Abstract
Lower respiratory tract infection (LRTI) is a broad terminology which includes acute bronchitis, pneumonia, acute exacerbations of chronic obstructive pulmonary disease/chronic bronchitis (AECB), and acute exacerbation of bronchiectasis. Acute LRTIs (ALRTIs) are one of the common clinical problems in community and hospital settings. Management of community-acquired pneumonia (CAP) and AECB may pose challenges because of diagnostic difficulty in differentiating infections caused by typical and atypical microorganisms and rising rates of antimicrobial resistance. Beta-lactam antibiotics, macrolides, and fluoroquinolones are routinely prescribed medicines for the management of ALRTIs. Macrolides are time-tested and effective agents for the treatment of LRTIs. Clarithromycin, a macrolide, offers several benefits in the management of ALRTIs. In this article, we discuss the management approach of LRTIs with focus on clarithromycin in the management of mild-to-moderate LRTIs (CAP and AECB), i.e., in outpatient settings.
KEY WORDS: Acute exacerbation of chronic bronchitis, clarithromycin, community-acquired pneumonia, macrolides
INTRODUCTION
According to the Global Burden of Disease 2015 study (GBD 2015), chronic obstructive pulmonary disease (COPD) and lower respiratory tract infections (LRTIs) represent the third and fourth most common causes of death, respectively, after ischemic heart disease and cerebrovascular disease.[1] LRTI is a broad terminology which includes different diseases including acute bronchitis, pneumonia, and acute exacerbation of chronic lung diseases such as COPD or bronchiectasis. Annual incidence of pneumonia, one of the most important LRTIs, is reported to be 24.8 per 10,000 adults. The rates differ based on the age, with higher incidence observed in patients between 65 and 79 years of age (63.0/10,000 adults) and >80 years of age (164.3/10,000 adults).[2] Pneumococcal pneumonia is the most common cause of mortality due to lower respiratory infections. According to the GBD 2015 study, pneumococcal pneumonia is the most common cause of pneumonia responsible for over 15 lakh (95% uncertainty interval of 9.58–21.84 lakh) counterfactual deaths across the world in 2015. Children younger than 5 years are commonly affected, making it the highest contributor of death due to LRTIs in them.[1] Community-acquired pneumonia (CAP) and acute exacerbation of chronic bronchitis (AECB) are the two commonly encountered acute LRTIs in outpatient setting. The annual incidence of CAP is about 5–11 per 1,000 population with higher rates reported in the elderly population.[3] It is the leading cause of death due to infectious diseases in developed countries[4] and a significant cause of morbidity and mortality in developing countries,[5] with huge medical costs, those in the US alone exceeding $ 10 billion per annum.[3,6] The mortality rates of CAP progressively increase with the severity of illness. The rates of mortality in outpatients, hospitalized cases, and those admitted in Intensive Care Unit (ICU) are <5%, 10%, and >30%, respectively.[7] Smoking, chronic medical illness, and long-term bedridden condition increase the risk of mortality.[8] The disease also constitutes high burden in children.[9,10] The exact epidemiology of CAP in India is unknown.
In this article, the published literature on LRTIs and its management is reviewed with special focus on clarithromycin. Review articles, clinical trials, clinical practice guidelines, and experimental studies published from 2000 onward were considered for writing the review. “PubMed” and “Google Scholar” “Google” sites were used to fetch the articles using words “pneumonia” and “macrolides.”
TYPES OF LOWER RESPIRATORY TRACT INFECTIONS
Table 1 provides criteria for different types of LRTIs.[11]
Table 1.
Lower respiratory tract infections
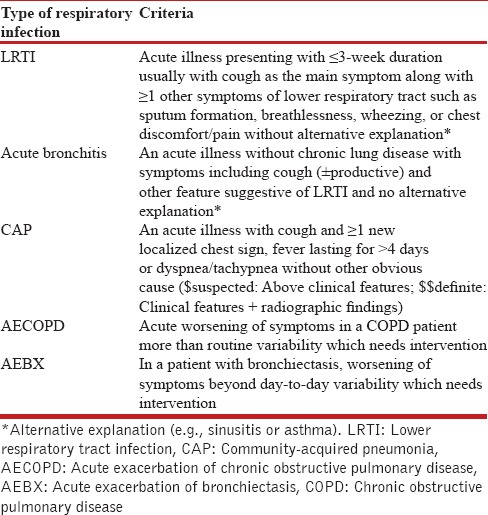
In routine clinical practice, it is difficult to differentiate between different diseases without diagnostic tests because of the considerable overlap in clinical presentation. Considering difficulties in performing full diagnostic workup in every patient, empirical and practical approach is necessary.[11]
COMMUNITY-ACQUIRED PNEUMONIA: CAUSATIVE AGENTS AND CLINICAL PRESENTATION
Pneumonia can be classified as “atypical” or “typical” type based on the patient presentation, clinical observations, causative pathogens, and course of the disease. Patients with atypical pneumonia have slightly different course than typical pneumococcal pneumonia. Otherwise, many clinical features of typical and atypical pneumonia are similar.[10] Typical pneumonia is associated with acute fever, chills, pleuritic chest pain, and productive cough whereas atypical CAP is commonly associated with myalgias, fever without chills, headache, and unproductive cough.[12]
It is important to differentiate infection from that of noninfectious disorders such as asthma, COPD, heart failure, or lung infarction. Due to their nature and ongoing treatment, these chronic noninfectious disorders can be identified based on clinical and medication history. Criteria in Table 1 are also useful for distinguishing LRTIs from other noninfectious disorders.
It is also important to differentiate pneumonia from other respiratory infections. Pneumonia is suspected based on one of the clinical features of the following: new localized chest sign, dyspnea, tachypnea, tachycardia (pulse rate more than 100/min), or fever for more than 4 days.[11]
Common bacterial pathogens responsible for typical pneumonia include Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, and Haemophilus influenzae[12] whereas microorganisms causing atypical CAP include Legionella pneumophila, Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Chlamydia psittaci.[10,13] S. pneumoniae is the primary cause of CAP in many cases. In the United States, pneumococcus was the most commonly reported bacterial pathogen but now is seen in only 10%–15% of inpatients.[14] Data from Western countries suggest the presence of S. pneumoniae in 24%–45% of the patients with CAP and severe CAP.[15] In India, the pattern of etiological agent of CAP varies with geographical distribution, for example, S. pneumoniae has been reported as the dominant bacterial agent (35.8%) of CAP in Shimla[16] whereas Pseudomonas aeruginosa predominated in another study in Srinagar.[17] Other pathogens including respiratory viruses are also gaining increasing recognition as etiological agents in CAP.[18] Atypical pathogens are present in up to 40% patients of CAP.[12] Legionella infection produces symptoms similar to other CAPs but with scanty sputum compared to other bacterial pneumonias. Gastrointestinal symptoms, headache, confusion, hemoptysis, dyspnea, and musculoskeletal complaints in the form of myalgia and arthralgia are possible symptoms in these patients.[10] Immunocompromised patients, smokers, and elderly people are more susceptible to severe Legionella infection.[13]
M. pneumoniae infections constitute about 15% and 27%–40% of CAPs in adults[16] and children, respectively.[19] The major symptoms of M. pneumoniae infection include fever, cough, sore throat, and coryza. Bullous myringitis, though not common, is a characteristic feature. Pharyngitis and crepitations may be observed on clinical examination.[10] CAP caused by C. pneumoniae may result in sore throat, nonproductive cough, hoarseness of voice, headache, pharyngitis, and wheezing.[10] Involvement of extrapulmonary organs, common with atypical infections, may help in clinical differentiation of atypical CAP from that of typical infections.[13] Mostly CAP is diagnosed based on the clinical history and physical examination. A weighted point system of syndromic diagnosis is useful for differentiating it from other pneumonia.[13] Radiological imaging and laboratory examination are useful diagnostic methods for confirmation of diagnosis.[10] Sometimes, additional diagnostic tests may be required. Other organisms must also be considered in light of the patient's risk factors and severity of illness. Information on the etiology of CAP is required for treatment and preventive purposes.
RADIOLOGICAL DIAGNOSIS OF COMMUNITY-ACQUIRED PNEUMONIA
Chest X-ray is the most common and useful investigation for the diagnosis of CAP. Posteroanterior (PA) and lateral view should be taken in adults suspected of pneumonia, whenever possible. Usually, chest X-ray in typical bacterial CAP shows the presence of segmental or lobar consolidation.[20] CAP caused by C. pneumoniae may show the presence of unilateral segmental patchy opacities.[10] Patients with atypical pneumonia often have localized or diffuse patchy infiltrates or ground-glass shadows on radiography.[12] Disproportional abnormalities on X-rays with symptoms[10] should raise the suspicion of atypical pneumonia.
LABORATORY INVESTIGATIONS IN SUSPECTED COMMUNITY-ACQUIRED PNEUMONIA
Typical pneumonia is often associated with elevations in total leukocyte count, erythrocyte sedimentation rate, and C-reactive protein (CRP). In atypical pneumonia, these investigations may be normal or slightly increased.[12]
CULTURE IN THE DIAGNOSIS OF PNEUMONIA
Sputum culture
Sputum stains and culture are relatively insensitive tests with limited specificity for the diagnosis of pneumococcal pneumonia. Diagnostic sensitivity of sputum culture is highly variable with range between 29% and 94%. On the one hand, in many cases, sputum culture may be negative, whereas on the other hand, there are chances of false-positive report because of the nasopharyngeal carriage.[21] Sputum examination is often negative for microorganisms, but leukocytes may be seen in atypical pneumonia.[10]
Blood and pleural culture
Clinical utility of blood and pleural culture in routine clinical practice is limited because of low yield of positive rates of S. pneumoniae due to reasons such as presence of pneumonia without bacteremia, autolysis, administration of antibiotics before sending sample for culture, or inadequate quantity of sample.[21] According to the NICE guidelines, microbiological tests should not be routinely offered to patients with low-severity CAP.[22]
OTHER DIAGNOSTIC METHODS
Additional investigations such as serological test, enzyme immunoassays, complement fixation method, polymerase chain reaction (PCR), and antigen detection may be required in some patients, especially if they do not respond to antibiotic therapy.[23] S. pneumoniae urinary antigen test can be an useful adjuvant test for the diagnosis of pneumococcal pneumonia.[24,25] Sensitivity, specificity, positive predictive value, and negative predictive value of this test have been reported to be 72.7%, 97.6%, 88.9%, and 93%, respectively.[25] Cold agglutinins may be elevated, but they disappear too quickly in M. pneumoniae infection. A study from Sri Lanka showed less reliability of cold agglutinin detection as compared to specific antibody detection by isotype ELISA for diagnosis of M. pneumoniae pneumonia because of its low sensitivity and positive predictive value.[26] Most atypical microorganisms are difficult to isolate; hence, indirect methods such as direct/indirect fluorescent antibody detection are often used for the diagnosis.[13] Multiplex PCR is gaining importance in the diagnosis of CAP. Short time for sample processing and ability to detect as many as 94.5% viral pathogens in comparison to viral culture are the main advantages of multiplex PCR method.[27] It can also detect atypical bacterial agents.[28] With wider availability, multiplex PCR has the potential to become the choice of investigation for diagnosis of pneumonia in future. However, despite comprehensive diagnostic workup, it is possible that no pathogen is detected in as much as 30%–60% cases with CAP.[29] A study performed by Jain et al.[2] showed the presence of pathogen only in 38% cases (n = 2259) with radiographic evidence of pneumonia and availability of specimens for both bacterial and viral testing.
MANAGEMENT OF COMMUNITY-ACQUIRED PNEUMONIA
Early identification of high-risk patients is important for the prevention of complications. A prognostic scoring method “CURB-65” (confusion, increase in blood urea nitrogen, increased respiratory rate, low blood pressure, and age of the patient above 65 years) is useful for this purpose. The CURB-65 scoring system has been validated in a hospital-based prospective study in Indian patients.[9] Pneumonia severity index (PSI) and SMART-COP rule are the other alternative methods of severity assessment in CAP.[30] Although there is not much to choose between these methods, some researchers tend to believe that PSI and SMART-COP are better. However, robust data comparing benefits of one over the other are limited.
The main goals of treatment of CAP are to eradicate the causative microorganisms, provide relief to the patient, and reduce the risk of hospitalization and reinfection. Despite extensive investigations, in many patients, the etiology of CAP remains undetected.[17] Thus, empirical treatment plays an important role in the management of CAP;[31] therefore, knowledge of potential causative agent is very important. The bacteria causing typical pneumonias usually respond to beta-lactam antibiotics. However, the atypical pathogens, being intracellular organisms, do not respond to beta-lactam antibiotics.[12]
The NICE guidelines[22] recommend considering a point-of-care CRP test in patients with symptoms of LRTI; in case, diagnosis is not made after clinical assessment or there is confusion regarding initiating antibiotic therapy. Decision to start antibiotic therapy can be made based on the level of CRP [Table 2].
Table 2.
Recommendations for initiating antibiotic therapy based on C-reactive protein level

Considering these challenges, empiric, broad-spectrum antibacterial therapy, providing coverage against common bacteria responsible for CAP as well as atypical microorganisms is often selected. Drug-related factors including pharmacological profile, safety profile, risk of drug interactions, and cost and patient-related factors such as severity of disease, comorbidities, clinical presentation, and local resistance pattern[31] are important while selecting an empirical therapy. In this regard, macrolides represent useful agents for the treatment of CAP caused by typical as well as atypical bacteria.
In several trials, macrolides have been shown to be efficacious in the treatment of LRTIs, including acute bronchitis, AECB, and CAP.[32]
ROLE OF MACROLIDES IN THE MANAGEMENT OF COMMUNITY-ACQUIRED PNEUMONIA
Macrolides offer several benefits in the management of respiratory infections because of their complementary action along with primary antimicrobial activity. Due to high tissue penetration, anti-inflammatory properties, and action on immune system, macrolides can reduce inflammatory responses.[33] Efficacy of azithromycin and clarithromycin have been proved in CAP in patients who need hospitalization.[32]
Clarithromycin has a broad spectrum of antibacterial activity, improved pharmacokinetic and pharmacodynamic properties, and better tolerability compared to erythromycin. Tissue concentration and concentration in the alveolar macrophages are about 2–20 times and 400 times higher than serum levels, respectively.[33]
Efficacy and safety of clarithromycin has been demonstrated in several clinical trials in patients with respiratory diseases. However, head-to-head comparative trials between clarithromycin and azithromycin are limited. Bonvehi et al. in a prospective, randomized study had compared the efficacy and safety of clarithromycin versus amoxicillin/clavulanic acid in 327 patients with CAP due to penicillin-resistant and/or macrolide-resistant S. pneumoniae. Patients were treated with either clarithromycin 500 mg or amoxicillin/clavulanic acid 875 mg/125 mg twice daily for 7 days. The clinical cure rate (92% vs. 91%) and overall bacterial eradication rate (91% vs. 93%) were found to be similar in both the groups.[34]
Some patients especially those hospitalized because of CAP need treatment with combination of antimicrobials, often used as empirical treatment. A study compared the combination of beta-lactam antimicrobial with macrolide therapy versus fluoroquinolone monotherapy in patients with severe CAP. The results of this study showed better outcomes with combination therapy for severe cases. For class V pneumonia, severity index 14-day and 30-day mortality rate with combination therapy was 8.2% and 18.4%, respectively. The corresponding rates with fluoroquinolone monotherapy were 26.8% and 36.6%. The difference in mortality at both 14 and 30 days with combination therapy was significantly better compared with fluoroquinolone monotherapy (P = 0.02 and P = 0.05, respectively). There was no difference in mortality rates between two arms for less severe disease. However, a retrospective study design was the limitation of this study. Larger randomized clinical trials are required to provide exact place of combination therapy in the treatment of CAP.[35]
CHOICE AND DURATION OF EMPIRICAL ANTIMICROBIALS FOR COMMUNITY-ACQUIRED PNEUMONIA IN OUTPATIENT SETTINGS
Amoxicillin is a preferred antimicrobial agent for initial empiric therapy in outpatients. Macrolides (clarithromycin and azithromycin) are recommended for patients with hypersensitivity to penicillins. In India, consequent to the high prevalence, possibility of concomitant tuberculosis should be kept in mind while selecting pharmacological therapy for the management of respiratory infections. Inappropriate use of antimicrobials such as fluoroquinolones can increase the risk of fluoroquinolone-resistant M. tuberculosis. In addition, there could be a chance of masking active tuberculosis. Considering this problem, the Indian guidelines suggest to prefer macrolides such as clarithromycin/azithromycin over quinolone/doxycycline as initial empiric therapy in patients with CAP. Due to widespread resistance among pathogens causing CAP, doxycycline is not recommended for the treatment of CAP. In patients with comorbidities, combination therapy with respiratory fluoroquinolone/beta-lactam plus a macrolide or amoxicillin–clavulanate is preferred.[36] The usual dosage of clarithromycin for CAP in outpatient settings is 500 mg twice daily.
MACROLIDE IN THE MANAGEMENT OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE
COPD is an inflammatory condition. Moreover, infections can further increase the inflammatory response in acute exacerbations.[37] Macrolides (clarithromycin/azithromycin) are among the choices of antibiotics for the treatment of uncomplicated COPD.[38] Macrolides are useful agents for patients without risk factor, no cardiac disease, those with age <65 years, patients having FEV1 more than 50% predicted, and <3 exacerbations in a year.
Clarithromycin is considered as one of the standard therapies for the treatment of AECB.[39,40] The dose is 500 mg twice daily for 7 days. The other agents used are amoxicillin 500 mg tid and cefuroxime axetil 250 mg bid for 7 days. The respiratory fluoroquinolones such as gatifloxacin and gemifloxacin have been compared with these standard therapies in clinical trials. Clarithromycin is shown to be effective and well tolerated in the treatment of adults with AECB.[41]
In the GLOBE trial, clinical success rate and bacteriological success rate with clarithromycin were 84.6% and 73.1%, respectively.[39] The MOSAIC trial compared moxifloxacin with standard therapy. Clarithromycin was one of the antimicrobials among standard therapies.[40] A meta-analysis comparing macrolides, quinolones, and amoxicillin/clavulanate in AECB showed that amoxicillin/clavulanate is associated with a higher risk of diarrhea and other adverse effects in general.[42]
GLOBAL GUIDELINES ON USAGES OF ANTIMICROBIAL IN PNEUMONIA AND ACUTE EXACERBATION OF CHRONIC BRONCHITIS
According to the Infectious Diseases Society of America (IDSA)/American Thoracic Society Consensus guidelines, macrolides are the drug of choice (with strong recommendation; Level I evidence) for formerly healthy adult outpatients with CAP, with no risk factors for drug-resistant S. pneumoniae (DRSP) infection. In the presence of comorbidities or other risk factors for DRSP infection, either a respiratory fluoroquinolone (moxifloxacin, gemifloxacin, or levofloxacin) or combination of beta-lactam plus a macrolide or amoxicillin-clavulanate is preferred.[43] The IDSA/American Thoracic Society Consensus guidelines recommend the use of a macrolide (strong recommendation; Level I evidence) for the management of CAP in previously healthy adults, with no risk factors for DRSP infection. In patients with comorbidities or use of antimicrobials for the past 3 months or other risk factors for DRSP infection, combination of beta-lactam plus a macrolide is recommended (strong recommendation; Level I evidence).[43]
According to the NICE guidelines (December 2014),[22] amoxicillin is preferred over macrolide or tetracycline in low-severity CAP. The guideline suggests macrolide or tetracycline in case of allergy to penicillin. In patients with moderate-to-severe CAP, dual therapy with amoxicillin and macrolide is suggested. Similarly, in highly severe CAP, dual therapy with a beta-lactamases-table beta-lactam and macrolide is suggested.
According to the European Respiratory Society–The European Society for Clinical Microbiology and Infectious Diseases guideline, amoxicillin or tetracycline is recommended as the choice of antibiotic. Macrolide is recommended as an alternative in case of hypersensitivity to first-line agent.[44]
INDIAN GUIDELINE RECOMMENDATIONS
Indian guidelines also recommend the use of macrolide in the treatment of CAP.[45] According to the joint ICS/NCCP (I) guidelines for the management of CAP in adults, oral macrolides are one of the recommended agents for the treatment of outpatients without comorbidities. In outpatients with comorbidities, oral combination of beta-lactams plus macrolide is recommended. For hospitalized patients in non-ICU and ICU setting, without risk factor for Pseudomonas aeruginosa, combination of beta-lactam plus macrolide is recommended.[46] Empiric therapy should be guided by local bacterial pattern based on the microbiological results.
ADVANTAGES OF CLARITHROMYCIN
Clarithromycin offers the following advantages in the treatment of LRTIs [Table 3].
Table 3.
Advantages of clarithromycin for lower respiratory tract infections

Unlike other agents such as beta-lactams and fluoroquinolones, macrolides prevent the release of pro-inflammatory protein toxins and production of bacterial adhesins and biofilm, i.e., structure which provide protection to pathogen against antibiotic and host defense.[33,51] These actions offer advantage to macrolides in the form of less risk of triggering adverse inflammatory response.[33] Macrolides also reduce the expression of adhesion molecules which could be useful for decreasing airway inflammation.[50] Important actions of clarithromycin include stimulation of ciliary movements, improved mucociliary clearance, inhibition of chemotaxis in the respiratory pathway, and suppression of mucus production and release. Mucus production is suppressed due to inhibition of muc5ac gene expression. Protection of ciliated respiratory epithelium and downregulation of the pro-inflammatory activities of epithelial cells are the other benefits of clarithromycin. Clarithromycin increases Th1/Th2 ratio by reducing IL-4 and IL-5 production. In patients with chronic respiratory illness, clarithromycin can induce apoptosis in lymphocytes in the lungs.[33] Pneumolysin, an important virulence factor of S. pneumoniae which increases extrapulmonary spread, is inhibited by clarithromycin.[49]
SUMMARY
LRTIs are common problems in day-to-day clinical practice. Antimicrobial therapy is a principal management component for these diseases. It is often difficult to diagnose and differentiate atypical infections from that of typical infections. Clinical findings and radiological imaging may help to suspect atypical infections. In country like India, it is often difficult to confirm the atypical infection even in the laboratories because of the inherent properties of atypical bacteria, limited access to the sophisticated laboratory methods, and cost. In such a scenario, physician often resorts to empirical therapy with antimicrobial agents. With growing evidence of antimicrobial resistance, empirical treatment is becoming more difficult. The antimicrobial agent is often selected based on the patient profile, local resistance pattern, availability of the medicine, and cost. Macrolide is an effective therapy for patients with LRTIs, i.e., CAP caused by both typical and atypical bacteria and AECB. Clarithromycin offers advantages in the form of improved pharmacokinetics, pharmacodynamics, and higher tissue concentration. Apart from the primary antibacterial property, clarithromycin shows immunomodulatory properties and anti-inflammatory properties. Considering these advantages, clarithromycin seems to be a better antimicrobial agent for empirical use in patients with LRTIs. In some cases, especially those with severe illness, combination therapy may be required. In such patients, combination of macrolide and beta-lactam antibiotic is a suitable option.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. Anant Patil, who participated in writing this review article.
REFERENCES
- 1.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: A systematic analysis for the global burden of disease study 2015. Lancet. 2016;388:1459–544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med. 2015;373:415–27. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brar NK, Niederman MS. Management of community-acquired pneumonia: A review and update. Ther Adv Respir Dis. 2011;5:61–78. doi: 10.1177/1753465810381518. [DOI] [PubMed] [Google Scholar]
- 4.Torres A, Peetermans WE, Viegi G, Blasi F. Risk factors for community-acquired pneumonia in adults in Europe: A literature review. Thorax. 2013;68:1057–65. doi: 10.1136/thoraxjnl-2013-204282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon HK. Changes in the epidemiology and burden of community-acquired pneumonia in Korea. Korean J Intern Med. 2014;29:735–7. doi: 10.3904/kjim.2014.29.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonafede MM, Suaya JA, Wilson KL, Mannino DM, Polsky D. Incidence and cost of CAP in a large working-age population. Am J Manag Care. 2012;18:380–7. [PubMed] [Google Scholar]
- 7.Nair GB, Niederman MS. Community-acquired pneumonia: An unfinished battle. Med Clin North Am. 2011;95:1143–61. doi: 10.1016/j.mcna.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghimire M, Bhattacharya SK, Narain JP. Pneumonia in South-East Asia region: Public health perspective. Indian J Med Res. 2012;135:459–68. [PMC free article] [PubMed] [Google Scholar]
- 9.Shah BA, Ahmed W, Dhobi GN, Shah NN, Khursheed SQ, Haq I, et al. Validity of pneumonia severity index and CURB-65 severity scoring systems in community acquired pneumonia in an Indian setting. Indian J Chest Dis Allied Sci. 2010;52:9–17. [PubMed] [Google Scholar]
- 10.Prasad R. Community acquired pneumonia: Clinical manifestations. J Assoc Physicians India. 2012;60(Suppl):10–2. [PubMed] [Google Scholar]
- 11.Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, et al. Guidelines for the management of adult lower respiratory tract infections – Summary. Clin Microbiol Infect. 2011;17(Suppl 6):1–24. doi: 10.1111/j.1469-0691.2011.03602.x. [DOI] [PubMed] [Google Scholar]
- 12.Bedi RS. Community acquired pneumonia-typical or atypical? Lung India. 2006;23:130–1. [Google Scholar]
- 13.Cunha BA. The atypical pneumonias: Clinical diagnosis and importance. Clin Microbiol Infect. 2006;12(Suppl 3):12–24. doi: 10.1111/j.1469-0691.2006.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2014;371:1619–28. doi: 10.1056/NEJMra1312885. [DOI] [PubMed] [Google Scholar]
- 15.Khawaja A, Zubairi AB, Durrani FK, Zafar A. Etiology and outcome of severe community acquired pneumonia in immunocompetent adults. BMC Infect Dis. 2013;13:94. doi: 10.1186/1471-2334-13-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bansal S, Kashyap S, Pal LS, Goel A. Clinical and bacteriological profile of community acquired pneumonia in Shimla, Himachal Pradesh. Indian J Chest Dis Allied Sci. 2004;46:17–22. [PubMed] [Google Scholar]
- 17.Shah BA, Singh G, Naik MA, Dhobi GN. Bacteriological and clinical profile of community acquired pneumonia in hospitalized patients. Lung India. 2010;27:54–7. doi: 10.4103/0970-2113.63606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, et al. Community-acquired pneumonia requiring hospitalization among US children. N Engl J Med. 2015;372:835–45. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferwerda A, Moll HA, de Groot R. Respiratory tract infections by Mycoplasma pneumoniae in children: A review of diagnostic and therapeutic measures. Eur J Pediatr. 2001;160:483–91. doi: 10.1007/s004310100775. [DOI] [PubMed] [Google Scholar]
- 20.Franquet T. Imaging of pneumonia: Trends and algorithms. Eur Respir J. 2001;18:196–208. doi: 10.1183/09031936.01.00213501. [DOI] [PubMed] [Google Scholar]
- 21.Song JY, Eun BW, Nahm MH. Diagnosis of pneumococcal pneumonia: Current pitfalls and the way forward. Infect Chemother. 2013;45:351–66. doi: 10.3947/ic.2013.45.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical Guideline. Pneumonia in Adults: Diagnosis and Management. NICE. 2014. [Last accessed on 2017 Nov 09]. Available from: https://www.nice.org.uk/guidance/cg191/resources/pneumonia-in-adults-diagnosis-and-management-35109868127173nice.org.uk/guidance/cg191 .
- 23.Kashyap S, Sarkar M. Mycoplasma pneumonia: Clinical features and management. Lung India. 2010;27:75–85. doi: 10.4103/0970-2113.63611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murdoch DR, Anderson TP, Beynon KA, Chua A, Fleming AM, Laing RT, et al. Evaluation of a PCR assay for detection of Streptococcus pneumoniae in respiratory and nonrespiratory samples from adults with community-acquired pneumonia. J Clin Microbiol. 2003;41:63–6. doi: 10.1128/JCM.41.1.63-66.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ercis S, Ergin A, Sahin GO, Hasçelik G, Uzun O. Validation of urinary antigen test for Streptococcus pneumoniae in patients with pneumococcal pneumonia. Jpn J Infect Dis. 2006;59:388–90. [PubMed] [Google Scholar]
- 26.Wijesooriya WR, Sunil-Chandra NP, Parera J. Reliability of cold agglutinin test (CAT) for diagnosis of Mycoplasma pneumoniae pneumonia in hospitalized patients. Sri Lankan J Infect Dis. 2016;6:25–32. [Google Scholar]
- 27.Layman CP, Gordon SM, Elegino-Steffens DU, Agee W, Barnhill J, Hsue G, et al. Rapid multiplex PCR assay to identify respiratory viral pathogens: Moving forward diagnosing the common cold. Hawaii J Med Public Health. 2013;72:24–6. [PMC free article] [PubMed] [Google Scholar]
- 28.Pinar A, Bozdemir N, Kocagöz T, Alaçam R. Rapid detection of bacterial atypical pneumonia agents by multiplex PCR. Cent Eur J Public Health. 2004;12:3–5. [PubMed] [Google Scholar]
- 29.Ewig S, Torres A, Angeles Marcos M, Angrill J, Rañó A, de Roux A, et al. Factors associated with unknown aetiology in patients with community-acquired pneumonia. Eur Respir J. 2002;20:1254–62. doi: 10.1183/09031936.02.01942001. [DOI] [PubMed] [Google Scholar]
- 30.Importance of severity assessment: Community-acquired pneumonia. J Assoc Physicians India. 2013;61:14–9. [PubMed] [Google Scholar]
- 31.Lutfiyya MN, Henley E, Chang LF, Reyburn SW. Diagnosis and treatment of community-acquired pneumonia. Am Fam Physician. 2006;73:442–50. [PubMed] [Google Scholar]
- 32.Zuckerman JM, Qamar F, Bono BR. Review of macrolides (azithromycin, clarithromycin), ketolids (telithromycin) and glycylcyclines (tigecycline) Med Clin North Am. 2011;95:761–91. doi: 10.1016/j.mcna.2011.03.012. viii. [DOI] [PubMed] [Google Scholar]
- 33.Steel HC, Theron AJ, Cockeran R, Anderson R, Feldman C. Pathogen- and host-directed anti-inflammatory activities of macrolide antibiotics. Mediators Inflamm. 2012;2012:584262. doi: 10.1155/2012/584262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonvehi P, Weber K, Busman T, Shortridge D, Notario G. Comparison of clarithromycin and amoxicillin/clavulanic acid for community-acquired pneumonia in an era of drug-resistant Streptococcus pneumoniae. Clin Drug Investig. 2003;23:491–501. doi: 10.2165/00044011-200323080-00001. [DOI] [PubMed] [Google Scholar]
- 35.Lodise TP, Kwa A, Cosler L, Gupta R, Smith RP. Comparison of beta-lactam and macrolide combination therapy versus fluoroquinolone monotherapy in hospitalized veterans affairs patients with community-acquired pneumonia. Antimicrob Agents Chemother. 2007;51:3977–82. doi: 10.1128/AAC.00006-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Management of community-acquired pneumonia. J Assoc Physicians India. 2013;61(7 Suppl):20–3. [PubMed] [Google Scholar]
- 37.Martinez FJ, Curtis JL, Albert R. Role of macrolide therapy in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3:331–50. doi: 10.2147/copd.s681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siddiqi A, Sethi S. Optimizing antibiotic selection in treating COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2008;3:31–44. doi: 10.2147/copd.s1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson R, Schentag JJ, Ball P, Mandell L, 068 Study Group. A comparison of gemifloxacin and clarithromycin in acute exacerbations of chronic bronchitis and long-term clinical outcomes. Clin Ther. 2002;24:639–52. doi: 10.1016/s0149-2918(02)85139-6. [DOI] [PubMed] [Google Scholar]
- 40.Wilson R, Allegra L, Huchon G, Izquierdo JL, Jones P, Schaberg T, et al. Short-term and long-term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis. Chest. 2004;125:953–64. doi: 10.1378/chest.125.3.953. [DOI] [PubMed] [Google Scholar]
- 41.Weiss K, Vanjaka A. Canadian Clarithromycin Study Group on Bronchitis. An open-label, randomized, multicenter, comparative study of the efficacy and safety of 7 days of treatment with clarithromycin extended-release tablets versus clarithromycin immediate-release tablets for the treatment of patients with acute bacterial exacerbation of chronic bronchitis. Clin Ther. 2002;24:2105–22. doi: 10.1016/s0149-2918(02)80100-x. [DOI] [PubMed] [Google Scholar]
- 42.Siempos II, Dimopoulos G, Korbila IP, Manta K, Falagas ME. Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: A meta-analysis. Eur Respir J. 2007;29:1127–37. doi: 10.1183/09031936.00147806. [DOI] [PubMed] [Google Scholar]
- 43.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious diseases society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, et al. Guidelines for the management of adult lower respiratory tract infections – Full version. Clin Microbiol Infect. 2011;17(Suppl 6):E1–59. doi: 10.1111/j.1469-0691.2011.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parikh FS. Care Guidelines for Community Acquired Pneumonia (CAP). Ch. 131. [Last accessed on 2017 Mar 28]. Available from: http://www.apiindia.org/pdf/medicine_update_2007/131.pdf .
- 46.Gupta D, Agarwal R, Aggarwal AN, Singh N, Mishra N, Khilnani GC, et al. Guidelines for diagnosis and management of community- and hospital-acquired pneumonia in adults: Joint ICS/NCCP(I) recommendations. Lung India. 2012;29:S27–62. doi: 10.4103/0970-2113.99248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leung WK, Graham DY. Clarithromycin for Helicobacter pylori infection. Expert Opin Pharmacother. 2000;1:507–14. doi: 10.1517/14656566.1.3.507. [DOI] [PubMed] [Google Scholar]
- 48.Thibodeau KP, Viera AJ. Atypical pathogens and challenges in community-acquired pneumonia. Am Fam Physician. 2004;69:1699–706. [PubMed] [Google Scholar]
- 49.Feldman C, Anderson R. Non-antimicrobial activity of macrolides: Therapeutic potential in chronic inflammatory airway disorders. Clin Drug Investig. 2007;27:27–35. [Google Scholar]
- 50.Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010;23:590–615. doi: 10.1128/CMR.00078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amsden GW. Anti-inflammatory effects of macrolides – An underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother. 2005;55:10–21. doi: 10.1093/jac/dkh519. [DOI] [PubMed] [Google Scholar]


