Abstract
Spontaneous pneumothorax is a very common medical emergency. Patients are often treated without treating the underlying cause. Lymphangioleiomyomatosis (LAM) is a rare cystic lung disease. Until recently, diagnosis of LAM was a challenge with nearly 100% mortality in 10 years, but better understanding of the disease through research and better radiological techniques and newer drugs such as sirolimus has improved the survival in such patients. We are presenting a rare case of LAM presenting as a secondary spontaneous pneumothorax treated with sirolimus.
KEY WORDS: Lymphangioleiomyomatosis, pneumothorax, sirolimus
INTRODUCTION
Lymphangioleiomyomatosis (LAM) is a rare cystic lung disease. Patients with LAM often develop progressive breathlessness and recurrent pneumothorax. Extrapulmonary manifestations may include renal angiomyolipomas, meningiomas, and chylothorax. Treatment generally includes symptomatic and supportive management in the form of inhaled bronchodilators and antiestrogens. Inhibitors of mechanistic target of rapamycin (mTOR) such as rapamycin also known sirolimus had shown to be a promising drug for the treatment of LAM.
CASE REPORT
A 27-year-old immunocompetent, nonsmoker, female patient, homemaker by occupation, was referred to our department as a case of left-sided secondary spontaneous pneumothorax with intercostal drainage tube. She was on antitubercular drugs for the past 3 months prescribed from a local physician but showed no improvement. Air leak on coughing was present and pneumothorax did not resolve. On detailed history, she revealed that 2 years back, she had a similar episode of pneumothorax, for which she had received antitubercular therapy for 6 months but without any improvement. On examination, the patient was afebrile, clubbing was absent, auscultation revealed bilateral fine crepts diffusely. Sputum smear examination for acid-fast bacilli and cartridge-based nucleic acid amplification test for mycobacterium tuberculosis were negative. A high-resolution computed tomography (HRCT) scan of thorax was done [Figure 1] which showed thin-walled cysts diffusely involving the lung bilaterally. LAM was suspected in the patient. Lung biopsy was planned, for which the patient refused. Serological test for vascular endothelial growth factor-D (VEGF-D) was positive. Ultrasonography of abdomen [Figure 2] was done for evaluation of tuberous sclerosis which showed the presence of right renal cyst and left ovarian cyst with hemangioma in liver. Computed tomography (CT) head was done which was within normal limit. Dermatological evaluation was normal. A provisional diagnosis of LAM was made and the patient was started on sirolimus 2 mg once daily and inhaled bronchodilators. Hormonal therapy with antiestrogens was advised, for which the patient declined due to social beliefs. After the expansion of the lungs, intercostal drainage tube was removed. Post 6 months of treatment, a repeat HRCT was done with resolution of cysts [Figure 3]. The patient is clinically improved and is in regular follow-up.
Figure 1.
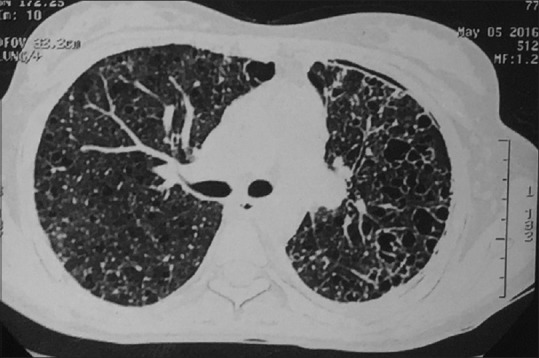
High-resolution computed tomography thorax at the level of right main bronchus showing bilateral thin-walled cystic lesions and left-sided pneumothorax
Figure 2.
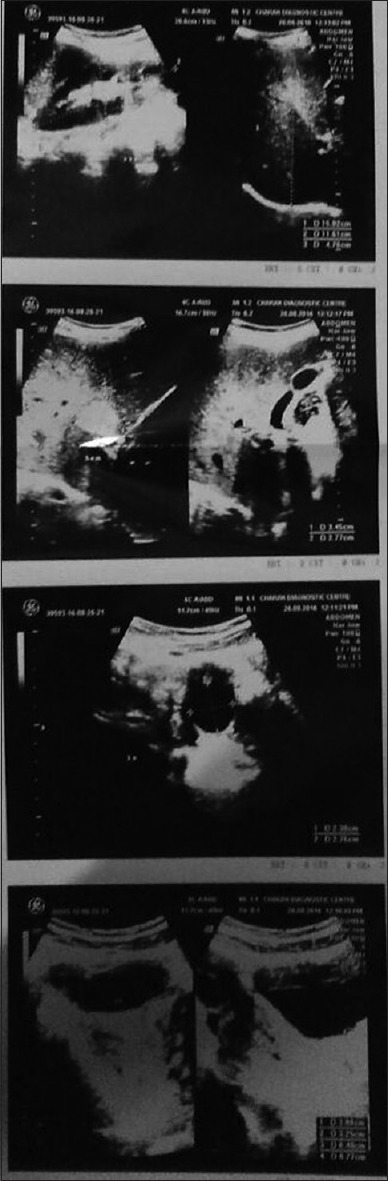
A hypoechoic space-occupying lesion is seen in segment V of liver, right kidney shows a cyst measuring approximately 15 mm × 10 mm at mid pole, left ovary shows a cyst measuring approximately 27 mm × 23 mm
Figure 3.
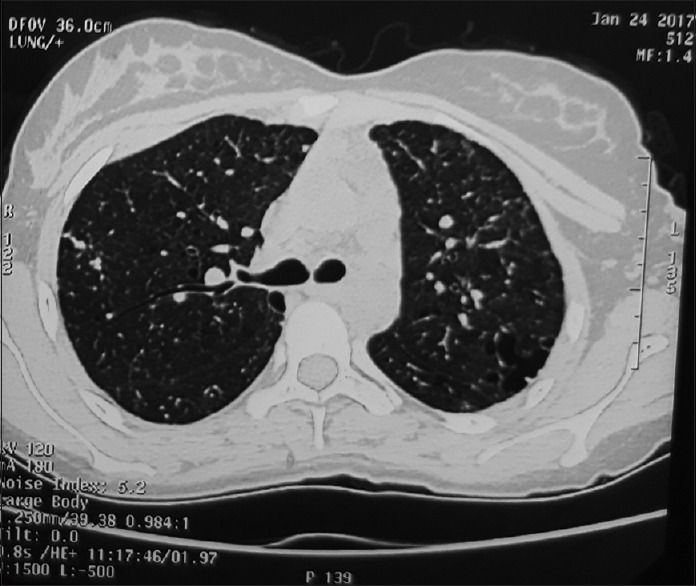
High-resolution computed tomography thorax at the level of right main bronchus showing decreased density of cysts as compared to previous image
DISCUSSION
LAM, a rare disease, occurs either sporadically or in association with tuberous sclerosis complex (TSC: TSC1 and TSC2) also known as congenital which is autosomal dominant genetic disorder.[1,2] LAM is less severe in TSC-LAM than sporadic LAM.[3] Sporadic LAM affects 1 in 400,000 adult females; LAM associated with TSC occurs in 30%–40% of adult females[4,5] and also in males and children. Patients with LAM often develop progressive breathlessness and recurrent pneumothorax.[1] Extrapulmonary manifestations may include renal angiomyolipomas, meningiomas, and chylothorax. Our patient did not have features associated with tuberous sclerosis or meningioma, but she had right renal cyst and left ovarian cyst along with hepatic hemangioma.
Cause of LAM is proposed to be proliferation of smooth muscle-like cells which leads to air trapping and cyst formation in alveoli. The two types of smooth cells are the spindle cells and epitheloid cells.[6] These cells stain positive for alpha actin, desmin and HMB-45, Melan-A, HMSA-1.[6] LAM cells also express estrogen and progesterone receptors. Our patients did not give consent for any biopsy and these tests could not be elicited on her. Such patients also have very high levels of serum VEGF-D which was also found in our patient.
Patients with pneumothorax generally present with sudden onset breathlessness and pleuritic chest pain. Generally, if pneumothorax is small, it can be managed conservatively by oxygen or closed aspiration. Massive pneumothorax may require tube thoracostomy or surgery.
As with our case, LAM usually presents with primary spontaneous pneumothorax and it usually occurs in women of child bearing age, it worsens during pregnancy, and estrogen use in any form may lead to worsening of symptoms. Diagnosis is made by tissue biopsy or in combination with HRCT thorax and characteristic history and clinical features suggestive of LAM.[3,4] HRCT feature characteristics of LAM are multiple (>10) thin-walled round well-defined air-filled cysts. Lung cysts are the hallmark lesion in LAM and are present in all patients. The cysts are generally round distributed evenly and are thin-walled. Patients presenting with LAM should also undergo thorough workup for tuberous sclerosis and abdominal lesions.
The diagnosis and consequences of further disease progression of a rare and an orphan disease such as LAM often leave the patients with a feeling of isolation. General management requires quitting of smoking if a patient is smoker. LAM often increases the chances of pneumothorax which is about 40% at the time of presentation and about 66% during the treatment. Patients of LAM should be warned of recurrence of pneumothorax and its sign and symptoms and to seek medical advice if it develops.
Administration of exogenous estrogen increases the chances of progression of pulmonary LAM. The female patients should be aware of using estrogen-containing pills as these can aggravate the pulmonary LAM.[1] Similarly, the patients should be properly explained that the pregnancy can increase the chances of pneumothorax in such patients.
There is not much left for the treatment of patients diagnosed with LAM. Treatment generally includes symptomatic and supportive management for complications due to LAM-like thoracotomy or intercostal tube placement for pneumothorax.
LAM involves mutation in TSC1 AND TSC2 proteins (also known as hamartin and tuberin, respectively). These are scaffolding proteins and involve mTOR signaling pathway. The mTOR pathway is activated in LAM patients.[7,8] Inhibitors of mTOR such as rapamycin, also known sirolimus, had shown to be a promising drug for the treatment of LAM. Two prospective open-label clinical trials suggest that the mTOR inhibitor sirolimus reduces angiomyolipoma volume.[9]
Other treatment options include Rho inhibitors, tyrosine kinase inhibitors, matrix metalloproteinase inhibitors, and antiestrogens.
CONCLUSION
Our patient was a healthy young female who was referred from a private hospital to our department as a case of secondary spontaneous pneumothorax assumed to be due to smear-negative pulmonary tuberculosis. On the basis of history, HRCT pattern, and serological test for VEGF-D, and a diagnosis of LAM was made. Ultrasonography of abdomen revealed cystic lesions in various abdominal organs including kidney, ovary, and liver. She was started on sirolimus and inhaled bronchodilators and is doing well.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Johnson S. Rare diseases 1. Lymphangioleiomyomatosis: Clinical features, management and basic mechanisms. Thorax. 1999;54:254–64. doi: 10.1136/thx.54.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franz DN, Brody A, Meyer C, Leonard J, Chuck G, Dabora S, et al. Mutational and radiographic analysis of pulmonary disease consistent with lymphangioleiomyomatosis and micronodular pneumocyte hyperplasia in women with tuberous sclerosis. Am J Respir Crit Care Med. 2001;164:661–8. doi: 10.1164/ajrccm.164.4.2011025. [DOI] [PubMed] [Google Scholar]
- 3.Ryu JH, Moss J, Beck GJ, Lee JC, Brown KK, Chapman JT, et al. The NHLBI lymphangioleiomyomatosis registry: Characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006;173:105–11. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urban T, Lazor R, Lacronique J, Murris M, Labrune S, Valeyre D, et al. Pulmonary lymphangioleiomyomatosis A study of 69 patients Groupe d'Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM“O“P) Medicine (Baltimore) 1999;78:321–37. doi: 10.1097/00005792-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Johnson SR, Tattersfield AE. Decline in lung function in lymphangioleiomyomatosis: Relation to menopause and progesterone treatment. Am J Respir Crit Care Med. 1999;160:628–33. doi: 10.1164/ajrccm.160.2.9901027. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Travis WD. Pulmonary lymphangioleiomyomatosis. Arch Pathol Lab Med. 2010;134:1823–8. doi: 10.5858/2009-0576-RS.1. [DOI] [PubMed] [Google Scholar]
- 7.Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM) J Biol Chem. 2002;277:30958–67. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- 8.Kenerson H, Folpe AL, Takayama TK, Yeung RS. Activation of the mTOR pathway in sporadic angiomyolipomas and other perivascular epithelioid cell neoplasms. Hum Pathol. 2007;38:1361–71. doi: 10.1016/j.humpath.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies DM, Johnson SR, Tattersfield AE, Kingswood JC, Cox JA, McCartney DL, et al. Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. N Engl J Med. 2008;358:200–3. doi: 10.1056/NEJMc072500. [DOI] [PubMed] [Google Scholar]


