Abstract
Background:
There is a paucity of literature regarding the microbial etiology of community-acquired pneumonia (CAP) in India. The current study was aimed to study the microbial etiology of hospitalized adults with CAP.
Methods:
The study was conducted in a 700-bedded North Indian hospital. Consecutive adults admitted with CAP over a period of 2 years from 2013 to 2015 were recruited for the study, and apart from clinical evaluation underwent various microbiological studies in the form of blood culture, sputum culture, urinary antigen for pneumococcus and Legionella, serology for Mycoplasma and Chlamydia and real-time reverse transcriptase polymerase chain reaction for influenza viruses. Radiographic studies were performed in all patients and repeated as required. The patients were treated with standard antibiotic/antiviral therapy and outcomes were recorded.
Results:
A total of 225 patients (median age: 59 years) were enrolled. Streptococcus pneumoniae was the most common organism found (30.5%), followed by Legionella pneumophila (17.5%), influenza viruses (15.4%), Mycoplasma pneumoniae (7.2%), Chlamydia pneumonia (5.5%), Mycobacterium tuberculosis (4.8%), Klebsiella pneumoniae (4.8%), methicillin-resistant Staphylococcus aureus (3.5%), Pseudomonas aeruginosa (3.1%), methicillin-sensitive S. aureus (1.7%), and Acinetobacter sp. (0.8%) with 4% of patients having multiple pathogens etiologies. High Pneumonia Severity Index score correlated with the severity and outcome of the CAP but was not predictive of any definite etiological pathogen. In-hospital mortality was 8%.
Conclusion:
Streptococcus pneumoniae, Legionella, and influenza constitute the most common etiological agents for north Indian adults with CAP requiring hospitalization. Appropriate antibiotic therapy and preventive strategies such as influenza and pneumococcal vaccination need to be considered in appropriate groups.
KEY WORDS: Atypical pathogens, microbiology, pneumonia, viruses
INTRODUCTION
Community-acquired pneumonia (CAP) is a common disease with significant morbidity and mortality worldwide.[1,2] The annual incidence of CAP varies from 5 to 11/1000 population, the rates being higher in the elderly.[3] The mortality rate for CAP is <5% for outpatient cases; it rises to 10% in admitted ward patients and can exceed 30% in patients admitted to Intensive Care Unit (ICU).[4] Patients with coexisting illnesses such as chronic obstructive pulmonary disease (COPD), diabetes mellitus, renal failure, congestive heart failure, coronary artery disease, malignancy, chronic neurological disease, and chronic liver disease have increased incidence of CAP with increased mortality.[3] The list of potential etiologic agents in CAP is extensive and includes bacteria, fungi, viruses, and protozoa. Although Pneumococcus remains the most commonly identified cause of CAP, the frequency with which it is implicated has declined,[5] and it is now detected in only about 10%–15% of inpatient cases in the United States[6,7] and thus other organisms must also be considered in light of the patient's risk factors and severity of illness.[8] It is considered useful to think of the etiological agent as a typical or an atypical organism. The former category includes Streptococcus pneumoniae, Haemophilus influenzae, and (in selected patients) Staphylococcus aureus and Gram-negative bacilli such as Klebsiella pneumoniae and Pseudomonas aeruginosa.[9,10,11] The “atypical” organisms include Mycoplasma pneumoniae, Chlamydia pneumoniae (mostly in outpatients), and Legionella spp. (mostly in inpatients) as well as respiratory viruses such as influenza viruses, adenoviruses, and respiratory syncytial viruses The frequency and importance of atypical pathogens have significant implications for therapy. These organisms are intrinsically resistant to all beta-lactam agents and must be treated with a macrolide, a fluoroquinolone, or a tetracycline. In the ~10%–15% of CAP cases that are polymicrobial, the etiology often includes a combination of typical and atypical pathogens.[12,13]
Differences in epidemiology of the pathogens causing CAP are crucial for decision about empiric antibiotic therapy which has a major influence on the prognosis of such patients. Data about the microbiological causes of CAP in developing countries, like India, are scant and hampered by the available data being old and limited by a small sample size and the detection methods having stayed confined to bacterial cultures. While some have reported Pneumococcus spp. as the dominant etiological agent,[8] others have reported Gram-negative bacilli as the most frequent pathogens.[11] Most of the studies conducted in India to look for etiological agents of pneumonia have been limited to find typical pathogens, and very scanty data are available for atypical pathogens. In the last few years, there have been further advances in the medical management of CAP with the development of novel immunoassays to detect S. pneumoniae and Legionella pneumophila antigens in urine. Use of the pneumococcal urinary antigen test (UAT) improves diagnostic yield by 23%–39%.[14,15] In a meta-analysis, the pooled sensitivity and specificity of Pneumococcal UAT was 74% (95% confidence interval [CI]: 72%–77%) and 94% (95% CI: 93%–95%), respectively, compared to traditional microbiology.[14] The Legionella UAT for the diagnosis of Legionnaire's disease has a sensitivity of around 70%–80% (generally higher than culture), a specificity of >99%, and will usually remain positive for days to weeks after effective treatment is initiated.[16] The availability of these rapid and sensitive techniques has facilitated the microbial diagnosis of pneumonia and provided new tools that may improve our epidemiological understanding of CAP. Hence, etiology of CAP can be looked at with wider array of diagnostic tools. Updated data about the incidence of pneumonia may be important to recognize changes in disease patterns, to assess the new etiological profile according to the new diagnostic technologies, to evaluate preventive interventions, and to allocate health care and research resources.[17,18,19,20] We herewith present our data of hospitalized patients with CAP seen over a 2-year period in Northern India.
METHODS
The study was conducted in Sher-i-Kashmir Institute of Medical Sciences (SKIMS), Kashmir, a 700-bedded tertiary care cum referral facility in North India. Adults (≥18 years, n = 225) clinically diagnosed to have CAP were selected using purposive sampling technique during a 24-month period, from November 1, 2013 to October 31, 2015. The exclusion criteria included patients’ age below 18 years of age or those who satisfied the criteria for a healthcare-associated pneumonia. CAP was defined as an acute illness associated with at least one of the following signs or symptoms: fever, new cough with or without sputum production, pleuritic chest pain, dyspnea, or altered breath sound on auscultation, plus a chest radiograph showing a new pulmonary infiltrate compatible with the presence of acute pneumonia appearing within 48 h of hospitalization.[1,2] Demographic data were recorded from all participants along with a history of any underlying comorbid illnesses such as diabetes mellitus, arterial hypertension, chronic lung disease, congestive heart failure, cerebrovascular disease, and any treatment received before the reporting to the hospital.
Severity of pneumonia at presentation was assessed by confusion, urea nitrogen, respiratory rate, blood pressure, age ≥ 65 years (CURB-65) score, and Pneumonia Severity Index (PSI)[9] was determined within the first 24 h after admission. All the patients underwent routine investigations in the form of complete blood count, routine serum biochemistry, radiography of the chest, electrocardiogram, and computed tomography of the chest when indicated. At least two blood cultures and one sputum culture were obtained at admission before the start of antimicrobial therapy. Induced sputum was obtained in patients in whom it was difficult to obtain routine sputum. Nasopharyngeal secretion samples for viral detection and urine samples for pneumococcal and L. pneumophila antigen detection were obtained in 24 h of admission. Blood samples were obtained for serological analysis of Mycoplasma pneumoniae and Chlamydophila pneumoniae.
Microbiologically adequate sputum (containing more than 25 polymorphonuclear cells and <10 epithelial cells per low power field) was subjected to Gram's staining and Ziehl–Neelson staining for acid-fast bacilli.[21] Quantitative cultures of sputum samples and qualitative cultures of nasopharyngeal samples as well as blood samples were performed in accordance with accepted methods and criteria.
Immunochromatographic membrane tests (Diagnostic Automation, Cortez diagnostics Inc., CA, USA) were performed on urine samples, for detection of pneumococcal antigens. ICT detects the S. pneumoniae C-polysaccharide which is found in the cell wall and is common to all serotypes.[20] Commercially available enzyme-linked immunosorbent assays (ELISA) were used according to the manufacturer's instructions for the detection of Legionella urinary antigen (Diagnostic Automation, Inc., Calabasas, CA, USA). Antibodies to M. pneumoniae and C. pneumoniae were detected by ELISA. Twin nasopharyngeal swabs were obtained and collected in viral transport medium, and detection of influenza viruses A and B was performed by real-time reverse transcriptase polymerase chain reaction (RT-PCR) using the CDC protocol.[14,15,16,22] Influenza A positive samples were further tested for A/H1N1 and A/H3N2 subtypes whereas Influenza B samples were tested for B/Victoria and B/Yamagata lineages.
Diagnostic criteria
An etiologic diagnosis were considered to be definitive if one of the following criteria were met:
Isolation of respiratory pathogen in a sterile specimen (blood and pleural fluid)
A single increased IgM titer for M. pneumoniae (≥1:16) or C. pneumoniae (≥1:10)
Positive urinary antigen for L. pneumophila type 1 or S. pneumoniae
A positive result for one respiratory virus by RT-PCR.
The results were analyzed with SPSS statistical software version 17.00 (IBM Analytics, USA). Appropriate statistical methods were employed which included t-tests for continuous variables and nonparametric tests for categorical variables. Univariate and multivariate analyses was employed to determine the factors associated with mortality among the patients. Values have been expressed as mean + standard deviation and P < 0.05 was considered statistically significant. All patients provided written informed consent. The study was approved by the Postgraduate Committee and the Institute Ethics Committee of the SKIMS.
RESULTS
Patient characteristics
The study population consisted of 119 (52.9%) males; age ranged from 18 to 93 years (median 59 years), 98 (43.6%) were current smokers and 32 (14.2%) were past smokers. Seventeen (7.6%) and 19 (8.4%) patients had previously been vaccinated against pneumococcus and influenza, respectively. At hospital admission, 152 patients (67.5%) had received antibiotic therapy prior to hospitalization. The comorbidities included hypertension (n = 92), COPD (n = 84), diabetes (n = 36), chronic kidney disease (n = 27), heart failure (n = 24), and chronic liver disease (n = 3). The patients presented with symptoms consisting in cough (89.8%), fever (86.2%), breathlessness (69.3%), chest pain (17.8%), and altered sensorium (19.1%). On physical examination, 33.8% had cyanosis and 18 (8%) patients had hypotension at presentation. Chest radiography revealed bilateral lung involvement in 12 (5.3%). The most common finding was consolidation in 108 (48%) followed by interstitial infiltrates in 93 (41%) patients and pleural effusion in 24 (10.6%). Out of 225 patients, 24 (10.6%) were admitted in ICU whereas 101 patients were managed in general pulmonary ward.
Microbiological profile
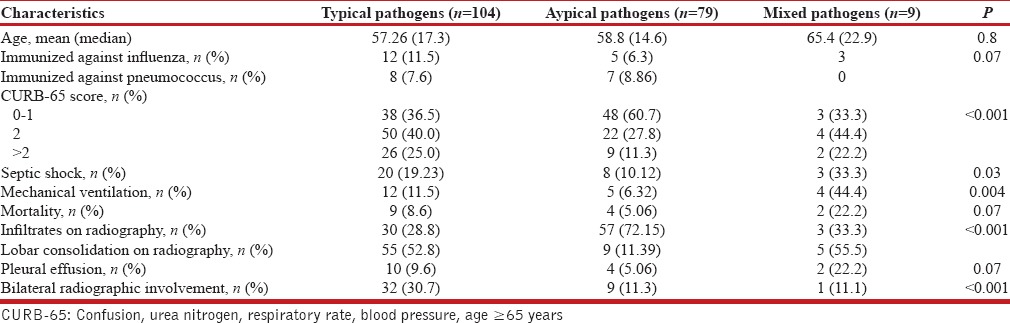
The frequency of various pathogens among the samples was tested is depicted in Table 1. S. pneumoniae was the most common detected pathogen (30.5%), among the tested patients followed by L. pneumophila (17.5%), influenza viruses (15.4%), M. pneumoniae (7.2%), C. pneumoniae (5.5%), Mycobacterium tuberculosis (4.8%), Klebsiella pneumonia (4.8%), methicillin-resistant S. aureus (MRSA) (3.5%), P. aeruginosa (3.1%), methicillin-sensitive S. aureus (1.7), and Acinetobacter baumannii (0.8%); 4% of patients had multiple pathogens. Table 2 summarizes the frequency of different etiological agents in different age groups. S. pneumoniae predominated in all the age groups. Polymicrobial etiology was found in nine patients. MRSA was found as co-pathogen in two patients who tested positive for influenza A. C. pneumoniae was identified as a co-pathogen in a patient detected positive for M. tuberculosis. Distribution of the pathogens with respect to the diagnostic methods used is shown in Table 3. No pathogen was detected in 63 patients. The characteristics of patients as per typical and atypical pathogens are shown in Table 4. Infections by typical pathogens were more severe with higher CURB-65 score at admission, more bilateral radiographic involvement, and had adverse clinical outcomes such as septic shock and need for mechanical ventilation as compared to atypical agents that were characterized by milder episodes.
Table 1.
Etiological pathogens
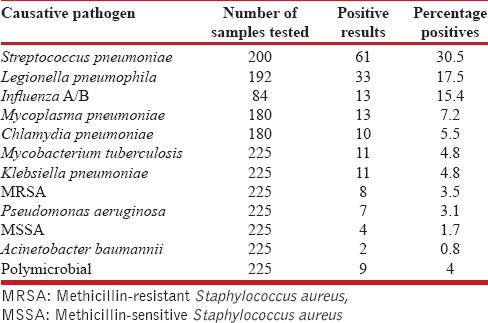
Table 2.
Etiology of pneumonia in relation to age
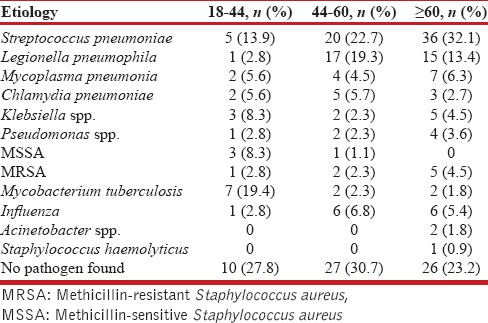
Table 3.
Distribution of pathogens and diagnostic tests
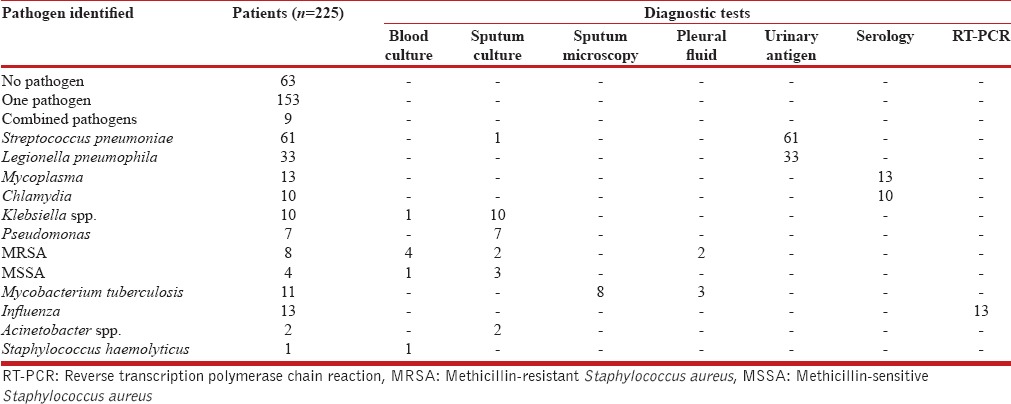
Table 4.
Characteristics of patients with community-acquired pneumonia according to etiologic categories
The average length of hospital stay was 9.6 (±10.65) days with an average ICU and ward stay of 7 and 3 days, respectively. Eighteen (8%) patients expired during hospitalization, 204 (90.6%) patients improved and were discharged, whereas three (1.3%) patients left against medical advice. On univariate analysis, the presence of hypotension at admission (P < 0.001), tachycardia ≥120 bpm (P = 0.002), use of mechanical ventilation (P < 0.001), CURB-65 score ≥2, and PSI Class ≥III (P < 0.001) were significantly different between survivors and nonsurvivors. On multivariate analysis, COPD, ICU admission, septic shock, CURB-65 >2, and PSI Class >III were identified as independent risk factors for mortality.
DISCUSSION
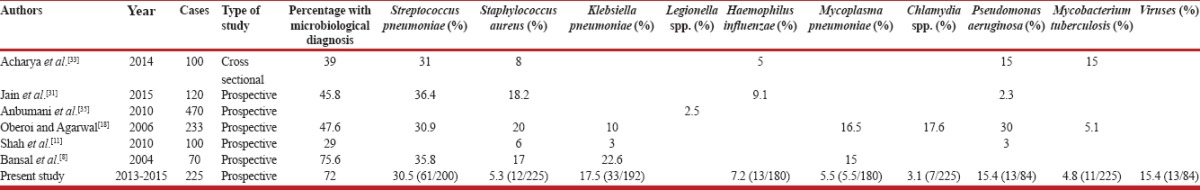
Our data gives a novel insight into the pathogens responsible for CAP among hospitalized patients in India depicting the changed paradigm in light of a higher microbiological yield clearly related to the use of a wider array of detection methods. The study depicts a different microbiological spectrum among CAP patients when compared to an earlier study from the same institution involving routine bacterial cultures in 100 patients.[11] While the changed spectrum can be attributed to the use of a wide array of diagnostic tests, variation of epidemiology over time also needs to be kept in consideration. At least one pathogen was found in 162 patients (72%), the yield being high compared to other similarly conducted studies.[8,11,23,24,25,26,27,28,29,30,31,32,33,34,35] A comparison of the yield and etiological spectrum in comparison to various international and national studies[8,11,23,24,25,26,27,28,29,30,31,32,33,34,35] is shown in Tables 5 and 6, respectively. A higher yield of the pathogens in our study calls for more routine use of these diagnostic modalities for arriving at the etiological diagnosis of CAP.
Table 5.
Comparison of the microbiological yield and distribution of various pathogens with previous studies
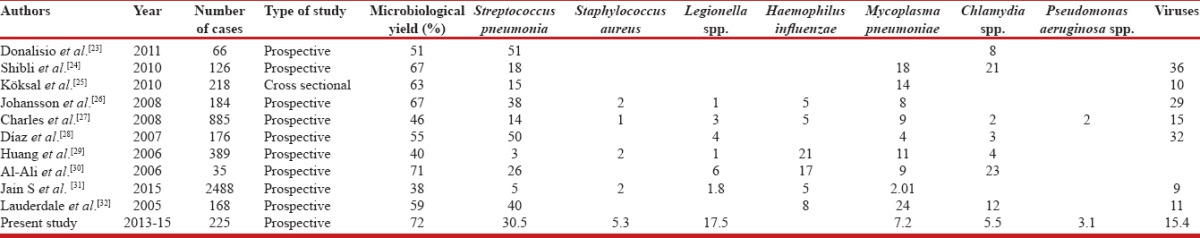
Table 6.
Comparison of the etiological profile with previous studies from India
S. pneumoniae was found to be the most common pathogen seen in about 30.5% of the tested patients. Such an yield is in conformity with studies earlier from Western countries where S. pneumoniae is reported in 24%–45% of the patients with CAP [Table 4]. Among the scant Indian studies that have addressed the etiological spectrum in adults have yielded varying results with S. pneumonia reported as the most common isolate in only one of the studies.[36] The high proportion of patients with L. pneumophila (17.5%), M. pneumoniae (7.2%), and Chlamydia (5.5%) in our patients emphasizes the role of atypical pathogens in the causation of pneumonia in our setting and is conformity with previous reports from Western countries [Table 4]. We found Legionella penumophila as the second most common pathogen. Legionella has been variously observed to be the causative organism among patients with CAP requiring hospitalization from developed countries to range from 2% to 9% [Tables 4 and 5]. The use of urinary antigen for the detection of Legionella increases the yield significantly.[24] Higher proportion of Legionella spp. remained unexplained. Pertinently Srinagar and adjacent areas are mostly supplied with water from treatment plants and an association has been seen between Legionella and Acanthamoeba that grow on biofilms of sand filters.[37,38]
Influenza viruses were detected in a 15.4% of the 84 patients who were tested for influenza. Logistic issues precluded routine testing for influenza and all pathogens in all cases. Influenza was also found as a co-pathogen in 33% of patients where pneumonia was polymicrobial in etiology (n = 9). Data regarding the contribution of influenza causing adult CAP in India are very limited. We have recently demonstrated influenza and other viruses as a cause of respiratory tract infection and acute exacerbation of COPD[39,40,41] in our patients, especially during winter months when the activity of respiratory viruses is high. Other investigators also have reported viruses as an increasingly recognized pathogen in the causation of CAP. Recently, Jain et al. detected viruses as the most common etiological agent of CAP in the US population.[31] Shibli et al. reported viral etiology in about 20% of their patients,[24] whereas Lauderdale et al. incriminated them in 11% of patient.[32] While our data are insufficient to recommend routine testing for viruses in CAP where the etiology is generally bacterial, it may be prudent to look for a viral etiology during times of documented heightened activity of influenza in a particular geographical area. Such inquiry could be suggested by surveillance studies available for various parts of a large land mass country like India.
M. tuberculosis was found in 4.4% of patients of CAP. About 19.4% cases of tuberculosis presenting as CAP belonged to the age group of 18–44 years indicating the burden of this disease on our young productive age group. Liam et al. isolated M. tuberculosis in 4.9% of CAP patients in Malaysia,[42] and Oberoi and Agarwal reported the pathogen in 5% of their cases from India.[18] The endemic nature and the heaviest burden of tuberculosis in the world argue for consideration of M. tuberculosis in the differential diagnosis of all patients with CAP in India. This is especially important if a predictable response to antimicrobial therapy is not forthcoming during therapy.
K. pneumoniae was isolated from 4.8% of patients, mostly from sputum culture. Klebsiella was found in 13.8% of patients admitted in ICU and in 4.8% of nonsurvivors. Studies reported during the past two decades from India have also reported a higher prevalence of K. pneumoniae among culture-positive pneumonias.[43] K. pneumoniae pneumonia which is common in European countries has an association with alcoholism.[44] The lower prevalence in our setting may be related to a lower use of alcohol in the community in comparison to other communities. P. aeruginosa was detected in 3.1% of patients. Its frequency increased with increasing age and it was associated with moderate (PSI III) to severe (PSI IV-V) CAP. Charles et al. report P. aeruginosa from 2% of patients.[27] In most of the studies, P. aeruginosa has been found more commonly in severe CAP.[13,45]
Mortality rate in our study was 8% which is lower than the previous study from this place.[11] Mortality due to CAP in various hospital-based studies has been variable. While the British Thoracic Society multicentric study recorded a surprisingly low mortality of 5.7%,[46] higher mortality ranging from 21% to 25% has been reported in other studies.[47,48] According to the study conducted by British Thoracic Society and the Public Health Laboratory Services, patients had a 21-fold increased risk of mortality if they had respiratory rate 30 breaths/min or more and diastolic blood pressure ≤60 mmHg.[46] A high PSI class in our cases was predictably associated with a higher mortality and calls for aggressive management of such cases.
Despite the fact that a number of patients had associated comorbidities that would have mandated routine influenza and pneumococcal vaccination, the uptake of vaccinations was poor in our cases which reflect a poor prescription rate for vaccinations as well as possible patient factors. We have recently reported a poor uptake of influenza and pneumococcal vaccination in patients with diabetes,[49] COPD,[50] and heart failure,[51] and hence we emphasize the routine use of influenza and pneumococcal vaccinations in high-risk groups.
Our study is limited by the fact that only inpatients were recruited, patients of immunosuppressive therapy were not included, and testing for uncommon pathogens such as Coxiella burnetii, Moraxella catarrhalis, and noninfluenza respiratory viruses was not performed. Quality of sputum has been evaluated as per the old method[21] and not by new method, in which in the presence of >10 epithelial cells per low power field, sample can be straightforward rejected unless pus cells are >100 and there is a single morphology on Gram staining.[52] However, the strengths of the study are its being the first study of its kind in Indian patients addressing a wide array of diagnostic tools for pathogen identification in cases of CAP. The study should serve as an impetus to further such studies in the developing world wherefrom data are scant and are desperately required to better understand the etiological spectrum of CAP so as to tailor appropriate antibiotic therapy and preventive strategies.
CONCLUSIONS
Streptococcus pneumoniae, Legionella, and influenza constitute the most common etiological agents in north Indian adults hospitalized with CAP. Further such studies need to be undertaken so that appropriate antibiotic ploicies based on local ecology of the etiological spectrum are devised as also appropriate vaccination strategies for the common vaccine preventable pathogens.
Financial support and sponsorship
The study was supported by a postgraduate grant from Sher-i-Kashmir Institute of Medical Sciences, Srinagar. The sponsor had no role in the collection, interpretation, analysis of data, or the final write up of the manuscript.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious diseases society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brar NK, Niederman MS. Management of community-acquired pneumonia: A review and update. Ther Adv Respir Dis. 2011;5:61–78. doi: 10.1177/1753465810381518. [DOI] [PubMed] [Google Scholar]
- 3.Colice GL, Morley MA, Asche C, Birnbaum HG. Treatment costs of community-acquired pneumonia in an employed population. Chest. 2004;125:2140–5. doi: 10.1378/chest.125.6.2140. [DOI] [PubMed] [Google Scholar]
- 4.Nair GB, Niederman MS. Community-acquired pneumonia: An unfinished battle. Med Clin North Am. 2011;95:1143–61. doi: 10.1016/j.mcna.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang GD, Fine M, Orloff J, Arisumi D, Yu VL, Kapoor W, et al. New and emerging etiologies for community-acquired pneumonia with implications for therapy. A prospective multicenter study of 359 cases. Medicine (Baltimore) 1990;69:307–16. doi: 10.1097/00005792-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Musher DM, Roig IL, Cazares G, Stager CE, Logan N, Safar H, et al. Can an etiologic agent be identified in adults who are hospitalized for community-acquired pneumonia: Results of a one-year study. J Infect. 2013;67:11–8. doi: 10.1016/j.jinf.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherwin RL, Gray S, Alexander R, McGovern PC, Graepel J, Pride MW, et al. Distribution of 13-valent pneumococcal conjugate vaccine Streptococcus pneumoniae serotypes in US adults aged ≥50 years with community-acquired pneumonia. J Infect Dis. 2013;208:1813–20. doi: 10.1093/infdis/jit506. [DOI] [PubMed] [Google Scholar]
- 8.Bansal S, Kashyap S, Pal LS, Goel A. Clinical and bacteriological profile of community acquired pneumonia in Shimla, Himachal Pradesh. Indian J Chest Dis Allied Sci. 2004;46:17–22. [PubMed] [Google Scholar]
- 9.Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, et al. BTS guidelines for the management of community acquired pneumonia in adults: Update 2009. Thorax. 2009;64(Suppl 3):iii1–55. doi: 10.1136/thx.2009.121434. [DOI] [PubMed] [Google Scholar]
- 10.Jokinen C, Heiskanen L, Juvonen H, Kallinen S, Karkola K, Korppi M, et al. Incidence of community-acquired pneumonia in the population of four municipalities in Eastern Finland. Am J Epidemiol. 1993;137:977–88. doi: 10.1093/oxfordjournals.aje.a116770. [DOI] [PubMed] [Google Scholar]
- 11.Shah BA, Singh G, Naik MA, Dhobi GN. Bacteriological and clinical profile of community acquired pneumonia in hospitalized patients. Lung India. 2010;27:54–7. doi: 10.4103/0970-2113.63606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capoor MR, Nair D, Aggarwal P, Gupta B. Rapid diagnosis of community acquired pneumonia using the Bac T alert 3 D system. Braz J Infect Dis. 2006;10:352–6. doi: 10.1590/s1413-86702006000500010. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-González A, Falguera M, Nogués A, Rubio-Caballero M. Is Streptococcus pneumoniae the leading cause of pneumonia of unknown etiology?A microbiologic study of lung aspirates in consecutive patients with community-acquired pneumonia. Am J Med. 1999;106:385–90. doi: 10.1016/s0002-9343(99)00050-9. [DOI] [PubMed] [Google Scholar]
- 14.Boulware DR, Daley CL, Merrifield C, Hopewell PC, Janoff EN. Rapid diagnosis of pneumococcal pneumonia among HIV-infected adults with urine antigen detection. J Infect. 2007;55:300–9. doi: 10.1016/j.jinf.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishida T, Hashimoto T, Arita M, Tojo Y, Tachibana H, Jinnai M, et al. A3-year prospective study of a urinary antigen-detection test for Streptococcus pneumoniae in community-acquired pneumonia: Utility and clinical impact on the reported etiology. J Infect Chemother. 2004;10:359–63. doi: 10.1007/s10156-004-0351-1. [DOI] [PubMed] [Google Scholar]
- 16.Helbig JH, Uldum SA, Bernander S, Lück PC, Wewalka G, Abraham B, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol. 2003;41:838–40. doi: 10.1128/JCM.41.2.838-840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fine MJ, Smith MA, Carson CA, Mutha SS, Sankey SS, Weissfeld LA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996;275:134–41. [PubMed] [Google Scholar]
- 18.Oberoi A, Agarwal A. Bacteriological profile, Serology and antibiotic Sensitivity pattern of microorganisms from community acquired Pneumonia. JK Sci. 2006;8:79–82. [Google Scholar]
- 19.Marston BJ, Plouffe JF, File TM, Jr, Hackman BA, Salstrom SJ, Lipman HB, et al. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance study in Ohio. The community-based pneumonia incidence study group. Arch Intern Med. 1997;157:1709–18. [PubMed] [Google Scholar]
- 20.Capelastegui A, España PP, Bilbao A, Gamazo J, Medel F, Salgado J, et al. Etiology of community-acquired pneumonia in a population-based study: Link between etiology and patients characteristics, process-of-care, clinical evolution and outcomes. BMC Infect Dis. 2012;12:134. doi: 10.1186/1471-2334-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musher DM, Montoya R, Wanahita A. Diagnostic value of microscopic examination of gram-stained sputum and sputum cultures in patients with bacteremic pneumococcal pneumonia. Clin Infect Dis. 2004;39:165–9. doi: 10.1086/421497. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Geneva: 2009. [Last accessed on 2010 Sep 28]. CDC Protocol of Real-Time RTPCR for Influenza A (H1N1) Available from: http://www.who.int/csr/resources/publications/swineflu/CDCrealtimeRTPCRprotocol_20090428.pdf . [Google Scholar]
- 23.Donalisio MR, Arca CH, Madureira PR. Clinical, epidemiological, and etiological profile of inpatients with community-acquired pneumonia at a general hospital in the Sumaremicro region of Brazil. J Bras Pneumol. 2011;37:200–8. doi: 10.1590/s1806-37132011000200010. [DOI] [PubMed] [Google Scholar]
- 24.Shibli F, Chazan B, Nitzan O, Flatau E, Edelstein H, Blondheim O, et al. Etiology of community-acquired pneumonia in hospitalized patients in Northern Israel. Isr Med Assoc J. 2010;12:477–82. [PubMed] [Google Scholar]
- 25.Köksal I, Ozlü T, Bayraktar O, Yılmaz G, Bülbül Y, Oztuna F, et al. Etiological agents of community-acquired pneumonia in adult patients in Turkey; a multicentric, cross-sectional study. Tuberk Toraks. 2010;58:119–27. [PubMed] [Google Scholar]
- 26.Johansson N, Kalin M, Tiveljung-Lindell A, Giske CG, Hedlund J. Etiology of community-acquired pneumonia: Increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202–9. doi: 10.1086/648678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charles PG, Whitby M, Fuller AJ, Stirling R, Wright AA, Korman TM, et al. The etiology of community-acquired pneumonia in Australia: Why penicillin plus doxycycline or a macrolide is the most appropriate therapy. Clin Infect Dis. 2008;46:1513–21. doi: 10.1086/586749. [DOI] [PubMed] [Google Scholar]
- 28.Díaz A, Barria P, Niederman M, Restrepo MI, Dreyse J, Fuentes G, et al. Etiology of community-acquired pneumonia in hospitalized patients in Chile: The increasing prevalence of respiratory viruses among classic pathogens. Chest. 2007;131:779–87. doi: 10.1378/chest.06-1800. [DOI] [PubMed] [Google Scholar]
- 29.Huang HH, Zhang YY, Xiu QY, Zhou X, Huang SG, Lu Q, et al. Community-acquired pneumonia in Shanghai, China: Microbial etiology and implications for empirical therapy in a prospective study of 389 patients. Eur J Clin Microbiol Infect Dis. 2006;25:369–74. doi: 10.1007/s10096-006-0146-7. [DOI] [PubMed] [Google Scholar]
- 30.Al-Ali MK, Batchoun RG, Al-Nour TM. Etiology of community-acquired pneumonia in hospitalized patients in Jordan. Saudi Med J. 2006;27:813–6. [PubMed] [Google Scholar]
- 31.Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. Community-acquired pneumonia requiring hospitalization among US Adults. N Engl J Med. 2015;373:415–27. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lauderdale TL, Chang FY, Ben RJ, Yin HC, Ni YH, Tsai JW, et al. Etiology of community acquired pneumonia among adult patients requiring hospitalization in Taiwan. Respir Med. 2005;99:1079–86. doi: 10.1016/j.rmed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 33.Acharya VK, Padyana M, Unnikrishnan B, Anand R, Acharya PR, Juneja DJ, et al. Microbiological profile and drug sensitivity pattern among community acquired pneumonia patients in tertiary care centre in Mangalore, Coastal Karnataka, India. J Clin Diagn Res. 2014;8:MC04–6. doi: 10.7860/JCDR/2014/7426.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain SK, Jain S, Trikha S. Study of clinical, radiological, and bacteriological profile of community-acquired pneumonia in hospitalized patients of Gajra Raja Medical College, Gwalior, Central India. Int J Sci Stud. 2014;2:96–100. [Google Scholar]
- 35.Anbumani S, Gururajkumar A, Chaudhury A. Isolation of Legionella pneumophila from clinical & environmental sources in a tertiary care hospital. Indian J Med Res. 2010;131:761–4. [PubMed] [Google Scholar]
- 36.Kulpati DD, Khastgir T. Reappraisal of pneumonias. J Assoc Physicians India. 1988;36:660–4. [PubMed] [Google Scholar]
- 37.Yu VL, Stout JE. Community-acquired legionnaires disease: Implications for underdiagnosis and laboratory testing. Clin Infect Dis. 2008;46:1365–7. doi: 10.1086/586742. [DOI] [PubMed] [Google Scholar]
- 38.Lau HY, Ashbolt NJ. The role of biofilms and protozoa in Legionella pathogenesis: Implications for drinking water. J Appl Microbiol. 2009;107:368–78. doi: 10.1111/j.1365-2672.2009.04208.x. [DOI] [PubMed] [Google Scholar]
- 39.Koul PA, Mir MA, Bali NK, Chawla-Sarkar M, Sarkar M, Kaushik S, et al. Pandemic and seasonal influenza viruses among patients with acute respiratory illness in Kashmir (India) Influenza Other Respir Viruses. 2011;5:e521–7. doi: 10.1111/j.1750-2659.2011.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koul P, Khan U, Bhat K, Saha S, Broor S, Lal R, et al. Recrudescent wave of A/H1N1pdm09 influenza viruses in winter 2012-2013 in Kashmir, India. PLoS Curr. 2013;5 doi: 10.1371/currents.outbreaks.f1241c3a2625fc7a81bf25eea81f66e6. pii: ecurrents.outbreaks.f1241c3a2625fc7a81bf25eea81f66e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koul PA, Mir H, Akram S, Potdar V, Chadha MS. Respiratory viruses in acute exacerbations of chronic obstructive pulmonary disease. Lung India. 2017;34:29–33. doi: 10.4103/0970-2113.197099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liam CK, Pang YK, Poosparajah S. Pulmonary tuberculosis presenting as community-acquired pneumonia. Respirology. 2006;11:786–92. doi: 10.1111/j.1440-1843.2006.00947.x. [DOI] [PubMed] [Google Scholar]
- 43.Madhu SV, Gupta U, Guleria JS, Talwar V. Clinical and bacteriological profile of hospitalized community acquired pneumonias – A preliminary study. Indian J Chest Dis Allied Sci. 1990;32:95–100. [PubMed] [Google Scholar]
- 44.Paganin F, Lilienthal F, Bourdin A, Lugagne N, Tixier F, Génin R, et al. Severe community-acquired pneumonia: Assessment of microbial aetiology as mortality factor. Eur Respir J. 2004;24:779–85. doi: 10.1183/09031936.04.00119503. [DOI] [PubMed] [Google Scholar]
- 45.Khawaja A, Zubairi AB, Durrani FK, Zafar A. Etiology and outcome of severe community acquired pneumonia in immunocompetent adults. BMC Infect Dis. 2013;13:94. doi: 10.1186/1471-2334-13-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Community-acquired pneumonia in adults in British hospitals in 1982-1983: A survey of aetiology, mortality, prognostic factors and outcome. The British thoracic society and the public health laboratory service. Q J Med. 1987;62:195–220. [PubMed] [Google Scholar]
- 47.Ortqvist A, Hedlund J, Grillner L, Jalonen E, Kallings I, Leinonen M, et al. Aetiology, outcome and prognostic factors in community-acquired pneumonia requiring hospitalization. Eur Respir J. 1990;3:1105–13. [PubMed] [Google Scholar]
- 48.Pachon J, Prados MD, Capote F, Cuello JA, Garnacho J, Verano A, et al. Severe community-acquired pneumonia. Etiology, prognosis, and treatment. Am Rev Respir Dis. 1990;142:369–73. doi: 10.1164/ajrccm/142.2.369. [DOI] [PubMed] [Google Scholar]
- 49.Koul PA, Bhat MA, Ali S, Rahim S, Ahmad S, Masoodi SR. Influenza and pneumococcal vaccination in patients with diabetes. J Diabetol. 2014;2:5. [Google Scholar]
- 50.Gnatiuc L, Buist AS, Kato B, Janson C, Aït-Khaled N, Nielsen R, et al. Gaps in using bronchodilators, inhaled corticosteroids and influenza vaccine among 23 high- and low-income sites. Int J Tuberc Lung Dis. 2015;19:21–30. doi: 10.5588/ijtld.14.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koul PA, Ali S, Mir H, Ahmad SJ, Bhat SA, Bhat MA, et al. Influenza vaccination in North Indian patients with heart failure. Indian Heart J. 2017;69:28–31. doi: 10.1016/j.ihj.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winn W, Jr, Allen S, Janda W, Koneman E. 6th ed. Philadelphia, USA: Lippincott, Williams and Wilkins Publications; 2006. Guidelines for collection, transport, processing, analysis and reporting of cultures from specific specimen sources. In: Koneman's Colour Atlas and Textbook of Microbiology; pp. 68–111. [Google Scholar]


