Abstract
Leiomyosarcomas are rare neoplasms of the smooth muscles. Primary pulmonary leiomyosarcomas, which constitute approximately 0.2%–0.5% of all primary lung malignancies, are extremely rare and highly lethal. They may originate from the smooth muscle cells of the bronchial wall, the blood vessels, or the pulmonary interstitium, and their rare occurrence, localization, and nonspecific clinical symptoms mean that correct diagnosis and proper management are often delayed. Here, we report a rapidly growing primary pulmonary leiomyosarcoma, which invaded the right atrium, vena cava superior, mediastinum, right hilar area, and left pulmonary artery within 4 months. On histopathology, a transthoracic needle biopsy of the mass confirmed leiomyosarcoma, and delayed presentation meant that there was a local spread to the neighboring structures at the time of diagnosis.
KEY WORDS: Diagnosis, lung neoplasms, primary pulmonary leiomyosarcoma
INTRODUCTION
Primary leiomyosarcomas of the lung are extremely rare and may arise from the smooth muscles of the pulmonary interstitium, bronchi, and blood vessels.[1] Due to their low incidence and localization, these tumors are often undetected and frequently misdiagnosed as pulmonary embolism, primary lung neoplasms, or cardiac neoplasms.[2] Clinical investigation is commonly nondiagnostic, and a definitive diagnosis is made by pathological examination of a tumor specimen. Here, we present a case of primary pulmonary leiomyosarcoma originating from the pulmonary artery, using clinical, imaging, and pathological findings.
CASE REPORT
A 70-year-old nonsmoking male presented with a 4-month history of progressive dyspnea, right-sided chest pain, and weight loss. He was initially diagnosed with pericardial effusion, approximately 4 months before the current presentation, and pericardial drainage (2.5 L) was subsequently carried out. Following drainage and a month of ibuprofen, no pericardial effusion was observed on echocardiography. He stated that he had no history of fever or hemoptysis or any other extrapulmonary symptoms or diseases. He was hypertensive and had a history of coronary artery stent. General physical examination was unremarkable. Examination of the respiratory system revealed diminished breath sounds in the right hemithorax, and initial laboratory evaluations were all normal except D-dimer, which was 3640 ng/ml. When he was admitted to the intensive care unit with severe orthopnea, due to his previous massive pleural and pericardial effusion and to exclude pulmonary thromboembolism, a bedside cardiopulmonary ultrasound (US) examination was performed. This surprisingly revealed a large immobile right atrial mass that was obstructing the tricuspid valve orifice and partially obstructing both the superior and inferior vena cava orifices [Figure 1]. The differential diagnoses were thrombus, atrial myxoma, or local invasion of a lung tumor. A chest computed tomography (CT) angiography examination showed a soft tissue mass extending from the right paratracheal area to the subcarinal area. This mass was obstructing the upper and intermediate bronchus, not distinguishable from both pulmonary arteries and invading the right atrium [Figure 2]. To confirm and stage the malignancy, a fluorine-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) scan was carried out. This showed that a heterogeneously ill-defined soft tissue lesion of 16 cm was encasing, and causing narrowing of, the right main bronchus and invading pulmonary arteries and the right atrium, with increased FDG uptake (maximum standardized uptake value: 11.1) [Figure 2]. No other pathological FDG uptake was detected. Fiberoptic bronchoscopy (FOB) revealed compression of the right main bronchus. Since insufficient tissue was obtained by repeated FOB and endobronchial US, an interventional radiologist performed a CT-guided tru-cut biopsy. Microscopic examination revealed a well-circumscribed malignant tumor, characterized by spindle cells with hyperchromatic nuclei and areas of markedly increased mitotic activity. The malignant spindle cells tested positive for vimentin, desmin, and smooth muscle actin [Figure 2] but tested negative for epithelial membrane antigen, pancytokeratin, cytokeratin 7, cytokeratin 20, S-100 antigen, CD31, CD34, MyoD1, and TTF-1. The tumor had no epithelial differentiation. Overall morphological features favored a high-grade sarcoma with evidence of smooth muscle differentiation, making this tumor a leiomyosarcoma. Since a needle biopsy, rather than a surgical biopsy, had been performed, mitoses could not be counted and the tumor origin could not be identified. We retrieved the CT images taken 4 months previously, and the radiological appearance was suggestive of a malignant tumor arising from the right pulmonary artery [Figure 3]. Since the mass was rapidly growing and was not suitable for surgery, the patient was instructed to follow up with an oncologist.
Figure 1.
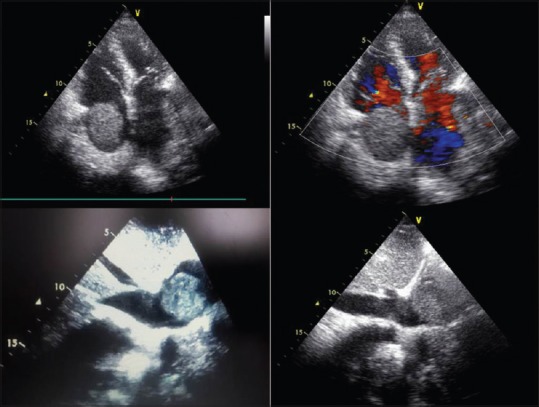
A transthoracic echocardiogram (apical 4-chamber view) showing a large echogenic immobile right atrial mass causing inferior vena cava obstruction
Figure 2.
I A Computerized tomography scan showing an ill-defined mass invading both pulmonary arteries and the right atrium, II fluorodeoxyglucose positron emission tomography/computed tomography scan showing a heterogeneously ill-defined mass lesion with increased fluorodeoxyglucose uptake. The central area of the mass is necrotic. III (A) Hematoxylin and eosin staining of the primary pulmonary leiomyosarcoma (B) positive for vimentin (C) positive for desmin and (D) positive for smooth muscle actin
Figure 3.

A computerized tomography image taken 4 months ago, showing a filling defect in the right main pulmonary artery
DISCUSSION
Primary pulmonary sarcomas are mesenchymal tumors that may originate from smooth muscle cells. They have been observed inside the bronchus (20%), in the lung parenchyma (70%) or in the pulmonary artery (10%),[3] and are considered rare, comprising only 0.5% of all lung malignancies. Leiomyosarcomas usually occur during the 6th decade of life or later, predominantly in men; however, pulmonary artery leiomyosarcomas usually occur a decade or so earlier, with a mean age at diagnosis of 50 years, and with equal frequency in men and women.[3]
Pulmonary artery leiomyosarcomas most frequently occur in the pulmonary trunk but can also occur in the right and left main pulmonary arteries, the pulmonary valve, and the right ventricular outflow tract. They predominantly grow intraluminally, with attachment to the wall in the direction of the blood flow.[4] Transmural spread of the tumor into adjacent lung parenchyma, bronchi, or lymph nodes occurs in approximately 50% of cases and may extend to the myocardium and mediastinum. Systemic metastases are usually found in approximately 20% of cases.[4] Our patient was diagnosed with advanced disease; the mass was obstructing the upper and intermediate bronchus, not distinguishable from both pulmonary arteries, and was invading the right atrium. It was a pulmonary artery tumor, according to previous CT images; when we retrieved the CT images that had been taken 4 months previously, we could estimate the origin as being the right main pulmonary artery. There were no distant metastases.
Many patients with pulmonary artery leiomyosarcoma present in a similar manner to those with other primary tumors of the lung (e.g., bronchogenic carcinoma), with symptoms including cough, dyspnea, hemoptysis, sputum, chest or back pain, and weight loss.[5] These symptoms are often misinterpreted as being related to pulmonary embolism, and a diagnosis of sarcoma is considered either not at all or too late.[6]
Pulmonary angiography may demonstrate abrupt interruption of the contrast as a pulmonary embolism in the early stage. However, CT may demonstrate vascular distension, extravascular invasion into adjacent structures, and inhomogeneous large necrotic masses in advanced disease.[4,6]
Increased uptake on FDG-PET in the area of the tumor can be helpful in differentiating a neoplasm from a pulmonary embolism, which does not show increased uptake.[7] The diagnosis can be confirmed by pathological and immunohistochemical examination. When actin, smooth muscle actin, and desmin immunohistochemical results are positive, it is presumed that the tumor originates from smooth muscle. When CD99 is negative, Ewing's sarcoma should not be considered. A negative endothelial membrane antigen result indicates that the tumor does not typically originate from epithelial tissue, and when S100 is negative, a tumor of the nervous tissue is not indicated.[8,9]
Without surgical intervention, the median survival time for patients with pulmonary artery leiomyosarcoma is approximately 1.5 months, and the survival rate is 6% at 5 years. Most patients die of decompensated right heart failure secondary to pulmonary outflow obstruction.[9]
The goals of treatment are to obtain local and systemic control of the sarcoma while preserving function and quality of life. If preoperative evaluation reveals no evidence of metastases, then treatment is surgical. Patients with large primary tumors may receive preoperative radiation treatment or chemotherapy or both.[9] Our patient was not suitable for surgery because of a giant mass with multiple invasions. In addition, he was not suitable for radiotherapy because of the size of his tumor and its proximity to his heart. He was instructed to follow up with an oncologist.
Point-of-care bedside cardiopulmonary US is a useful tool in the differential diagnosis of severe dyspnea in urgent pulmonary and cardiac conditions.[10] In this rare case, with delayed diagnosis, an examination of this type was very useful in revealing that the reason for the patient's severe dyspnea was a tumor that probably originated from the cardiac or vascular structures.
CONCLUSION
Pulmonary artery leiomyosarcoma is rare and grows rapidly. It is difficult to differentiate from other pulmonary tumors or benign condition. As in our case there can be a missed diagnosis at first visit. The widespread use of simple bedside US facilitates clinicians’ diagnosis and treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dixit R, Banerjee A, Panjabi M. Primary leiomyosarcoma of the lung. Lung India. 2007;24:153–5. [Google Scholar]
- 2.Adeli SH, Nemati B, Jandaghi M, Riahi MM, Hosseinzadeh F, Salarvand F. Pulmonary hypertension due to a pulmonary artery leiomyosarcoma: A case report. Case Rep Pulmonol. 2013;2013:160619. doi: 10.1155/2013/160619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sardenberg RA, Cangnaci Neto R, Cavalcanti F, Younes RN. High-grade primary pulmonary leiomyosarcoma. Einstein (Sao Paulo) 2011;9:523–6. doi: 10.1590/S1679-45082011RC1773. [DOI] [PubMed] [Google Scholar]
- 4.Kim JH, Gutierrez FR, Lee EY, Semenkovich J, Bae KT, Ylagan LR. Primary leiomyosarcoma of the pulmonary artery: A diagnostic dilemma. Clin Imaging. 2003;27:206–11. doi: 10.1016/s0899-7071(02)00568-5. [DOI] [PubMed] [Google Scholar]
- 5.Cordes BG, Collins BT, McDonald JW, Khosla A, Salimi Z. Fine needle aspiration biopsy of primary leiomyosarcoma arising from a pulmonary vein. Acta Cytol. 1999;43:523–6. [PubMed] [Google Scholar]
- 6.Hoffmeier A, Semik M, Fallenberg EM, Scheld HH. Leiomyosarcoma of the pulmonary artery – A diagnostic chameleon. Eur J Cardiothorac Surg. 2001;20:1049–51. doi: 10.1016/s1010-7940(01)00939-3. [DOI] [PubMed] [Google Scholar]
- 7.Kessler A, Son H. Pulmonary artery angiosarcoma on 18F-FDG PET/CT masquerading as pulmonary embolism. Clin Nucl Med. 2015;40:82–4. doi: 10.1097/RLU.0000000000000614. [DOI] [PubMed] [Google Scholar]
- 8.Arnold LM, 3rd, Burman SD, O-Yurvati AH. Diagnosis and management of primary pulmonary leiomyosarcoma. J Am Osteopath Assoc. 2010;110:244–6. [PubMed] [Google Scholar]
- 9.Shen W, Chen J, Wei S, Wang X, Li X, Zhou Q. Primary pulmonary leiomyosarcoma. J Chin Med Assoc. 2014;77:49–51. doi: 10.1016/j.jcma.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Bouhemad B, Zhang M, Lu Q, Rouby JJ. Clinical review: Bedside lung ultrasound in critical care practice. Crit Care. 2007;11:205. doi: 10.1186/cc5668. [DOI] [PMC free article] [PubMed] [Google Scholar]



