Abstract
A subset of non-small cell lung carcinoma (NSCC) harbor active mutations of epidermal growth factor receptor (EGFR). In these, EGFR tyrosine kinase inhibitors (EGFR-TKIs) are recommended as the first-line treatment. Though drug resistance is inevitable, histological transformation to small cell lung carcinoma (SCLC) is a rare mechanism for acquired resistance. Here we report one such rare case of histological transformation of pulmonary adenocarcinoma to small cell lung carcinoma in 46 year old male treated with Gefitinib.
KEY WORDS: Acquired resistance, epidermal growth factor receptor, histological transformation, nonsmall cell lung carcinoma, small cell lung carcinoma, tyrosine kinase inhibitor
INTRODUCTION
The nonsmall cell lung carcinoma (NSCC) harbors active mutations of epidermal growth factor receptor (EGFR). Majority of the EGFR mutations are located in tyrosine kinase binding domain (exon 18–21). The exon 19 deletion and exon 21 L858R point mutation encompasses almost 90% of the active mutations.[1] Current treatment guidelines recommends EGFR tyrosine kinase inhibitors (EGFR-TKIs) as the first-line treatment for a subset of EGFR-mutated NSCC.[2] In spite of a high rate of therapeutic response to EGFR-TKIs, drug resistance is inevitable and occurs on an average within a year.[3] Acquired resistance occurs through emergence of a secondary mutation of T790M and activation of bypass signal transduction pathway. Histological transformation to small cell lung carcinoma (SCLC) is a rare mechanism.[1] Here, we report one such rare case. A comprehensive literature search has shown very few cases mostly in the form of case reports documented in the recent years. The largest series has been reported from Korea comprising of six cases.[4] To the best of our knowledge, such histological transformation has not been described in Indian literature till date.
CASE REPORT
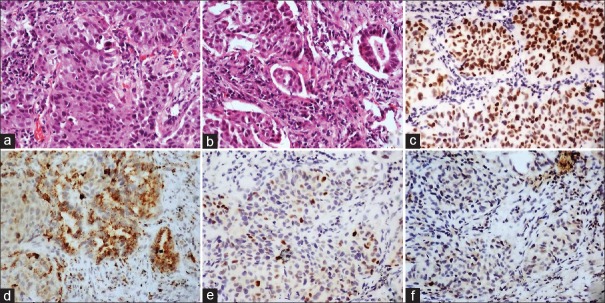
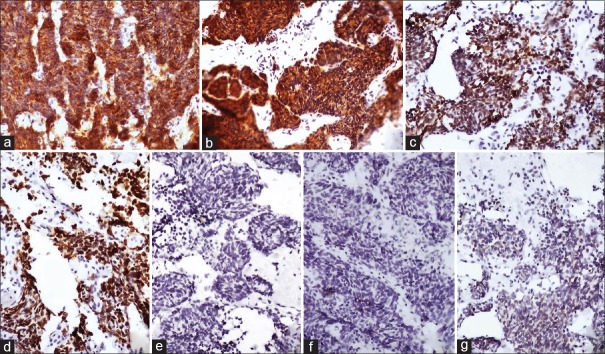
A 46-year-old male, a known smoker presented with backache for 1 month. There was no loss of sensation or weakness of the lower limbs. There was no history of cough shortness of breath, chest pain, headache, vomiting, and seizures. He was evaluated at an outside hospital. Magnetic resonance imaging of spine showed altered signal intensities which were hypointense on T1 and hyperintense on T2/STIR in the body, pedicle and lamina of L5, and D9 vertebral bodies and bilateral iliac bones suggestive of metastasis. On further workup, whole body fluorodeoxyglucose (FDG) positron emission tomography (PET) computed tomography (CT) scan showed increased uptake in large lobulated mass lesion with surrounding spiculations in the posterior segment of the right upper lobe, abutting major fissure. There was nodular pleural thickening on the right side with minimal loculated pleural effusion. On examination, basal crepts were heard on the right side. Laboratory parameters were within normal limits. Core biopsy of the lung was done. Histopathology was suggestive of adenocarcinoma (ADC) showing predominantly solid pattern with focal acinar formation [Figure 1a and b]. Immunohistochemistry (IHC) showed diffuse positivity for ADC markers thyroid transcription factor 1 (TTF1) and napsin A. Among the squamous cell markers, P63 stained some of tumor cell nuclei while p40 was negative [Figure 1c-f]. A diagnosis of pulmonary ADC was thus confirmed on IHC. He was started on chemotherapy with pemetrexed, carboplatin and zoledronate. Subsequently, EGFR analysis done on formalin fixed paraffin-embedded tissue block revealed exon 19 mutation. After completion of 4 cycles of chemotherapy, he was started on switch maintenance with gefitinib and was continued for a period of 1 year. On follow-up, PET CT was done to assess the disease response. Compared to the previous PET CT, there was an increase in size and FDG uptake of primary right lung lesion, pleural deposit, and effusion. He also developed ill-defined enhancing lesions in bilateral frontal lobes and left cerebellum. On examination, there was multiple level IV lymph nodes in the right cervical region. The breath sounds were reduced on the right side. There were no focal neurological deficits. In view of progressive disease, a repeat biopsy of the right pleural-based lesion was done. Biopsy revealed round to polygonal cells in sheets, nests, and trabeculae. The cells had scant cytoplasm, high N: C ratio, and hyperchromatic nuclei with molding and spindling [Figure 2]. On IHC, the cells showed diffuse strong positivity for TTF1, chromogranin and synaptophysin. Ki-67 showed increased labeling (>90%) in tumor cells [Figure 3a-d]. Napsin A and p40 were distinctly negative while p63 showed weak nuclear staining in some tumor cells [Figure 3e-g]. Biopsy features this time were thus suggestive of small cell carcinoma. There was no evidence of ADC component in this biopsy. In view of histological transformation from nonsmall cell to small cell carcinoma, the previous biopsy was again reviewed. It did not show any evidence of small cell component. Repeat EGFR analysis on this second biopsy also revealed exon 19 deletion similar to the initial biopsy ruling out a second de novo primary. The tumor was negative for T790M mutation. He was started on chemotherapy with 6 cycles of carboplatin and etoposide for small cell component and cranial radiotherapy for brain metastasis. Gefitinib was discontinued. At 3 months follow up, he showed symptomatic improvement.
Figure 1.
(a and b) Section show infiltrating lesion comprised of polygonal cells arranged is predominantly in solid nests and islands with focal acinar (black arrow) pattern (H and E; ×100). (c) Thyroid transcription factor-1 showing diffuse strong nuclear positivity in tumor cells (Poly-HRP; ×100). (d) Napsin A showing granular cytoplasmic positivity in tumor cells (Poly-HRP; ×100). (e) P63 showing positivity in nuclei of some tumor cells (Poly-HRP; ×100) and (f) p40 negative in tumor cells (Poly-HRP; ×100)
Figure 2.
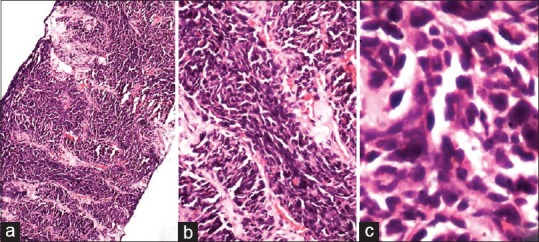
(a-c) Section show infiltrating lesion comprised of sheets and trabeculae of polygonal cells. These cells have high N:C ratio with scant cytoplasm. Nuclei show evidence moulding and spindling with inconspicuous nucleoli (H and E; [a] ×40, [b] ×100, [c] ×400)
Figure 3.
Immunohistochemistry findings. (a) Chromogranin and (b) synaptophysin showing diffuse strong cytoplasmic positivity in tumor cells (Poly-HRP; ×100). (c) Thyroid transcription factor-1 showing diffuse nuclear positivity in tumor cells (Poly-HRP; ×100). (d) Ki-67 highlighting very high proliferative activity in tumor cells (Poly-HRP; ×100). (e) Napsin A and (f) p40 negative in tumor cells (Poly-HRP; ×100). (g) P63 showing weak nuclear staining in some of the tumor cells (Poly-HRP; ×100)
DISCUSSION
There are various mechanisms of acquired resistance to EGFR inhibitors. Acquired resistance arising from T790M mutations and bypass of EGFR signaling through MET and HER2 amplification accounts for 50%–60% and 15%–20% of resistant cases, respectively.[5,6,7] The former increases the affinity of EGFR receptor for ATP leading to continued EGFR signaling even in the presence of the inhibitor.[8] Histological transformation of NSCC to SCLC has been recently described as a rare mechanism of acquired resistance accounting for 3% of cases.[6] It was first described in 2006 in a 45-year-old woman with EGFR-mutant ADC following treatment with erlotinib.[9] The absence of T790M mutation in the repeat biopsy following treatment with EGFR-TKI points toward histological transformation responsible for acquired resistance in this case. Although the exact mechanism is not clear, it can be possibly attributed to the alteration of morphology from NSCC, mostly ADC, to high-grade neuroendocrine phenotype (SCLC).[10] The combined small cell carcinomas which may not be apparent on the initial diagnostic biopsy can also lead to sampling errors, especially in small biopsies. In such cases, sampling of different histological areas in the initial and repeat biopsy may be misleading. The SCLC component may persist and dominate the histology following successful treatment of the ADC component by EGFR inhibitor. Thus, genomic sequencing of EGFR mutation in original tumor and transformed SCLC at the time of resistance helps in resolving such issues.[4] Similar to other reported cases, the repeat biopsy following treatment retained the original EGFR-activating mutation in the present case ruling out an independent second primary malignancy.[5] Ahn et al. in their series of six cases of ADC reported histologic transformation to SCLC following TKI therapy in four cases which had EGFR activating mutations.[4] Jiang et al. in his meta-analysis of 18 cases noted EGFR mutations in 16 cases of which 61% had exon 19 deletion and 28% had exon 21 point mutation. Among these, 11 SCLC retained the same mutation as their ADC counterparts.[11]
The possibility of histological transformation to SCLC should be suspected in cases with clinical and radiological progression of disease following treatment with TKIs. A repeat biopsy should be performed in all such cases to confirm progression to SCLC. The current case showed radiological evidence of increase in size and uptake of the original lesion as well as new lesion in the brain when compared with the initial radiological findings at diagnosis. It is important to identify small cell component on histology in view of good therapeutic response to targeted SCLC chemotherapy regimen.[12]
Furthermore, Norkowski et al. reported small cell transformation in EGFR-mutant tumors before treatment with TKIs. This suggests that EGFR inhibition is not solely responsible for phenotypic change in EGFR-mutant cases.[13] Similar histological transformation has also been reported in cases of acquired resistance to crizotinib in anaplastic lymphoma kinase rearranged lung tumors.[14] Large cell neuroendocrine carcinoma transformation has also been described in literature as an acquired resistance mechanism to EGFR TKIs and as well as crizotinib.[15,16]
CONCLUSION
This case highlights the importance of additional biopsy in all cases of progressive disease following treatment with EGFR-TKIs to rule out possibility of an acquired resistance. Identification of histologic transformation to small cell component is important as therapy can be switched to etoposide and cisplatin against SCLC.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lee JC, Jang SH, Lee KY, Kim YC. Treatment of non-small cell lung carcinoma after failure of epidermal growth factor receptor tyrosine kinase inhibitor. Cancer Res Treat. 2013;45:79–85. doi: 10.4143/crt.2013.45.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moiseenko VM, Protsenko SA, Semenov II, Moiseenko FV, Levchenko EV, Barchuk AS, et al. Effectiveness of gefitinib (Iressa) as first-line therapy for inoperable non-small-cell lung cancer with mutated EGFR gene (phase II study) Vopr Onkol. 2010;56:20–3. [PubMed] [Google Scholar]
- 3.Engelman JA, Jänne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–9. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- 4.Ahn S, Hwang SH, Han J, Choi YL, Lee SH, Ahn JS, et al. Transformation to small cell lung cancer of pulmonary adenocarcinoma: Clinicopathologic analysis of six cases. J Pathol Transl Med. 2016;50:258–63. doi: 10.4132/jptm.2016.04.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240–7. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 8.Yun CH, Mengwasser KE, Toms AV, Woo MS, Greulich H, Wong KK, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008;105:2070–5. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zakowski MF, Ladanyi M, Kris MG. Memorial Sloan-Kettering Cancer Center Lung Cancer OncoGenome Group. EGFR mutations in small-cell lung cancers in patients who have never smoked. N Engl J Med. 2006;355:213–5. doi: 10.1056/NEJMc053610. [DOI] [PubMed] [Google Scholar]
- 10.Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: Molecular drivers and cells of origin. Lancet Oncol. 2015;16:e165–72. doi: 10.1016/S1470-2045(14)71180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang SY, Zhao J, Wang MZ, Huo Z, Zhang J, Zhong W, et al. Small-cell lung cancer transformation in patients with pulmonary adenocarcinoma: A case report and review of literature. Medicine (Baltimore) 2016;95:e2752. doi: 10.1097/MD.0000000000002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim WJ, Kim S, Choi H, Chang J, Shin HJ, Park CK, et al. Histological transformation from non-small cell to small cell lung carcinoma after treatment with epidermal growth factor receptor-tyrosine kinase inhibitor. Thorac Cancer. 2015;6:800–4. doi: 10.1111/1759-7714.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norkowski E, Ghigna MR, Lacroix L, Le Chevalier T, Fadel É, Dartevelle P, et al. Small-cell carcinoma in the setting of pulmonary adenocarcinoma: New insights in the era of molecular pathology. J Thorac Oncol. 2013;8:1265–71. doi: 10.1097/JTO.0b013e3182a407fa. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto S, Ikushima S, Ono R, Awano N, Kondo K, Furuhata Y, et al. Transformation to small-cell lung cancer as a mechanism of acquired resistance to crizotinib and alectinib. Jpn J Clin Oncol. 2016;46:170–3. doi: 10.1093/jjco/hyv173. [DOI] [PubMed] [Google Scholar]
- 15.Kogo M, Shimizu R, Uehara K, Takahashi Y, Kokubo M, Imai Y, et al. Transformation to large cell neuroendocrine carcinoma as acquired resistance mechanism of EGFR tyrosine kinase inhibitor. Lung Cancer. 2015;90:364–8. doi: 10.1016/j.lungcan.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Caumont C, Veillon R, Gros A, Laharanne E, Bégueret H, Merlio JP, et al. Neuroendocrine phenotype as an acquired resistance mechanism in ALK-rearranged lung adenocarcinoma. Lung Cancer. 2016;92:15–8. doi: 10.1016/j.lungcan.2015.12.001. [DOI] [PubMed] [Google Scholar]