On the basis that plaque-induced gingivitis always precedes the occurrence and recurrence of periodontitis, the mainstay of primary and secondary prevention of both diseases is the control of supragingival plaque. Scaling and root planing are still considered the gold standard and forms the cornerstone of nonsurgical periodontal therapy. Maintenance and prevention of recurrence can be achieved by chemical plaque control.
The European Workshop on Periodontology in 1996 defined, the agents used in chemical supragingival plaque control as antiplaque, antigingivitis, plaque reducing, and antimicrobial agents. All of them have been shown to benefit gingivitis by altering the quantity/quality of plaque. At present, most antiplaque agents are antimicrobial and prevent the bacterial proliferation of plaque development. The most studied and effective chemical plaque control agent for plaque inhibition and the prevention of gingivitis is chlorhexidine (CHX). Long-term oral use has been related to a number of local side effects such as brown discoloration of teeth and restoration and taste perturbation. On the contrary, in practice today is the use of herbal mouth rinses. They have been shown to be effective in control of plaque with fewer/no reported side effects.
The oxidative process is essential for life and death of a cell. Molecular oxygen has the ability to unpair and leave free radicals which are unstable. This unstable radical is highly reactive and causes the formation of reactive oxygen species (ROS). ROS are generated from enzymatic and nonenzymatic sources. These reactive metabolites are selectively neutralized by body's defense mechanism. Lipopolysaccharides and DNA from periodontal pathogens stimulate the innate immune response and causes recruitment and activation of hyperresponsive polymorphonuclear leukocytes (PMNs) and thus speeds up the production of ROS. Periodontal tissue destruction leads to overproduction of lipid peroxides, an inflammatory mediator, and oxidized proteins. These products further activate macrophages, neutrophils, and fibroblasts to generate more ROS. Certainly, free radicals and other reactive species have extremely short half-life in vivo (10)−6 to (10)−9 and simply cannot be measured directly. In vitro, systems called spin traps are used to measure radical species, but there are currently no suitable spin traps/probes available for in vivo measurement of ROS production in humans. Hence, measurement of ROS is done by measuring the concentration of biomarkers of tissue destruction. Reactive oxidative metabolites (ROMs) is one such method of evaluating total oxidative stress in the plasma.[3]
Turmeric, known as “Indian saffron,” has been in use for thousands of years in Indian medicine, cuisine as well as for the religious purposes. The bright yellow-colored rhizome is a product of Curcuma longa, with curcumin (CMN) as its active ingredient. CMN has a plethora of beneficial properties including analgesic, anti-inflammatory, antioxidant, antiseptic, and antimicrobial which can be exploited to treat various diseases. The anti-inflammatory effect has been explored in the treatment of gingivitis, both as a local application in the form of a gel or as a mouthwash.[4,5] Although improvement in clinical parameters has been noted, biochemical markers for the anti-inflammatory effect have not been studied. Therefore, the aim of the study was to examine and compare the anti-plaque and anti-gingivitis effect of CHX and indigenously prepared CMN mouthwash on clinical parameters as well as the anti-inflammatory effect of both on reactive oxygen metabolites level in the saliva of gingivitis participants.
MATERIALS AND METHODS
A total of thirty participants were recruited for the study. Ethical committee approval was procured from the institution. The study protocol, orientation about the products (both CHX and CMN mouth rinse), timeline of the study, and the probable risks associated with the study were elucidated to the participants, and an oral as well as a written consent was procured. Following a detailed medical and dental history, systemically healthy participants with a minimum of twenty teeth and with chronic gingivitis were included for the study. The exclusion criteria were the presence of any systemic disease, pregnancy and lactation, allergy to either CHX and/or turmeric, participants on antibiotics for the past 3 months, participants with a history of oral prophylaxis within 3 months previous to this study, mentally challenged participants, and habits like mouth breathing, smoking that might alter the result of the study.
Preparation of curcumin mouthwash
Raw material
The mouthwash was prepared at the Department of Pharmacology, ACS Medical College and Research Centre. The raw material, turmeric rhizome was collected from bio organic farm, Thiruvallur consisting of 5% CMN. The solvents used were HPLC grade obtained from Nanjing Hanbang Chemicals Co. Ltd., (Jiangsu, China).
Curcumin extract
The turmeric root was ground into powder using mortar and was air dried to remove moisture in the ground powder. A known amount of turmeric powder (100 g) was weighed accurately and was soaked in 99% ethanol for 48 h. The filtrate that was procured was a nice amber colored ethanol solution. The ethanol was allowed to evaporate by air drying and was placed in a microwave at 200 F, till a dry extract enriched in CMN was obtained.
Curcumin mouthwash
Preparation of the mouthwash was in accordance with the previous study by Waghmare et al. 2011.[6] CMN mouthwash was prepared by dissolving 10 mg of CMN extract in 100 ml of distilled water and 0.005% of flavoring agent (peppermint oil) and the pH was adjusted to 4.
Study design and clinical protocol
This study was designed as a single-blind, randomized, three groups, and placebo-controlled experiment using three different mouthwashes [Figure 1]. The sample size was calculated based on the gingivitis prevalence in Mogappair population from a study in 2011[7] and using the sample size formula: N = 4qp/L2 Where q is 95% confidence level, p is prevalence, L is allowable error. To eliminate bias, diagnosis, scaling, and recording of findings was done by a single examiner. The thirty participants (18 females and 12 males; age group 25–60 years) who satisfied the inclusion/exclusion criteria were randomly allocated to one of the following three groups.
Figure 1.

Study design flowchart. CHX – Chlorhexidine, TUM – Turmeric, PI – Plaque index, GI – Gingival index, ROM – Reactive oxygen metabolite
Group I – Control (saline)
Group II – CHX mouthwash 0.2% (Rexidin; Warren, Indoco Remedies Ltd, India)
Group III – CMN mouthwash 0.1%.
The study data were entered into a standard pro forma. At baseline, all the three groups underwent meticulous scaling and polishing and were assigned to the respective mouthwash. Before scaling, all the participants were assessed clinically for plaque index (PI)[8] and gingival index (GI)[9] and unstimulated saliva was collected for ROM estimation. After thorough rinsing, to remove any debris, the saliva was collected 10 min later to avoid sample dilution. Whole saliva (2 ml) was collected in disposable, sterile, clean tubes, and centrifuged immediately to remove cell debris at 1000 rpm for 10 min at 4°C. The supernatant was removed and stored in small aliquots at −80°C until analysis.
The participants were advised to follow their regular brushing protocol. After brushing, the participants were advised to use 10 ml of the mouthwash without dilution, swish for 1 min, twice every day for 4 weeks. Participants were also instructed to refrain from consuming anything for ½ h after rinsing. The compliance of the participants was verified by providing them with a medication sheet, explaining the time and dose. The medication sheet had to be filled by the participants after using the mouthwash. Clinical assessment of PI and GI and saliva collection for ROM was performed again at the end of 4 weeks.
Primary and secondary endpoints
The primary outcome measure was the differences in the mean PI and GI scores from baseline to 4 weeks[10] and the secondary outcome analyzed was the changes in the ROM levels from baseline to 4 weeks.
Measurement of reactive oxygen metabolites[3]
The measurement of salivary ROM level (whole oxidant capacity of saliva against N-N-diethyl paraphenylenediamine in the acidic buffer) was performed using a spectrophotometer. About 20 μl salivary sample and 1 ml acetate buffer (pH–4.8) were gently mixed in a cuvette and 20 μl chromogenic substrate (N, N-diethyl-p-phenylenediamine) was added. After mixing, the cuvette was immediately incubated in the thermostatic block of the analyzer for 5 min at 37°C. The 505 nm absorbance was recorded. The Carratelli unit is used for measurement. It was established that 1 CARRU corresponds to 0.08 mg/dl hydrogen peroxide.
Test principle
The ROMs test uses the principle of Fenton's reaction: by mixing a biological sample with an acidic buffer (Reagent R1), the newly created transition metal ion (iron or copper) catalyzes the breakdown of hydroperoxyl (ROO+) and alkoxyl (RO+). By adding a chromogen (N, N-diethyl-p-phenylenediamine, Reagent R2) having the ability to donate an electron and change color when oxidized by free radicals, and using photometric reading available with the FRAS 4 dedicated analytical equipment, it becomes possible to quantify the level of hydroperoxides available in the sample.
Statistical analysis
The normality tests Kolmogorov-Smirnov and Shapiro-Wilk's tests results revealed that the variables followed a normal distribution. Therefore, to analyze the data parametric methods were applied. To compare the mean values between groups one-way ANOVA was applied followed by Bonferroni post hoc tests for multiple pair-wise comparisons. To compare mean values between baseline and 4 weeks paired t-test was applied. To analyze the data statistical software was used (IBM, SPSS Statistics for Windows, Version 22.0, Armonk, NY, USA: IBM Corp. Released 2013) significance level is fixed as 5% (α = 0.05).
RESULTS
The ROM levels, PI, and GI mean values show a statistical significance from baseline to 4 weeks, indicating reduction in PI, GI scores as well as mean levels of ROM [Table 1]. On intragroup comparison of PI, GI, and ROM from baseline to 4 weeks [Table 2], the control group showed statistical significance for PI, whereas ROM levels and GI showed no significance. The CHX group had significant values (P < 0.05) for both PI and GI, but no significance was noted in ROM values from baseline to 4 weeks. Table 3 shows the intergroup comparison of PI, GI, and ROM levels at baseline and at 4 weeks by one-way ANOVA. Significance was evident at end of 4 weeks for all the three parameters, namely, PI, GI, and ROM levels. Table 4 shows Bonferroni post hoc tests for multiple comparison.
Table 1.
Comparisons in plaque index, gingival index, and reactive oxygen metabolite levels from baseline to 4 weeks*
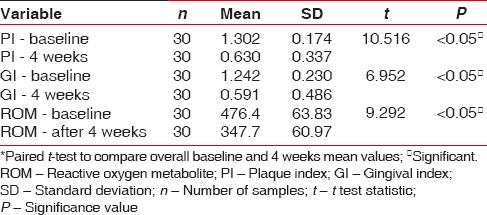
Table 2.
Intragroup comparison of plaque index, gingival index, and reactive oxygen metabolite levels from baseline to 4 weeks*
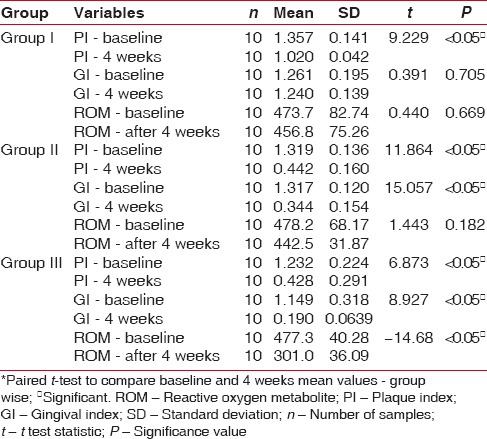
Table 3.
Intergroup comparison of plaque index, gingival index, and reactive oxygen metabolite levels at baseline and at 4 weeks*
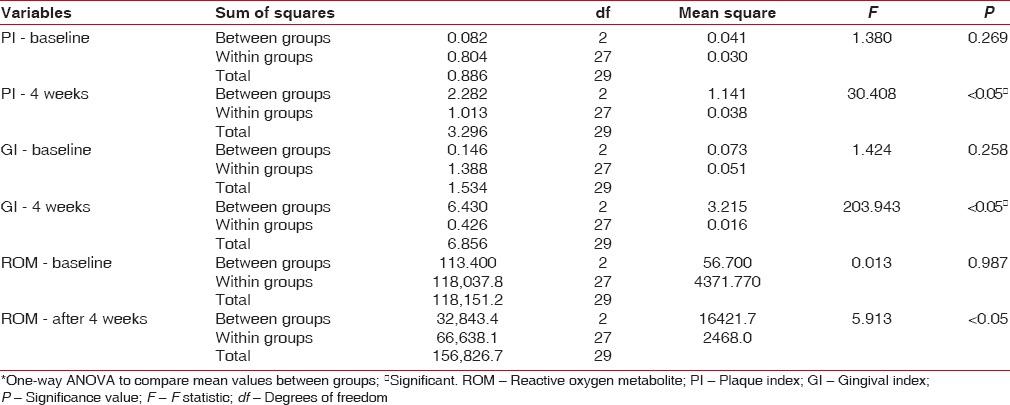
Table 4.
Bonferroni post hoc tests for multiple comparisons
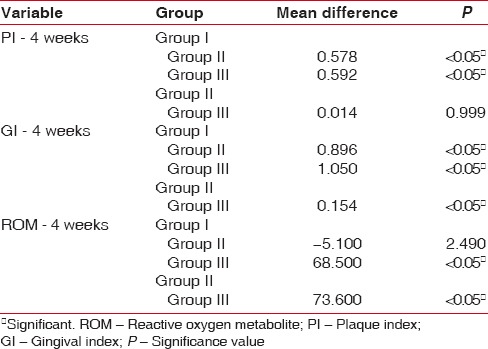
A statistically significant difference was seen for PI between control and CHX as well as control and CMN group. However, no significance was seen between CHX and CMN group. Similarly, for GI, it was significant among all the groups, whereas for ROM, the significant pairs were the control and CMN group as well as the CHX and CMN group.
DISCUSSION
Ayurvedic medicine has its origin in ancient Indian culture – the medicinal use of plants to treat various diseases. In the recent years, interest in herbal alternatives to treat gingivitis has skyrocketed as these naturally occurring compounds not only achieve the desired effect but also have no significant side effects.
As with many other oral health diseases, it is proven that bacterial laden dental plaque is the culprit behind gingival inflammation and the effect of plaque control on gingivitis and periodontitis is well documented. In short-term investigations, chemical plaque control (oral rinsing twice a day with 0.2% CHX gluconate solution) has been effective in the prevention of human gingivitis.[11] However, certain unwarranted side effects preclude its use for long term.
The effect of CHX on plaque and its property of slow release have made it as the panacea in the adjunctive treatment to gingivitis. CHX being cationic prevents pellicle formation and is bacteriostatic at lower concentrations and bactericidal at higher concentrations.[12]
CMN, a polyphenolic compound, is a secondary metabolite isolated from the rhizomes of turmeric, and exhibits a number of therapeutic effects, including anti-cancer properties. It also protects against free-radical damage because it is a strong antioxidant.[13]
The antibacterial effect of CMN is well documented and includes a plethora of microorganisms.[14] Mohammed et al.[15] have shown that the antimicrobial activity of CMN may be useful for controlling dental biofilms. Waghmare et al.[6] have reported a significant reduction in the total microbial count with the use of turmeric mouthwash, making it a suitable candidate as an antiplaque agent.
The anti-inflammatory effects of CMN have been well studied in many medical conditions. It can be effective in improving inflammation of rheumatoid arthritis (RA) and reducing clinical manifestation of RA.[16] Exhaustive remission of anterior uveitis was noticed with the use of CMN.[17] CMN was proven beneficial in irritable bowel syndrome (IBS) treatment[18] and also works as a reducing agent to delayed graft rejection after kidney transplant surgery.[19] It is natural therefore to exploit the anti-inflammatory effect in the treatment of gingival inflammation.
The anti-inflammatory mechanism of action of CMN could be due to the blockage of arachidonic acid metabolism,[20] namely, (1) selective inhibition in the synthesis of prostaglandin E2 and thromboxane occurs without altering the production of prostacyclin; (2) inhibition of arachidonic acid metabolism through lipoxygenase and scavenging of free-radicals generated in this pathway; and (3) decreased expression of inflammatory cytokines interleukin (IL)-1b, IL-6, and tumor necrosis factor-alpha.
In the present study, the objective was to evaluate the anti-plaque and anti-gingivitis effect of CMN on clinical parameters using PI, GI, and biochemical parameter, namely, the ROM and compare it with the conventionally used CHX mouth rinse and saline mouth rinse which was maintained as the control group. Collection of saliva is less invasive and less tedious than GCF, which requires considerable skill and would be ideal in reflecting periodontal inflammation. Since all were gingivitis participants, saliva was considered rather than GCF. In all the groups, taken together, there is overall reduction in PI, GI and ROM levels [Table 1]. On individual group comparison between baseline and 4 weeks, significant independent reduction in PI was seen in all the three groups and reduction GI scores are seen in both CHX and CMN groups and only in CMN group, reduction in ROM levels are seen at 4 weeks interval [Table 2]. The anti-plaque activity of 0.2% CHX is well established in many studies,[21,22] and our results are also consistent with the previous studies. The intergroup comparison of PI, GI, and ROM levels at baseline and at 4 weeks, also showed significance between the groups for all the three parameters [Table 3]. However, Bonferroni post hoc tests for multiple comparisons [Table 4], show nonsignificance between PI of CHX and CMN, denote that both were comparable in the anti-plaque property but significant for GI, indicating CMN having better anti-inflammatory effects than CHX at 4 weeks. Our results are in concordance with Mali et al.,[4] wherein improved results in clinical indices in both 1% CMN mouthwash as well as 0.2% CHX mouthwash were reported. However, our results differ from Singh et al. 2015,[23] wherein reduction in PI at end of 2nd and 3rd week was better in CHX group. Similarly, the decrease of mean GI was evidently higher in Group CHX than Group CMN. The difference between our study and theirs could be due to the formulation used – gel versus mouthwash in our study, which may have influenced the results.
About 1% CMN solution was shown to resolve signs and symptoms of inflammation better than CHX and saline irrigation as a subgingival irrigant and reduces the inflammatory edema and vascular engorgement of connective tissue, causing shrinkage.[24]
Few studies have compared the gel formulations of turmeric with CHX in periodontitis participants. Jaswal et al. 2014[25] did a comparative evaluation of single application of 2% whole turmeric gel versus 1% CHX gel in chronic periodontitis patients and concluded that the experimental local drug delivery system containing 2% whole turmeric gel helped in reduction of probing depth and gain of clinical attachment levels. In addition to clinical parameters, the effect on periodontal pathogens was also noted by Bhatia et al. 2014.[26] The locally delivered 1% CMN gel was more effective in inhibiting the growth of oral bacteria (Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum, and Capnocytophaga) when used as an adjunct to SRP in the treatment of chronic periodontitis.
PMNs are the primary host defense modulator in gingival inflammation and ROS are generated by PMNs, as a result of the inflammatory tissue response mechanisms. Reactive oxygen metabolites (ROM), a measure of ROS has been found to be useful for the evaluation of oxidative status. This test measures the level of generic peroxide, which in turn reflects the level of free radicals from which they were formed. Uma Sudhakar et al. 2015,[27] showed increased ROM levels in saliva of chronic gingivitis and periodontitis patients, indicating significant oxidative stress in gingival inflammation. In the present study, overall ROM levels, reduced at the end of 4 weeks [Table 1]. Although ROM level reduction was seen in control and CHX group, it was not significant [Table 2], whereas it was significant in CMN group. The evaluation of ROM is novel, reflecting the anti-inflammatory and anti-oxidant effect of CMN; however, a similar study with ROM estimation was not found for comparison. Our results have shown a significant decrease in ROM levels at the end of 4 weeks only in the CMN group and not in the control or CHX groups, therefore, this additional benefit can be attributed to anti-inflammatory effect of CMN only.
The therapeutic potential of CMN is being explored and recently, it has been shown that a series of chemically modified CMCs with increased solubility and zinc-binding activity, while retaining, or further enhancing, their effects better than the parent compound CMN, on pro-inflammatory cytokines and MMPs in in vitro, in cell culture, and in an animal model of periodontal inflammation.[28]
Both CHX and CMN groups had good patient compliance and no adverse effects were reported in both the groups. However, the limitations of the study were the shorter duration of the clinical trial and smaller sample size. Additional studies are required corroborate whether the effect is clinically and statistically significant over a longer duration of time.
CONCLUSION
Within the limitations of the study, CMN mouthwash, as a plaque control agent was found to be comparable in plaque reduction as that of CHX and better in reducing gingival inflammation as evident clinically and in the reduction of ROM. The anti-inflammatory and antioxidant effects of CMN should be ascertained with further longitudinal studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors are thankful to the Department of Pharmacology, ACS Medical College and Research Institute for preparing the CMN mouthwash.
REFERENCES
- 1.Loe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol. 1965;36:177–87. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- 2.Theilade E, Wright WH, Jensen SB, Löe H. Experimental gingivitis in man. II. A longitudinal clinical and bacteriological investigation. J Periodontal Res. 1966;1:1–3. doi: 10.1111/j.1600-0765.1966.tb01842.x. [DOI] [PubMed] [Google Scholar]
- 3.Cesarone MR, Belcaro G, Carratelli M, Cornelli U, De Sanctis MT, Incandela L, et al. A simple test to monitor oxidative stress. Int Angiol. 1999;18:127–30. [PubMed] [Google Scholar]
- 4.Mali AM, Behal R, Gilda SS. Comparative evaluation of 0.1% turmeric mouthwash with 0.2% chlorhexidine gluconate in prevention of plaque and gingivitis: A clinical and microbiological study. J Indian Soc Periodontol. 2012;16:386–91. doi: 10.4103/0972-124X.100917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kandwal A, Mamgain RK, Mamgain P. Comparative evaluation of turmeric gel with 2% chlorhexidine gluconate gel for treatment of plaque induced gingivitis: A randomized controlled clinical trial. Ayu. 2015;36:145–50. doi: 10.4103/0974-8520.175537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waghmare PF, Chaudhari AU, Karhadkar VM, Jamkhande AS. Comparative evaluation of turmeric and chlorhexidine gluconate mouthwash in prevention of plaque formation and gingivitis: A clinical and microbiological study. J Contemp Dent Pract. 2011;12:221–4. doi: 10.5005/jp-journals-10024-1038. [DOI] [PubMed] [Google Scholar]
- 7.Gopalakrishnan S, Jayakumar P, Sudhakar U, Shankarram V. Prevalence of gingivitis and periodontitis in Mogappair population, Chennai, Tamilnadu. Int J Contemp Dent. 2011;2:83–8. [Google Scholar]
- 8.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 9.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38:610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 10.Vangipuram S, Jha A, Bhashyam M. Comparative efficacy of aloe vera mouthwash and chlorhexidine on periodontal health: A randomized controlled trial. J Clin Exp Dent. 2016;8:e442–e447. doi: 10.4317/jced.53033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathur S, Mathur T, Srivastava R, Khatri R. Chlorhexidine: The gold standard in chemical plaque control. Natl J Physiol Pharm Pharmacol. 2011;1:45–50. [Google Scholar]
- 12.Addy M. Chlorhexidine compared with other locally delivered antimicrobials. A short review. J Clin Periodontol. 1986;13:957–64. doi: 10.1111/j.1600-051x.1986.tb01434.x. [DOI] [PubMed] [Google Scholar]
- 13.Bernd A, Ramirez-Bosca A, Huber H, Diaz Alperi J, Thaci D, Sewell A, et al. In vitro studies on the immunomodulating effects of polypodium leucotomos extract on human leukocyte fractions. Arzneimittelforschung. 1995;45:901–4. [PubMed] [Google Scholar]
- 14.Moghadamtousi SZ, Kadir HA, Hassandarvish P, Tajik H, Abubakar S, Zandi K, et al. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res Int 2014. 2014 doi: 10.1155/2014/186864. 186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohammed NA, Habil NY. Evaluation of antimicrobial activity of curcumin against two oral bacteria. Automation, Control Intell Syst. 2015;3:18–21. [Google Scholar]
- 16.Deodhar SD, Sethi R, Srimal RC. Preliminary study on antirheumatic activity of curcumin (diferuloyl methane) Indian J Med Res. 1980;71:632–4. [PubMed] [Google Scholar]
- 17.Lal B, Kapoor AK, Asthana OP, Agrawal PK, Prasad R, Kumar P, et al. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res. 1999;13:318–22. doi: 10.1002/(SICI)1099-1573(199906)13:4<318::AID-PTR445>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Bundy R, Walker AF, Middleton RW, Booth J. Turmeric extract may improve irritable bowel syndrome symptomology in otherwise healthy adults: A pilot study. J Altern Complement Med. 2004;10:1015–8. doi: 10.1089/acm.2004.10.1015. [DOI] [PubMed] [Google Scholar]
- 19.Shoskes D, Lapierre C, Cruz-Correa M, Muruve N, Rosario R, Fromkin B, et al. Beneficial effects of the bioflavonoids curcumin and quercetin on early function in cadaveric renal transplantation: A randomized placebo controlled trial. Transplantation. 2005;80:1556–9. doi: 10.1097/01.tp.0000183290.64309.21. [DOI] [PubMed] [Google Scholar]
- 20.Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, Conney AH, et al. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res. 1991;51:813–9. [PubMed] [Google Scholar]
- 21.Tepe JH, Leonard GJ, Singer RE, Gray JA, Gibberman BP, Mulvihill JE. The long-term effect of chlorhexidine on plaque, gingivitis, sulcus depth, gingival recession, and loss of attachment in beagle dogs. J Periodontal Res. 1983;18:452–8. doi: 10.1111/j.1600-0765.1983.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 22.Subhash G, Das AK. The effect of chlorhexidine gluconate solution on gingivitis and its best mode of application. J Indian Dent Assoc. 1985;57:501–4. [PubMed] [Google Scholar]
- 23.Singh V, Pathak AK, Pal M, Sareen S, Goel K. Comparative evaluation of topical application of turmeric gel and 0.2% chlorhexidine gluconate gel in prevention of gingivitis. Natl J Maxillofac Surg. 2015;6:67–71. doi: 10.4103/0975-5950.168238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suhag A, Dixit J, Dhan P. Role of curcumin as a subgingival irrigant: A pilot study. Perio. 2007;4:115–21. [Google Scholar]
- 25.Jaswal R, Dhawan S, Grover V, Malhotra R. Comparative evaluation of single application of 2% whole turmeric gel versus 1% chlorhexidine gel in chronic periodontitis patients: A pilot study. J Indian Soc Periodontol. 2014;18:575–80. doi: 10.4103/0972-124X.142445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatia M, Urolagin SS, Pentyala KB, Urolagin SB, K B M, Bhoi S. Novel therapeutic approach for the treatment of periodontitis by curcumin. J Clin Diagn Res. 2014;8:ZC65–9. doi: 10.7860/JCDR/2014/8231.5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sudhakar U, Ramakrishnan T, Rekha A, Tamizhchelvan H, Ram VS, Kannadasan K, et al. Prevalence of reactive oxygen metabolite levels in plasma, GCF and saliva in chronic periodontitis, chronic gingivitis and healthy periodontium – A biochemical study. Biosci Biotech Res Asia. 2015;12:1659–63. [Google Scholar]
- 28.Gu Y, Lee HM, Napolitano N, Clemens M, Zhang Y, Sorsa T, et al. 4-methoxycarbonyl curcumin: A unique inhibitor of both inflammatory mediators and periodontal inflammation. Mediators Inflamm 2013. 2013:329740. doi: 10.1155/2013/329740. [DOI] [PMC free article] [PubMed] [Google Scholar]


